Long-Term Soft-Food Rearing in Young Mice Alters Brain Function and Mood-Related Behavior
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
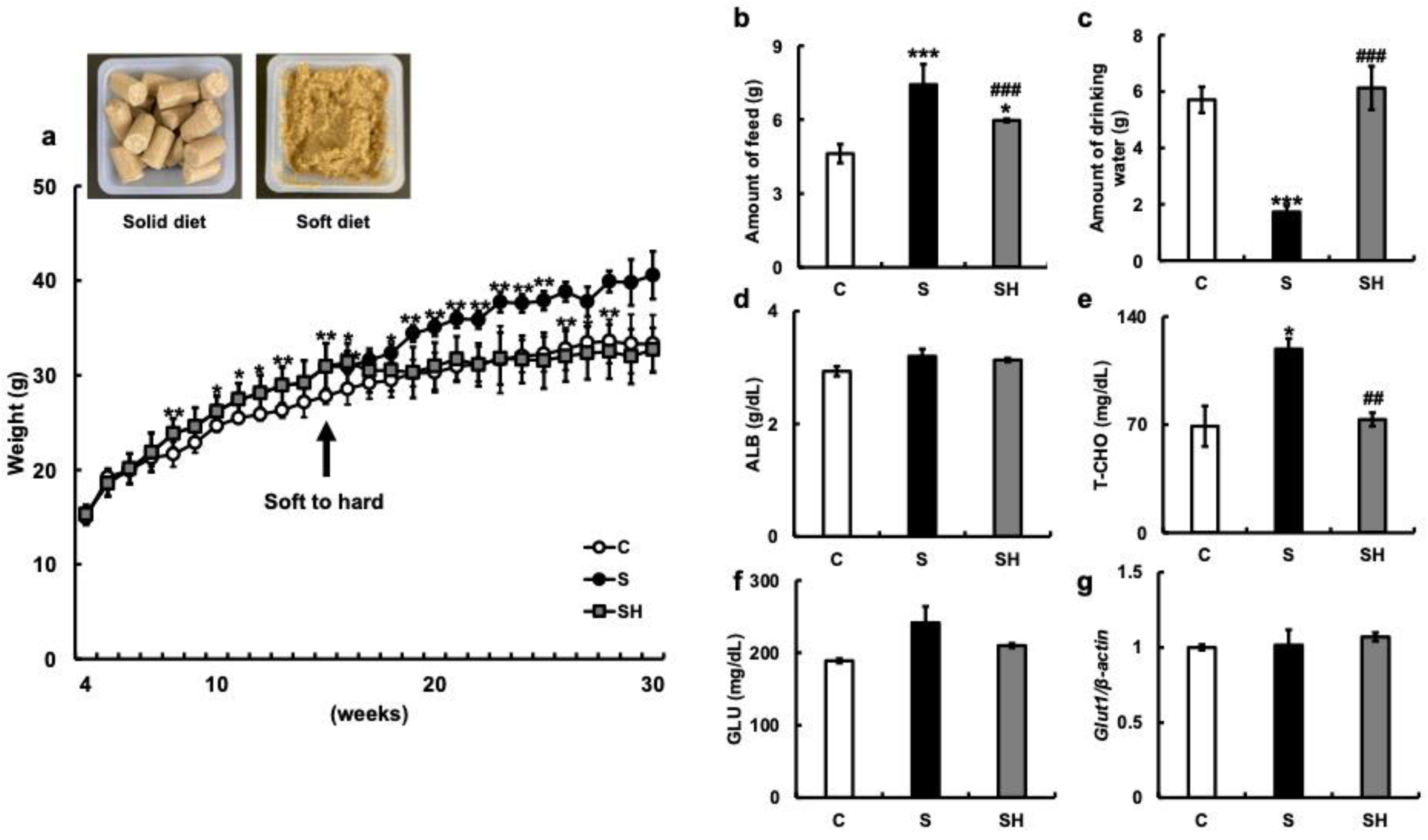
2.2. Body Weight and General Health
2.3. Blood Tests
2.3.1. Preparation of Blood Serum
2.3.2. Serum Biochemistry Test
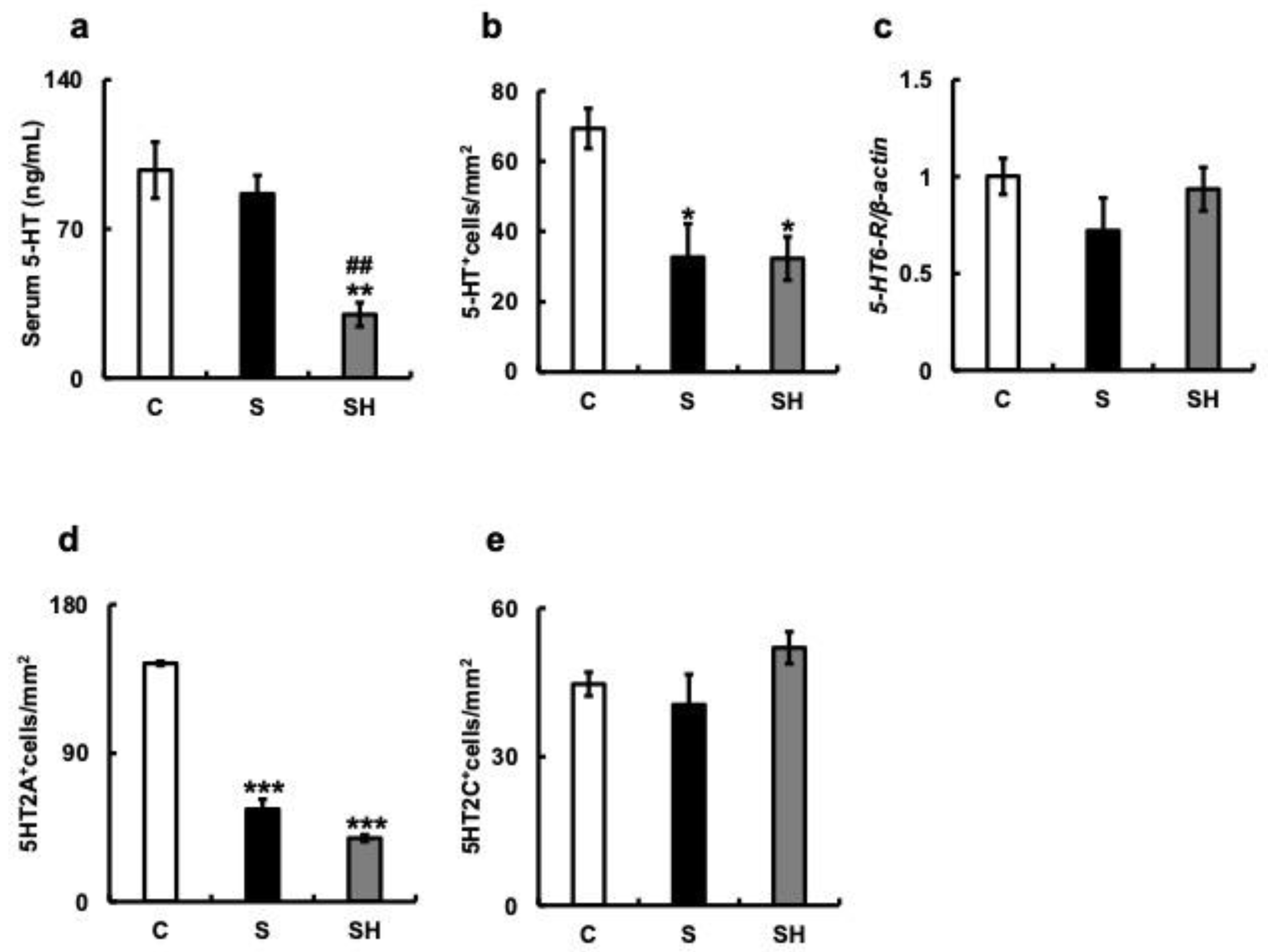
2.3.3. Serum 5-Hydroxytryptamine (5-HT) Test
2.4. Behavioral Testing
2.4.1. Y-Maze Test
2.4.2. Motor Skill Learning Test
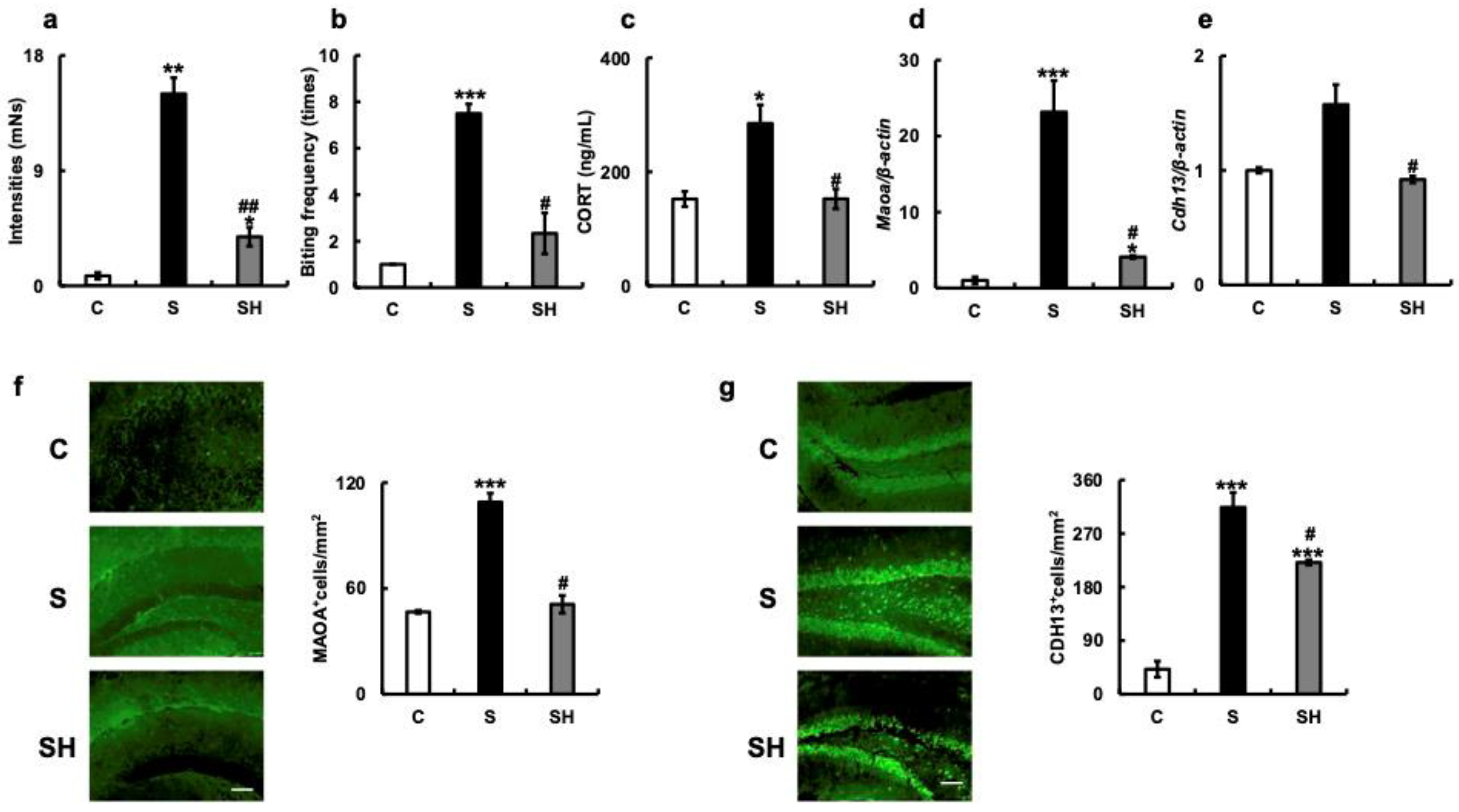
2.4.3. Measurement of Aggressive Biting Behavior (ABB)
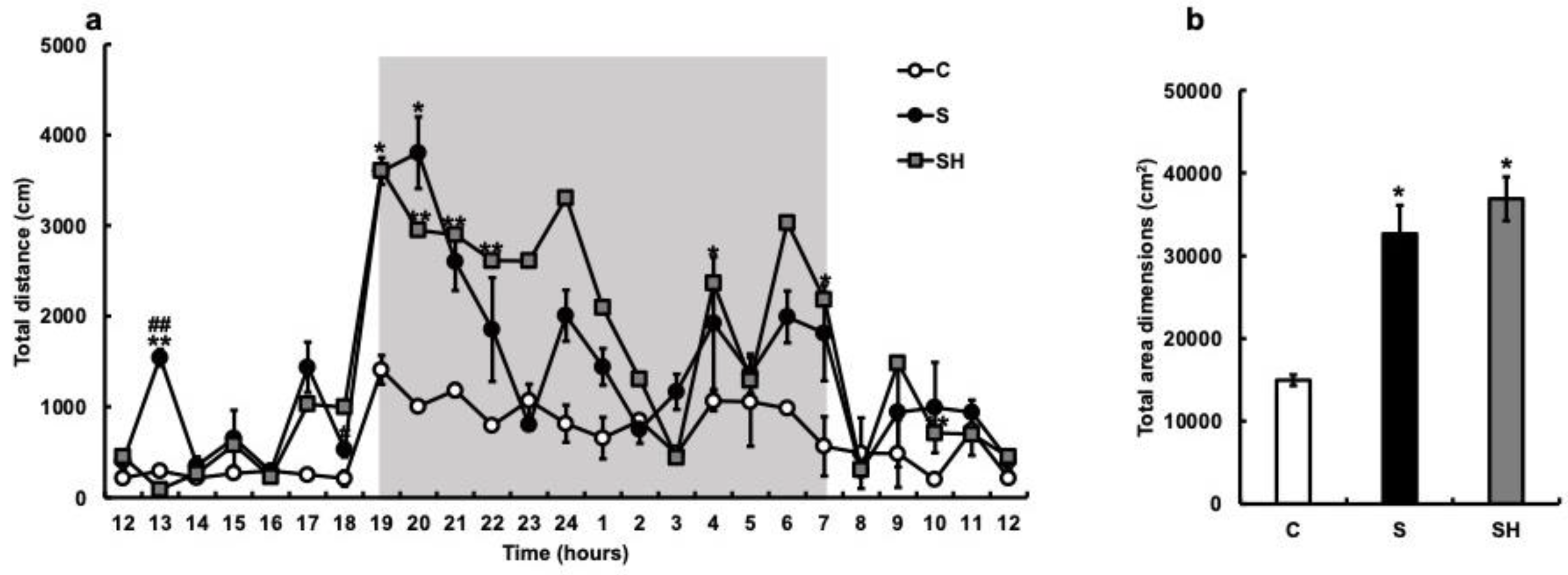
2.4.4. Twenty-Four-Hour Locomotor Activity
2.5. Real-Time PCR
2.6. Immunohistochemistry
2.7. Statistical Analyses
3. Results
3.1. Changes in Body Weight and Serum Composition Due to Soft Feeding
3.2. Effects of Soft-Food Rearing on Memory and Behavior
3.3. Twenty-Four-Hour Locomotor Activity
3.4. ABB toward an Inanimate Object
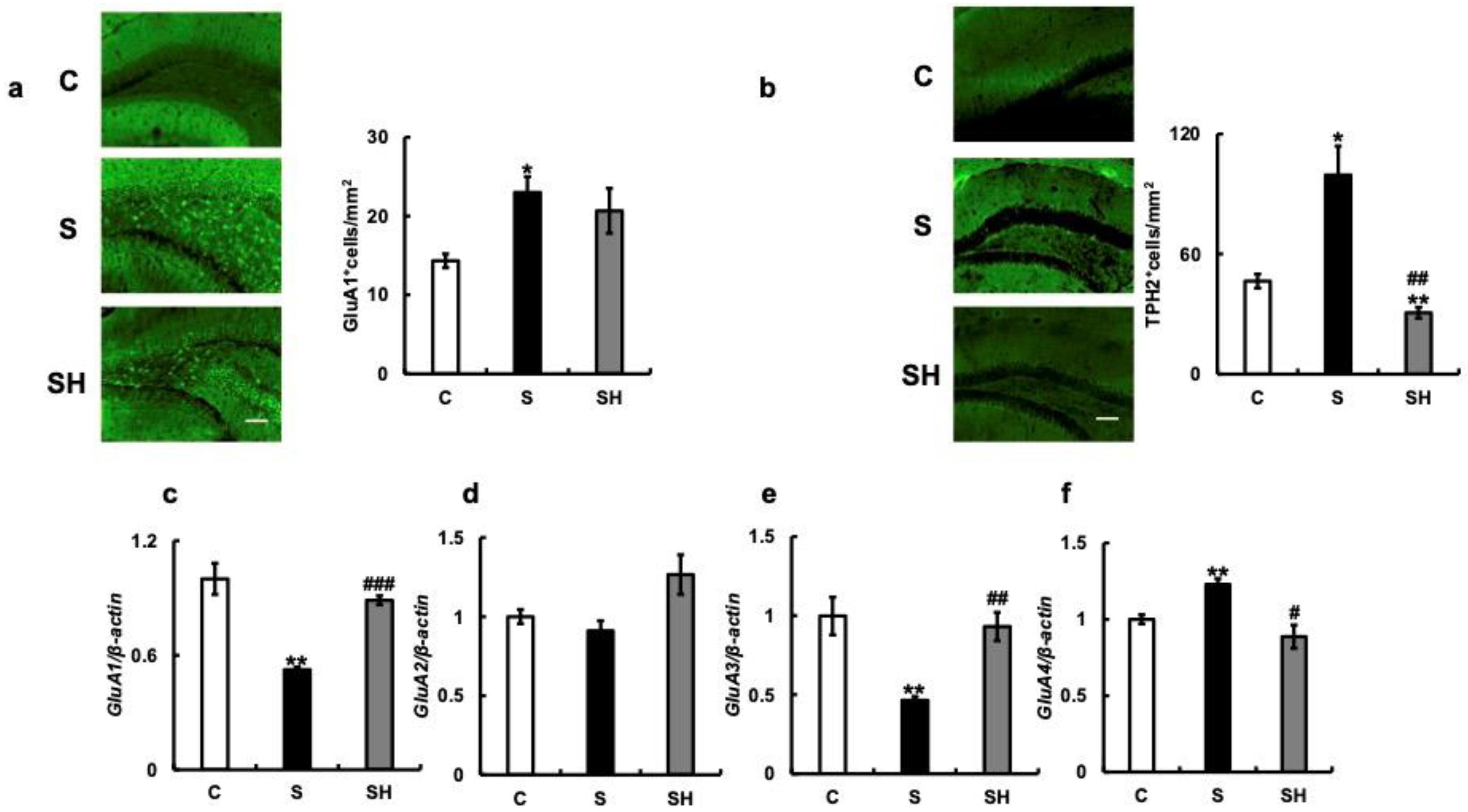
3.5. Effects of Soft-Food Rearing on the Expression of Serotonin-Related Molecules
3.6. Effects of Soft-Food Rearing on AMPA Receptor-Related Molecule Expression in the Hippocampus
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Healthy Diet. Available online: https://www.who.int/news-room/fact-sheets/detail/healthy-diet (accessed on 11 April 2023).
- Galiniak, S.; Aebisher, D.; Bartusik-Aebisher, D. Health benefits of resveratrol administration. Acta Biochim. Pol. 2019, 66, 13–21. [Google Scholar] [CrossRef] [Green Version]
- Sunkara, A.; Raizner, A. Supplemental vitamins and minerals for cardiovascular disease prevention and treatment. Methodist. DeBakey Cardiovasc. J. 2019, 15, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Forde, C.G.; Bolhuis, D. Interrelations between food form, texture, and matrix influence energy intake and metabolic responses. Curr. Nutr. Rep. 2022, 11, 124–132. [Google Scholar] [CrossRef] [PubMed]
- van der Bilt, A.; Engelen, L.; Pereira, L.J.; van der Glas, H.W.; Abbink, J.H. Oral physiology and mastication. Physiol. Behav. 2006, 89, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Kubo, K.Y.; Iinuma, M.; Azuma, K. Mastication as a stress-coping behavior. BioMed Res. Int. 2015, 2015, 876409. [Google Scholar] [CrossRef] [PubMed]
- Hori, K.; Uehara, F.; Yamaga, Y.; Yoshimura, S.; Okawa, J.; Tanimura, M.; Ono, T. Reliability of a novel wearable device to measure chewing frequency. J. Prosthodont. Res. 2021, 65, 340–345. [Google Scholar] [CrossRef]
- Hamada, Y.; Hayashi, N. Chewing increases postprandial diet-induced thermogenesis. Sci. Rep. 2021, 11, 23714. [Google Scholar] [CrossRef]
- Onyper, S.V.; Carr, T.L.; Farrar, J.S.; Floyd, B.R. Cognitive advantages of chewing gum. Now you see them, now you don’t. Appetite 2011, 57, 321–328. [Google Scholar] [CrossRef]
- Pedersen, A.M.L.; Sørensen, C.E.; Proctor, G.B.; Carpenter, G.H.; Ekström, J. Salivary secretion in health and disease. J. Oral Rehabil. 2018, 45, 730–746. [Google Scholar] [CrossRef]
- Tada, A.; Miura, H. Association of mastication and factors affecting masticatory function with obesity in adults: A systematic review. BMC Oral Health 2018, 18, 76. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto, T.; Hirayama, A. Effects of soft-diet feeding on synaptic density in the hippocampus and parietal cortex of senescence-accelerated mice. Brain Res. 2001, 902, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, M.; Niijima-Yaoita, F.; Yoneda, H.; Chiba, K.; Tsuchiya, S.; Hagiwara, Y.; Sasaki, K.; Sugawara, S.; Endo, Y.; Tan-No, K.; et al. Long-term feeding on powdered food causes hyperglycemia and signs of systemic illness in mice. Life Sci. 2014, 103, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Yoshino, F.; Yoshida, A.; Hori, N.; Ono, Y.; Kimoto, K.; Onozuka, M.; Lee, M.C. Soft-food diet induces oxidative stress in the rat brain. Neurosci. Lett. 2012, 508, 42–46. [Google Scholar] [PubMed]
- Ono, Y.; Yamamoto, T.; Kubo, K.Y.; Onozuka, M. Occlusion and brain function: Mastication as a prevention of cognitive dysfunction. J. Oral Rehabil. 2010, 37, 624–640. [Google Scholar] [CrossRef] [PubMed]
- Mori, D.; Katayama, T.; Miyake, H.; Fujiwara, S.; Kubo, K.-Y. Occlusal disharmony leads to learning deficits associated with decreased cellular proliferation in the hippocampal dentate gyrus of SAMP8 mice. Neurosci. Lett. 2013, 534, 228–232. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, M.; Tada, H.; Wang, J.; Yamada, M.; Kurosawa, M.; Satoh, A.; Ogiso, N.; Shikama, Y.; Matsushita, K. Molar loss induces hypothalamic and hippocampal astrogliosis in aged mice. Sci. Rep. 2022, 12, 6409. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, F.B.; Pereira Fernandes, L.d.M.; Noronha, P.A.; dos Santos, M.A.; Gomes-Leal, W.; Ferraz Maia, C.d.S.; Lima, R.R. Masticatory deficiency as a risk factor for cognitive dysfunction. Int. J. Med. Sci. 2014, 2, 209–214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frota de Almeida, M.N.; de Siqueira Mendes, F.d.C.C.; Gurgel Felício, A.P.; Falsoni, M.; Ferreira de Andrade, M.L.; Bento-Torres, J.; da Costa Vasconcelos, P.F.; Perry, V.H.; Picanço-Diniz, C.W.; Kronka Sosthenes, M.C. Spatial memory decline after masticatory deprivation and aging is associated with altered laminar distribution of CA1 astrocytes. BMC Neurosci. 2012, 13, 23. [Google Scholar] [CrossRef] [Green Version]
- Aguayo-Mendoza, M.; Santagiuliana, M.; Ong, X.; Piqueras-Fiszman, B.; Scholten, E.; Stieger, M. How addition of peach gel particles to yogurt affects oral behavior, sensory perception and liking of consumers differing in age. Food Res. Int. 2020, 134, 109213. [Google Scholar] [CrossRef]
- Wallace, R.; Lombardi, K.; Backer, C.D.; Costello, L.; Devine, A. Sharing is caring: A study of food-sharing practices in Australian early childhood education and care services. Nutrients 2020, 12, 229. [Google Scholar] [CrossRef] [Green Version]
- Okihara, H.; Ito, J.-I.; Kokai, S.; Ishida, T.; Hiranuma, M.; Kato, C.; Yabushita, T.; Ishida, K.; Ono, T.; Michikawa, M. Liquid diet induces memory impairment accompanied by a decreased number of hippocampal neurons in mice. J. Neurosci. Res. 2014, 92, 1010–1017. [Google Scholar] [CrossRef] [PubMed]
- Fukushima-Nakayama, Y.; Ono, T.; Hayashi, M.; Inoue, M.; Wake, H.; Ono, T.; Nakashima, T. Reduced mastication impairs memory function. J. Dent. Res. 2017, 96, 1058–1066. [Google Scholar] [CrossRef] [PubMed]
- Kubo, K.-Y.; Ichihashi, Y.; Kurata, C.; Iinuma, M.; Mori, D.; Katayama, T.; Miyake, H.; Fujiwara, S.; Tamura, Y. Masticatory function and cognitive function. Okajimas Folia Anat. Jpn. 2010, 87, 135–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Connor, D.F.; Newcorn, J.H.; Saylor, K.E.; Amann, B.H.; Scahill, L.; Robb, A.S.; Jensen, P.S.; Vitiello, B.; Findling, R.L.; Buitelaar, J.K. Maladaptive aggression: With a focus on impulsive aggression in children and adolescents. J. Child. Adolesc. Psychopharmacol. 2019, 29, 576–591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raaijmakers, M.A.; Posthumus, J.A.; van Hout, B.A.; van Engeland, H.; Matthys, W. Cross-sectional study into the costs and impact on family functioning of 4-year-old children with aggressive behavior. Prev. Sci. 2011, 12, 192–200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laninga-Wijnen, L.; Harakeh, Z.; Steglich, C.; Dijkstra, J.K.; Veenstra, R.; Vollebergh, W. The norms of popular peers moderate friendship dynamics of adolescent aggression. Child. Dev. 2017, 88, 1265–1283. [Google Scholar] [CrossRef] [PubMed]
- Taubner, S.; Zimmermann, L.; Ramberg, A.; Schröder, P. Mentalization mediates the relationship between early maltreatment and potential for violence in adolescence. Psychopathology 2016, 49, 236–246. [Google Scholar] [CrossRef]
- Khayyatzadeh, S.S.; Mehramiz, M.; Mirmousavi, S.J.; Mazidi, M.; Ziaee, A.; Kazemi-Bajestani, S.M.R.; Ferns, G.A.; Moharreri, F.; Ghayour-Mobarhan, M. Adherence to a dash-style diet in relation to depression and aggression in adolescent girls. Psychiatry Res. 2018, 259, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Kalantari, N.; Doaei, S.; Gordali, M.; Rahimzadeh, G.; Gholamalizadeh, M. The association between dairy intake, simple sugars and body mass index with expression and extent of anger in female students. Iran. J. Psychiatry 2016, 11, 43–50. [Google Scholar]
- O’Neill, J.; Brock, C.; Olesen, A.E.; Andresen, T.; Nilsson, M.; Dickenson, A.H. Unravelling the mystery of capsaicin: A tool to understand and treat pain. Pharmacol. Rev. 2012, 64, 939–971. [Google Scholar] [CrossRef] [Green Version]
- Wahl, F.; Allix, M.; Plotkine, M.; Boulu, R.G. Neurological and behavioral outcomes of focal cerebral ischemia in rats. Stroke 1992, 23, 267–272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuchiiwa, T.; Kuchiiwa, S. Evaluation of aggressiveness of female mice using a semi-automated apparatus for measurement of aggressive biting behavior toward an inanimate object. J. Neurosci. Methods 2016, 257, 179–184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamasaki, N.; Maekawa, M.; Kobayashi, K.; Kajii, Y.; Maeda, J.; Soma, M.; Takao, K.; Tanda, K.; Ohira, K.; Toyama, K.; et al. Alpha-CaMKII deficiency causes immature dentate gyrus, a novel candidate endophenotype of psychiatric disorders. Mol. Brain 2008, 1, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiuccariello, L.; Houle, S.; Miler, L.; Cooke, R.G.; Rusjan, P.M.; Rajkowska, G.; Levitan, R.D.; Kish, S.J.; Kolla, N.J.; Ou, X.; et al. Elevated monoamine oxidase a binding during major depressive episodes is associated with greater severity and reversed neurovegetative symptoms. Neuropsychopharmacology 2014, 39, 973–980. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
- Kanda, Y. Investigation of the freely available easy-to-use software “EZR” for medical statistics. Bone Marrow Transplant. 2013, 48, 452–458. [Google Scholar] [CrossRef] [Green Version]
- Napoleão, A.; Fernandes, L.; Miranda, C.; Marum, A.P. Effects of calorie restriction on health span and insulin resistance: Classic calorie restriction diet vs. ketosis-inducing diet. Nutrients 2021, 13, 1302. [Google Scholar] [CrossRef]
- Karl, T.; Pabst, R.; von Hörsten, S. Behavioral phenotyping of mice in pharmacological and toxicological research. Exp. Toxicol. Pathol. 2003, 55, 69–83. [Google Scholar] [CrossRef]
- Soreff, S.M.; Gupta, V.; Wadhwa, R.; Arif, H. Aggression. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Hamon, M.; Blier, P. Monoamine neurocircuitry in depression and strategies for new treatments. Prog. Neuropsychopharmacol. Biol. Psychiatry 2013, 45, 54–63. [Google Scholar] [CrossRef]
- Gescher, D.M.; Kahl, K.G.; Hillemacher, T.; Frieling, H.; Kuhn, J.; Frodl, T. Epigenetics in personality disorders: Today’s insights. Front. Psychiatry 2018, 9, 579. [Google Scholar] [CrossRef] [Green Version]
- Yanowitch, R.; Coccaro, E.F. The neurochemistry of human aggression. Adv. Genet. 2011, 75, 151–169. [Google Scholar] [PubMed]
- Coccaro, E.F.; Fanning, J.R.; Phan, K.L.; Lee, R. Serotonin and impulsive aggression. CNS Spectr. 2015, 20, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Kolla, N.J.; Bortolato, M. The role of monoamine oxidase A in the neurobiology of aggressive, antisocial, and violent behavior: A tale of mice and men. Prog. Neurobiol. 2020, 194, 101875. [Google Scholar] [CrossRef]
- Qiu, M.; Zhang, C.; Dai, Y.; Zhang, L.; Wang, Y.; Peng, W.; Chen, Y.; Wen, C.; Li, H.; Zhu, T. MRNA levels of MAOA and 5-HT 2 A receptor in patients with pathological internet use: Correlations with comorbid symptoms. Front. Psychiatry 2021, 12, 667699. [Google Scholar] [CrossRef] [PubMed]
- Grunewald, M.; Johnson, S.; Lu, D.; Wang, Z.; Lomberk, G.; Albert, P.R.; Stockmeier, C.A.; Meyer, J.H.; Urrutia, R.; Miczek, K.A.; et al. Mechanistic role for a novel glucocorticoid-KLF11 (TIEG2) protein pathway in stress-induced monoamine oxidase A expression. J. Biol. Chem. 2012, 287, 24195–24206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Q.; Cole, D.A. Aggressive outbursts among adults with major depressive disorder: Results from the collaborative psychiatric epidemiological surveys. J. Psychiatr. Res. 2021, 135, 325–331. [Google Scholar] [CrossRef]
- Tiihonen, J.; Rautiainen, M.R.; Ollila, H.M.; Repo-Tiihonen, E.; Virkkunen, M.; Palotie, A.; Pietiläinen, O.; Kristiansson, K.; Joukamaa, M.; Lauerma, H.; et al. Genetic background of extreme violent behavior. Mol. Psychiatry 2015, 20, 786–792. [Google Scholar] [CrossRef] [Green Version]
- Ciranna, L. Serotonin as a modulator of glutamate- and GABA-mediated neurotransmission: Implications in physiological functions and in pathology. Curr. Neuropharmacol. 2006, 4, 101–114. [Google Scholar] [CrossRef] [Green Version]
- Lin, X.; Huang, L.; Huang, H.; Ke, Z.; Chen, Y. Disturbed relationship between glucocorticoid receptor and 5-HT1AR/5-HT2AR in ADHD rats: A correlation study. Front. Neurosci. 2022, 16, 1064369. [Google Scholar] [CrossRef]
- Zhang, G.; Stackman, R.W. The role of serotonin 5-HT2A receptors in memory and cognition. Front. Pharmacol. 2015, 6, 225. [Google Scholar] [CrossRef] [Green Version]
- Muguruza, C.; Miranda-Azpiazu, P.; Díez-Alarcia, R.; Morentin, B.; González-Maeso, J.; Callado, L.F.; Meana, J.J. Evaluation of 5-HT2A and MGlu2/3 receptors in postmortem prefrontal cortex of subjects with major depressive disorder: Effect of antidepressant treatment. Neuropharmacology 2014, 86, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Pritchard, A.L.; Harris, J.; Pritchard, C.W.; Coates, J.; Haque, S.; Holder, R.; Bentham, P.; Lendon, C.L. Role of 5HT2A and 5HT2C polymorphisms in behavioural and psychological symptoms of Alzheimer’s disease. Neurobiol. Aging 2008, 29, 341–347. [Google Scholar] [CrossRef]
- Porter, R.J.; Gallagher, P.; Watson, S.; Young, A.H. Corticosteroid-serotonin interactions in depression: A review of the human evidence. Psychopharmacology 2004, 173, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Gorlova, A.; Ortega, G.; Waider, J.; Bazhenova, N.; Veniaminova, E.; Proshin, A.; Kalueff, A.V.; Anthony, D.C.; Lesch, K.-P.; Strekalova, T. Stress-induced aggression in heterozygous TPH2 mutant mice is associated with alterations in serotonin turnover and expression of 5-HT6 and AMPA subunit 2A receptors. J. Affect. Disord. 2020, 272, 440–451. [Google Scholar] [CrossRef]
- Lesch, K.-P.; Araragi, N.; Waider, J.; van den Hove, D.; Gutknecht, L. Targeting brain serotonin synthesis: Insights into neurodevelopmental disorders with long-term outcomes related to negative emotionality, aggression and antisocial behaviour. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2012, 367, 2426–2443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strekalova, T.; Svirin, E.; Waider, J.; Gorlova, A.; Cespuglio, R.; Kalueff, A.; Pomytkin, I.; Schmitt-Boehrer, A.G.; Lesch, K.-P.; Anthony, D.C. Altered behaviour, dopamine and norepinephrine regulation in stressed mice heterozygous in TPH2 gene. Prog. Neuropsychopharmacol. Biol. Psychiatry 2021, 108, 110155. [Google Scholar] [CrossRef] [PubMed]
- Sachs, B.D.; Jacobsen, J.P.R.; Thomas, T.L.; Siesser, W.B.; Roberts, W.L.; Caron, M.G. The effects of congenital brain serotonin deficiency on responses to chronic fluoxetine. Transl. Psychiatry 2013, 3, e291. [Google Scholar] [CrossRef] [Green Version]
- Vogel, S.; Schwabe, L. Stress, aggression, and the balance of approach and avoidance. Psychoneuroendocrinology 2019, 103, 137–146. [Google Scholar] [CrossRef]
- Conway, C.C.; Keenan-Miller, D.; Hammen, C.; Lind, P.A.; Najman, J.M.; Brennan, P.A. Coaction of stress and serotonin transporter genotype in predicting aggression at the transition to adulthood. J. Clin. Child. Adolesc. Psychol. 2012, 41, 53–63. [Google Scholar] [CrossRef] [Green Version]
- Amidfar, M.; de Oliveira, J.; Kucharska, E.; Budni, J.; Kim, Y.-K. The role of CREB and BDNF in neurobiology and treatment of Alzheimer’s disease. Life Sci. 2020, 257, 118020. [Google Scholar] [CrossRef]
- Duman, R.S.; Monteggia, L.M. A neurotrophic model for stress-related mood disorders. Biol. Psychiatry 2006, 59, 1116–1127. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, E.; Hashimoto, K.; Okamura, N.; Koike, K.; Komatsu, N.; Kumakiri, C.; Nakazato, M.; Watanabe, H.; Shinoda, N.; Okada, S.; et al. Alterations of serum levels of brain-derived neurotrophic factor (BDNF) in depressed patients with or without antidepressants. Biol. Psychiatry 2003, 54, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Karege, F.; Vaudan, G.; Schwald, M.; Perroud, N.; La Harpe, R. Neurotrophin levels in postmortem brains of suicide victims and the effects of antemortem diagnosis and psychotropic drugs. Brain Res. Mol. Brain Res. 2005, 136, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Martinowich, K.; Lu, B. Interaction between BDNF and serotonin: Role in mood disorders. Neuropsychopharmacology 2008, 33, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Kernie, S.G. BDNF regulates eating behavior and locomotor activity in mice. EMBO J. 2000, 19, 1290–1300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lyons, W.E.; Mamounas, L.A.; Ricaurte, G.A.; Coppola, V.; Reid, S.W.; Bora, S.H.; Wihler, C.; Koliatsos, V.E.; Tessarollo, L. Brain-derived neurotrophic factor-deficient mice develop aggressiveness and hyperphagia in conjunction with brain serotonergic abnormalities. Proc. Natl. Acad. Sci. USA 1999, 96, 15239–15244. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto, T.; Hirayama, A.; Hosoe, N.; Furube, M.; Hirano, S. Soft-diet feeding inhibits adult neurogenesis in hippocampus of mice. Bull. Tokyo Dent. Coll. 2009, 50, 117–124. [Google Scholar] [CrossRef] [Green Version]
- Jacobi, E.; von Engelhardt, J. Diversity in AMPA receptor complexes in the brain. Curr. Opin. Neurobiol. 2017, 45, 32–38. [Google Scholar] [CrossRef]
- Adamczyk, A.; Mejias, R.; Takamiya, K.; Yocum, J.; Krasnova, I.N.; Calderon, J.; Cadet, J.L.; Huganir, R.L.; Pletnikov, M.V.; Wang, T. GluA3-deficiency in mice is associated with increased social and aggressive behavior and elevated dopamine in striatum. Behav. Brain Res. 2012, 229, 265–272. [Google Scholar] [CrossRef] [Green Version]
- Gorlova, A.; Pavlov, D.; Anthony, D.C.; Ponomarev, E.D.; Sambon, M.; Proshin, A.; Shafarevich, I.; Babaevskaya, D.; Lesch, K.-P.; Bettendorff, L.; et al. Thiamine and benfotiamine counteract ultrasound-induced aggression, normalize AMPA receptor expression and plasticity markers, and reduce oxidative stress in mice. Neuropharmacology 2019, 156, 107543. [Google Scholar] [CrossRef]
- Bartholow, B.D. The aggressive brain: Insights from neuroscience. Curr. Opin. Psychol. 2018, 19, 60–64. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, M.R.; Potenza, M.N. Recent insights into the neurobiology of impulsivity. Curr. Addict. Rep. 2014, 1, 309–319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walker, S.E.; Papilloud, A.; Huzard, D.; Sandi, C. The link between aberrant hypothalamic–pituitary–adrenal axis activity during development and the emergence of aggression—Animal studies. Neurosci. Biobehav. Rev. 2018, 91, 138–152. [Google Scholar] [CrossRef] [PubMed]

| Name | Forward | Reverse |
|---|---|---|
| Mouse Glut1 | TCAACACGGCCTTCACTG | CACGATGCTCAGATAGGACATC |
| Mouse Fos (c-Fos) | GGGGACAGCCTTTCCTACTA | CTGTCACCGTGGGGATAAAG |
| Mouse Rbfox3 (NeuN) | CACCACTCTCTTGTCCGTTTGC | GGCTGAGCATATCTGTAAGCTGC |
| Mouse Bdnf | TGCAGGGGCATAGACAAAAGG | CTTATGAATCGCCAGCCAATTCTC |
| Mouse Maoa | TCAATGTAGCCACTCCACTGT | TTGGGGATAAAGTGAAGCTGA |
| Mouse Cdh13 | GGCAATTGACAGTGGCAACC | TGCAGGAGCACACTTGTACC |
| Mouse 5-HT6-R | GCATAGCTCAGGCCGTATGT | CACCACTGTGAGAGGTCCAC |
| Mouse GluA1 | GGACAACTCAAGCGTCCAGA | GTCGGTAGGAATAGCCCACG |
| Mouse GluA2 | GCGTGGAAATAGAAAGGGCC | ACTCCAGTACCCAATCTTCCG |
| Mouse GluA3 | ACCATCAGCATAGGTGGACTT | ACGTGGTAGTTCAAATGGAAGG |
| Mouse GluA4 | GGCCAGGGAATTGACATGGA | CCTTTCGAGGTCCTGTGCTT |
| Mouse β-actin | TGTGATGGTGGGAATGGGTCAGAA | TGTGGTGCCAGATCTTCTCCATGT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Furukawa, M.; Tada, H.; Raju, R.; Wang, J.; Yokoi, H.; Yamada, M.; Shikama, Y.; Matsushita, K. Long-Term Soft-Food Rearing in Young Mice Alters Brain Function and Mood-Related Behavior. Nutrients 2023, 15, 2397. https://doi.org/10.3390/nu15102397
Furukawa M, Tada H, Raju R, Wang J, Yokoi H, Yamada M, Shikama Y, Matsushita K. Long-Term Soft-Food Rearing in Young Mice Alters Brain Function and Mood-Related Behavior. Nutrients. 2023; 15(10):2397. https://doi.org/10.3390/nu15102397
Chicago/Turabian StyleFurukawa, Masae, Hirobumi Tada, Resmi Raju, Jingshu Wang, Haruna Yokoi, Mitsuyoshi Yamada, Yosuke Shikama, and Kenji Matsushita. 2023. "Long-Term Soft-Food Rearing in Young Mice Alters Brain Function and Mood-Related Behavior" Nutrients 15, no. 10: 2397. https://doi.org/10.3390/nu15102397
APA StyleFurukawa, M., Tada, H., Raju, R., Wang, J., Yokoi, H., Yamada, M., Shikama, Y., & Matsushita, K. (2023). Long-Term Soft-Food Rearing in Young Mice Alters Brain Function and Mood-Related Behavior. Nutrients, 15(10), 2397. https://doi.org/10.3390/nu15102397






