Milk Allergen Micro-Array (MAMA) for Refined Detection of Cow’s-Milk-Specific IgE Sensitization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cow-Milk-Allergic Patients, Sera and PBMC Samples
2.2. ImmunoCAP ISAC Measurements
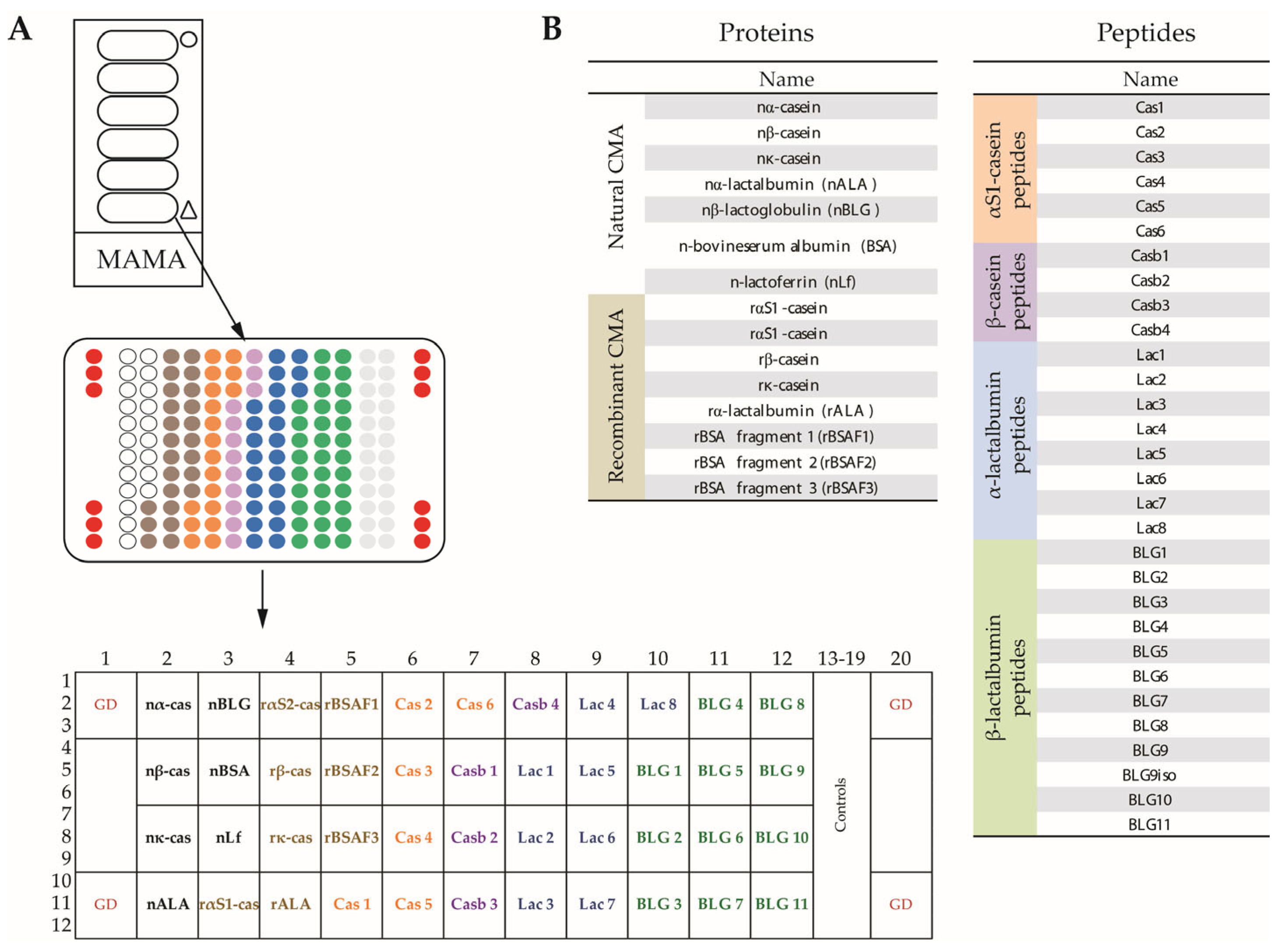
2.3. Milk Allergen Micro-Array (MAMA)
2.4. Statistical Analyses
3. Results
3.1. Characterization of CM-Allergic Patients
3.2. Creation of MAMA
3.3. MAMA, but Not ImmunoCAP, or Skin Testing with CM Extracts Identifies all Patients with CMA According to a Sampson Score of 1–5
3.4. All CM-Allergic Patients with a Sampson Grade of 4–5 (but Bot Those with a Score of 1–3) Show Casein-Specific IgE Reactivity
3.5. The Use of MAMA Reveals IgE Reactivity to Cryptic Milk-Allergen-Derived Peptides
3.6. IgE Levels to CM Allergens and Allergen-Derived Peptides Are Higher in Patients with Anaphylactic Reactions to CM than in Patients without Anaphylactic Symptoms
3.7. CM-Allergic Patients Who Develop Tolerance after an Elimination Diet Show a Drop of Allergen-Specific IgE When Using MAMA
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MAMA | Milk allergen micro-array |
| CM | Cow´s milk |
| CMA | Cow’s milk allergy |
| BSA | Bovine serum albumin |
| HSA | Human serum albumin |
| AIT | Allergen-specific immunotherapy |
| OIT | Oral immunotherapy |
| GI | Gastrointestinal symptoms |
| BA | Bronchial asthma |
| AR | Allergic rhinitis |
| AD | Atopic dermatitis |
| FA | Food allergy |
| SPT | Skin prick test |
| MV | Median value |
| ISU | ISAC standardized units |
| ISU-E | IgE level presented in ISAC standardized units |
| A4–5 | Patients with milk-related anaphylaxis with a Sampson score of 4–5 |
| A1–3 | Patients with milk-related anaphylaxis with a Sampson score of 1–3 |
| NA | Patients without anaphylaxis |
| na-cas | natural alpha casein |
| nALA | natural alpha lactalbumin |
| nBLG | natural betalagtoglobulin |
References
- Arasi, S.; Cafarotti, A.; Fiocchi, A. Cow’s milk allergy. Curr. Opin. Allergy Clin. Immunol. 2022, 22, 181–187. [Google Scholar] [CrossRef]
- Östblom, E.; Lilja, G.; Pershagen, G.; van Hage, M.; Wickman, M. Phenotypes of food hypersensitivity and development of allergic diseases during the first 8 years of life. Clin. Exp. Allergy 2008, 38, 1325–1332. [Google Scholar] [CrossRef]
- Valenta, R.; Hochwallner, H.; Linhart, B.; Pahr, S. Food Allergies: The Basics. Gastroenterology 2015, 148, 1120–1131.e4. [Google Scholar] [CrossRef] [PubMed]
- Fiocchi, A.; Brozek, J.; Schünemann, H.; Bahna, S.L.; von Berg, A.; Beyer, K.; Bozzola, M.; Bradsher, J.; Compalati, E.; Ebisawa, M.; et al. World Allergy Organization (WAO) Diagnosis and Rationale for Action against Cow’s Milk Allergy (DRACMA) Guidelines. World Allergy Organ. J. 2010, 3, 57–161. [Google Scholar] [CrossRef] [PubMed]
- Canani, R.B.; Ruotolo, S.; Discepolo, V.; Troncone, R. The diagnosis of food allergy in children. Curr. Opin. Pediatr. 2008, 20, 584–589. [Google Scholar] [CrossRef] [PubMed]
- Lieberman, J.A.; Sicherer, S.H. Diagnosis of Food Allergy: Epicutaneous Skin Tests, In Vitro Tests, and Oral Food Challenge. Curr. Allergy Asthma Rep. 2011, 11, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Perry, T.T.; Matsui, E.C.; Conover-Walker, M.K.; Wood, R.A. Risk of oral food challenges. J. Allergy Clin. Immunol. 2004, 114, 1164–1168. [Google Scholar] [CrossRef]
- Sporik, R.; Hill, D.J.; Hosking, C.S. Specificity of allergen skin testing in predicting positive open food challenges to milk, egg and peanut in children. Clin. Exp. Allergy 2000, 30, 1541–1546. [Google Scholar] [CrossRef]
- Hochwallner, H.; Schulmeister, U.; Swoboda, I.; Balic, N.; Geller, B.; Nystrand, M.; Harlin, A.; Thalhamer, J.; Scheiblhofer, S.; Niggemann, B.; et al. Microarray and allergenic activity assessment of milk allergens. Clin. Exp. Allergy 2010, 40, 1809–1818. [Google Scholar] [CrossRef]
- Sampson, H.A.; Ho, D.G. Relationship between food-specific IgE concentrations and the risk of positive food challenges in children and adolescents. J. Allergy Clin. Immunol. 1997, 100, 444–451. [Google Scholar] [CrossRef]
- Cuomo, B.; Indirli, G.C.; Bianchi, A.; Arasi, S.; Caimmi, D.; Dondi, A.; La Grutta, S.; Panetta, V.; Verga, M.C.; Calvani, M. Specific IgE and skin prick tests to diagnose allergy to fresh and baked cow’s milk according to age: A systematic review. Ital. J. Pediatr. 2017, 43, 93. [Google Scholar] [CrossRef] [PubMed]
- Hochwallner, H.; Schulmeister, U.; Swoboda, I.; Spitzauer, S.; Valenta, R. Cow’s milk allergy: From allergens to new forms of diagnosis, therapy and prevention. Methods 2014, 66, 22–33. [Google Scholar] [CrossRef] [PubMed]
- Matricardi, P.M.; Kleine-Tebbe, J.; Hoffmann, H.J.; Valenta, R.; Hilger, C.; Hofmaier, S.; Aalberse, R.C.; Agache, I.; Asero, R.; Ballmer-Weber, B.; et al. EAACI Molecular Allergology User’s Guide. Pediatr. Allergy Immunol. 2016, 27, S23. [Google Scholar] [CrossRef] [PubMed]
- Valenta, R.; Lidholm, J.; Niederberger, V.; Hayek, B.; Kraft, D.; Grönlund, H. The recombinant allergen-based concept of component-resolved diagnostics and immunotherapy (CRD and CRIT). Clin. Exp. Allergy 1999, 29, 896–904. [Google Scholar] [CrossRef] [PubMed]
- Linhart, B.; Freidl, R.; Elisyutina, O.; Khaitov, M.; Karaulov, A.; Valenta, R. Molecular Approaches for Diagnosis, Therapy and Prevention of Cow’s Milk Allergy. Nutrients 2019, 11, 1492. [Google Scholar] [CrossRef] [PubMed]
- Cerecedo, I.; Zamora, J.; Shreffler, W.G.; Lin, J.; Bardina, L.; Dieguez, M.C.; Wang, J.; Muriel, A.; de la Hoz, B.; Sampson, H.A. Mapping of the IgE and IgG4 sequential epitopes of milk allergens with a peptide microarray–based immunoassay. J. Allergy Clin. Immunol. 2008, 122, 589–594. [Google Scholar] [CrossRef] [PubMed]
- Savilahti, E.; Kuitunen, M. Allergenicity of cow milk proteins. J. Pediatr. 1992, 121, S12–S20. [Google Scholar] [CrossRef]
- Karsonova, A.V.; Riabova, K.A.; Khaitov, M.R.; Elisyutina, O.G.; Ilina, N.; Fedenko, E.S.; Fomina, D.S.; Beltyukov, E.; Bondarenko, N.L.; Evsegneeva, I.V.; et al. Milk-Specific IgE Reactivity Without Symptoms in Albumin-Sensitized Cat Allergic Patients. Allergy Asthma Immunol. Res. 2021, 13, 668–670. [Google Scholar] [CrossRef]
- Ansotegui, I.J.; Melioli, G.; Canonica, G.W.; Gómez, R.M.; Jensen-Jarolim, E.; Ebisawa, M.; Luengo, O.; Caraballo, L.; Passalacqua, G.; Poulsen, L.K.; et al. A WAO—ARIA—GA2LEN consensus document on molecular-based allergy diagnosis (PAMD@): Update 2020. World Allergy Organ. J. 2020, 13, 100091. [Google Scholar] [CrossRef]
- Lin, J.; Bardina, L.; Shreffler, W.G.; Andreae, D.A.; Ge, Y.; Wang, J.; Bruni, F.M.; Fu, Z.; Han, Y.; Sampson, H.A. Development of a novel peptide microarray for large-scale epitope mapping of food allergens. J. Allergy Clin. Immunol. 2009, 124, 315–322.e3. [Google Scholar] [CrossRef]
- Beyer, K.; Jarvinen, K.-M.; Bardina, L.; Mishoe, M.; Turjanmaa, K.; Niggemann, B.; Ahlstedt, S.; Venemalm, L.; Sampson, H.A. IgE-binding peptides coupled to a commercial matrix as a diagnostic instrument for persistent cow’s milk allergy. J. Allergy Clin. Immunol. 2005, 116, 704–705. [Google Scholar] [CrossRef] [PubMed]
- Sackesen, C.; Suárez-Fariñas, M.; Silva, R.; Lin, J.; Schmidt, S.; Getts, R.; Gimenez, G.; Yilmaz, E.A.; Cavkaytar, O.; Buyuktiryaki, B.; et al. A new Luminex-based peptide assay to identify reactivity to baked, fermented, and whole milk. Allergy 2019, 74, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Savilahti, E.M.; Rantanen, V.; Lin, J.S.; Karinen, S.; Saarinen, K.M.; Goldis, M.; Mäkelä, M.J.; Hautaniemi, S.; Savilahti, E.; Sampson, H.A. Early recovery from cow’s milk allergy is associated with decreasing IgE and increasing IgG4 binding to cow’s milk epitopes. J. Allergy Clin. Immunol. 2010, 125, 1315–1321.e9. [Google Scholar] [CrossRef] [PubMed]
- Caubet, J.-C.; Nowak-Wegrzyn, A.; Moshier, E.; Godbold, J.; Wang, J.; Sampson, H.A. Utility of casein-specific IgE levels in predicting reactivity to baked milk. J. Allergy Clin. Immunol. 2013, 131, 222–224.e4. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Lin, J.; Bardina, L.; Goldis, M.; Nowak-Wegrzyn, A.; Shreffler, W.G.; Sampson, H.A. Correlation of IgE/IgG4 milk epitopes and affinity of milk-specific IgE antibodies with different phenotypes of clinical milk allergy. J. Allergy Clin. Immunol. 2010, 125, 695–702. [Google Scholar] [CrossRef] [PubMed]
- Sicherer, S.H.; A Sampson, H. 9. Food allergy. J. Allergy Clin. Immunol. 2006, 117, 470–475. [Google Scholar] [CrossRef]
- Muraro, A.; Roberts, G.; Clark, A.; Eigenmann, P.; Halken, S.; Lack, G.; Moneret-Vautrin, A.; Niggemann, B.; Rancé, F.; EAACI Task Force on Anaphylaxis in Children. The management of anaphylaxis in childhood: Position paper of the European academy of allergology and clinical immunology. Allergy 2007, 62, 857–871. [Google Scholar] [CrossRef]
- Dribin, T.E.; Schnadower, D.; Spergel, J.M.; Campbell, R.L.; Shaker, M.; Neuman, M.I.; Michelson, K.A.; Capucilli, P.S.; Camargo, C.A., Jr.; Brousseau, D.C.; et al. Severity grading system for acute allergic reactions: A multidisciplinary Delphi study. J. Allergy Clin. Immunol. 2021, 148, 173–181. [Google Scholar] [CrossRef]
- Gattinger, P.; Niespodziana, K.; Stiasny, K.; Sahanic, S.; Tulaeva, I.; Borochova, K.; Dorofeeva, Y.; Schlederer, T.; Sonnweber, T.; Hofer, G.; et al. Neutralization of SARS-CoV-2 requires antibodies against conformational receptor-binding domain epitopes. Allergy 2022, 77, 230–242. [Google Scholar] [CrossRef]
- Lupinek, C.; Wollmann, E.; Baar, A.; Banerjee, S.; Breiteneder, H.; Broecker, B.M.; Bublin, M.; Curin, M.; Flicker, S.; Garmatiuk, T.; et al. Advances in allergen-microarray technology for diagnosis and monitoring of allergy: The MeDALL allergen-chip. Methods 2014, 66, 106–119. [Google Scholar] [CrossRef]
- Niespodziana, K.; Stenberg-Hammar, K.; Megremis, S.; Cabauatan, C.R.; Napora-Wijata, K.; Vacal, P.C.; Gallerano, D.; Lupinek, C.; Ebner, D.; Schlederer, T.; et al. PreDicta chip-based high resolution diagnosis of rhinovirus-induced wheeze. Nat. Commun. 2018, 9, 2382. [Google Scholar] [CrossRef]
- Holm, S. A simple sequentially rejective multiple test procedure. Scand. J. Stat. 1979, 6, 65–70. [Google Scholar]
- Schulmeister, U.; Hochwallner, H.; Swoboda, I.; Focke-Tejkl, M.; Geller, B.; Nystrand, M.; Härlin, A.; Thalhamer, J.; Scheiblhofer, S.; Keller, W.; et al. Cloning, Expression, and Mapping of Allergenic Determinants of αS1-Casein, a Major Cow’s Milk Allergen. J. Immunol. 2009, 182, 7019–7029. [Google Scholar] [CrossRef] [PubMed]
- Hochwallner, H.; Schulmeister, U.; Swoboda, I.; Focke-Tejkl, M.; Civaj, V.; Balic, N.; Nystrand, M.; Härlin, A.; Thalhamer, J.; Scheiblhofer, S.; et al. Visualization of clustered IgE epitopes on α-lactalbumin. J. Allergy Clin. Immunol. 2010, 125, 1279–1285.e9. [Google Scholar] [CrossRef] [PubMed]
- Ahrens, B.; De Oliveira, L.C.L.; Grabenhenrich, L.; Schulz, G.; Niggemann, B.; Wahn, U.; Beyer, K. Individual cow’s milk allergens as prognostic markers for tolerance development? Clin. Exp. Allergy 2012, 42, 1630–1637. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.; Workman, L.; Schuyler, A.J.; Rifas-Shiman, S.L.; McGowan, E.C.; Oken, E.; Gold, D.R.; Hamilton, R.G.; Platts-Mills, T.A. Allergen sensitization in a birth cohort at midchildhood: Focus on food component IgE and IgG4 responses. J. Allergy Clin. Immunol. 2018, 141, 419–423.e5. [Google Scholar] [CrossRef] [PubMed]
- Lupinek, C.; Wollmann, E.; Valenta, R. Monitoring Allergen Immunotherapy Effects by Microarray. Curr. Treat. Options Allergy 2016, 3, 189–203. [Google Scholar] [CrossRef]
- van Hage, M.; Hamsten, C.; Valenta, R. ImmunoCAP assays: Pros and cons in allergology. J. Allergy Clin. Immunol. 2017, 140, 974–977. [Google Scholar] [CrossRef]





| Patients | Number | Gender | Age | CM-Related Symptoms | SPT CM | Total IgE | sIgE to CM | Other Allergy |
|---|---|---|---|---|---|---|---|---|
| n % of total | f/m (%) | Years [Q1–Q3] (min–max) | Type: n | n | kU/L [Q1–Q3] (min–max), n | kUA/L [Q1–Q3] (min-max), n | Type: n | |
| Total CM | 80 | 28/52 | MV: 3.1 | Skin: 62 | pos: 27 | MV: 99 | MV: 4.6 | BA: 18 |
| allergic | 100% | (35/65%) | [2.0–5.5] | GI: 42 | neg: 6 | [34.1–649] | [0.9–24.3] | AR: 28 |
| (0.5–12) | Sys: 41 | nd: 46 | (6.7–7372) n = 67 | (0–100) n = 57 | FA: 52 | |||
| With anaphylaxis | 41 | 14/27 | MV: 5.0 | Skin: 29 | pos: 16 | MV: 212 | MV: 20.4 | BA: 12 |
| 51.25% | (34.2/65.8%) | [2.9–8.5] | GI: 20 | neg: 1 | [45.9–705] | [8–93.9] | AR: 18 | |
| (1–12) | Sys: 41 | nd: 24 | (6.7–7372) n = 36 | (0.12–100) n = 26 | FA: 29 | |||
| A4-5 | 20 | 7/13 | MV: 4.9 | Skin: 14 | pos: 3 | MV: 257.5 | MV: 46.6 | BA: 8 |
| Sampson score 4-5 | 25% | (35/65%) | [2.9–8.8] | GI: 9 | neg: 0 | [95–791.3] | [20–98] | AR: 9 |
| (2.3–12) | Sys: 20 | nd: 17 | (6.7–7372) n = 18 | (6–100) n = 12 | FA: 15 | |||
| A1-3 | 21 | 7/14 | MV: 5.0 | Skin: 15 | pos: 13 | MV: 95 | MV: 13.3 | BA: 4 |
| Sampson score 1-3 | 26.25% | (33.3/66.7%) | [2.5–8.5] | GI: 11 | neg: 1 | [43.3–699.3] | [2.1–46.1] | AR: 9 |
| (1–11.2) | Sys: 21 | nd: 7 | (20.2–4030) n = 18 | (0.12–100) n = 14 | FA: 14 | |||
| NA | 39 | 14/25 | MV: 2.5 | Skin: 33 | pos: 11 | MV: 68.5 | MV: 1.3 | BA: 6 |
| Without | 48.75% | (35.9/64.1%) | [1.3–3.9] | GI: 22 | neg: 5 | [23.3–311] | [0.4–3.6] | AR: 10 |
| anaphylaxis | (0.5–6) | Sys: 0 | nd: 22 | (7.5–1661) n = 31 | (0–100) n = 31 | FA: 23 |
| Time | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Patient ID | Gender | Age (Months) | Difference (Months) | Tolerance Development | Tolerance to Milk | Symptoms | Eosinophils | Total IgE | ||
| Severity | Other | % | ×109/L | (kU/L) | ||||||
| 1 | f | 34 | 18 | no | 5 | BA, AR, GI | 8 | 0.68 | 440 | |
| 1a | 52 | no | no | 5 | BA, AR, GI | 5 | 0.6 | 440 | ||
| 25 | m | 44 | 18 | no | 3 | AD, BA | 5 | 0.4 | 39.5 | |
| 25a | 62 | no | no | 3 | AD, BA | 5 | 0.4 | 39.5 | ||
| 65 | m | 72 | 9 | baked | 0 | AD, AR, GI | 6.3 | 0.42 | nd | |
| 65a | 81 | no | baked | 0 | AD, AR, GI | 6.3 | 0.42 | nd | ||
| 66 | m | 16 | 18 | baked | 0 | AD, AR, GI | 2 | 0.02 | 248.7 | |
| 66a | 34 | no | baked | 0 | AD, AR, GI | 2 | 0.13 | 127.8 | ||
| 11 | m | 58 | 11 | no | 4 | AD, AR, GI | 8 | 0.67 | nd | |
| 11a | 69 | no | no | 4 | AD, AR, GI | 5 | 0.4 | nd | ||
| 36 | f | 35 | 18 | no | 2 | AR, GI | 3.6 | 0.3 | 32.3 | |
| 36a | 53 | yes | baked | 0 | AR | 3.6 | 0.3 | 32.3 | ||
| 32 | m | 134 | 9 | no | 2 | AR, GI | 3.7 | 0.3 | 48.1 | |
| 32a | 143 | yes | baked | 0 | 0 | nd | nd | nd | ||
| 38 | f | 88 | 18 | no | 2 | AD, AR, GI | 3 | 0.2 | 82.5 | |
| 38a | 106 | yes | baked | 0 | 0 | nd | nd | nd | ||
| 67 | m | 30 | 18 | baked | 0 | AD, GI | 4 | 0.24 | 78.8 | |
| 67a | 48 | yes | fermented | 0 | AD | 4.6 | 0.3 | 58.6 | ||
| 70 | m | 67 | 12 | baked | 0 | AD, BA, AR, GI | 8.8 | 1 | nd | |
| 70a | 79 | yes | fermented | 0 | BA, AR | 11.6 | 1.27 | nd | ||
| 30 | m | 21 | 8 | no | 3 | AD, GI | 8 | 0.68 | nd | |
| 30a | 29 | yes | baked | 0 | AD | 3.2 | 0.3 | nd | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garib, V.; Trifonova, D.; Freidl, R.; Linhart, B.; Schlederer, T.; Douladiris, N.; Pampura, A.; Dolotova, D.; Lepeshkova, T.; Gotua, M.; et al. Milk Allergen Micro-Array (MAMA) for Refined Detection of Cow’s-Milk-Specific IgE Sensitization. Nutrients 2023, 15, 2401. https://doi.org/10.3390/nu15102401
Garib V, Trifonova D, Freidl R, Linhart B, Schlederer T, Douladiris N, Pampura A, Dolotova D, Lepeshkova T, Gotua M, et al. Milk Allergen Micro-Array (MAMA) for Refined Detection of Cow’s-Milk-Specific IgE Sensitization. Nutrients. 2023; 15(10):2401. https://doi.org/10.3390/nu15102401
Chicago/Turabian StyleGarib, Victoria, Daria Trifonova, Raphaela Freidl, Birgit Linhart, Thomas Schlederer, Nikolaos Douladiris, Alexander Pampura, Daria Dolotova, Tatiana Lepeshkova, Maia Gotua, and et al. 2023. "Milk Allergen Micro-Array (MAMA) for Refined Detection of Cow’s-Milk-Specific IgE Sensitization" Nutrients 15, no. 10: 2401. https://doi.org/10.3390/nu15102401






