RAASi Therapy Attenuates the Association between 24-h Urinary Potassium Excretion and Dietary Potassium Intake in CKD Patients
Abstract
:1. Introduction
2. Subjects and Methods
2.1. Biochemistry
2.2. Assessment of Dietary Intake
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lichtenstein, A.H.; Appel, L.J.; Brands, M.; Carnethon, M.; Daniels, S.; Franch, H.A.; Franklin, B.; Kris-Etherton, P.; Harris, W.S.; Howard, B.; et al. Diet and lifestyle recommendations revision 2006: A scientific statement from the American Heart Association Nutrition Committee. Circulation 2006, 114, 82–96. [Google Scholar] [CrossRef] [PubMed]
- Dyer, A.R.; Elliott, P.; Shipley, M. Urinary electrolyte excretion in 24 hours and blood pressure in the INTERSALT Study. II. Estimates of electrolyte-blood pressure associations corrected for regression dilution bias. The INTERSALT Cooperative Research Group. Am. J. Epidemiol. 1994, 139, 940–951. [Google Scholar] [CrossRef] [PubMed]
- Weaver, C.M. Potassium and health. Adv. Nutr. 2013, 4, 368S–377S. [Google Scholar] [CrossRef] [PubMed]
- Aburto, N.J.; Hanson, S.; Gutierrez, H.; Hooper, L.; Elliott, P.; Cappuccio, F.P. Effect of increased potassium intake on cardiovascular risk factors and disease: Systematic review and meta-analyses. BMJ 2013, 346, f1378. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Liu, T.; Kuklina, E.V.; Flanders, W.D.; Hong, Y.; Gillespie, C.; Chang, M.H.; Gwinn, M.; Dowling, N.; Khoury, M.J.; et al. Sodium and potassium intake and mortality among U.S. adults: Prospective data from the Third National Health and Examination Survey. Arch. Intern. Med. 2011, 171, 1183–1191. [Google Scholar] [CrossRef]
- Chang, H.Y.; Hu, Y.W.; Yue, C.S.J.; Wen, Y.W.; Yeh, W.T.; Hsu, L.S.; Tsai, S.Y.; Pan, W.H. Effect of potassium-enriched salt on cardiovascular mortality and medical expenses of elderly men. Am. J. Clin. Nutr. 2006, 83, 1289–1296. [Google Scholar] [CrossRef]
- Neal, B.; Wu, Y.; Feng, X.; Zhang, R.; Zhang, Y.; Shi, J.; Zhang, J.; Tian, M.; Huang, L.; Li, Z.; et al. Effect of Salt Substitution on Cardiovascular Events and Death. N. Engl. J. Med. 2021, 385, 1067–1077. [Google Scholar] [CrossRef]
- Narasaki, Y.; You, A.S.; Malik, S.; Moore, L.W.; Bross, R.; Cervantes, M.K.; Daza, A.; Kovesdy, C.P.; Nguyen, D.V.; Kalantar-Zadeh, K.; et al. Dietary potassium intake, kidney function, and survival in a nationally representative cohort. Am. J. Clin. Nutr. 2022, 116, 1123–1134. [Google Scholar] [CrossRef]
- Mun, K.H.; Im Yu, G.; Choi, B.Y.; Kim, M.K.; Shin, M.; Shin, D.H. Association of dietary potassium intake with the development of chronic kidney disease and renal function in patients with mildly decreased kidney function: The Korean Multi-Rural Communities Cohort Study. Med. Sci. Monit. 2019, 25, 1061. [Google Scholar] [CrossRef]
- Nagata, T.; Sobajima, H.; Ohashi, N.; Hirakawa, A.; Katsuno, T.; Yasuda, Y.; Matsuo, S.; Tsuboi, N.; Maruyama, S. Association between 24 h urinary sodium and potassium excretion and estimated glomerular filtration rate (eGFR) decline or death in patients with diabetes mellitus and eGFR more than 30 ml/min/1.73m2. PLoS ONE 2016, 11, e0152306. [Google Scholar] [CrossRef]
- Sharma, S.; McFann, K.; Chonchol, M.; De Boer, I.H.; Kendrick, J. Association between dietary sodium and potassium intake with chronic kidney disease in US adults: A cross-sectional study. Am. J. Nephrol. 2013, 37, 526–533. [Google Scholar] [CrossRef] [PubMed]
- Elfassy, T.; Zhang, L.; Raij, L.; Bibbins-Domingo, K.; Lewis, C.E.; Allen, N.B.; Liu, K.J.; Peralta, C.A.; Odden, M.C.; Zeki Al Hazzouri, A. Results of the CARDIA study suggest that higher dietary potassium may be kidney protective. Kidney Int. 2020, 98, 87–194. [Google Scholar] [CrossRef] [PubMed]
- Smyth, A.; Dunkler, D.; Gao, P.; Teo, K.K.; Yusuf, S.; O’Donnell, M.J.; Mann, J.F.; Clase, C.M.; ONTARGET and TRANSCEND investigators. The relationship between estimated sodium and potassium excretion and subsequent renal outcomes. Kidney Int. 2014, 86, 1205–1212. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.W.; Park, J.T.; Yoo, T.; Lee, J.; Chung, W.; Lee, K.; Chae, D.; Ahn, C.; Kang, S.; Choi, K.H.; et al. Urinary potassium excretion and progression of CKD. Clin. J. Am. Soc. Nephrol. 2019, 14, 330–340. [Google Scholar] [CrossRef] [PubMed]
- De Nicola, L.; Di Lullo, L.; Paoletti, E.; Cupisti, A.; Bianchi, S. Chronic hyperkalemia in non-dialysis CKD: Controversial issues in nephrology practice. J. Nephrol. 2018, 31, 653–664. [Google Scholar] [CrossRef]
- Ramos, C.I.; González-Ortiz, A.; Espinosa-Cuevas, A.; Avesani, C.M.; Carrero, J.J.; Cuppari, L. Does dietary potassium intake associate with hyperkalemia in patients with chronic kidney disease? Nephrol. Dial. Transplant. 2021, 36, 2049–2057. [Google Scholar] [CrossRef]
- Palmer, B.F.; Clegg, D.J. Physiology and pathophysiology of potassium homeostasis. Adv. Physiol. Educ. 2016, 40, 480–490. [Google Scholar] [CrossRef]
- De Nicola, L.; Garofalo, C.; Borrelli, S.; Minutolo, R. Recommendations on nutritional intake of potassium in CKD: It’s now time to be more flexible! Kidney Int. 2022, 102, 700–703. [Google Scholar] [CrossRef]
- Borrelli, S.; De Nicola, L.; Minutolo, R.; Conte, G.; Chiodini, P.; Cupisti, A.; Santoro, D.; Calabrese, V.; Giannese, D.; Garofalo, C.; et al. Current Management of Hyperkalemia in Non-Dialysis CKD: Longitudinal Study of Patients Receiving Stable Nephrology Care. Nutrients 2021, 13, 942. [Google Scholar] [CrossRef]
- Clase, C.M.; Carrero, J.J.; Ellison, D.H.; Grams, M.E.; Hemmelgarn, B.R.; Jardine, M.J.; Kovesdy, C.P.; Kline, G.A.; Lindner, G.; Obrador, G.T.; et al. Potassium homeostasis and management of dyskalemia in kidney diseases: Conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int. 2020, 97, 42–61. [Google Scholar] [CrossRef]
- Wei, K.Y.; Gritter, M.; Vogt, L.; de Borst, M.H.; Rotmans, J.I.; Hoorn, E.J. Dietary potassium and the kidney: Lifesaving physiology. Clin. Kidney J. 2020, 13, 952–968. [Google Scholar] [CrossRef] [PubMed]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F., 3rd; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A New Equation to Estimate Glomerular Filtration Rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef]
- Maroni, B.J.; Steinman, T.I.; Mitch, W.E. A method for estimating nitrogen intake of patients with chronic renal failure. Kidney Int. 1985, 27, 58–65. [Google Scholar] [CrossRef]
- D’Alessandro, C.; Giannese, D.; Avino, M.; Cupisti, A. Energy Requirement for Elderly CKD Patients. Nutrients 2021, 13, 3396. [Google Scholar] [CrossRef] [PubMed]
- Banca Dati di Composizione degli Alimenti per Studi Epidemiologi in Italia; Istituto Europeo di Oncologia. Revisione 2022. Available online: www.bda-ieo-it (accessed on 18 May 2023).
- Tinker, L.F.; Huang, Y.; Johnson, K.C.; Carbone, L.D.; Snetselaar, L.; Van Horn, L.; Manson, J.E.; Liu, S.; Mossavar-Rahmani, Y.; Prentice, R.L.; et al. Estimating 24-Hour Urinary Excretion of Sodium and Potassium Is More Reliable from 24-Hour Urine Than Spot Urine Sample in a Feeding Study of US Older Postmenopausal Women. Curr. Dev. Nutr. 2021, 5, nzab125. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, C.; Abreu, S.; Padrão, P.; Pinho, O.; Graça, P.; Breda, J.; Santos, R.; Moreira, P. Sodium and potassium urinary excretion and dietary intake: A cross-sectional analysis in adolescents. Food Nutr. Res. 2016, 60, 29442. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Rodríguez, E.; Ortega, R.M.; Andrés Carvajales, P.; González-Rodríguez, L.G. Relationship between 24 h urinary potassium and diet quality in the adult Spanish population. Public Health Nutr. 2015, 18, 850–859. [Google Scholar] [CrossRef]
- Mente, A.; Irvine, E.J.; Honey, R.J.; Logan, A.G. Urinary potassium is a clinically useful test to detect a poor quality diet. J. Nutr. 2009, 139, 743–749. [Google Scholar] [CrossRef]
- Tasevska, N.; Runswick, S.A.; Bingham, S.A. Urinary potassium is as reliable as urinary nitrogen for use as a recovery biomarker in dietary studies of free living individuals. J. Nutr. 2006, 136, 1334–1340. [Google Scholar] [CrossRef]
- Lobene, A.J.; Stremke, E.R.; McCabe, G.P.; Moe, S.M.; Moorthi, R.N.; Hill Gallant, K.M. Spot Urine Samples to Estimate Na and K Intake in Patients with Chronic Kidney Disease and Healthy Adults: A Secondary Analysis from a Controlled Feeding Study. J. Ren. Nutr. 2021, 31, 602–610. [Google Scholar] [CrossRef]
- Turban, S.; Juraschek, S.P.; Miller, E.R., 3rd; Anderson, C.A.M.; White, K.; Charleston, J.; Appel, L.J. Randomized Trial onthe Effects of Dietary Potassium on Blood Pressure and Serum Potassium Levels in Adults with Chronic Kidney Disease. Nutrients 2021, 13, 2678. [Google Scholar] [CrossRef] [PubMed]
- Suh, S.H.; Song, S.H.; Oh, T.R.; Choi, H.S.; Kim, C.S.; Bae, E.H.; Oh, K.H.; Lee, J.; Han, S.H.; Kim, Y.H.; et al. Association of Urinary Potassium Excretion with Blood Pressure Variability and Cardiovascular Outcomes in Patients with Pre-Dialysis Chronic Kidney Disease. Nutrients 2021, 13, 4443. [Google Scholar] [CrossRef] [PubMed]
- Leonberg-Yoo, A.K.; Tighiouart, H.; Levey, A.S.; Beck, G.J.; Sarnak, M.J. Urine Potassium Excretion. Kidney Failure, and Mortality in CKD. Am. J. Kidney Dis. 2017, 69, 341–349. [Google Scholar] [CrossRef] [PubMed]
- D’Alessandro, C.; Cumetti, A.; Pardini, E.; Mannucci, C.; Serio, P.; Morganti, R.; Cupisti, A. Prevalence and correlates of hyperkalemia in a renal nutrition clinic. Intern Emerg. Med. 2021, 16, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Inker, L.A.; Grams, M.E.; Levey, A.S.; Coresh, J.; Cirillo, M.; Collins, J.F.; Gansevoort, R.T.; Gutierrez, O.M.; Hamano, T.; Heine, G.H.; et al. CKD Prognosis Consortium. Relationship of Estimated GFR and Albuminuria to Concurrent Laboratory Abnormalities: An Individual Participant Data Meta-analysis in a Global Consortium. Am. J. Kidney Dis. 2019, 73, 206–217. [Google Scholar] [CrossRef]
- Kovesdy, C.P. Management of hyperkalaemia in chronic kidney disease. Nat. Rev. Nephrol. 2014, 10, 653–662. [Google Scholar] [CrossRef]
- St-Jules, D.E.; Goldfarb, D.S.; Sevick, M.A. Nutrient Non-equivalence: Does Restricting High-Potassium Plant Foods Help to Prevent Hyperkalemia in Hemodialysis Patients? J. Ren. Nutr. 2016, 26, 282–287. [Google Scholar] [CrossRef]
- Ceccanti, C.; Guidi, L.; D’Alessandro, C.; Cupisti, A. Potassium Bioaccessibility in Uncooked and Cooked Plant Foods: Results from a Static In Vitro Digestion Methodology. Toxins 2022, 14, 668. [Google Scholar] [CrossRef]
- Cupisti, A.; Kovesdy, C.P.; D’Alessandro, C.; Kalantar-Zadeh, K. Dietary Approach to Recurrent or Chronic Hyperkalaemia in Patients with Decreased Kidney Function. Nutrients 2018, 10, 261. [Google Scholar] [CrossRef]
- Kalantar-Zadeh, K.; Fouque, D. Nutritional Management of Chronic Kidney Disease. N. Engl. J. Med. 2017, 377, 1765–1776. [Google Scholar] [CrossRef]
- Noori, N.; Kalantar-Zadeh, K.; Kovesdy, C.P.; Murali, S.B.; Bross, R.; Nissenson, A.R.; Kopple, J.D. Dietary potassium intake and mortality in long-term hemodialysis patients. Am. J. Kidney Dis. 2010, 56, 338–347. [Google Scholar] [CrossRef] [PubMed]
- Bakris, G.L.; Siomos, M.; Richardson, D.; Janssen, I.; Bolton, W.K.; Hebert, L.; Agarwal, R.; Catanzaro, D. ACE inhibition or angiotensin receptor blockade: Impact on potassium in renal failure. VAL-K Study Group. Kidney Int. 2000, 58, 2084–2092. [Google Scholar] [CrossRef] [PubMed]
- Espinel, E.; Joven, J.; Gil, I.; Sune, P.; Renedo, B.; Fort, J.; Seron, D. Risk of hyperkalemia in patients with moderate chronic kidney disease initiating angiotensin converting enzyme inhibitors or angiotensin receptor blockers: A randomized study. BMC Res. Notes 2013, 6, 306–310. [Google Scholar] [CrossRef] [PubMed]
- Malta, D.; Arcand, J.; Ravindran, A.; Floras, V.; Allard, J.P.; Newton, G.E. Adequate intake of potassium does not cause hyperkalemia in hypertensive individuals taking medications that antagonize the renin angiotensin aldosterone system. Am. J. Clin. Nutr. 2016, 104, 990–994. [Google Scholar] [CrossRef]
- Pecoits-Filho, R.; Fliser, D.; Tu, C.; Zee, J.; Bieber, B.; Wong, M.M.Y.; Port, F.; Combe, C.; Lopes, A.A.; Reichel, H.; et al. Prescription of renin-angiotensin-aldosterone system inhibitors (RAASi) and its determinants in patients with advanced CKD under nephrologist care. J. Clin. Hypertens. 2019, 21, 991–1001. [Google Scholar] [CrossRef]
- Rossignol, P.; Ruilope, L.M.; Cupisti, A.; Ketteler, M.; Wheeler, D.C.; Pignot, M.; Cornea, G.; Schulmann, T.; Lund, L.H. Recurrent hyperkalaemia management and use of renin-angiotensin-aldosterone system inhibitors: A European multi-national targeted chart review. Clin. Kidney J. 2019, 13, 714–719. [Google Scholar] [CrossRef]
- Gibson, R.S.; Charrondiere, U.R.; Bell, W. Measurement Errors in Dietary Assessment Using Self-Reported 24-Hour Recalls in Low-Income Countries and Strategies for Their Prevention. Adv. Nutr. 2017, 15, 980–991. [Google Scholar] [CrossRef]
| RAASi | RAASi | ||
|---|---|---|---|
| NO | YES | p | |
| Age, years | 59.4 ± 14.0 | 61.1 ± 13.3 | 0.508 |
| Weight, kg | 77.8 ± 13.8 | 77.0 ± 15.5 | 0.743 |
| BMI, kg/m2 | 28.5 (25.7–30.2) | 25.4 (24.0–28.9) | 0.022 |
| BUN, mg/dL | 28.7 (24.7–39.0) | 34.3 (25.7–45.8) | 0.056 |
| sCreatinine, mg/dL | 1.60 (1.36–2.27) | 1.83 (1.54–2.55) | 0.058 |
| eGFR, mL/min/1.73 m2 | 42.0 (28.5–49.6) | 32.8 (24.0–43.8) | 0.052 |
| sSodium, mEq/L | 141 (139–143) | 142 (140–143) | 0.072 |
| sPotassium, mEq/L | 4.6 (4.2–4.8) | 4.7 (4.3–5.0) | 0.103 |
| sCalcium, mg/dL | 9.6 (9.3–10.1) | 9.5 (9.2–9.9) | 0.202 |
| sPhosphate, mg/dL | 3.4 ± 0.7 | 3.3 ± 0.6 | 0.626 |
| sBicarbonate, mEq/L | 25.0 (23.9–28.3) | 24.0 (22.1–26.0) | 0.059 |
| sAlbumin, g/dL | 4.1 (3.88–4.35) | 4.2 (2.75–4.39) | 0.825 |
| Hemoglobin, g/dL | 13.3 ± 1.6 | 13.3 ± 1.8 | 0.950 |
| PTH, pg/mL | 47.0 (34.0–62.4) | 69.8 (45.1–114) | 0.011 |
| uCreatinine, mg/24-h | 1186 (933–1431) | 1200 (1002–1538) | 0.481 |
| uUrea, g/24-h | 19.2 (15.1–24.0) | 18.4 (15.0–23.0) | 0.718 |
| uSodium, mEq/24-h | 149 (105–200) | 134 (101–170) | 0.147 |
| uPotassium, mEq/24-h | 60.0 (49.2–72.0) | 56.0 42.5–68.9) | 0.228 |
| RAASi | RAASi | ||
|---|---|---|---|
| NO | YES | p | |
| Energy, Kcal | 1833 (1402–2156) | 1833 (1464–2063) | 0.903 |
| Protein, g | 69.4 ± 21.6 | 62.7 ± 17.4 | 0.084 |
| Lipids, g | 60.5 (48.5–75.0) | 63.8 (48.2–76.8) | 0.604 |
| Carbohydrates, g | 248 (170–285) | 231 (190–295) | 0.942 |
| Sodium, mg | 948 (632–1691) | 1109 (719–1658) | 0.440 |
| Potassium, mg | 2077 ± 592 | 2284 ± 656 | 0.063 |
| Calcium, mg | 521 ± 230 | 502 ± 225 | 0.634 |
| Phosphorus, mg | 970 ± 280 | 958 ± 362 | 0.827 |
| Iron, mg | 8.0 (6.25–10.6) | 8.7 (6.55–10.6) | 0.546 |
| Fibers, g | 16.0 (10.4–20.2) | 16.8 (12.3–19.3) | 0.482 |
| RAASi Group | |||||
|---|---|---|---|---|---|
| uK | sK | dK | eGFR | ||
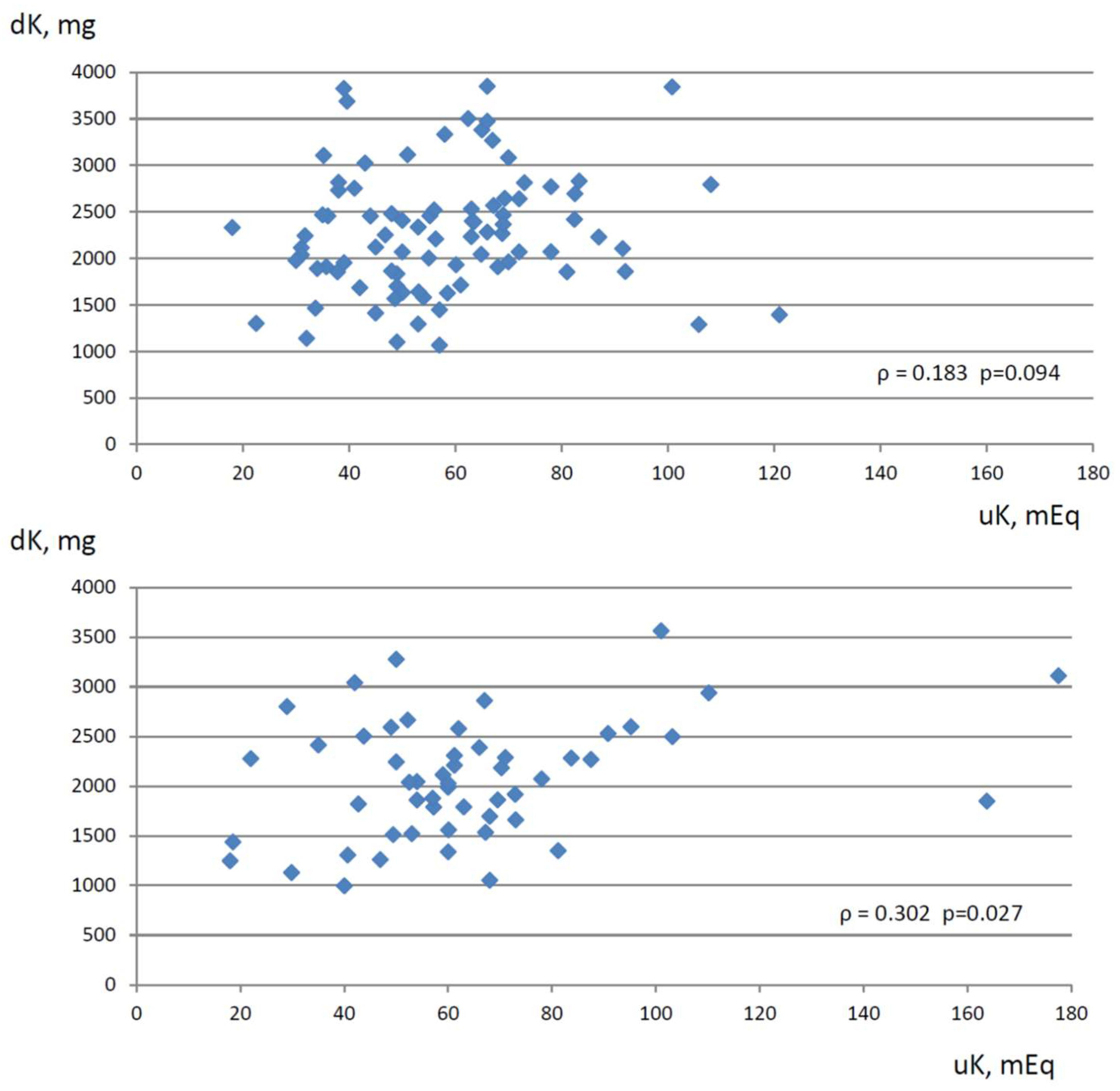
| No RAASi Group | uK | xxxxxx | 0.042 | 0.183 | 0.154 |
| sK | 0.063 | xxxxxx | −0.045 | −0.232 ^ | |
| dK | 0.302 * | −0.072 | xxxxxx | 0.056 | |
| eGFR | 0.343 *** | −0.322 ** | 0.216 | xxxxxx | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giannese, D.; D’Alessandro, C.; Pellegrino, N.; Panichi, V.; Cupisti, A. RAASi Therapy Attenuates the Association between 24-h Urinary Potassium Excretion and Dietary Potassium Intake in CKD Patients. Nutrients 2023, 15, 2454. https://doi.org/10.3390/nu15112454
Giannese D, D’Alessandro C, Pellegrino N, Panichi V, Cupisti A. RAASi Therapy Attenuates the Association between 24-h Urinary Potassium Excretion and Dietary Potassium Intake in CKD Patients. Nutrients. 2023; 15(11):2454. https://doi.org/10.3390/nu15112454
Chicago/Turabian StyleGiannese, Domenico, Claudia D’Alessandro, Nicola Pellegrino, Vincenzo Panichi, and Adamasco Cupisti. 2023. "RAASi Therapy Attenuates the Association between 24-h Urinary Potassium Excretion and Dietary Potassium Intake in CKD Patients" Nutrients 15, no. 11: 2454. https://doi.org/10.3390/nu15112454






