Relationships between the Intakes of Human Milk Components and Body Composition of Breastfed Infants: A Systematic Review
Abstract
:1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Eligibility Criteria and Selection of Articles
2.3. Data Extraction
2.4. Quality Assessment
3. Results
3.1. Synthesis
3.2. Participant Charcteristics and Stage of Lactation
3.3. Determination of 24 h Milk Intake
3.4. Methodology of Human Milk Sampling and Storage
3.5. Methodology of Human Milk Analysis
3.6. Methodology of Measuring Infant Body Composition and Growth Parameters
3.7. Statistical Analysis
3.8. Risk of Bias
3.9. Twenty-Four-Hour Intakes of Macronutrients and Infant Body Composition
3.9.1. Protein

3.9.2. Carbohydrates
3.9.3. Fat
3.10. Twenty-Four-Hour Intakes of Bioactive Molecules and Infant Body Composition
3.10.1. Metabolic Hormones
3.10.2. Immunomodulatory Proteins
3.10.3. Human Milk Oligosaccharides
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Author, Year, Country | Study Type, Time Postpartum, Sample Size | Sample Type, Collection, Analyses | 24 h Milk Intake and Body Composition Methods | Component Concentration | Component 24 h Intake | Relationship with Anthropometry or Body Composition | ß (SE) or r | p-Value |
|---|---|---|---|---|---|---|---|---|
| Olga et al., 2022 United Kingdom [34] | LS, Birth–12 m, n = 94 (complete measurements n = 47) EBF to 6 w + (n = 70) EBF to 3 m + (n = 60) EBF to 6 m + (n = 22) | SHM/WHM, post-feed sampling DUMAS method (total nitrogen for protein calculation), 1H-NMR (carbohydrates and triglycerides) | 4–6 w: 780 ± 160 mL/24 h (450–1260) (n = 70) EBF at 3 m, 4–6 w: 800 ± 160 mL/24 h (n = 60) 24 h MI: dose-to-the-mother deuterium-oxide (2H2O) turnover BC: anthropometry and skinfold thickness (triceps, subscapular, flank, quadriceps) | (n = 59) Carbohydrates, kcal/100 mL 6 w: 25.1 ± 1.5 | (n = 47) Carbohydrates, g/24 h 6 w: 50.8 ± 11.7 | No concentrations and BC analysis | NR | NR |
| Carbohydrate intake at 6 w | ||||||||
| Weight gain: ↑0–6 w; | 0.04 (0.01) a | <0.001 b | ||||||
| ↓3–12 m | –0.03 (0.01) | 0.025 b | ||||||
| BMI gain: ↑0–6 w | 0.05 (0.01) | 0.002 b | ||||||
| Skinfold gain: ↑0–6 w; | 0.06 (0.01) | <0.001 b | ||||||
| ↓3–12 m | –0.04 (0.01) | 0.009 | ||||||
| Carbohydrate intake and SDS 0–3 m | ||||||||
| ↑Weight SDS | 0.02 (0.01) | 0.002 b | ||||||
| ↑BMI SDS | 0.02 (0.01) | 0.008 b | ||||||
| Carbohydrate intake and SDS 3–12 m | ||||||||
| ↓Weight SDS | –0.02 (0.01) | 0.030 | ||||||
| ↓Length SDS | –0.02 (0.01) | 0.040 | ||||||
| Protein, kcal/100 mL 6 w: 4.6 ± 0.9 | Protein, g/24 h 6 w: 9.1 ± 2.1 | Protein intake at 6 w | ||||||
| Weight gain: ↑0–6 w | 0.22 (0.04) | <0.001 b | ||||||
| BMI gain: ↑0–6 w | 0.22 (0.08) | 0.010 | ||||||
| Skinfold gain: ↑0–6 w; | 0.28 (0.07) | <0.001 b | ||||||
| ↓3–12 m | –0.19 (0.07) | 0.006 b | ||||||
| Protein intake and SDS 0–3 m | ||||||||
| ↑Weight SDS | 0.05 (0.02) | 0.010 | ||||||
| ↑BMI SDS | 0.06 (0.03) | 0.040 | ||||||
| Protein intake and SDS 3–12 m | NS | |||||||
| Fat, kcal/100 mL 6 w: 35.3 ± 19.0 | Fat, g/24 h 6 w: 29.6 ± 17.4 | Fat intake at 6 w | ||||||
| Length gain: ↓0–6 w; | –0.02 (0.01) | 0.020 | ||||||
| ↑6 w–3 m; | 0.01 (0.01) | 0.010 | ||||||
| BMI gain: ↑0–6 w | 0.03 (0.01) | 0.020 | ||||||
| Fat intake and SDS 0–3 m | NS | |||||||
| Fat intake and SDS 3–12 m | NS | |||||||
| Gridneva et al., 2022 Australia [35] | LS, 2–12 m, n = 20, 2 m (n = 14) 5 m (n = 20) 9 m (n = 18) 12 m (n = 18) EBF to 5 m | SHM, pre-/post-feed, morning sampling d Bradford assay (casein, total, and whey protein), enzymatic spectrophotometry (Kuhn and Lowenstein, lactose), deproteinisation with trichloroacetic acid, dehydration by sulfuric acid (TCH) | 5 m: 819 ± 205 (498–1185) (n = 17) 9 m: 478 ± 154 (300–775) (n = 8) 12 m: 451 ± 216 (255–795) (n = 8) 24 h MI: pre-/post-feed test weighing infant Regional BC: US scans of anterior upper arm and thigh | Total protein, whey protein and casein in Gridneva et al., 2018 (a) [42] below TCH and lactose in Gridneva et al., 2019 [41] below | Total protein, whey protein and casein in Gridneva et al., 2018 (a) [42] below TCH and lactose in Gridneva et al., 2019 [41] below | All macronutrient intakes (overall) | NR | NS |
| Total protein concentration | ||||||||
| Mid-thigh lean area: ↓2, 5 m, ↑9 m, ↓12 m | NA e | 0.030 c | ||||||
| Whey protein concentration | ||||||||
| Mid-thigh lean area: ↓2, 5 m, ↑9 m, ↓12 m | NA e | 0.008 c | ||||||
| Casein concentration | ||||||||
| Mid-thigh fat area: ↑2 m; ↓5, 9, 12 m | NA e | 0.039 c | ||||||
| TCH concentration | NR | NS | ||||||
| Lactose concentration | ||||||||
| Mid-arm lean area: ↓2 m; ↑5, 9, ↓12 m | NA e | 0.040 c | ||||||
| Cheema et al., 2021 Australia [37] | CS 3 m, n = 57 EBF to 5 m | SHM, pre-/post-feed sampling Enzymatic- spectrophotometry (Kuhn and Lowenstein method, lactose, glucose) | 3 m: 793 ± 176 (512–1305) (n = 45) 24 h MI: pre-/post-feed test-weighing infant BC: anthropometry and BIS | (n = 57) Lactose, g/L 2 m: 86.56 ± 7.91 (69.91–106.09) | (n = 45) Lactose, g/24 h 3 m: 68.35 ± 16.63 (39.91–129.27) | Lactose concentration | NR | NS |
| Lactose intake | ||||||||
| ↑Weight | 0.017 (0.005) | 0.001 | ||||||
| ↑Length | 0.047 (0.017) | 0.010 | ||||||
| ↑FFM (BIS) | 0.011 (0.003) | 0.001 | ||||||
| ↑FFMI (BIS) | 0.026 (0.009) | 0.009 | ||||||
| ↑FM (BIS) | 0.005 (0.002) | 0.011 | ||||||
| ↑FMI (BIS) | 0.014 (0.006) | 0.028 | ||||||
| ↑WAZ | 0.017 (0.006) | 0.007 | ||||||
| Glucose, g/L 2 m: 0.26 ± 0.09 (0.09–0.47) | Glucose, g/24 h 3 m: 0.20 ± 0.09 (0.05–0.49) | Glucose concentration | NR | NS | ||||
| Glucose intake | ||||||||
| ↑HC | 4.304 (1.885) | 0.028 | ||||||
| Gridneva et al., 2021 Australia [38] | LS, 2–12 m, n = 20, 2 m (n = 15) 5 m (n = 20) 9 m (n = 19) 12 m (n = 18) EBF to 5 m | SHM, pre-/post-feed, morning sampling d Bradford assay (casein, total and whey protein), enzymatic spectrophotometry (Kuhn and Lowenstein method, lactose), SHM deproteinised with trichloroacetic acid, dehydrated by sulfuric acid (TCH) | 5 m: 819 ± 205 (498–1185) (n = 17) 9 m: 478 ± 154 (300–775) (n = 8) 12 m: 451 ± 216 (255–795) (n = 8) 24 h MI: pre-/post-feed test weighing infant Regional BC: US scans of upper abdomen (preperitoneal and subcutaneous fat thickness) | Casein, whey, and total protein in Gridneva et al., 2018 (a) [42] below | Casein, whey, and total protein in Gridneva et al., 2018 (a) [42] below | Total protein concentration | ||
| Visceral/subcutaneous-abdominal depths ratio: ↑2, 5, 9 m; ↓12 m | NA e | 0.006 c | ||||||
| Preperitoneal fat area: ↓2 m; ↑5 m; ↓9, 12 m | NA e | 0.013 c | ||||||
| Total protein intake (overall) | ||||||||
| ↑Subcutaneous-abdominal fat area | 0.053 (0.021) | 0.013 b | ||||||
| Whey protein concentration and intake | NR | NS | ||||||
| Casein concentration | NR | NS | ||||||
| Casein intake (overall) | ||||||||
| ↑Subcutaneous-abdominal depth | 1.34 (0.555) | 0.021 | ||||||
| Lactose and TCH in Gridneva et al., 2019 [41] below | Lactose and TCH in Gridneva et al., 2019 [41] below | ↑Subcutaneous-abdominal fat area | 0.235 (0.103) | 0.027 | ||||
| Lactose concentration | NR | NS | ||||||
| Lactose intake (overall) | ||||||||
| ↑Subcutaneous-abdominal depth | 0.054 (0.023) | 0.021 | ||||||
| ↑Subcutaneous-abdominal fat area | 0.012 (0.005) | 0.013 b | ||||||
| TCH concentration | NR | NS | ||||||
| TCH intake (overall) | ||||||||
| ↑Subcutaneous-abdominal depth | 0.047 (0.018) | 0.005 | ||||||
| ↑Subcutaneous-abdominal fat area | 0.009 (0.003) | 0.004 b | ||||||
| George et al., 2021 Australia [39] | LS, Birth–6 m (n = 11) EBF | WHM, pre-feed, morning and evening sampling Liquid chromatography-mass spectrometry) (targeted lipidomics analysis of 166 human milk fat globule membrane (MFGM) lipids) | 3 m: 727 ± 164 (473–894) (n = 11) 24 h MI: pre-/post-feed test weighing infant BC: anthropometry | See paper (Supplementary Table S1) for concentrations [39] | 10 lipid species with the highest mean intakes (μmol/24 h) at 3 m: PI 36:2: 17.15 ± 10.35 SM 40:1: 13.64 ± 6.51 SM 36:1: 11.05 ± 4.95 PC 36:2: 10.27 ± 7.03 SM 42:2: 9.71 ± 4.28 SM 34:1: 8.24 ± 3.92 PI 38:4: 7.21 ± 3.71 PI 36:1: 7.17 ± 4.15 PI 38:3: 7.10 ± 0.26 PC 34:1: 6.35 ± 4.26 See paper for the rest of intakes (Supplementary Table S2) and full correlation results (Supplementary Table S3) [39] | Lipid concentrations | NA | NA |
| Lipids intake—strong positive correlations (r > 0.70) with infant anthropometry at 1, 3, and 6 m found for 99/166 MFGM lipid species | ||||||||
| For 10 lipid species with the highest mean intakes: | ||||||||
| PI 36:2: ↑Weight at 3 m | 0.76 | NA | ||||||
| SM 40:1: ↑Weight at 3 m | 0.68 | NA | ||||||
| SM 36:1: ↑Weight at 3 m | 0.59 | NA | ||||||
| PC 36:2: ↑Weight at 3 m | 0.74 | NA | ||||||
| SM 42:2: ↑Weight at 3 m | 0.71 | NA | ||||||
| SM 34:1: ↑Weight at 3 m | 0.65 | NA | ||||||
| PI 38:4: ↑Weight at 3 m | 0.75 | NA | ||||||
| PI 36:1: ↑Weight at 3 m | 0.72 | NA | ||||||
| PI 38:3: ↑Weight at 3 m | 0.65 | NA | ||||||
| PC 34:1: ↑Weight at 3 m | 0.72 | NA | ||||||
| 1 m strong lipid intake correlations: | r > 0.80 | NA | ||||||
| ↑HC: LPC 15:0, LPC 17:1, PC 31:0, PC-O 32:0 | ||||||||
| ↑HCZ: SM 31:1 | ||||||||
| ↑HC and HCZ: Cer d18:1/25:0, Cer d19:1/22:0, Cer d19:1/24:0, LPC 14:0, PC 35:2, PE 32:1, PE 35:2, PE 40:5 | ||||||||
| 3 m strong lipid intake correlations: | r > 0.80 | NA | ||||||
| ↑HC: Cer d19:1/24:0 | ||||||||
| 6 m strong lipid intake correlations: | r > 0.80 | NA | ||||||
| ↑WLZ: PI 38:5 | ||||||||
| ↑HC: LPC 17:0, PC 31:0, PC 33:0, PC-O 32:0, PC-O 34:0, PE 35:1 | ||||||||
| ↑HCZ: Cer d19:1/24:1, SM 31:1 | ||||||||
| ↑HC and HCZ: Cer d18:1/23:0, Cer d18:2/23:0, Cer d19:1/22:0, Cer d19:1/24:0, LPC 14:0, LPC 15:0, LPC 16:1, LPC 17:1, PC 33:1, PC-O 34:1, SM 43:1 | ||||||||
| Gridneva et al., 2019 Australia [41] | LS, 2–12 m, n = 20, 2 m (n = 15) 5 m (n = 20) 9 m (n = 19) 12 m (n = 18) EBF to 5 m | SHM, pre-/post-feed, morning sampling d Enzymatic- spectrophotometry (Kuhn and Lowenstein method, lactose), mid-infrared spectroscopy and UV spectrophotometry (TCH) | 5 m: 819 ± 205 (498–1185) (n = 17) 9 m: 502 ± 158 (300–775) (n = 8) 12 m: 446 ± 200 (255–795) (n = 8) 24 h MI: re-/post-feed test weighing infant BC: anthropometry, BIS, and US skinfolds BC equations | TCH, g/L 2 m: 86.7 ± 9.2 (67.1–97.5) (n = 15) 5 m: 80.7 ± 7.9 (69.3–94.1) (n = 20) 9 m: 87.8 ± 11.1 (60.9–105.6) (n = 19) 12 m: 88.4 ± 21.2 (56.9–126.9) (n = 14) | TCH, g/24 h 2–5 m: 63.2 ± 15.0 (42.9–97.2) (n = 17) 9 m: 44.8 ± 15.2 (21.2–69.6) (n = 8) 12 m: 40.7 ± 29.8 (22.2–100.9) (n = 8) | TCH concentration | ||
| ↑Length | 0.047 (0.013) | <0.001 b | ||||||
| ↑Weight | 0.013 (0.004) | 0.003 b | ||||||
| BMI ↑2 m; ↓5, 9, 12 m | NA e | 0.044 c | ||||||
| ↑FFM (BIS) | 0.020 (0.004) | <0.001 b | ||||||
| ↑FFM (US2FS) | 0.009 (0.004) | 0.032 | ||||||
| ↑FFM (US4FS) | 0.010 (0.004) | 0.020 b | ||||||
| ↑FFMI (BIS) | 0.022 (0.007) | 0.002 b | ||||||
| FM (BIS) ↑2 m; ↓5, 9, 12 m | NA e | 0.016 b,c | ||||||
| %FM (BIS) ↑2 m; ↓5, 9, 12 m | NA e | 0.001 b,c | ||||||
| FMI (BIS) ↑2 m; ↓5, 9, 12 m | NA e | 0.001 b,c | ||||||
| FM/FFM (BIS) ↑2 m; ↓5, 9, 12 m | NA e | 0.003 b,c | ||||||
| TCH intake (overall) | ||||||||
| BMI ↑2–5 m, ↓9, 12 m | NA e | 0.019 b,c | ||||||
| ↓FFMI (US4SF) | –0.034 (0.007) | <0.001 b | ||||||
| ↑FM (US2FS) | 0.011 (0.004) | 0.006 b | ||||||
| ↑FM (US4FS) | 0.014 (0.004) | <0.001 b | ||||||
| ↑%FM (US2FS) | 0.010 (0.036) | 0.005 b | ||||||
| %FM (US4FS) ↑2–5 m; ↓9 m; ↑12 m | NA e | 0.016 b,c | ||||||
| ↑FMI (US2FS) | 0.025 (0.009) | 0.003 b | ||||||
| FMI (US4FS) ↑2–5 m; ↓9 m; ↑12 m | NA e | 0.038 b,c | ||||||
| ↑FM/FFM (US2FS) | 0.002 (0.001) | 0.004 b | ||||||
| FM/FFM (US4FS) ↑2–5 m; ↓9 m; ↑12 m | NA e | 0.007 b,c | ||||||
| TCH intake between 2 and 5 m | ||||||||
| ↑ΔLength 2–12 m | 0.074 (0.026) | 0.023 | ||||||
| ↑Weight 2–5 m | 0.018 (0.008) | 0.039 | ||||||
| ↑ΔBMI 2–5 m | 0.040 (0.016) | 0.037 | ||||||
| ↑ΔFFM (BIS) 2–5 m | 0.009 (0.004) | 0.045 | ||||||
| ↑ΔFFM (US4FS) 2–9 m | 0.034 (0.013) | 0.038 | ||||||
| ↑ΔFFM (US4FS) 2–12 m | 0.045 (0.016) | 0.024 | ||||||
| ↑ΔFM (US2FS) 2–5 m | 0.024 (0.008) | 0.019 | ||||||
| ↑ΔFMI (US2FS) 2–5 m | 0.050 (0.018) | 0.022 | ||||||
| ↓ΔFMI (US4FS) 9–12 m | –0.015 (0.006) | 0.031 | ||||||
| ↓Δ%FM (US4FS) 2–12 m | –0.371 (0.139) | 0.032 | ||||||
| ↓ΔFM/FFM (BIS) 2–12 m | –0.005 (0.002) | 0.040 | ||||||
| ↓ΔFM/FFM (US4FS) 2–12 m | –0.007 (0.003) | 0.034 | ||||||
| TCH intake at 9 m | ||||||||
| ↓ΔBMI 5–12 m | –0.081 (0.029) | 0.037 | ||||||
| ↑ΔFFM (US2FS) 2–9 m | 0.107 (0.021) | 0.037 | ||||||
| ↓ΔFM (BIS) 5–12 m | –0.033 (0.010) | 0.018 | ||||||
| ↓ΔFMI (BIS) 5–12 m | –0.077 (0.028) | 0.040 | ||||||
| TCH intake at 12 m | ||||||||
| ↓ΔFFMI (US2FS) 5–12 m | –0.038 (0.014) | 0.038 | ||||||
| ↓ΔFFMI (US2FS) 9–12 m | –0.021 (0.007) | 0.033 | ||||||
| ↓ΔFFMI (US4FS) 5–12 m | 0.031 (0.009) | 0.029 | ||||||
| Lactose, g/L 2 m: 64.5 ± 4.1 (59.1–77.9) (n = 15) 5 m: 64.3 ± 5.9 (53.5–70.6) (n = 20) 9 m: 65.3 ± 5.3 (57.6–79.0) (n = 19) 12 m: 66.9 ± 4.0 (60.1–79.3) (n = 14) | Lactose, g/24 h 2–5 m: 51.2 ± 14.5 (32.6–83.6) (n = 17) 9 m: 34.0 ± 11.0 (19.6–51.3) (n = 8) 12 m: 28.7 ± 12.1 (18.0–51.4) (n = 8) | Lactose concentration | ||||||
| ↑Length | 0.065 (0.034) | 0.047 | ||||||
| Lactose intake (overall) | ||||||||
| BMI: ↑2–5, 9 m; ↓12 m | NA e | 0.011 b,c | ||||||
| FFMI (US4SF): ↑2–5, 9 m; ↓12 m | NA e | 0.015 b,c | ||||||
| ↑FM (US2SF) | 0.014 (0.006) | 0.008 b | ||||||
| ↑FM (US4SF) | 0.015 (0.005) | 0.004 b | ||||||
| ↑%FM (US2SF | 0.128 (0.050) | 0.019 b | ||||||
| ↑%FM (US4 FF) | 0.156 (0.048) | 0.001 b | ||||||
| ↑FMI (BIS) | 0.027 (0.013) | 0.045 | ||||||
| ↑FMI (US2SF) | 0.032 (0.012) | 0.005 b | ||||||
| ↑FMI (US4SF) | 0.038 (0.012) | <0.001 b | ||||||
| ↑FM/FFM (US2SF) | 0.003 (0.001) | 0.012 b | ||||||
| ↑FM/FFM (US4SF) | 0.003 (0.001) | <0.001 b | ||||||
| Lactose intake between 2 and 5 m | ||||||||
| ↑ΔLength 2–12 m | 0.100 (0.032) | 0.016 | ||||||
| ↑ΔBMI 2–5 m | 0.049 (0.019) | 0.035 | ||||||
| ↑ΔFFM (BIS) 2–12 m | 0.035 (0.012) | 0.025 | ||||||
| ↑ΔFFM (US2FS) 2–9 m | 0.024 (0.009) | 0.029 | ||||||
| ↑ΔFFM (US4FS) 2–9 m | 0.043 (0.012) | 0.009 | ||||||
| ↑ΔFFMI (US4FS) 2–12 m | 0.088 (0.035) | 0.044 | ||||||
| ↑ΔFM (US2FS) 2–5 m | 0.029 (0.010) | 0.014 | ||||||
| ↓ΔFMI (BIS) 5–12 m | –0.045 (0.017) | 0.023 | ||||||
| ↑ΔFMI (US2FS) 2–5 m | 0.057 (0.023) | 0.036 | ||||||
| ↓ΔFM/FFM (BIS) 2–12 m | –0.006 (0.002) | 0.032 | ||||||
| ↓ΔFM/FFM (BIS) 5–12 m | –0.004 (0.002) | 0.034 | ||||||
| ↑ΔFM/FFM (US2FS) 2–5 m | 0.007 (0.003) | 0.036 | ||||||
| Lactose intake at 9 m | ||||||||
| ↓ΔFM (BIS) 5–12 m | –0.037 (0.014) | 0.045 | ||||||
| Lactose intake at 12 m | ||||||||
| ↓ΔFFMI (US2FS) 5–12 m | –0.097 (0.018) | 0.003 | ||||||
| ↓ΔFFMI (US4FS) 5–12 m | –0.080 (0.007) | 0.0004 b | ||||||
| ↑ΔFM (US2FS) 2–12 m | 0.036 (0.010) | 0.032 | ||||||
| Gridneva et al., 2018 (a) Australia [42] | LS, 2–12 m, n = 20, 2 m (n = 13) 5 m (n = 20) 9 m (n = 18) 12 m (n = 13) EBF to 5 m | SHM, pre-/post-feed, morning sampling d Kunz and Lonnerdal (casein and whey protein fraction separation) Bradford assay (protein) | 5 m: 818.8 ± 204.9 (498–1185) (n = 17) 9 m: 478 ± 154 (300–775) (n = 8) 12 m: 451.1 ± 215.7 (255–795) (n = 8) 24 h MI: pre-/post-feed test weighing infant BC: anthropometry, BIS, and US skinfolds BC equations | Total protein, g/L 2 m: 11.03 ± 1.40 (7.60–12.32) (n = 15) 5 m: 11.90 ± 4.31 (7.93–24.16) (n = 20) 9 m: 9.69 ± 1.12 (7.25–14.96) (n = 19) 12 m: 10.72 ± 2.84 (5.89–16.80) (n = 15) | Total protein, g/24 h 2–5 m: 9.19 ± 3.82 (4.51–20.34) (n = 17) 9 m: 5.24 ± 1.84 (2.18–7.48) (n = 8) 12 m: 4.18 ± 2.11 (1.93–7.23) (n = 8) | Total protein concentration | ||
| ↓FFMI (BIS) | –0.034 (0.022) | 0.032 | ||||||
| Total protein intake (overall) | NR | NS | ||||||
| Total protein intake between 2 and 5 m | ||||||||
| ↑ΔHC 9–12 m | 0.09 (0.04) | 0.046 | ||||||
| ↑ΔLength 2–9 m | 0.40 (0.15) | 0.025 | ||||||
| ↑ΔLength 2–12 m | 0.57 (0.17) | 0.011 | ||||||
| ↑ΔBMI 2–5 m | 0.29 (0.09) | 0.013 | ||||||
| ↑ΔFFM (BIS) 2–12 m | 0.18 (0.07) | 0.041 | ||||||
| ↑ΔFFM (BIS) 9–12 m | 0.08 (0.03) | 0.041 | ||||||
| ↑ΔFFM (US4FS) 2–9 m | 0.19 (0.07) | 0.037 | ||||||
| ↑ΔFFMI (BIS) 2–5 m | 0.14 (0.06) | 0.042 | ||||||
| ↑ΔFFMI (US4FS) 2–5 m | 0.27 (0.09) | 0.023 | ||||||
| ↑ΔFM (US2FS) 2–5 m | 0.13 (0.06) | 0.049 | ||||||
| ↑ΔFMI (BIS) 2–5 m | 0.17 (0.07) | 0.044 | ||||||
| ↓ΔFMI (US2FS) 9–12 m | –0.07 (0.03) | 0.029 | ||||||
| Total protein intake at 9 m | ||||||||
| ↓ΔHC 9–12 m | –0.30 (0.09) | 0.021 | ||||||
| ↓ΔFFMI (BIS) 2–5 m | –0.36 (0.02) | 0.004 | ||||||
| Total protein intake at 12 m | ||||||||
| ↓ΔBMI 9–12 m | –0.30 (0.09) | 0.021 | ||||||
| ↓ΔFFMI (US2FS) 5–12 m | –0.48 (0.18) | 0.045 | ||||||
| ↓ΔFFMI (US4FS) 5–12 m | –0.50 (0.09) | 0.006 | ||||||
| ↑ΔFM (US2FS) 2–12 m | 0.21 (0.05) | 0.024 | ||||||
| ↑ΔFM (US2FS) 5–12 m | 0.14 (0.06) | 0.043 | ||||||
| ↑Δ%FM (US2SF) 5–12 m | 1.60 (0.59) | 0.036 | ||||||
| ↑ΔFMI (US2FS) 2–12 m | 0.38 (0.08) | 0.045 | ||||||
| Whey protein, g/L 2 m: 6.44 ± 1.62 (4.12–9.08) (n = 15) 5 m: 5.43 ± 0.90 (3.82–7.38) (n = 20) 9 m: 5.43 ± 0.93 (3.94–9.40) (n = 19) 12 m: 7.61 ± 1.85 (4.49–9.76) (n = 15) | Whey protein, g/24 h 2–5 m: 4.23 ± 1.14 (2.65–6.76) (n = 17) 9 m: 3.0 2 ±1.11 (1.70–4.64) (n = 8) 12 m: 2.78 ± 1.34 (1.15–4.40) (n = 8) | Whey protein concentration | NR | NS | ||||
| Whey protein intake (overall) | ||||||||
| ↓FFMI (BIS) | –0.319 (0.121) | 0.004 | ||||||
| ↑FM (US2SF) | 0.148 (0.062) | 0.024 | ||||||
| ↑%FM (US2SF) | 1.330 (0.554) | 0.033 | ||||||
| ↑%FM (U42 SF) | 1.190 (0.565) | 0.038 | ||||||
| ↑FMI (US2SF) | 0.336 (0.135) | 0.016 | ||||||
| ↑FMI (US4SF) | 0.309 (0.143) | 0.034 | ||||||
| Whey protein intake between 2 and 5 m | ||||||||
| ↑ΔLength 2–12 m | 1.01 (0.35) | 0.024 | ||||||
| ↑ΔBMI 2–5 m | 0.58 (0.19) | 0.017 | ||||||
| ↑ΔFFMI (US4FS) 2–5 m | 0.65 (0.21) | 0.016 | ||||||
| ↑ΔFM (US2FS) 2–5 m | 0.29 (0.12) | 0.032 | ||||||
| ↑ΔFMI (US2FS) 2–5 m | 0.61 (0.25) | 0.042 | ||||||
| Whey protein intake at 9 m | NR | NS | ||||||
| Whey protein intake at 12 m | ||||||||
| ↓ΔFFMI (US4FS) 5–12 m | –0.62 (0.22) | 0.049 | ||||||
| ↑ΔFM (US2SF) 2–12 m | 0.26 (0.06) | 0.025 | ||||||
| Casein, g/L 2 m: 1.24 ± 0.24 (0.69–1.57) (n = 15) 5 m: 1.51 ± 0.44 (0.78–3.45) (n = 20) 9 m: 1.11 ± 0.38 (0.49–2.00) (n = 19) 12 m: 1.07 ± 0.35 (0.65–1.87) (n = 15) | Casein, g/24 h 2–5 m: 1.45 ± 0.82 (0.56–3.63) (n = 17) 9 m: 0.60 ± 0.23 (0.17–0.95) (n = 8) 12 m: 0.54 ± 0.34 (0.24–1.19) (n = 8) | Casein–whey ratio | ||||||
| ↑%FM (US4 FF) | 9.09 (4.62) | 0.046 | ||||||
| Casein concentration | NR | NS | ||||||
| Casein intake (overall) | ||||||||
| HC: ↓2–5 m; ↑9 m; ↓12 m | NA e | 0.046 c | ||||||
| BMI: ↑2–5 m; ↓9, 12 m | NA e | 0.047 c | ||||||
| ↓FFM (US4SF) | –0.456 (0.144) | 0.003 b | ||||||
| ↓FFMI (US4SF) ↓2–5, 9, 12 m | NA e | 0.022 b,c | ||||||
| ↑FM (US4SF) | 0.390 (0.100) | <0.001 b | ||||||
| ↑%FM (US4SF) | 4.220 (0.932) | <0.001 b | ||||||
| ↑FMI (US2SF) | 0.517 (0.248) | 0.048 | ||||||
| ↑FMI (US4SF) | 0.789 (0.240) | 0.001 b | ||||||
| Casein intake between 2 and 5 m | ||||||||
| ↓ΔHC 2–5 m | –1.17 (0.39) | 0.016 | ||||||
| ↓ΔFM (US4FS) 2–9 m | –0.47 (0.17) | 0.031 | ||||||
| ↓ΔFM (US4FS) 2–12 m | –0.49 (0.20) | 0.040 | ||||||
| ↓Δ%FM (BIS) 2–12 m | –4.48 (1.61) | 0.027 | ||||||
| ↓Δ%FM (US2FS) 2–9 m | –3.62 (1.50) | 0.042 | ||||||
| ↓Δ%FM (US4FS) 2–9 m | –6.67 (1.36) | 0.002 | ||||||
| ↓Δ%FM (US4FS) 2–12 m | –6.27 (2.42) | 0.036 | ||||||
| ↓ΔFMI (US4FS) 2–9 m | –1.29 (0.32) | 0.005 | ||||||
| ↓ΔFMI (US4FS) 2–12 m | –1.44 (0.50) | 0.028 | ||||||
| Casein intake at 9 m | ||||||||
| ↓ΔHC 5–12 m | –1.78 (0.62) | 0.036 | ||||||
| ↓ΔHC 9–12 m | –2.40 (0.74) | 0.023 | ||||||
| Casein intake at 12 m | ||||||||
| ↑ΔHC 2–12 m | 4.01 (0.60) | 0.022 | ||||||
| ↓ΔBMI between 9 and 12 m | –2.09 (0.61) | 0.019 | ||||||
| ↓ΔFFM (US4FS) 2–12 m | –3.77 (0.73) | 0.014 | ||||||
| ↓ΔFFMI (US2FS) 9–12 m | –2.13 (0.45) | 0.005 | ||||||
| ↓ΔFFMI (US4FS) 5–12 m | –2.84 (0.87) | 0.031 | ||||||
| ↓ΔFFMI (US4FS) 9–12 m | –2.40 (0.78) | 0.028 | ||||||
| ↑Δ%FM (US4FS) 2–12 m | 33.56 (8.59) | 0.030 | ||||||
| Kon et al., 2014 Russian Federation [44] | CS, 1, 2 or 3 m, n = 103, 1 m (n = 32) 2 m (n = 34) 3 m (n = 33) LWG (<500 g/m, n = 18) NWG (500–1000 g/m, n = 40) HWG (>1000 g/m, n = 45) EBF | SHM, midstream morning sampling Van de Kamer method (fat), Kjeldahl and Lowry technique (protein) | Combined mean (calculated): 1 m: 736 (n = 32) 2 m: 826 (n = 34; no LWG) 3 m: 891 (n = 33) LWG 1 m: 555 ± 32 (n = 9) 2 m: NA 3 m: 753 ± 53 (n = 5) NWG 1 m: 849 ± 96 (n = 11) 2 m: 752 ± 93 (n = 14) 3 m: 896 ± 42 (n = 15) HWG 1 m: 768 ± 41 (n = 12) 2 m: 878 ± 37 (n = 20) 3 m: 937 ± 70 (n = 13) 24 h MI: pre-/post-feed test weighing infant BC: weight gain | Fat, g/100 g LWG 1 m: 4.08 ± 0.45 (n = 9) 2 m: NA (n = 4, excluded) 3 m: 2.51 ± 0.26 (n = 5) NWG 1 m: 4.39 ± 0.84 (n = 11) 2 m: 4.43 ± 0.52 (n = 14) 3 m: 3.76 ± 0.47 (n = 15) HWG 1 m: 4.51 ± 0.57 (n = 12) 2 m: 3.65 ± 0.36 (n = 20) 3 m: 3.80 ± 0.25 (n = 13) Protein g/100 g LWG 1 m: 1.77 ± 0.22 (n = 9) 2 m: NA (n = 4, excluded) 3 m: 1.50 ± 0.09 (n = 5) NWG 1 m: 1.70 ± 0.10 (n = 11) 2 m: 1.68 ± 0.14 (n = 14) 3 m: 1.32 ± 0.08 (n = 15) HWG 1 m: 1.66 ± 0.16 (n = 12) 2 m: 1.77 ± 0.12 (n = 20) 3 m: 1.50 ± 0.20 (n = 13) | Fat, g/24 h LWG 1 m: 24.6 ± 4.4 (n = 9) 2 m: NA (n = 4, excluded) 3 m: 19.1 ± 2.8 (n = 5) NWG 1 m: 34.4 ± 3.6 (n = 11) 2 m: 34.7 ± 6.5 (n = 14) 3 m: 35.3 ± 4.4 (n = 15) HWG 1 m: 38.6 ± 6.6 (n = 12) 2 m: 31.3 ± 2.9 (n = 20) 3 m: 36.2 ± 2.5 (n = 13) Protein, g/24 h LWG 1 m: 10.2 ± 1.4 (n = 9) 2 m: NA (n = 4, excluded) 3 m: 11.3 ± 1.2 (n = 5) NWG 1 m: 15.2 ± 2.4 (n = 11) 2 m: 12.1 ± 1.8 (n = 14) 3 m: 11.7 ± 1.1 (n = 15) HWG 1 m: 12.2 ± 1.1 (n = 12) 2 m: 15.2 ± 1.2 (n = 20) 3 m: 14.1 ± 2.7 (n = 13) | Fat concentration | ||
| No significant difference between LWG, NWG, and HWG groups at any time point | Concentrations by group and time point are on the left | NS | ||||||
| Fat intake | ||||||||
| No significant difference between LWG, NWG, and HWG groups at 2 and 3 m | Intakes by group and time point are on the left | NS | ||||||
| ↑Fat intake at 3 m in the HWG group compared with the LWG group | <0.05 | |||||||
| Protein concentration | ||||||||
| No significant difference between LWG, NWG, and HWG groups at any time point | Concentrations by group and time point are on the left | NS | ||||||
| Protein intake | ||||||||
| No significant difference between LWG, NWG, and HWG groups at any time point | Intakes by group and time point are on the left | NS | ||||||
| Mitoulas et al., 2002 Australia [29] | LS, 1–12 m, n = 17, 1 m (n = 17) 2 m (n = 17) 4 m (n = 17) 6 m (n = 15) 9 m (n = 6) 12 m (n = 5) EBF to 4 m | WHM (fat), SHM (lactose and protein), pre-/post-feed sampling Colorimetric spectrophotometry Stern and Shapiro (fat), enzymatic-spectrophotometry (Kuhn and Lowenstein method, lactose), Bradford assay (protein) | 24 h MI averaged by the breast: 1 m: 416 ± 24 (n = 34) 2 m: 408 ± 23 (n = 34) 4 m: 421 ± 20 (n = 34) 6 m: 413 ± 25 (n = 30) 9 m: 354 ± 47 (n = 12) 12 m: 252 ± 51 (n = 10) 1–12 m: 399 ± 11 (n = 154) 24 h MI: pre-/post-feed test weighing the mother, corrected for insensible water loss BC: anthropometry | Fat, g/L 1 m: 39.9 ± 1.4 (n = 17) 2 m: 35.2 ± 1.4 (n = 17) 4 m: 35.4 ± 1.4 (n = 16) 6 m: 37.3 ± 1.4 (n = 14) 9 m: 40.7 ± 1.7 (n = 6) 12 m: 40.9 ± 3.3 (n = 5) Lactose, g/L 1 m: 59.7 ± 0.8 (n = 9) 2 m: 60.4 ± 1.1 (n = 9) 4 m: 62.6 ± 1.3 (n = 8) 6 m: 62.5 ± 1.7 (n = 8) 9 m: 62.8 ± 1.5 (n = 6) 12 m: 61.4 ± 2.9 (n = 5) Protein, g/L 1 m: 10.5 ± 0.4 (n = 9) 2 m: 9.6 ± 0.37 (n = 9) 4 m: 9.33 ± 0.42 (n = 9) 6 m: 8.03 ± 0.38 (n = 8) 9 m: 8.34 ± 0.45 (n = 6) 12 m: 8.34 ± 0.57 (n = 5) | Intake per breast Fat, g/24 h 1 m: 16.4 ± 1.2 (n = 17) 2 m: 14.2 ± 0.95 (n = 17) 4 m: 14.3 ± 0.6 (n = 16) 6 m: 15.7 ± 0.9 (n = 14) 9 m: 14.3 ± 1.9 (n = 6) 12 m: 10.4 ± 2.0 (n = 5) Lactose, g/24 h 1 m: 22.9 ± 2.0 (n = 9) 2 m: 25.4 ± 2.2 (n = 9) 4 m: 27.0 ± 2.2 (n = 8) 6 m: 25.1 ± 2.1 (n = 8) 9 m: 22.4 ± 3.2 (n = 6) 12 m: 17.4 ± 3.5 (n = 5) Protein, g/24 h 1 m: 4.0 ± 0.4 (n = 9) 2 m: 4.05 ± 0.41 (n = 9) 4 m: 3.83 ± 0.23 (n = 9) 6 m: 3.13 ± 0.2 (n = 8) 9 m: 2.94 ± 0.39 (n = 6) 12 m: 2.14 ± 0.41 (n = 5) | Fat concentration and intake | ||
| 1–6 m Weight | NR | NS | ||||||
| 1–6 m Weight gain | NR | NS | ||||||
| Lactose concentration and intake | ||||||||
| 1–6 m Weight | NR | NS | ||||||
| 1–6 m Weight gain | NR | NS | ||||||
| Protein concentration and intake 1–6 m Weight 1–6 m Weight gain | NR NR | NS NS | ||||||
| Heinig et al., 1993 United States of America [45] | LS, 3–18 m, n = 60, 3 m (n = 71) 6 m (n = 56) 9 m (n = 46) 12 m (n = 40) 15 m (n = NR) 18 m (n = NR) EBF to 4 m | WHM, 24 h alternate breast expression Kjeldahl and Lowry methods (protein), modified Folch extraction (lipid), Dahlquist lactase assay (lactose) | 24 h MI, uncorrected: 3 m: 768 ± 127 6 m: 732 ± 165 9 m: 611 ± 211 12 m: 413 ± 246 Corrected for insensible water loss: 3 m: 812 ± 133 6 m: 769 ± 171 9 m: 646 ± 217 12 m: 448 ± 251 24 h MI: pre-/post-feed test-weighing infant and 4 d weighed food record BC: total body water (isotope-dilution method) and anthropometry | Protein, g/24 h 3 m: 8.7 6 m: 8.1 9 m: 8.4 12 m: 10.0 Fat concentration, stable and averaged throughout 3–12 m: 37 g/L Lactose concentration, stable and averaged throughout 3–12 m: 74 g/L | Protein, g/24 h 3 m: 6.8 ± 1.1 (n = 71) 6 m: 5.9 ± 1.3 (n = 56) 9 m: 5.0 ± 1.6 (n = 46) 12 m: 3.8 ± 1.9 (n = 40) Protein intake from human milk % 3 m: 100% 6 m: 78 ± 20% 9 m: 46 ± 25% 12 m: 22 ± 17% Lactose and fat intakes NR, only used for calculation of energy intake | No concentrations and BC analysis | NR | NR |
| Total protein intake | ||||||||
| ↑Weight gain 3–6 m | 0.30 | <0.01 | ||||||
| ↑Lean body mass gain 3–6 m | 0.34 | <0.01 | ||||||
| ↑Weight gain 6–9 m | 0.34 | <0.01 | ||||||
| ↑Lean body mass gain 6–9 m | 0.34 | <0.05 | ||||||
| ↑Lean body mass gain 12–15 m | 0.33 | <0.05 | ||||||
| Human Milk Macronutrient | Time Postpartum | Sample Size | Macronutrient Intake, g/24 h | Comments | Author, Year |
|---|---|---|---|---|---|
| Total protein | 1 m | n = 9 n = 11 n = 12 | 10.2 ± 1.4 a 15.2 ± 2.4 12.2 ± 1.1 | LWG NWG HWG | Kon et al., 2014 [44] |
| n = 9 | 4.0 ± 0.4 | Intake per breast | Mitoulas et al., 2002 [29] | ||
| 6 w | n = 47 | 9.1 ± 2.1 | Olga et al., 2022 [34] | ||
| 2 m | n = 4 n = 14 n = 20 | NA 12.1 ± 1.8 15.2 ± 1.2 | LWG NWG HWG | Kon et al., 2014 [44] | |
| n = 9 | 4.05 ± 0.41 | Intake per breast | Mitoulas et al., 2002 [29] | ||
| 3 m | n = 5 n = 15 n = 13 | 11.3 ± 1.2 11.7 ± 1.1 14.1 ± 2.7 | LWG NWG HWG | Kon et al., 2014 [44] | |
| n = 71 | 6.8 ± 1.1 | Protein intake from human milk at 3 m: 100% | Heinig et al., 1993 [45] | ||
| 2–5 m | n = 17 | 9.19 ± 3.82 (4.51–20.34) | Gridneva et al., 2018 (a) [42] | ||
| 4 m | n = 9 | 3.83 ± 0.23 | Intake per breast | Mitoulas et al., 2002 [29] | |
| 6 m | n = 8 | 3.13 ± 0.2 | Intake per breast | Mitoulas et al., 2002 [29] | |
| n = 56 | 5.9 ± 1.3 | Protein intake from human milk at 6 m: 78 ± 20% | Heinig et al., 1993 [45] | ||
| 9 m | n = 8 | 5.24 ± 1.84 (2.18–7.48) | Gridneva et al., 2018 (a) [42] | ||
| n = 6 | 2.94 ± 0.39 | Intake per breast | Mitoulas et al., 2002 [29] | ||
| n = 46 | 5.0 ± 1.6 | Protein intake from human milk at 9 m: 46 ± 25% | Heinig et al., 1993 [45] | ||
| 12 m | n = 8 | 4.18 ± 2.11 (1.93–7.23) | Gridneva et al., 2018 (a) [42] | ||
| n = 5 | 2.14 ± 0.41 | Intake per breast | Mitoulas et al., 2002 [29] | ||
| n = 40 | 3.8 ± 1.9 | Protein intake from human milk at 12 m: 22 ± 17% | Heinig et al., 1993 [45] | ||
| Casein | 2–5 m | n = 17 | 1.45 ± 0.82 (0.56–3.63) | Gridneva et al., 2018 (a) [42] | |
| 9 m | n = 8 | 0.60 ± 0.23 (0.17–0.95) | Gridneva et al., 2018 (a) [42] | ||
| 12 m | n = 8 | 0.54 ± 0.34 (0.24–1.19) | Gridneva et al., 2018 (a) [42] | ||
| Whey protein | 2–5 m | n = 17 | 4.23 ± 1.14 (2.65–6.76) | Gridneva et al., 2018 (a) [42] | |
| 9 m | n = 8 | 3.0 2 ± 1.11 (1.70–4.64) | Gridneva et al., 2018 (a) [42] | ||
| 12 m | n = 8 | 2.78 ± 1.34 (1.15–4.40) | Gridneva et al., 2018 (a) [42] | ||
| Total carbohydrates | 2–5 m | n = 17 | 63.2 ± 15.0 (42.9–97.2) | Gridneva et al., 2019 [41] | |
| 9 m | n = 8 | 44.8 ± 15.2 (21.2–69.6) | Gridneva et al., 2019 [41] | ||
| 12 m | n = 8 | 40.7 ± 29.8 (22.2–100.9) | Gridneva et al., 2019 [41] | ||
| Lactose | 1 m | n = 9 | 22.9 ± 2.0 | Intake per breast | Mitoulas et al., 2002 [29] |
| 6 w | n = 47 | 50.8 ± 11.7 | Olga et al., 2022 [34] | ||
| 2 m | n = 9 | 25.4 ± 2.2 | Intake per breast | Mitoulas et al., 2002 [29] | |
| 3 m | n = 45 | 68.35 ± 16.63 (39.91–129.27) | Cheema et al., 2021 [37] | ||
| 2–5 m | n = 17 | 51.2 ± 14.5 (32.6–83.6) | Gridneva et al., 2019 [41] | ||
| 4 m | n = 8 | 27.0 ± 2.2 | Intake per breast | Mitoulas et al., 2002 [29] | |
| 6 m | n = 8 | 25.1 ± 2.1 | Intake per breast | Mitoulas et al., 2002 [29] | |
| 9 m | n = 8 | 34.0 ± 11.0 (19.6–51.3) | Gridneva et al., 2019 [41] | ||
| n = 6 | 22.4 ± 3.2 | Intake per breast | Mitoulas et al., 2002 [29] | ||
| 12 m | n = 8 | 28.7 ± 12.1 (18.0–51.4) | Gridneva et al., 2019 [41] | ||
| n = 5 | 17.4 ± 3.5 | Intake per breast | Mitoulas et al., 2002 [29] | ||
| Glucose | 3 m | n = 45 | 0.20 ± 0.09 (0.05–0.49) | Cheema et al., 2021 [37] | |
| Fat | 1 m | n = 9 n = 11 n = 12 | 24.6 ± 4.4 34.4 ± 3.6 38.6 ± 6.6 | LWG NWG HWG | Kon et al., 2014 [44] |
| n = 17 | 16.4 ± 1.2 | Intake per breast | Mitoulas et al., 2002 [29] | ||
| 6 w | n = 47 | 29.6 ± 17.4 | Olga et al., 2022 [34] | ||
| 2 m | n = 4 n = 14 n = 20 | NA 34.7 ± 6.5 31.3 ± 2.9 | LWG NWG HWG | Kon et al., 2014 [44] | |
| n = 17 | 14.2 ± 0.95 | Intake per breast | Mitoulas et al., 2002 [29] | ||
| 3 m | n = 5 n = 15 n = 13 | 19.1 ± 2.8 35.3 ± 4.4 36.2 ± 2.5 | LWG NWG HWG | Kon et al., 2014 [44] | |
| 4 m | n = 16 | 14.3 ± 0.6 | Intake per breast | Mitoulas et al., 2002 [29] | |
| 6 m | n = 14 | 15.7 ± 0.9 | Intake per breast | Mitoulas et al., 2002 [29] | |
| 9 m | n = 6 | 14.3 ± 1.9 | Intake per breast | Mitoulas et al., 2002 [29] | |
| 12 m | n = 5 | 10.4 ± 2.0 | Intake per breast | Mitoulas et al., 2002 [29] | |
| MFGM lipids | 1 m 3 m | n = 11 n = 11 | See paper for intakes of 166 lipids at 1 m and 3 m [39] | George et al., 2021 [39] |
| Author, Year, Country | Study Type, Time Postpartum, Sample Size | Sample Type, Collection, Analyses | 24 h Milk Intake and Body Composition Methods | Component Concentration | Component 24 h Intake | Association with Anthropometry or Body Composition | ß (SE) or r | p-Value |
|---|---|---|---|---|---|---|---|---|
| Gridneva et al., 2022 Australia [35] | LS, 2–12 m, n = 20, 2 m (n = 14) 5 m (n = 20) 9 m (n = 18) 12 m (n = 18) EBF to 5 m | SHM (WHM for adiponectin and leptin), pre-/post-feed, morning sampling d Enzymatic spectrophotometry (lactose); deproteinisation with trichloroacetic acid, dehydration by sulfuric acid (TCH) for calculation of total HMO concentration, Selsted and Martinez method (lysozyme), ELISA (adiponectin, whole milk leptin, lactoferrin, sIgA) | 5 m: 819 ± 205 (498–1185) (n = 17) 9 m: 478 ± 154 (300–775) (n = 8) 12 m: 451 ± 216 (255–795) (n = 8) 24 h MI: pre-/post-feed test weighing infant Regional BC: US scans of anterior upper arm and thigh | Adiponectin and whole milk leptin in Gridneva et al., 2018 (b) [43] below Total HMOs in Gridneva et al., 2019 [41] below Lactoferrin, lysozyme and sIgA in Gridneva et al., 2020 [40] below | Adiponectin and whole milk leptin in Gridneva et al., 2018 (b) [43] below Total HMOs in Gridneva et al., 2019 [41] below Lactoferrin, lysozyme and sIgA in Gridneva et al., 2020 [40] below | Adiponectin concentration | ||
| ↓Mid-thigh fat area | NA e | 0.028 c | ||||||
| Adiponectin intake (overall) | NR | NS | ||||||
| Whole milk leptin concentration | NR | NS | ||||||
| Whole milk leptin intake (overall) | ||||||||
| Mid-arm lean area: ↑2 m; ↓5 m; ↑9 m; ↓12 m | NA e | 0.046 c | ||||||
| ↓Mid-thigh lean area | −0.06 (0.03) | 0.025 | ||||||
| Total HMO concentration | NR | NS | ||||||
| Total HMO intake (overall) | ||||||||
| Mid-arm fat area: ↓2 m; ↑5, 9, 12 m | NA e | 0.004 b,c | ||||||
| Lactoferrin concentration | ||||||||
| Mid-arm lean area: ↓2, 5 m; ↑9, 12 m | NA e | 0.029 c | ||||||
| Mid-thigh lean area: ↓2, 5 m; ↑9 m; ↓12 m | NA e | 0.040 c | ||||||
| Lactoferrin intake (overall) | ||||||||
| sIgA concentration | NR | NS | ||||||
| Mid-arm lean area: ↓2 m; ↑5, 9, 12 m | NA e | 0.007 c | ||||||
| Mid-arm fat area: ↑2, 5 m; ↓9, 12 m | NA e | 0.041 c | ||||||
| sIgA intake (overall) | NR | NS | ||||||
| Lysozyme concentration | NR | NS | ||||||
| Lysozyme intake (overall) | ||||||||
| Mid-arm fat area: ↑2, 5, 9 m; ↓12 m | NA e | 0.001 b,c | ||||||
| Cheema et al., 2022 Australia [36] | CS, 3 m, n = 60 EBF to 5 m Maternal secretor status: secretor (n = 49), non-secretor (n = 11) | WHM, pre-/post-feed sampling HPLC (19 individual HMOs: 2′-fucosyllactose (2′FL), 3′-sialyllactose (3′SL), 3-fucosyllactose (3 FL), 6′-sialyllactose (6′SL), difucosyllactose (DFLac), difucosyllacto-n-hexaose (DFLNH), difucosyllacto-n-tetrose (DFLNT), disialyllacto-n-hexaose (DSLNH), disialyllacto-n-tetraose (DSLNT), fucodisialyllacto-n-hexaose (FDSLNH), fucosyllacto-n-hexaose (FLNH), lacto-n-fucopentaose (LNFP I), lacto-n-fucopentaose II (LNFP II), lacto-n-fucopentaose III (LNFP III), lacto-n-hexaose (LNH), lacto-n-neotetraose (LNnT), lacto-n-tetrose (LNT), sialyl-lacto-n-tetraose b (LSTb), sialyl-lacto-n-tetraose c (LSTc) | 3 m: 785 ± 172 (512–1305) (n = 47) 24 h MI: pre-/post-feed test-weighing infant BC: anthropometry and BIS | HMOs, μg/mL Individual HMO concentrations in entire cohort and by maternal secretor status in Cheema et al., 2022 [36] | HMOs, μg/24 h Individual HMO intakes in entire cohort and by maternal secretor status in Cheema et al., 2022 [36] | For results by maternal secretor status, see Cheema et al., 2022 [36]; in entire cohort: | ||
| 2′FL concentration | NR | NS | ||||||
| 2′FL intake | ||||||||
| ↑Weight | 0.00 (0.00) | 0.03 | ||||||
| ↑FM | 0.02 (0.01) | 0.04 | ||||||
| 3 FL concentration | NR | NS | ||||||
| 3 FL intake | ||||||||
| ↑Weight | 0.09 (0.03) | 0.01 | ||||||
| ↑Length | 0.20 (0.08) | 0.01 | ||||||
| ↑WAZ | 0.10 (0.04) | 0.01 | ||||||
| ↑LAZ | 0.08 (0.04) | 0.04 | ||||||
| ↑Log FFM | 0.01 (00.0) | 0.01 | ||||||
| ↑Log FFMI | 0.01 (00.0) | 0.04 | ||||||
| Log (DFLac) concentration | NR | NS | ||||||
| Log (DFLac) intake | ||||||||
| ↑Weight | 0.33 (0.14) | 0.02 | ||||||
| ↑BMI | 0.51 (0.23) | 0.03 | ||||||
| ↑BMIAZ | 0.28 (0.13) | 0.04 | ||||||
| ↑Log FFM | 0.05 (0.02) | 0.02 | ||||||
| ↑Log FFMI | 0.04 (0.01) | 0.02 | ||||||
| Log (DFLNH) concentration | ||||||||
| ↑ Weight | 0.24 (0.11) | 0.03 | ||||||
| ↑Length | 1.1 (0.36) | <0.001 | ||||||
| ↑LAZ | 0.44 (0.17) | 0.01 | ||||||
| ↑Log FFM | 0.04 (0.02) | 0.02 | ||||||
| Log (DFLNH) intake | ||||||||
| ↑Weight | 0.28 (0.11) | 0.01 | ||||||
| ↑Length | 1.22 (0.35) | <0.001 | ||||||
| ↑WAZ | 0.28 (0.13) | 0.03 | ||||||
| ↑LAZ | 0.48 (0.17) | 0.01 | ||||||
| ↑Log FFM | 0.04 (0.02) | 0.01 | ||||||
| DFLNT concentration | NR | NS | ||||||
| DFLNT intake | ||||||||
| ↑BMI | 0.12 (0.05) | 0.04 | ||||||
| ↑ BMIAZ | 0.07 (0.03) | 0.03 | ||||||
| ↑Log FFMI | 0.01 (0.00) | 0.03 | ||||||
| FLNH concentration | ||||||||
| ↓Weight | –0.03 (0.01) | 0.04 | ||||||
| FLNH intake | NR | NS | ||||||
| Log (LNFP III) concentration | ||||||||
| ↓%FM | –1.6 (0.64) | 0.02 | ||||||
| ↓FM/FFM | –0.03 (0.01) | 0.02 | ||||||
| Log (LNFP III) intake | NR | NS | ||||||
| Log (LNnT) concentration | ||||||||
| ↓Length | –1.11 (0.5) | 0.03 | ||||||
| ↓LAZ | –0.51 (0.23) | 0.03 | ||||||
| Log (LNnT) intake | NR | NS | ||||||
| LSTb concentration | NR | NS | ||||||
| LSTb intake | ||||||||
| ↑BMI | 0.83 (0.39) | 0.04 | ||||||
| Cheema et al., 2021 Australia [37] | CS, 3 m, n = 57 EBF to 5 m | WHM, pre-/post-feed sampling ELISA (insulin) | 3 m: 793 ± 176 (512–1305) (n = 45) 24 h MI: pre-/post-feed test-weighing infant BC: anthropometry and BIS | Insulin, ng/mL 2 m: 1.48 ± 1.14 (0.46–5.90) (n = 57) | Insulin, ng/24 h 3 m: 1.16 ± 0.78 (0.30–4.17) (n = 45) | Insulin concentration | NR | NS |
| Insulin intake | NR | NS | ||||||
| Gridneva et al., 2021 Australia [38] | LS, 2–12 m, n = 20, 2 m (n = 15) 5 m (n = 20) 9 m (n = 19) 12 m (n = 18) EBF to 5 m | SHM (WHM for adiponectin and leptin), pre-/post-feed, morning sampling d Enzymatic spectrophotometry (lactose); deproteinisation with trichloroacetic acid, dehydration by sulfuric acid (TCH) for calculation of total HMO concentration, Selsted and Martinez method (lysozyme), ELISA (adiponectin, whole milk leptin, lactoferrin, sIgA) | 5 m: 819 ± 205 (498–1185) (n = 17) 9 m: 478 ± 154 (300–775) (n = 8) 12 m: 451 ± 216 (255–795) (n = 8) 24 h MI: pre-/post-feed test-weighing infant Regional BC: US scans of upper abdomen (preperitoneal and subcutaneous fat thickness) | Adiponectin and whole milk leptin in Gridneva et al., 2018 (b) [43] below Total HMOs in Gridneva et al., 2019 [41] below Lactoferrin, lysozyme, and sIgA in Gridneva et al., 2020 [40] below | Adiponectin and whole milk leptin in Gridneva et al., 2018 (b) [43] below Total HMOs in Gridneva et al., 2019 [41] below Lactoferrin, lysozyme, and sIgA in Gridneva et al., 2020 [40] below | Adiponectin concentration | ||
| Subcutaneous-abdominal depth: ↓2 m; ↑5 m; ↓9, 12 m | NA e | 0.041 c | ||||||
| Adiponectin intake (overall) | ||||||||
| Subcutaneous-abdominal depth: ↓2 m; ↑5 m; ↓9, 12 m | NA e | 0.027 c | ||||||
| Whole milk leptin concentration | NR | NS | ||||||
| Whole milk leptin intake (overall) | NR | NS | ||||||
| Total HMO concentration | NR | NS | ||||||
| Total HMO intake (overall) | NR | NS | ||||||
| Lactoferrin concentration | ||||||||
| Visceral depth: ↓2 m; ↑5, 9 m; ↓12 m | NA e | 0.003 b,c | ||||||
| Lactoferrin intake (overall) | ||||||||
| Preperitoneal/subcutaneous-abdominal fat areas ratio: ↓2 m; ↑5 m; ↓9 m; ↑12 m | NA e | 0.028 c | ||||||
| Lysozyme concentration | ||||||||
| ↓Visceral depth | –32.5 (14.1) | 0.019 | ||||||
| Lysozyme intake (overall) | NR | NS | ||||||
| sIgA concentration | ||||||||
| Preperitoneal fat area: ↓2 m; ↑5 m; ↓9, 12 m | NA e | 0.049 c | ||||||
| sIgA intake (overall) | ||||||||
| Subcutaneous-abdominal depth: ↓2 m; ↑5 m; ↓9, 12 m | NA e | 0.029 c | ||||||
| ↑Subcutaneous-abdominal fat area | 0.742 (0.379) | 0.042 | ||||||
| Gridneva et al., 2020 Australia [40] | LS, 2–12 m, n = 20, 2 m (n = 14) 5 m (n = 20) 9 m (n = 18) 12 m (n = 18) EBF to 5 m | SHM, pre-/post-feed, morning sampling d Selsted and Martinez method (lysozyme), ELISA (lactoferrin and sIgA) | 5 m: 819 ± 205 (498–1185) (n = 17) 9 m: 502 ± 158 (300–775) (n = 8) 12 m: 446 ± 200 (255–795) (n = 8) 24 h MI: pre-/post-feed test-weighing infant BC: anthropometry, BIS, and US skinfolds BC equations | Lactoferrin, g/L 2 m: 0.44 ± 0.22 (0.03–1.04) (n = 14) 5 m: 0.41 ± 0.17 (0.07–0.75) (n = 20) 9 m: 0.59 ± 0.25 (0.29–1.16) (n = 19) 12 m: 0.63 ± 0.20 (0.30–1.02) (n = 15) | Lactoferrin, g/24 h 2–5 m: 0.29 ± 0.12 (0.06–0.51) (n = 17) 9 m: 0.31 ± 0.21 (0.13–0.74) (n = 8) 12 m: 0.25 ± 0.10 (0.10–0.36) (n = 8) | Lactoferrin concentration | NR | NS |
| Lactoferrin intake (overall) | ||||||||
| ↓FFMI (US4SF) | –3.130 (1.040) | 0.002 b | ||||||
| Lactoferrin intake between 2 and 5 m | ||||||||
| ↓ΔWeight 9–12 m | –0. 871 (0.316) | 0.015 | ||||||
| ↓ΔFFM (US2FS) 9–12 m | –1.052 (0.465) | 0.041 | ||||||
| ↑ΔFFMI (US4FS) 2–5 m | 3.832 (1.546) | 0.042 | ||||||
| Lactoferrin intake at 9 m | ||||||||
| ↑ΔWeight 2–19 m | 2.154 (0.562) | 0.031 | ||||||
| Lactoferrin intake at 12 m | ||||||||
| ↑ΔFM (BIS) 2–12 m | 3.529 (0.587) | 0.027 | ||||||
| ↑ΔFMI (BIS) 2–12 m | 8.065 (0.652) | 0.007 | ||||||
| ↑Δ%FM (BIS) 2–12 m | 41.532 (9.350) | 0.047 | ||||||
| Lysozyme, g/L 2 m: 0.11 ± 0.11 (0.13–0.46) (n = 14) 5 m: 0.1 1 ± 0.05 (0.08–0.29) (n = 20) 9 m: 0.14 ± 0.02 (0.10–0.16) (n = 19) 12 m: 0.26 ± 0.21 (0.12–0.65) (n = 15) | Lysozyme, g/24 h 2–5 m: 0.09 ± 0.07 (0.06–0.34) (n = 17) 9 m: 0.06 ± 0.02 (0.04–0.10) (n = 8) 12 m: 0.08 ± 0.05 (0.05–0.17) (n = 8) | Lysozyme concentration | ||||||
| FMI (BIS): ↓2 m; ↑5, 9 m; ↓12 m | NA e | 0.026 c | ||||||
| FM/FFM (US4SF): ↓2 m; ↑5, 9 m; ↓12 m | NA e | 0.045 c | ||||||
| Lysozyme intake (overall) | ||||||||
| ↑FM (US2SF) | 1.350 (1.140) | 0.037 | ||||||
| ↑FM (US4SF) | 2.990 (1.250) | 0.004 b | ||||||
| ↑%FM (US4SF) | 24.90 (11.20) | 0.030 | ||||||
| ↑FM/FFM (BIS) | 8.510 (3.450) | 0.018 | ||||||
| ↑FMI (US4SF) | 8.010 (2.80) | 0.004 b | ||||||
| ↑FM/FFM (BIS) | 0.546 (0.261) | 0.043 | ||||||
| ↑ FM/FFM (US4SF) | 0.457 (0.203) | 0.028 | ||||||
| Lysozyme intake between 2 and 5 m | ||||||||
| ↑ΔWeight 2–5 m | 18.226 (6.038) | 0.017 | ||||||
| ↑ΔWeight 2–9 m | 25.390 (10.229) | 0.038 | ||||||
| ↑ΔBMI 2–5 m | 43.300 (9.247) | 0.002 | ||||||
| ↑ΔFFM (US4SF) 2–9 m | 20.817 (7.992) | 0.035 | ||||||
| ↑ΔFFM (US4SF) 2–12 m | 25.039 (10.134) | 0.043 | ||||||
| ↑ΔFFMI (US4SF) 2–5 m | 33.548 (8.580) | 0.006 | ||||||
| ↑ΔFM (BIS) 2–5 m | 12.699 (3.623) | 0.010 | ||||||
| ↑ΔFMI (BIS) 2–5 m | 28.902 (7.779) | 0.008 | ||||||
| ↑ΔFMI (US2SF) 2–5 m | 29.835 (11.250) | 0.033 | ||||||
| Lysozyme intake at 9 m | NR | NS | ||||||
| Lysozyme intake at 12 m | ||||||||
| ↓ΔFFM (US2SF) 5–12 m | –9.658 (2.578) | 0.013 | ||||||
| ↓ΔFFMI (US2FS) 5–12 m | –44.270 (3.902) | 0.0003 b | ||||||
| ↓ΔFM/FFM (BIS) | –2.948 (1.053) | 0.049 | ||||||
| sIgA, g/L 2 m: 0.4 6 ± 0.21 (0.08–0.93) (n = 14) 5 m: 0.50 ± 0.18 (0.15–0.81) (n = 20) 9 m: 0.62 ± 0.21 (0.23–1.08) (n = 19) 12 m: 0.71 ± 0.20 (0.34–1.02) (n = 15) | sIgA, g/24 h 2–5 m: 0.38 ± 0.18 (0.15–0.80) (n = 17) 9 m: 0.28 ± 0.12 (0.10–0.48) (n = 8) 12 m: 0.25 ± 0.09 (0.14–0.38) (n = 8) | sIgA concentration | ||||||
| ↓FM (US4SF) | –0.606 (0.298) | 0.045 | ||||||
| sIgA intake (overall) | ||||||||
| ↓ FFM (US2SF) | –1.590 (0.602) | 0.012 | ||||||
| ↓ FFM (US4SF) | –1.640 (0.703) | 0.034 | ||||||
| FFMI (BIS): ↑2–5 m; ↓9, 12 m | NA e | 0.019 c | ||||||
| ↓FFMI (US4SF) | –3.520 (1.090) | 0.008 | ||||||
| ↑%FM (US2SF) | 9.390 (4.150) | 0.046 | ||||||
| ↑FM/FFM (US2SF) | 0.206 (0.081) | 0.021 | ||||||
| sIgA intake between 2 and 5 m | ||||||||
| ↑ΔBMI 2–5 m | 3.771 (1.286) | 0.019 | ||||||
| ↑ΔFFMI (BIS) 2–5 m | 1.859 (0.732) | 0.035 | ||||||
| ↑ΔFM (US2SF) 2–5 m | 2.147 (0.617) | 0.007 | ||||||
| ↑Δ%FM (US2SF) 2–5 m | 22.978 (8.156) | 0.020 | ||||||
| ↑ΔFMI (US2SF) 2–5 m | 4.522 (1.461) | 0.015 | ||||||
| ↑ΔFM/FFM (US2SF) 2–5 m | 0.503 (0.167) | 0.015 | ||||||
| sIgA intake at 9 m | ||||||||
| ↑ΔHC 5–9 m | 2.644 (0.796) | 0.021 | ||||||
| sIgA intake at 12 m | ||||||||
| ↑ΔFM (BIS) 2–12 m | 4.239 (0.530) | 0.015 | ||||||
| ↑ΔFM (US4SF) 2–12 m | 3.962 (1.146) | 0.041 | ||||||
| ↑Δ%FM (BIS) 2–12 m | 51.094 (5.864) | 0.013 | ||||||
| ↑ΔFMI (BIS) 2–12 m | 9.589 (0.699) | 0.005 | ||||||
| ↑ΔFMI (US2SF) 2–12 m | 9.002 (1. 957) | 0.038 | ||||||
| ↑ΔFM/FFM (BIS) 2–12 m | 0.850 (0.124) | 0.021 | ||||||
| Gridneva et al., 2019 Australia [41] | LS, 2–12 m, n = 20, 2 m (n = 15) 5 m (n = 20) 9 m (n = 19) 12 m (n = 18) EBF up to 5 m | SHM pre-/post-feed, morning sampling d Enzymatic spectrophotometry (lactose); deproteinisation with trichloroacetic acid, dehydration by sulfuric acid (TCH) for calculation of total HMO concentration | 5 m: 819 ± 205 (498–1185) (n = 17) 9 m: 502 ± 158 (300–775) (n = 8) 12 m: 446 ± 200 (255–795) (n = 8) 24 h MI: re-/post-feed test-weighing infant BC: anthropometry, BIS, and US skinfolds BC equations | Total HMO, g/L 2 m: 22.3 ± 10.7 (0–35.8) (n = 15) 5 m: 16.4 ± 9.9 (2.3–29.9) (n = 20) 9 m: 22.5 ± 9.2 (0–36.9) (n = 19) 12 m: 21.4 ± 22.3 (3.0–62.2) (n = 14) | Total HMO, g/24 h 2–5 m: 12.0 ± 6.0 (2.0–21.6) (n = 17) 9 m: 10.8 ± 5.4 (0–15.7) (n = 8) 12 m: 12.0 ± 18.5 (1.5–49.5) (n = 8) | Total HMO concentration | ||
| ↑Length | 0.031 (0.014) | 0.036 | ||||||
| ↑Weight | 0.009 (0.004) | 0.038 | ||||||
| BMI: ↑2 m; ↓5, 9, 12 m | NA e | 0.027 c | ||||||
| ↑FFM (BIS) | 0.015 (0.004) | < 0.001 b | ||||||
| ↑FFMI (BIS) | 0.018 (0.007) | 0.008 b | ||||||
| FM (BIS): ↑2 m; ↓5, 9, 12 m | NA e | 0.039 c | ||||||
| %FM (BIS): ↑2 m; ↓5, 9, 12 m | NA e | 0.005 b,c | ||||||
| FMI (BIS): ↑2 m; ↓5, 9, 12 m | NA e | 0.003 b,c | ||||||
| FM/FFM (BIS): ↑2 m; ↓5, 9, 12 m | NA e | 0.006 b,c | ||||||
| Total HMO intake (overall) | ||||||||
| ↑FM (US4SF) | 0.020 (0.008) | 0.010 | ||||||
| ↑%FM (US4 FF) | 0.168 (0.074) | 0.025 | ||||||
| ↓FMI (BIS) | –0.024 (0.017) | 0.049 | ||||||
| ↑FMI (US4SF) | 0.040 (0.018) | 0.034 | ||||||
| ↓FM/FFM (BIS) | –0.002 (0.001) | 0.024 | ||||||
| ↑FM/FFM (US4SF) | 0.003 (0.001) | 0.027 | ||||||
| Total HMO intake between 2 and 5 m | ||||||||
| ↑ΔHC 5–12 m | 0.047 (0.018) | 0.023 | ||||||
| Total HMO intake at 9 m | ||||||||
| ↓ΔBMI 2–12 m | –0.401 (0.082) | 0.040 | ||||||
| ↓ΔFFMI (BIS) 5–12 m | –0.110 (0.043) | 0.049 | ||||||
| ↓ΔFFMI (US4SF) 9–12 m | –0.169 (0.058) | 0.032 | ||||||
| ↓ΔFMI (BIS) 5–12 m | –0.294 (0.097) | 0.029 | ||||||
| Total HMO intake at 12 m | ||||||||
| ↓Δ%FM (BIS) 5–12 m | –0.184 (0.070) | 0.047 | ||||||
| ↓ΔFMI (BIS) 5–12 m | –0.052 (0.017) | 0.032 | ||||||
| ↓ΔFM/FFM (BIS) 5–12 m | –0.004 (0.001) | 0.021 | ||||||
| Gridneva et al., 2018 (b) Australia [43] | LS, 2–12 m, n = 20, 2 m (n = 13) 5 m (n = 20) 9 m (n = 18) 12 m (n = 13) EBF to 5 m | WHM (adiponectin), SHM and WHM (leptin), pre-/post-feed, morning sampling d ELISA (adiponectin, whole and skim milk leptin) | 5 m: 819 ± 205 (498–1185) (n = 17) 9 m: 502 ± 158 (300–775) (n = 8) 12 m: 446 ± 200 (255–795) (n = 8) 24 h MI: pre-/post-feed test-weighing infant BC: anthropometry, BIS, and US skinfolds BC equations | Adiponectin, ng/mL 2 m: 11.14 ± 5.79 (6.61–21.56) (n = 15) 5 m: 8.42 ± 1.69 (6.18–22.58) (n = 20) 9 m: 8.44 ± 1.33 (6.41–12.86) (n = 18) 12 m: 11.22 ± 4.22 (5.66–19.38) (n = 15) | Adiponectin, ng/24 h 2–5 m: 7976 ± 4480 (3771–22439) (n = 17) 9 m: 4446 ± 1645 (2142–6673) (n = 8) 12 m: 3922 ± 1431 (2511–6352) (n = 8) | Adiponectin concentration | ||
| ↓FFM (US4SF) | –0.032 (0.015) | 0.025 | ||||||
| Adiponectin intake (overall) | ||||||||
| HC: ↓2–5 m; ↑9 m; ↓12 m | NA e | 0.026 c | ||||||
| BMI: ↑2–5 m; ↓9, 12 m | NA e | 0.016 c | ||||||
| ↓FFM (US4SF) | –0.0001 (0.00002) | 0.005 b | ||||||
| ↓FFMI (US4SF) | –0.0001 (0.00004) | 0.009 b | ||||||
| ↑FM (US4SF) | 0.004 (0.001) | <0.001 b | ||||||
| ↑%FM (US4SF) | 0.0007 (0.0002) | <0.001 b | ||||||
| ↑FMI (US2SF) | 0.0001 (0.00004) | 0.039 | ||||||
| ↑FMI (US4SF) | 0.0001 (0.00004) | <0.001 b | ||||||
| Adiponectin intake between 2 and 5 m | ||||||||
| ↑ΔLength 5–9 m | 0.0002 (0.0001) | 0.010 | ||||||
| ↑ΔFFM (US4SF) 2–9 m | 0.0001 (0.00002) | 0.036 | ||||||
| ↑ΔFFM (US4SF) 2–12 m | 0.0001 (0.00003) | 0.043 | ||||||
| ↓ΔFFM (US4SF) 5–9 m | –0.0001 (0.00002) | 0.011 | ||||||
| ↑ΔFFM (US4SF) 5–12 m | 0.0001 (0.00003) | 0.018 | ||||||
| ↑ΔFFMI (BIS) 2–12 m | 0.0001 (0.00004) | 0.029 | ||||||
| ↑ΔFFMI (US2SF) 2–12 m | 0.0001 (0.00004) | 0.026 | ||||||
| ↑ΔFFMI (US4SF) 2–12 m | 0.0002 (0.00004) | 0.009 | ||||||
| ↓ΔFM (US4SF) 5–12 m | –0.0001 (0.00002) | 0.049 | ||||||
| ↓Δ%FM (US4SF) 2–9 m | –0.001 (0.0002) | 0.044 | ||||||
| ↓Δ%FM (US4SF) 5–9 m | –0.001 (0.0003) | 0.044 | ||||||
| ↓Δ%FM (US4SF) 5–12 m | –0.001 (0.0002) | 0.007 | ||||||
| ↓Δ%FM (US4SF) 9–12 m | –0.0001 (0.0002) | 0.047 | ||||||
| Adiponectin intake at 9 m | ||||||||
| ↓ΔHC 9–12 m | –0.0003 (0.0001) | 0.017 | ||||||
| Adiponectin intake at 12 m | ||||||||
| ↓ΔFFMI (US4SF) 5–12 m | –0.001 (0.0002) | 0.020 | ||||||
| ↑ΔFM (BIS) 2–12 m | 0.001 (0.0001) | 0.018 | ||||||
| ↑ΔFM (US2SF) 2–12 m | 0.001 (0.0001) | 0.012 | ||||||
| ↑Δ%FM (BIS) 2–12 m | 0.007 (0.001) | 0.011 | ||||||
| ↑Δ%FM (US2SF) 2–12 m | 0.009 (0.002) | 0.013 | ||||||
| ↑ΔFMI (US2SF) 2–12 m | 0.001 (0.0001) | 0.005 | ||||||
| Whole milk leptin, ng/mL 2 m: 0.50 ± 0.18 (0.24–0.77) (n = 15) 5 m: 0.49 ± 0.17 (0.23–0.71) (n = 20) 9 m: 0.56 ± 0.11 (0.42–0.67) (n = 18) 12 m: 0.50 ± 0.11 (0.34–0.74) (n = 15) | Whole milk leptin, ng/24 h 2–5 m: 362 ± 173 (162–841) (n = 17) 9 m: 280 ± 73 (132–349) (n = 8) 12 m: 219 ± 90 (122–350) (n = 8) | Whole milk leptin concentration | ||||||
| FFM (BIS): ↓2, 5 m; ↑9, 12 m | NA e | 0.016 c | ||||||
| Whole milk leptin intake (overall) | ||||||||
| FFMI (US4SF): ↑2–5 m; ↓9, 12 m | NA e | 0.036 c | ||||||
| Whole milk leptin intake between 2 and 5 m | ||||||||
| ↑ΔLength 2–9 m | 0.008 (0.003) | 0.022 | ||||||
| Whole milk leptin intake at 9 m | ||||||||
| ↑ΔLength 2–12 m | 0.041 (0.004) | 0.007 | ||||||
| Whole milk leptin intake at 12 m | ||||||||
| ↓ΔBMI 9–12 m | –0.008 (0.006) | 0.019 | ||||||
| ↓ΔFFMI (US2SF) 5–12 m | –0.012 (0.004) | 0.040 | ||||||
| ↓ΔFFMI (US4SF) 5–12 m | –0.012 (0.002) | 0.007 | ||||||
| ↑ΔFM (BIS) 2–12 m | 0.005 (0.001) | 0.046 | ||||||
| ↑ΔFM (US2SF) 2–12 m | 0.005 (0.0003) | 0.0006 b | ||||||
| ↑Δ%FM (BIS) 2–12 m | 0.065 (0.015) | 0.049 | ||||||
| ↑Δ%FM (US2SF) 2–12 m | 0.073 (0.004) | 0.0004 b | ||||||
| ↑ΔFMI (US2SF) 2–12 m | 0.012 (0.002) | 0.018 | ||||||
| Skim milk leptin, ng/mL 2 m: 0.34 ± 0.20 (0.20–0.84) (n = 15) 5 m: 0.26 ± 0.08 (0.20–0.40) (n = 20) 9 m: 0.21 ± 0.02 (0.19–0.27) (n = 18) 12 m: 0.21 ± 0.03 (0.19–0.40) (n = 15) | Skim milk leptin, ng/24 h 2–5 m: 200 ± 81 (106–402) (n = 17) 9 m: 114 ± 38 (62–172) (n = 8) 12 m: 93 ± 36 (51–159) (n = 8) | Skim milk leptin concentration | ||||||
| ↓ΔHC | –1.85 (0.84) | 0.028 | ||||||
| Skim milk leptin intake (overall) | ||||||||
| BMI: ↑2–5 m; ↓9, 12 m | NA e | 0.004 b,c | ||||||
| FFMI (US4SF): ↓2–5, 9, 12 m | NA e | 0.012 b,c | ||||||
| ↑FM (BIS) | 0.003 (0.001) | 0.025 | ||||||
| ↑FM (US2SF): ↑2–5 m; ↓9 m; ↑12 m | NA e | 0.007 b,c | ||||||
| ↑FM (US4SF) | 0.004 (0.001) | <0.001 b | ||||||
| ↑%FM (US2SF) | 0.029 (0.009) | 0.001 b | ||||||
| ↑%FM (US4SF) | 0.031 (0.009) | 0.002 b | ||||||
| ↑FMI (BIS) | 0.005 (0.003) | 0.038 | ||||||
| ↑FMI (US2SF) | 0.008 (0.002) | <0.001 b | ||||||
| ↑FMI (US4SF) | 0.008 (0.002) | <0.001 b | ||||||
| Skim milk leptin intake between 2 and 5 m | ||||||||
| ↑ΔWeight 2–9 m | 0.005 (0.002) | 0.015 | ||||||
| ↑ΔWeight 2–12 m | 0.005 (0.002) | 0.026 | ||||||
| ↑ΔBMI 2–5 m | 0.006 (0.002) | 0.021 | ||||||
| ↑ΔFFM (BIS) 2–12 m | 0.005 (0.001) | 0.013 | ||||||
| ↑ΔFFM (US2SF) 2–9 m | 0.003 (0.001) | 0.012 | ||||||
| ↑ΔFFM (US4SF) 2–9 m | 0.004 (0.001) | 0.010 | ||||||
| ↑ΔFFM (US4SF) 2–12 m | 0.005 (0.002) | 0.018 | ||||||
| ↑ΔFM (BIS) 2–5 m | 0.002 (0.001) | 0.044 | ||||||
| ↑ΔFM (US2SF) 2–5 m | 0.003 (0.001) | 0.029 | ||||||
| ↓Δ%FM (US4SF) 5–12 m | –0.027 (0.012) | 0.036 | ||||||
| ↑ΔFMI (US2SF) 2–5 m | 0.007 (0.003) | 0.047 | ||||||
| ↓ΔFMI (US4SF) 5–12 m | –0.007 (0.003) | 0.026 | ||||||
| Skim milk leptin intake at 9 m | ||||||||
| ↑ΔWeight 2–9 m | 0.026 (0.004) | 0.007 | ||||||
| ↑ΔWeight 2–12 m | 0.033 (0.008) | 0.025 | ||||||
| ↑ΔBMI 2–12 m | 0.10 (0.02) | 0.035 | ||||||
| ↑ΔFM (BIS) 2–9 m | 0.052 (0.012) | 0.046 | ||||||
| Skim milk leptin intake at 12 m | ||||||||
| ↓ΔFFMI (US2SF) 5–12 m | –0.031 (0.007) | 0.005 | ||||||
| ↓ΔFFMI (US4SF) 5–12 m | –0.025 (0.002) | 0.0004 b | ||||||
| ↑ΔFM (US2SF) 2–12 m | 0.010 (0.002) | 0.022 | ||||||
| ↑Δ%FM (US2SF) 2–12 m | 0.135 (0.041) | 0.046 | ||||||
| Kon et al., 2014 Russian Federation [44] | CS, 1, 2, or 3 m, n = 103, 1 m (n = 32) 2 m (n = 34) 3 m (n = 33) LWG (<500 g/m, n = 18) NWG (500–1000 g/m, n = 40) HWG (>1000 g/m, n = 45) EBF | SHM, midstream morning sampling ELISA | Combined mean (calculated): 1 m: 736 (n = 32) 2 m: 826 (n = 34; no LWG) 3 m: 891 (n = 33) LWG 1 m: 555 ± 32 (n = 9) 2 m: NA 3 m: 753 ± 53 (n = 5) NWG 1 m: 849 ± 96 (n = 11) 2 m: 752 ± 93 (n = 14) 3 m: 896 ± 42 (n = 15) HWG 1 m: 768 ± 41 (n = 12) 2 m: 878 ± 37 (n = 20) 3 m: 937 ± 70 (n = 13) 24 h MI: pre-/post-feed test-weighing infant BC: weight gain | Adiponectin, μg/mL LWG 1 m: 1.06 ± 0.10 (n = 9) 2 m: NA (n = 4, excluded) 3 m: 1.09 ± 0.15 (n = 5) NWG 1 m: 1.14 ± 0.09 (n = 11) 2 m: 1.04 ± 0.0 (n = 14) 3 m: 1.14 ± 0.08 (n = 15) HWG 1 m: 1.10 ± 0.09 (n = 12) 2 m: 1.15 ± 0.08 (n = 20) 3 m: 1.12 ± 0.10 (n = 13) Ghrelin, ng/mL LWG 1 m: 0.77 ± 0.22 (n = 9) 2 m: NA (n = 4, excluded) 3 m: 8.24 ± 4.76 (n = 5) NWG 1 m: 7.52 ± 2.63 (n = 11) 2 m: 5.06 ± 2.49 (n = 14) 3 m: 0.71 ± 0.19 (n = 15) HWG 1 m: 2.32 ± 1.19 (n = 12) 2 m: 6.52 ± 1.87 (n = 20) 3 m: 3.05 ± 1.9 (n = 13) IGF-1, ng/mL LWG 1 m: 3.95 ± 1.86 (n = 9) 2 m: NA (n = 4, excluded) 3 m: 3.15 ± 1.22 (n = 5) NWG 1 m: 3.07 ± 1.55 (n = 11) 2 m: 3.21 ± 0.74 (n = 14) 3 m: 7.13 ± 1.29 (n = 15) HWG 1 m: 7.92 ± 3.72 (n = 12) 2 m: 6.97 ± 2.05 (n = 20) 3 m: 12.20 ± 2.41 (n = 13) Skim milk leptin, ng/mL LWG 1 m: 1.63 ± 0.27 (n = 9) 2 m: NA (n = 4, excluded) 3 m: 1.35 ± 0.31 (n = 5) NWG 1 m: 1.55 ± 0.17 (n = 11) 2 m: 1.83 ± 0.23 (n = 14) 3 m: 3.29 ± 0.70 (n = 15) HWG 1 m: 1.53 ± 0.29 (n = 12) 2 m: 2.20 ± 0.28 (n = 20) 3 m: 3.57 ± 1.37 (n = 13) | Adiponectin, μg/24 h LWG 1 m: 569 ± 56 (n = 9) 2 m: NA (n = 4, excluded) 3 m: 852 ± 130 (n = 5) NWG 1 m: 918 ± 202 (n = 11) 2 m: 736 ± 130 (n = 14) 3 m: 898 ± 133 (n = 15) HWG 1 m: 810 ± 59 (n = 12) 2 m: 1030 ± 82 (n = 20) 3 m: 1051 ± 116 (n = 13) Ghrelin, ng/24 h LWG 1 m: 428 ± 246 (n = 9) 2 m: NA (n = 4, excluded) 3 m: 6288 ± 3855 (n = 5) NWG 1 m: 845 ± 445 (n = 11) 2 m: 5343 ± 3938 (n = 14) 3 m: 345 ± 112 (n = 15) HWG 1 m: 715 ± 279 (n = 12) 2 m: 2304 ± 1197 (n = 20) 3 m: 488 ± 234 (n = 13) IGF-1, ng/24 h LWG 1 m: 1789 ± 1175 (n = 9) 2 m: NA (n = 4, excluded) 3 m: 2252 ± 822 (n = 5) NWG 1 m: 2894 ± 1493 (n = 11) 2 m: 2898 ± 804 (n = 14) 3 m: 6289 ± 1059 (n = 15) HWG 1 m: 5653 ± 2666 (n = 12) 2 m: 5690 ± 1567 (n = 20) 3 m: 11351 ± 2990 (n = 13) Skim milk leptin, ng/24 h LWG 1 m: 860 ± 225 (n = 9) 2 m: NA (n = 4, excluded) 3 m: 1096 ± 150 (n = 5) NWG 1 m: 1291 ± 619 (n = 11) 2 m: 1237 ± 272 (n = 14) 3 m: 3463 ± 1232 (n = 15) HWG 1 m: 1584 ± 420 (n = 12) 2 m: 2777 ± 567 (n = 20) 3 m: 4979 ± 1959 (n = 13) | Adiponectin concentration | ||
| No significant difference between LWG, NWG, and HWG groups at any time point | Concentrations by group and time point are on the left | NS | ||||||
| Adiponectin intake | ||||||||
| ↑Adiponectin intake at 1 m in the HWG group compared with the LWG group | Intakes by group and time point are on the left | <0.05 | ||||||
| Ghrelin concentration | ||||||||
| ↑Ghrelin concentration at 1 m in the HWG group compared with the NWG and LWG groups | <0.05 | |||||||
| Ghrelin intake | ||||||||
| No significant difference between the LWG, NWG, and HWG groups at any time point | NS | |||||||
| IGF-1 concentration | ||||||||
| ↑IGF-1 concentration at 3 m in the HWG group compared with the LWG group | <0.05 | |||||||
| IGF-1 intake | ||||||||
| ↑IGF-1 intake at 3 m in the HWG group compared with the LWG group | <0.05 | |||||||
| ↑IGF-1 intake at 3 m in the NWG group compared with the LWG group | <0.05 | |||||||
| Skim milk leptin concentration | ||||||||
| ↑Skim milk leptin concentration at 3 m in NWG compared with LWG group | <0.05 | |||||||
| Skim milk leptin intake | ||||||||
| ↑Skim milk leptin intake at 2 m in HWG compared with NWG group | <0.05 | |||||||
| Human Milk Bioactive Molecules | Time Postpartum | Sample Size | Human Milk Sample Type | 24 h Intake of Bioactive Molecules | Comments | Author, Year |
|---|---|---|---|---|---|---|
| Adiponectin | 1 m | n = 9 n = 11 n = 12 | Skim milk | 569 ± 56 μg/24 h a 918 ± 202 810 ± 59 | LWG NWG HWG | Kon et al., 2014 [44] |
| 2 m | n = 4 n = 14 n = 20 | Skim milk | NA 736 ± 130 μg/24 h 1030 ± 82 | LWG NWG HWG | Kon et al., 2014 [44] | |
| 3 m | n = 5 n = 15 n = 13 | Skim milk | 852 ± 130 μg/24 h 898 ± 133 1051 ± 116 | LWG NWG HWG | Kon et al., 2014 [44] | |
| 2–5 m | n = 17 | Whole milk | 7976 ± 4480 (3771–22,439) ng/24 h | Gridneva et al., 2018 (b) [43] | ||
| 9 m | n = 8 | Whole milk | 4446 ± 1645 (2142–6673) ng/24 h | Gridneva et al., 2018 (b) [43] | ||
| 12 m | n = 8 | Whole milk | 3922 ± 1431 (2511–6352) ng/24 h | Gridneva et al., 2018 (b) [43] | ||
| Whole milk leptin | 2–5 m | n = 17 | Whole milk | 362 ± 173 (162–841) ng/24 h | Gridneva et al., 2018 (b) [43] | |
| 9 m | n = 8 | Whole milk | 280 ± 73 (132–349) ng/24 h | Gridneva et al., 2018 (b) [43] | ||
| 12 m | n = 8 | Whole milk | 219 ± 90 (122–350) ng/24 h | Gridneva et al., 2018 (b) [43] | ||
| Skim milk leptin | 1 m | n = 9 n = 11 n = 12 | Skim milk | 860 ± 225 ng/24 h 1291 ± 619 1584 ± 420 | LWG NWG HWG | Kon et al., 2014 [44] |
| 2 m | n = 4 n = 14 n = 20 | Skim milk | NA 1237 ± 272 ng/24 h 2777 ± 567 | LWG NWG HWG | Kon et al., 2014 [44] | |
| 3 m | n = 5 n = 15 n = 13 | Skim milk | 1096 ± 150 ng/24 h 3463 ± 1232 4979 ± 1959 | LWG NWG HWG | Kon et al., 2014 [44] | |
| 2–5 m | n = 17 | Skim milk | 200 ± 81 (106–402) ng/24 h | Gridneva et al., 2018 (b) [43] | ||
| 9 m | n = 8 | Skim milk | 114 ± 38 (62–172) ng/24 h | Gridneva et al., 2018 (b) [43] | ||
| 12 m | n = 8 | Skim milk | 93 ± 36 (51–159) ng/24 h | Gridneva et al., 2018 (b) [43] | ||
| Insulin | 3 m | n = 45 | Whole milk | 1.16 ± 0.78 (0.30–4.17) ng/24 h | Cheema et al., 2021 [37] | |
| Ghrelin | 1 m | n = 9 n = 11 n = 12 | Skim milk | 428 ± 246 ng/24 h 845 ± 445 715 ± 279 | LWG NWG HWG | Kon et al., 2014 [44] |
| 2 m | n = 4 n = 14 n = 20 | Skim milk | NA 5343 ± 3938 ng/24 h 2304 ± 1197 | LWG NWG HWG | Kon et al., 2014 [44] | |
| 3 m | n = 5 n = 15 n = 13 | Skim milk | 6288 ± 3855 ng/24 h 345 ± 112 488 ± 234 | LWG NWG HWG | Kon et al., 2014 [44] | |
| IGF-1 | 1 m | n = 9 n = 11 n = 12 | Skim milk | 1789 ± 1175 ng/24 h 2894 ± 1493 5653 ± 2666 | LWG NWG HWG | Kon et al., 2014 [44] |
| 2 m | n = 4 n = 14 n = 20 | Skim milk | NA 2898 ± 804 ng/24 h 5690 ± 1567 | LWG NWG HWG | Kon et al., 2014 [44] | |
| 3 m | n = 5 n = 15 n = 13 | Skim milk | 2252 ± 822 ng/24 h 6289 ± 1059 11,351 ± 2990 | LWG NWG HWG | Kon et al., 2014 [44] | |
| Lactoferrin | 2–5 m | n = 17 | Skim milk | 0.29 ± 0.12 (0.06–0.51) g/24 h | Gridneva et al., 2020 [40] | |
| 9 m | n = 8 | Skim milk | 0.31 ± 0.21 (0.13–0.74) g/24 h | Gridneva et al., 2020 [40] | ||
| 12 m | n = 8 | Skim milk | 0.25 ± 0.10 (0.10–0.36) g/24 h | Gridneva et al., 2020 [40] | ||
| Lysozyme | 2–5 m | n = 17 | Skim milk | 0.09 ± 0.07 (0.06–0.34) g/24 h | Gridneva et al., 2020 [40] | |
| 9 m | n = 8 | Skim milk | 0.06 ± 0.02 (0.04–0.10) g/24 h | Gridneva et al., 2020 [40] | ||
| 12 m | n = 8 | Skim milk | 0.08 ± 0.05 (0.05–0.17) g/24 h | Gridneva et al., 2020 [40] | ||
| sIgA | 2–5 m | n = 17 | Skim milk | 0.38 ± 0.18 (0.15–0.80) g/24 h | Gridneva et al., 2020 [40] | |
| 9 m | n = 8 | Skim milk | 0.28 ± 0.12 (0.10–0.48) g/24 h | Gridneva et al., 2020 [40] | ||
| 12 m | n = 8 | Skim milk | 0.25 ± 0.09 (0.14–0.38) g/24 h | Gridneva et al., 2020 [40] | ||
| HMOs | 3 m | n = 47 | Whole milk | See paper for intakes of 19 individual HMOs [36] | In entire cohort and by maternal secretor status | Cheema et al., 2022 [36] |
| Total HMOs | 2–5 m | n = 17 | Skim milk | 12.0 ± 6.0 (2.0–21.6) g/24 h | Gridneva et al., 2019 [41] | |
| 9 m | n = 8 | Skim milk | 10.8 ± 5.4 (0–15.7) g/24 h | Gridneva et al., 2019 [41] | ||
| 12 m | n = 8 | Skim milk | 12.0 ± 18.5 (1.5–49.5) g/24 h | Gridneva et al., 2019 [41] |
References
- Perrella, S.; Gridneva, Z.; Lai, C.T.; Stinson, L.; George, A.; Bilston-John, S.; Geddes, D. Human milk composition promotes optimal infant growth, development and health. Semin. Perinatol. 2021, 45, 151380. [Google Scholar] [CrossRef] [PubMed]
- Christian, P.; Smith, E.R.; Lee, S.E.; Vargas, A.J.; Bremer, A.A.; Raiten, D.J. The need to study human milk as a biological system. Am. J. Clin. Nutr. 2021, 113, 1063–1072. [Google Scholar] [CrossRef]
- Geddes, D.T.; Gridneva, Z.; Perrella, S.L.; Mitoulas, L.R.; Kent, J.C.; Stinson, L.F.; Lai, C.T.; Sakalidis, V.S.; Twigger, A.J.; Hartmann, P.E. 25 years of research in human lactation: From discovery to translation. Nutrients 2021, 13, 3071. [Google Scholar] [CrossRef]
- Hennet, T.; Borsig, L. Breastfed at Tiffany’s. Trends Biochem. Sci. 2016, 41, 508–518. [Google Scholar] [CrossRef] [PubMed]
- Wells, J.C.K.; Shirley, M.K. Body composition and the monitoring of non-communicable chronic disease risk. Glob. Health Epidemiol. Genom. 2016, 1, e18. [Google Scholar] [CrossRef]
- World Health Organization. Noncommunicable Diseases Progress Monitor. 2020. Available online: https://apps.who.int/iris/handle/10665/330805 (accessed on 24 June 2022).
- Victora, C.; Horta, B.; De Mola, C.; Quevedo, L.; Pinheiro, R.; Gigante, D.; Gonçalves, H.; Colugnati, F. Association between breastfeeding and intelligence, educational attainment, and income at 30 years of age: A prospective birth cohort study from Brazil. Lancet Glob. Health 2015, 3, e199–e205. [Google Scholar] [CrossRef]
- Victora, C.G.; Bahl, R.; Barros, A.J.D.; Franca, G.V.A.; Horton, S.; Krasevec, J.; Murch, S.; Sankar, M.J.; Walker, N.; Rollins, N.C.; et al. Breastfeeding in the 21st century: Epidemiology, mechanisms, and lifelong effect. Lancet 2016, 387, 475–490. [Google Scholar] [CrossRef]
- Eidelman, A. Breastfeeding and the use of human milk: An analysis of the American Academy of Pediatrics 2012 Breastfeeding Policy Statement. Breastfeed. Med. 2012, 7, 323–324. [Google Scholar] [CrossRef]
- Vestergaard, M.; Obel, C.; Henriksen, T.; Sørensen, H.; Skajaa, E.; Ostergaard, J. Duration of breastfeeding and developmental milestones during the latter half of infancy. Acta Paediatr. 1999, 88, 1327–1332. [Google Scholar] [CrossRef] [PubMed]
- Koletzko, B.; Brands, B.; Chourdakis, M.; Cramer, S.; Grote, V.; Hellmuth, C.; Kirchberg, F.; Prell, C.; Rzehak, P.; Uhl, O.; et al. The power of programming and the EarlyNutrition project: Opportunities for health promotion by nutrition during the first thousand days of life and beyond. Ann. Nutr. Metab. 2014, 64, 187–196. [Google Scholar] [CrossRef]
- Ratnasingham, A.; Eiby, Y.; Dekker Nitert, M.; Donovan, T.; Lingwood, B.E. Review: Is rapid fat accumulation in early life associated with adverse later health outcomes? Placenta 2017, 54, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Owen, C.; Martin, R.; Whincup, P.; Smith, G.; Cook, D. Effect of infant feeding on the risk of obesity across the life course: A quantitative review of published evidence. Pediatrics 2005, 115, 1367–1377. [Google Scholar] [CrossRef] [PubMed]
- Arenz, S.; Rückerl, R.; Koletzko, B.; von Kries, R. Breast-feeding and childhood obesity—A systematic review. Int. J. Obes. 2004, 28, 1247–1256. [Google Scholar] [CrossRef]
- Horta, B.L.; Loret de Mola, C.; Victora, C.G. Long-term consequences of breastfeeding on cholesterol, obesity, systolic blood pressure and type 2 diabetes: A systematic review and meta-analysis. Acta Paediatr. 2015, 104, 30–37. [Google Scholar] [CrossRef]
- Druet, C.; Stettler, N.; Sharp, S.; Simmons, R.K.; Cooper, C.; Smith, G.D.; Ekelund, U.; Levy-Marchal, C.; Jarvelin, M.R.; Kuh, D.; et al. Prediction of childhood obesity by infancy weight gain: An individual-level meta-analysis. Paediatr. Perinat. Epidemiol. 2011, 26, 19–26. [Google Scholar] [CrossRef]
- Luque, V.; Closa-Monasterolo, R.; Escribano, J.; Ferre, N. Early programming by protein intake: The effect of protein on adiposity development and the growth and functionality of vital organs. Nutr. Metab. Insights 2015, 8, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Koletzko, B.; von Kries, R.; Closa, R.; Escribano, J.; Scaglioni, S.; Giovannini, M.; Beyer, J.; Demmelmair, H.; Gruszfeld, D.; Dobrzanska, A.; et al. Lower protein in infant formula is associated with lower weight up to age 2 y: A randomized clinical trial. Am. J. Clin. Nutr. 2009, 89, 1836–1845. [Google Scholar] [CrossRef]
- Gidrewicz, D.A.; Fenton, T.R. A systematic review and meta-analysis of the nutrient content of preterm and term breast milk. BMC Pediatr. 2014, 14, 216. [Google Scholar] [CrossRef]
- Fields, D.A.; Schneider, C.R.; Pavela, G. A narrative review of the associations between six bioactive components in breast milk and infant adiposity. Obesity 2016, 24, 1213–1221. [Google Scholar] [CrossRef]
- Butte, N.; Garza, C.; Smith, E.; Nichols, B. Human milk intake and growth in exclusively breast-fed infants. J. Pediatr. 1984, 104, 187–195. [Google Scholar] [CrossRef]
- Kent, J.C.; Mitoulas, L.R.; Cregan, M.D.; Ramsay, D.T.; Doherty, D.A.; Hartmann, P.E. Volume and frequency of breastfeedings and fat content of breast milk throughout the day. Pediatrics 2006, 117, e387–e395. [Google Scholar] [CrossRef] [PubMed]
- Dewey, K.; Lonnerdal, B. Milk and nutrient intake of breast-fed infants from 1 to 6 months: Relation to growth and fatness. J. Pediatr. Gastroenterol. Nutr. 1983, 2, 497–506. [Google Scholar] [CrossRef]
- Arthur, P.; Hartmann, P.; Smith, M. Measurement of the milk intake of breast-fed infants. J. Pediatr. Gastroenterol. Nutr. 1987, 6, 758–763. [Google Scholar] [CrossRef]
- Kent, J.; Hepworth, A.; Sherriff, J.; Cox, D.; Mitoulas, L.; Hartmann, P. Longitudinal changes in breastfeeding patterns from 1 to 6 months of lactation. Breastfeed. Med. 2013, 8, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Rattigan, S.; Ghisalberti, A.V.; Hartmann, P.E. Breast-milk production in Australian women. Br. J. Nutr. 1981, 45, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Kent, J.; Mitoulas, L.; Cox, D.B.; Owens, R.; Hartmann, P. Breast volume and milk production during extended lactation in women. Exp. Physiol. 1999, 84, 435–447. [Google Scholar] [CrossRef]
- Rios-Leyvra, M.; Yao, Q. The volume of breast milk intake in infants and young children: A systematic review and meta-analysis. Breastfeed. Med. 2023, 18, 188–197. [Google Scholar] [CrossRef]
- Mitoulas, L.R.; Kent, J.C.; Cox, D.B.; Owens, R.A.; Sherriff, J.L.; Hartmann, P.E. Variation in fat, lactose and protein in human milk over 24 h and throughout the first year of lactation. Br. J. Nutr. 2002, 88, 29–37. [Google Scholar] [CrossRef]
- Dewey, K.; Heinig, M.; Nommsen, L.; Lonnerdal, B. Maternal versus infant factors related to breast milk intake and residual milk volume: The DARLING study. Pediatrics 1991, 87, 829–837. [Google Scholar] [CrossRef]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A.; PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan-a web and mobile app for systematic reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef]
- National Institute for Clinical Excellence. Methodological Checklists for Cohort Studies. Available online: https://www.nice.org.uk/ (accessed on 24 June 2022).
- Olga, L.; Vervoort, J.; van Diepen, J.A.; Gross, G.; Petry, C.J.; Prentice, P.M.; Chichlowski, M.; von Tol, E.A.F.; Hughes, I.A.; Dunger, D.B.; et al. Associations between breast milk intake volume, macronutrient intake, and infant growth in a longitudinal birth cohort: The Cambridge Baby Growth and Breastfeeding Study (CBGS-BF). Br. J. Nutr. 2022, 1–27. [Google Scholar] [CrossRef]
- Gridneva, Z.; Rea, A.; Lai, C.T.; Tie, W.J.; Kugananthan, S.; Warden, A.H.; Perrella, S.L.; Murray, K.; Geddes, D.T. Human milk macronutrients and bioactive molecules and development of regional fat depots in Western Australian infants during the first 12 months of lactation. Life 2022, 12, 493. [Google Scholar] [CrossRef]
- Cheema, A.S.; Gridneva, Z.; Furst, A.J.; Roman, A.S.; Trevenen, M.L.; Turlach, B.A.; Lai, C.T.; Stinson, L.F.; Bode, L.; Payne, M.S.; et al. Human milk oligosaccharides and bacterial profile modulate infant body composition during exclusive breastfeeding. Int. J. Mol. Sci. 2022, 23, 2865. [Google Scholar] [CrossRef]
- Cheema, A.S.; Stinson, L.F.; Rea, A.; Lai, C.T.; Payne, M.S.; Murray, K.; Geddes, D.T.; Gridneva, Z. Human milk lactose, insulin, and glucose relative to infant body composition during exclusive breastfeeding. Nutrients 2021, 13, 3724. [Google Scholar] [CrossRef]
- Gridneva, Z.; Rea, A.; Lai, C.T.; Tie, W.J.; Kugananthan, S.; Murray, K.; Hartmann, P.E.; Geddes, D.T. Development of visceral and subcutaneous-abdominal adipose tissue in breastfed infants during first year of lactation. Nutrients 2021, 13, 3294. [Google Scholar] [CrossRef]
- George, A.D.; Gay, M.C.L.; Selvalatchmanan, J.; Torta, F.; Bendt, A.K.; Wenk, M.R.; Murray, K.; Wlodek, M.E.; Geddes, D.T. Healthy breastfeeding infants consume different quantities of milk fat globule membrane lipids. Nutrients 2021, 13, 2951. [Google Scholar] [CrossRef]
- Gridneva, Z.; Lai, C.T.; Rea, A.; Tie, W.J.; Ward, L.C.; Murray, K.; Hartmann, P.E.; Geddes, D.T. Human milk immunomodulatory proteins are related to development of infant body composition during the first year of lactation. Pediatr. Res. 2020, 89, 911–921. [Google Scholar] [CrossRef]
- Gridneva, Z.; Rea, A.; Tie, W.J.; Lai, C.T.; Kugananthan, S.; Ward, L.C.; Murray, K.; Hartmann, P.E.; Geddes, D.T. Carbohydrates in human milk and body composition of term infants during the first 12 months of lactation. Nutrients 2019, 11, 1472. [Google Scholar] [CrossRef]
- Gridneva, Z.; Tie, W.J.; Rea, A.; Lai, C.T.; Ward, L.C.; Murray, K.; Hartmann, P.E.; Geddes, D.T. Human milk casein and whey protein and infant body composition over the first 12 months of lactation. Nutrients 2018, 10, 1332. [Google Scholar] [CrossRef]
- Gridneva, Z.; Kugananthan, S.; Rea, A.; Lai, C.T.; Ward, L.C.; Murray, K.; Hartmann, P.E.; Geddes, D.T. Human milk adiponectin and leptin and infant body composition over the first 12 months of lactation. Nutrients 2018, 10, 1125. [Google Scholar] [CrossRef] [PubMed]
- Kon, I.Y.; Shilina, N.M.; Gmoshinskaya, M.V.; Ivanushkina, T.A. The study of breast milk IGF-1, leptin, ghrelin and adiponectin levels as possible reasons of high weight gain in breast-fed infants. Ann. Nutr. Metab. 2014, 65, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Heinig, M.J.; Nommsen, L.A.; Peerson, J.M.; Lonnerdal, B.; Dewey, K.G. Energy and protein intakes of breast-fed and formula-fed infants during the first year of life and their association with growth velocity: The DARLING Study. Am. J. Clin. Nutr. 1993, 58, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Saben, J.; Sims, C.; Abraham, A.; Bode, L.; Andres, A. Human milk oligosaccharide concentrations and infant intakes are associated with maternal overweight and obesity and predict infant growth. Nutrients 2021, 13, 446. [Google Scholar] [CrossRef]
- Saben, J.L.; Sims, C.R.; Pack, L.; Lan, R.; Borsheim, E.; Andres, A. Infant intakes of human milk branched chain amino acids are negatively associated with infant growth and influenced by maternal body mass index. Pediatr. Obes. 2022, 17, e12876. [Google Scholar] [CrossRef]
- Sims, C.; Lipsmeyer, M.; Turner, D.; Andres, A. Human milk composition differs by maternal BMI in the first 9 months postpartum. Am. J. Clin. Nutr. 2020, 112, 548–557. [Google Scholar] [CrossRef]
- Gridneva, Z.; Rea, A.; Hepworth, A.R.; Ward, L.C.; Lai, C.T.; Hartmann, P.E.; Geddes, D.T. Relationships between breastfeeding patterns and maternal and infant body composition over the first 12 months of lactation. Nutrients 2018, 10, 45. [Google Scholar] [CrossRef]
- Khan, S.; Hepworth, A.R.; Prime, D.K.; Lai, C.T.; Trengove, N.J.; Hartmann, P.E. Variation in fat, lactose, and protein composition in breast milk over 24 hours: Associations with infant feeding patterns. J. Hum. Lact. 2013, 29, 81–89. [Google Scholar] [CrossRef]
- George, A.D.; Gay, M.C.L.; Murray, K.; Muhlhausler, B.S.; Wlodek, M.E.; Geddes, D.T. Human milk sampling protocols affect estimation of infant lipid intake. J. Nutr. 2020, 150, 2924–2930. [Google Scholar] [CrossRef]
- Scanlon, K.S.; Alexander, M.P.; Serdula, M.K.; Davis, M.K.; Bowman, B.A. Assessment of infant feeding: The validity of measuring milk intake. Nutr. Rev. 2002, 60, 235–251. [Google Scholar] [CrossRef]
- Coward, W.A.; Cole, T.J.; Sawyer, M.B.; Prentice, A.M. Breast-milk intake measurement in mixed-fed infants by administration of deuterium oxide to their mothers. Hum. Nutr. Clin. Nutr. 1982, 36, 141–148. [Google Scholar]
- Kent, J.C.; Prime, D.K.; Garbin, C.P. Principles for maintaining or increasing breast milk production. J. Obstet. Gynecol. Neonatal Nurs. 2012, 41, 114–121. [Google Scholar] [CrossRef]
- Koletzko, B. Human milk lipids. Ann. Nutr. Metab. 2016, 69, 28–40. [Google Scholar] [CrossRef]
- Guo, M. Human Milk Biochemistry and Infant Formula. In Manufacturing Technology; Elsevier: Cambrdige, UK, 2014. [Google Scholar]
- Anderson, N.K.; Beerman, K.A.; McGuire, M.A.; Dasgupta, N.; Grinari, J.M.; Williams, J.; McGuire, M.K. Dietary fat type influences total milk fat content in lean women. J. Nutr. 2005, 135, 416–421. [Google Scholar] [CrossRef]
- Nommsen, L.A.; Lovelady, C.A.; Heinig, M.; Lonnerdal, B.; Dewey, K.G. Determinants of energy, protein, lipid, and lactose concentrations in human milk during the first 12 mo of lactation: The DARLING Study. Am. J. Clin. Nutr. 1991, 53, 457–465. [Google Scholar] [CrossRef]
- Van de Kamer, J.H.; ten Bokkel Huinink, H.; Weyers, H.A. Rapid method for the determination of fat in feces. J. Biol. Chem. 1949, 177, 347–355. [Google Scholar] [CrossRef]
- Kugananthan, S.; Lai, C.T.; Gridneva, Z.; Mark, P.J.; Geddes, D.T.; Kakulas, F. Leptin levels are higher in whole compared to skim human milk, supporting a cellular contribution. Nutrients 2016, 8, 711. [Google Scholar] [CrossRef]
- Andreas, N.J.; Hyde, M.J.; Gale, C.; Parkinson, J.R.C.; Jeffries, S.; Holmes, E.; Modi, N. Effect of maternal body mass index on hormones in breast milk: A systematic review. PLoS ONE 2014, 9, e115043. [Google Scholar] [CrossRef]
- Kugananthan, S.; Gridneva, Z.; Lai, C.T.; Hepworth, A.R.; Mark, P.J.; Kakulas, F.; Geddes, D.T. Associations between maternal body composition and appetite hormones and macronutrients in human milk. Nutrients 2017, 9, 252. [Google Scholar] [CrossRef]
- Fields, D.A.; George, B.; Williams, M.; Whitaker, K.; Allison, D.B.; Teague, A.; Demerath, E.W. Associations between human breast milk hormones and adipocytokines and infant growth and body composition in the first 6 months of life. Pediatr. Obes. 2017, 12, 78–85. [Google Scholar] [CrossRef]
- Jevitt, C.; Hernandez, I.; Groër, M. Lactation complicated by overweight and obesity: Supporting the mother and newborn. J. Midwifery Womens Health 2007, 52, 606–613. [Google Scholar] [CrossRef] [PubMed]
- Perez, M.R.; de Castro, L.S.; Chang, Y.S.; Sañudo, A.; Marcacine, K.O.; Amir, L.H.; Ross, M.G.; Coca, K.P. Breastfeeding practices and problems among obese women compared with nonobese women in a Brazilian hospital. Womens Health Rep. 2021, 2, 219–226. [Google Scholar] [CrossRef]
- Ward, L.; Poston, L.; Godfrey, K.; Koletzko, B. Assessing early growth and adiposity: Report from an Early Nutrition Academy workshop. Ann. Nutr. Metab. 2013, 63, 120–130. [Google Scholar] [CrossRef] [PubMed]
- Andres, A.; Casey, P.H.; Cleves, M.A.; Badger, T.M. Body fat and bone mineral content of infants fed breast milk, cow’s milk formula, or soy formula during the first year of life. J. Pediatr. 2013, 163, 49–54. [Google Scholar] [CrossRef]
- Chen, J.-R.; Samuel, H.A.; Shlisky, J.; Sims, C.R.; Lazarenko, O.P.; Williams, D.K.; Andres, A.; Badger, T.M. A longitudinal observational study of skeletal development between ages 3 mo and 6 y in children fed human milk, milk formula, or soy formula. Am. J. Clin. Nutr. 2023. [Google Scholar] [CrossRef] [PubMed]
- Lingwood, B.E.; van Leeuwen, A.M.S.; Carberry, A.E.; Fitzgerald, E.C.; Callaway, L.K.; Colditz, P.B.; Ward, L.C. Prediction of fat-free mass and percentage of body fat in neonates using biolelectrical impedance analysis and anthropometric measures: Validation against PEA POD. Br. J. Nutr. 2012, 107, 1545–1552. [Google Scholar] [CrossRef]
- Gridneva, Z.; Hepworth, A.R.; Ward, L.C.; Lai, C.T.; Hartmann, P.E.; Geddes, D.T. Determinants of body composition in breastfed infants using bioimpedance spectroscopy and ultrasound skinfolds–methods comparison. Pediatr. Res. 2016, 81, 423–433. [Google Scholar] [CrossRef]
- Molinari, C.E.; Casadio, Y.S.; Hartmann, B.T.; Livk, A.; Bringans, S.; Arthur, P.G.; Hartmann, P.E. Proteome mapping of human skim milk proteins in term and preterm milk. J. Proteome Res. 2012, 11, 1696–1714. [Google Scholar] [CrossRef]
- Ballard, O.; Morrow, A.L. Human milk composition: Nutrients and bioactive factors. Pediatr. Clin. North Am. 2013, 60, 49–74. [Google Scholar] [CrossRef]
- Casadio, Y.S.; Williams, T.M.; Lai, C.T.; Olsson, S.E.; Hepworth, A.R.; Hartmann, P.E. Evaluation of a mid-infrared analyzer for the determination of the macronutrient composition of human milk. J. Hum. Lact. 2010, 26, 376–383. [Google Scholar] [CrossRef]
- Bode, L.; Jantscher-Krenn, E. Structure-function relationships of human milk oligosaccharides. Adv. Nutr. 2012, 3, 383S–391S. [Google Scholar] [CrossRef]
- Kozyrskyj, A.L.; Kalu, R.; Koleva, P.T.; Bridgman, S.L. Fetal programming of overweight through the microbiome: Boys are disproportionately affected. J. Dev. Orig. Health Dis. 2016, 7, 25–34. [Google Scholar] [CrossRef]
- Bode, L. Human milk oligosaccharides: Every baby needs a sugar mama. Glycobiology 2012, 22, 1147–1162. [Google Scholar] [CrossRef]
- Karatas, Z.; Aydogdu, S.D.; Dinleyici, E.C.; Colak, O.; Dogruel, N. Breastmilk ghrelin, leptin, and fat levels changing foremilk to hindmilk: Is that important for self-control of feeding? Eur. J. Pediatr. 2011, 170, 1273–1280. [Google Scholar] [CrossRef]
- Kierson, J.; Dimatteo, D.; Locke, R.; Mackley, A.; Spear, M. Ghrelin and cholecystokinin in term and preterm human breast milk. Acta Pediatr. 2006, 95, 991–995. [Google Scholar] [CrossRef]
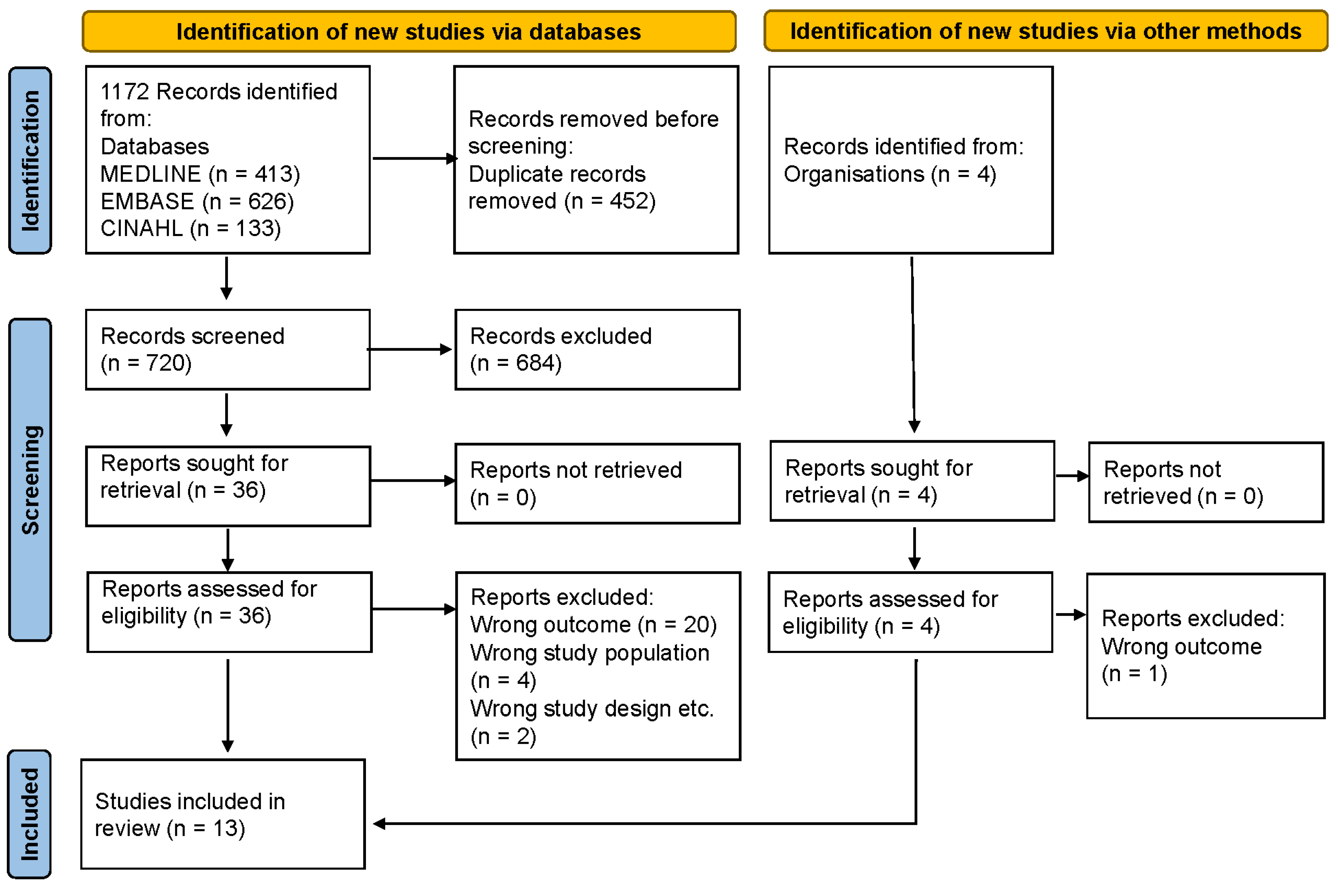
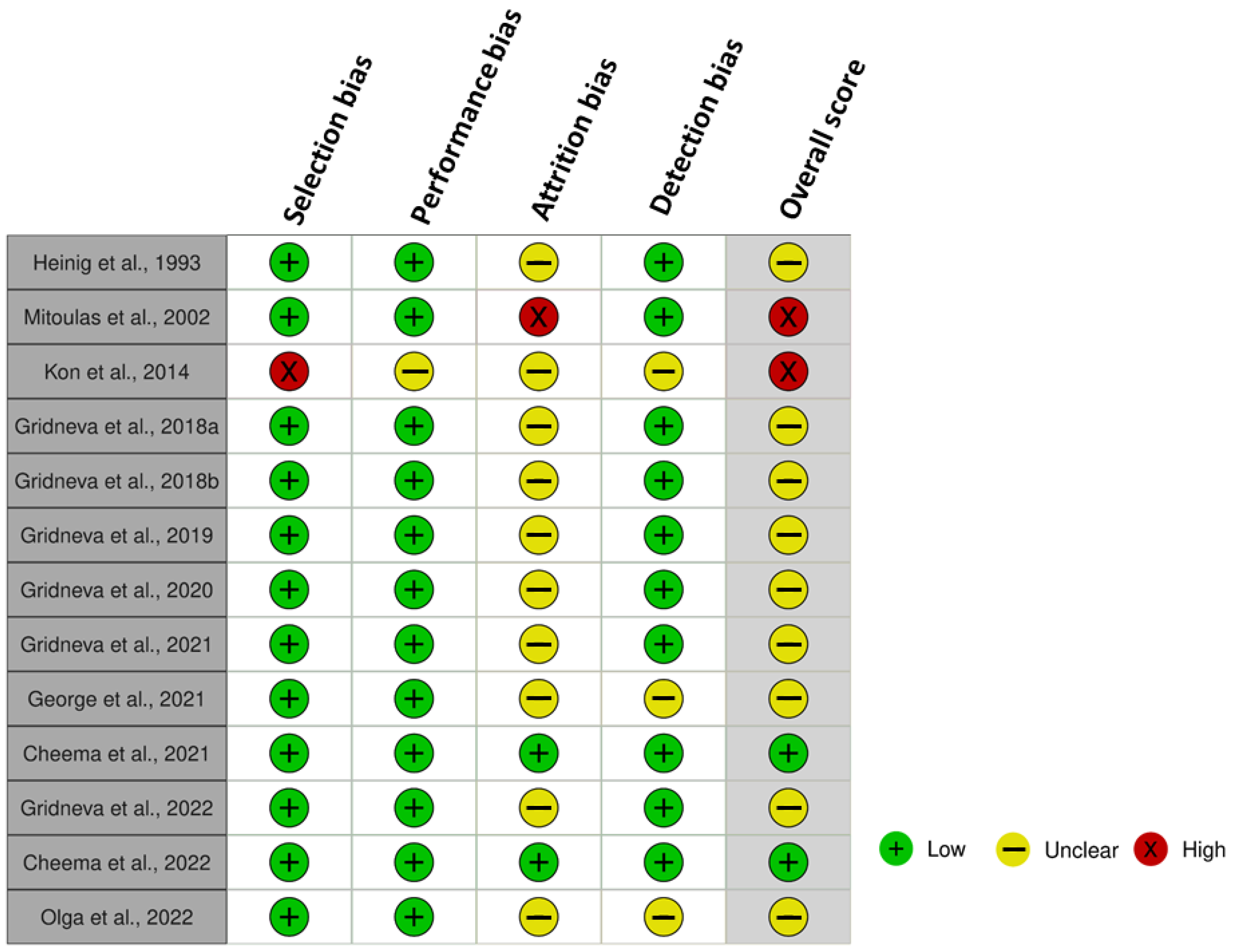

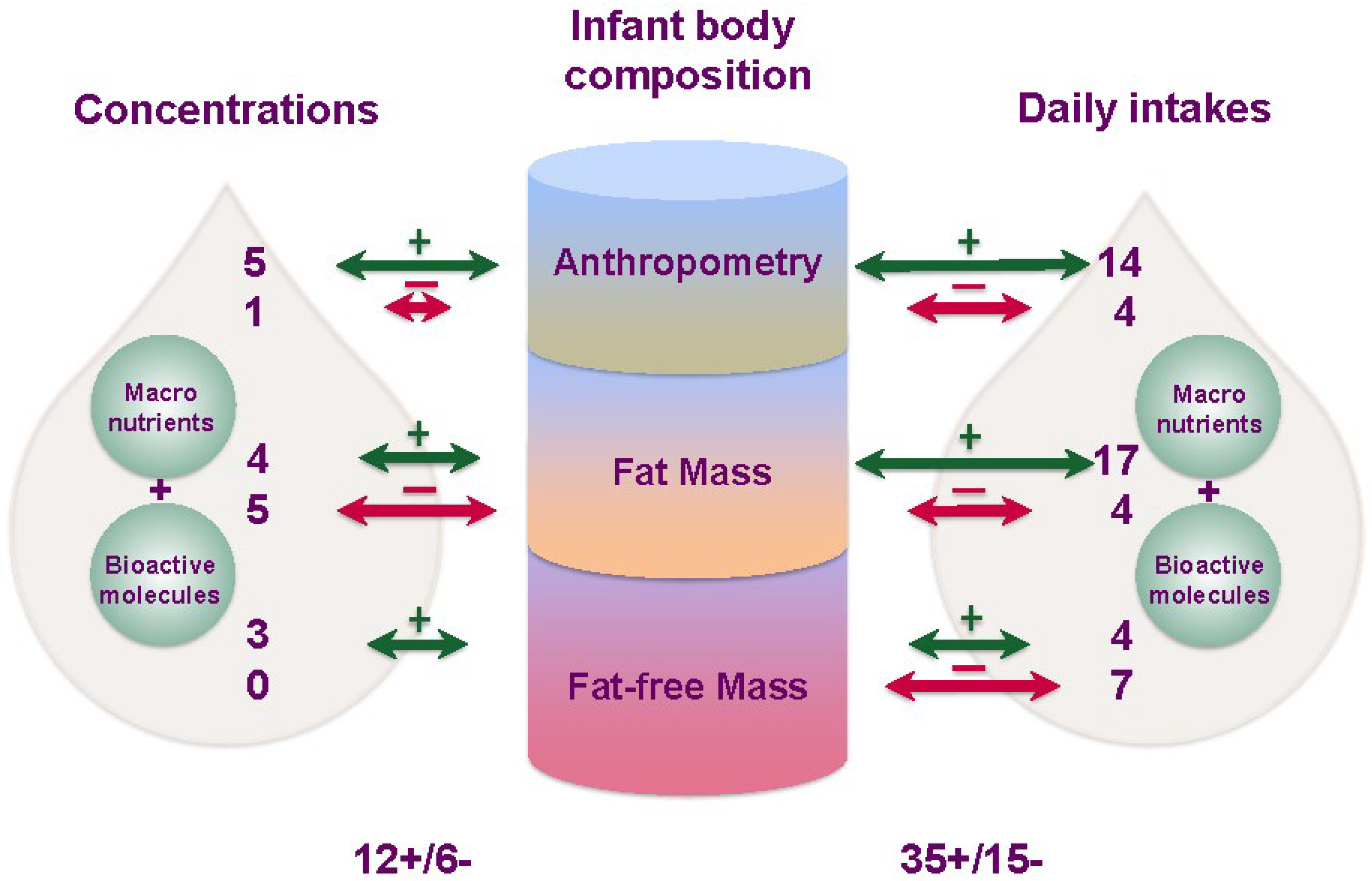
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Norrish, I.; Sindi, A.; Sakalidis, V.S.; Lai, C.T.; McEachran, J.L.; Tint, M.T.; Perrella, S.L.; Nicol, M.P.; Gridneva, Z.; Geddes, D.T. Relationships between the Intakes of Human Milk Components and Body Composition of Breastfed Infants: A Systematic Review. Nutrients 2023, 15, 2370. https://doi.org/10.3390/nu15102370
Norrish I, Sindi A, Sakalidis VS, Lai CT, McEachran JL, Tint MT, Perrella SL, Nicol MP, Gridneva Z, Geddes DT. Relationships between the Intakes of Human Milk Components and Body Composition of Breastfed Infants: A Systematic Review. Nutrients. 2023; 15(10):2370. https://doi.org/10.3390/nu15102370
Chicago/Turabian StyleNorrish, Isabella, Azhar Sindi, Vanessa S. Sakalidis, Ching Tat Lai, Jacki L. McEachran, Mya Thway Tint, Sharon L. Perrella, Mark P. Nicol, Zoya Gridneva, and Donna T. Geddes. 2023. "Relationships between the Intakes of Human Milk Components and Body Composition of Breastfed Infants: A Systematic Review" Nutrients 15, no. 10: 2370. https://doi.org/10.3390/nu15102370
APA StyleNorrish, I., Sindi, A., Sakalidis, V. S., Lai, C. T., McEachran, J. L., Tint, M. T., Perrella, S. L., Nicol, M. P., Gridneva, Z., & Geddes, D. T. (2023). Relationships between the Intakes of Human Milk Components and Body Composition of Breastfed Infants: A Systematic Review. Nutrients, 15(10), 2370. https://doi.org/10.3390/nu15102370





