Antioxidant and Antiaging Activity of Fermented Coix Seed Polysaccharides on Caenorhabditis elegans
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
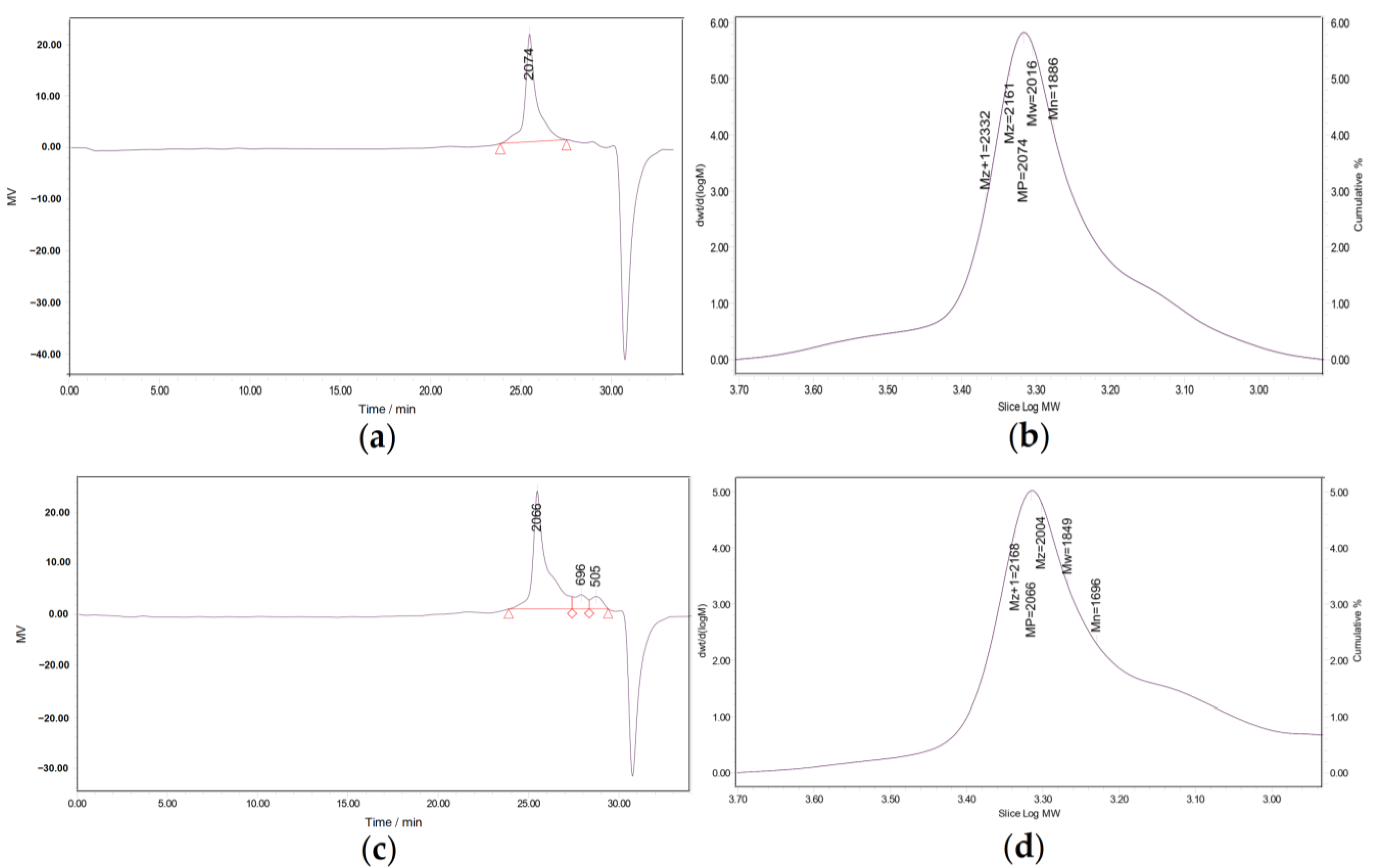
2.3. Molecular Weight Determination of Polysaccharides
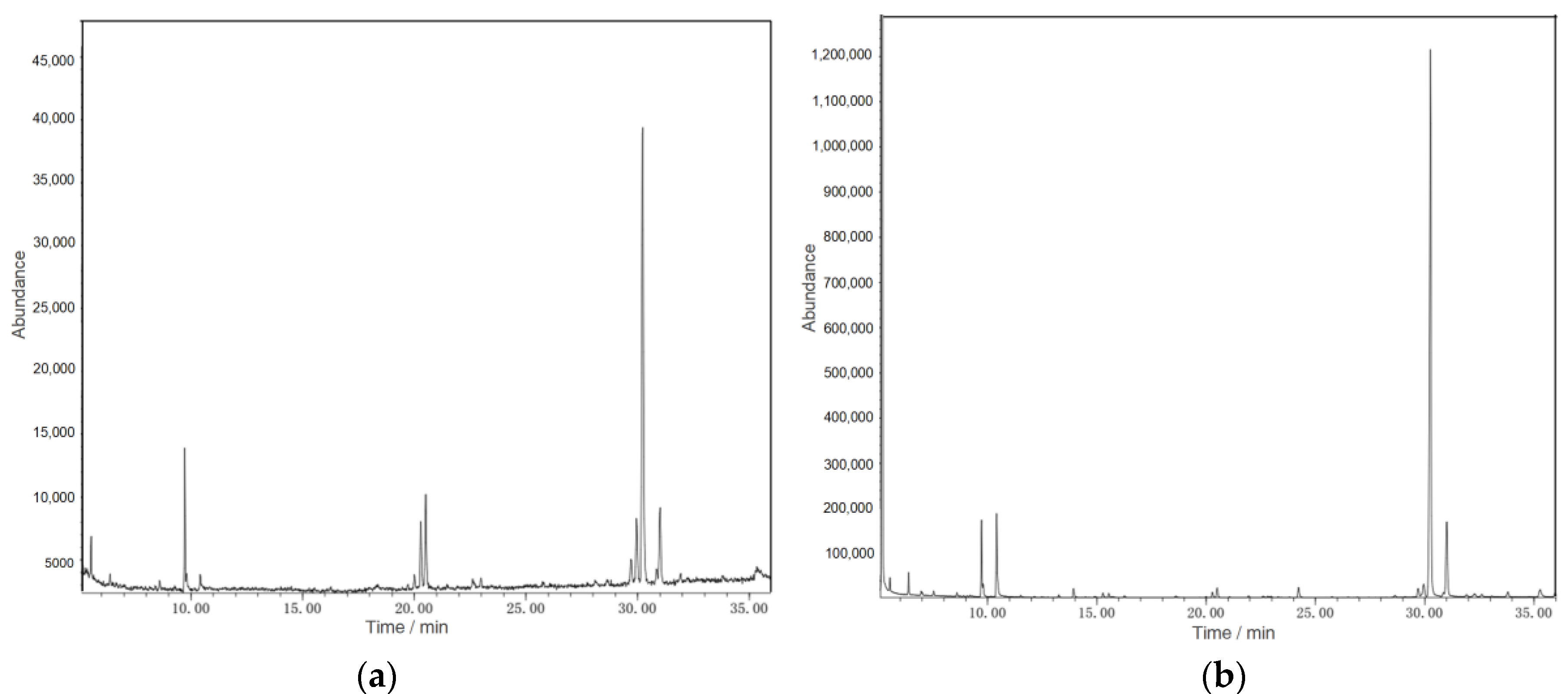
2.4. Analysis of Polysaccharide Components
2.5. Antioxidant Ability Detection In Vitro
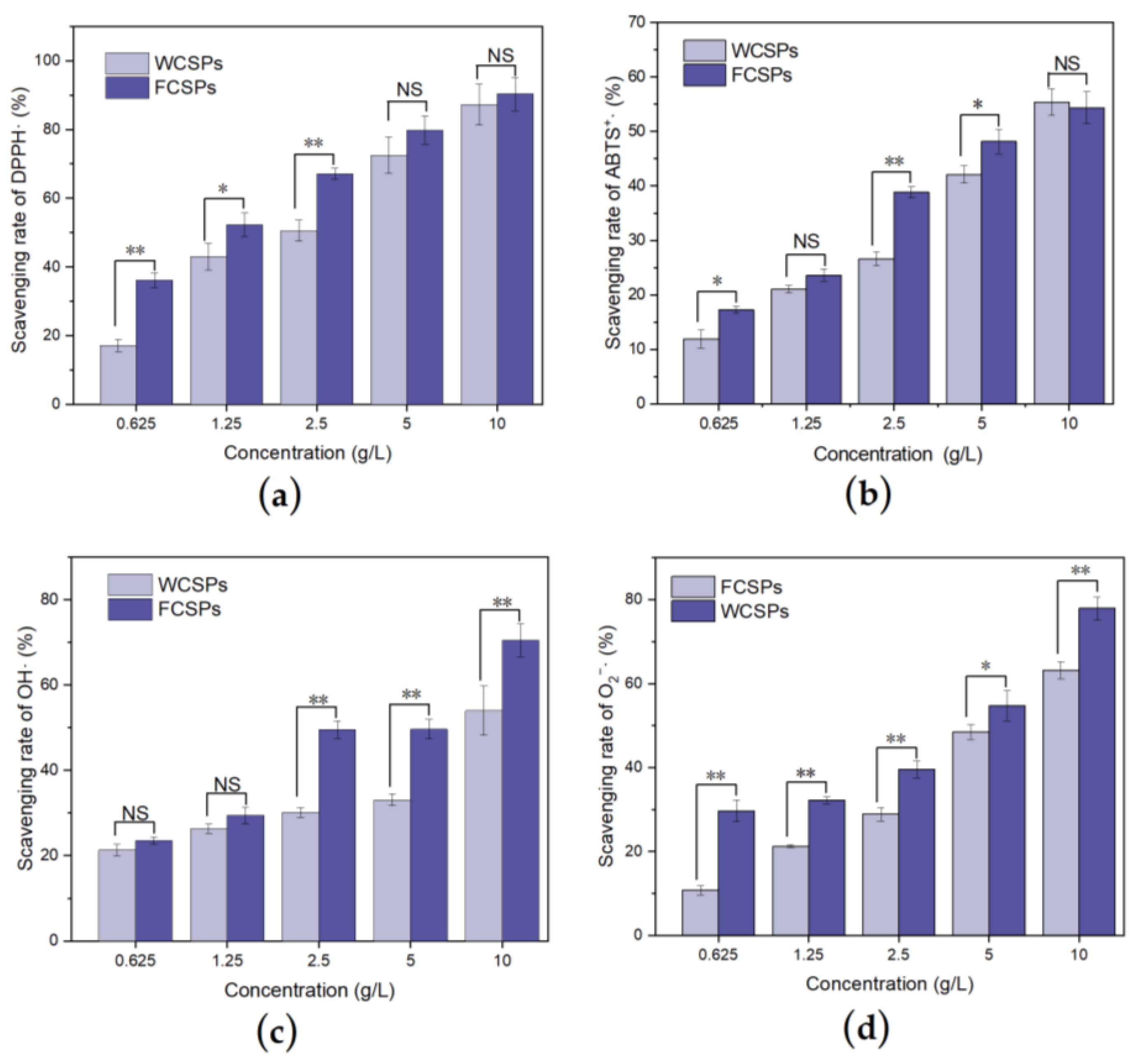
2.5.1. Detection of the DPPH Radical (DPPH·) Scavenging Rate
2.5.2. Detection of the ABTS Radical (ABTS+·) Scavenging Rate
2.5.3. Detection of the Hydroxyl Radical (·OH) Scavenging Rate
2.5.4. Detection of the Superoxide Anion (O2−·) Scavenging Rate
2.6. Antiaging Activity Detection In Vivo
2.6.1. Culture and Synchronization of C. elegans
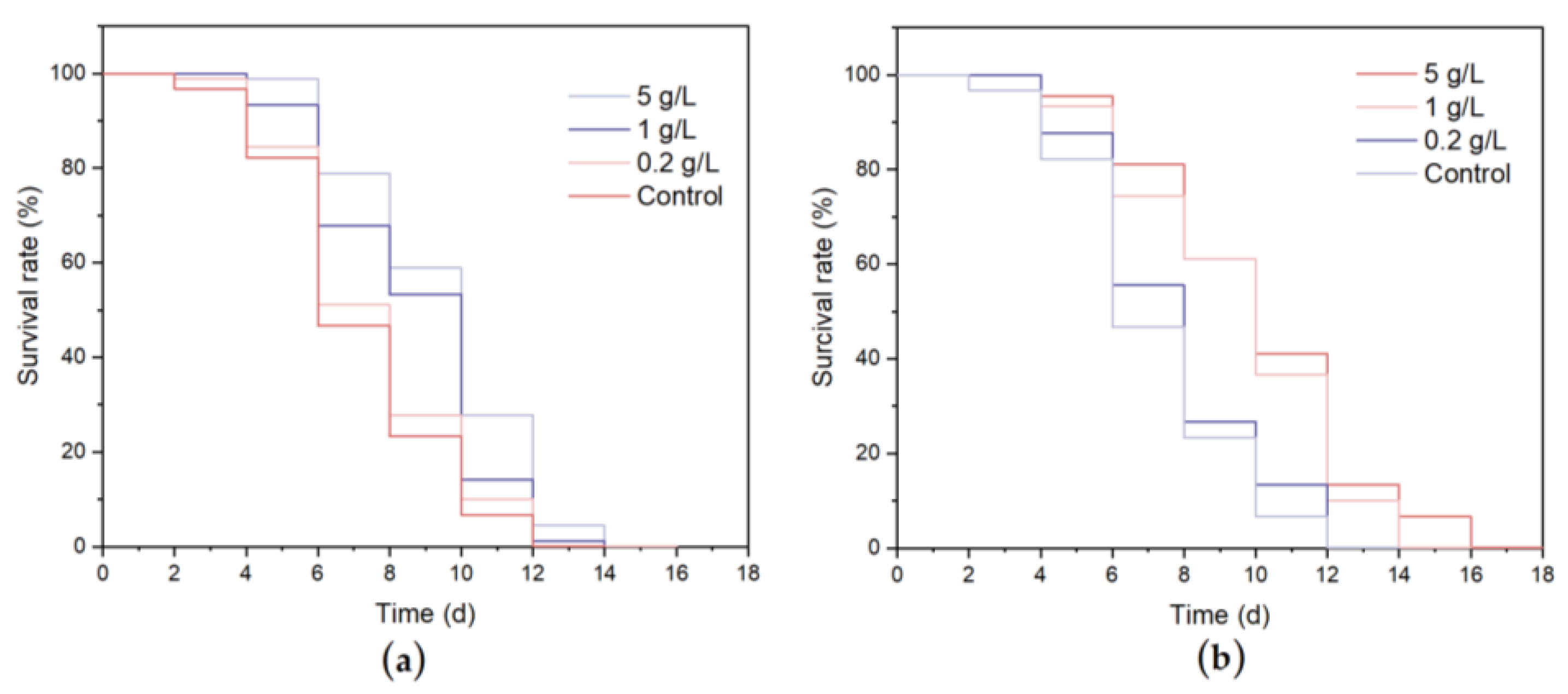
2.6.2. Effects of WCSPs and FCSPs on the Longevity of C. elegans
2.6.3. Effects of WCSPs and FCSPs on the Oviposition Ability of C. elegans
2.6.4. Effects of WCSPs and FCSPs on the Anti-Heat Stress Ability of C. elegans
2.6.5. Effects of WCSPs and FCSPs on the Antioxidant Stress Ability of C. elegans
2.6.6. Detection of Antioxidant Enzyme Activity and MDA Content
2.6.7. Gene Detection via qRT-PCR
2.7. Statistical Analysis
3. Results
3.1. Polysaccharide Composition of WCSPs and FCSPs
3.2. Molecular Weight Detection
3.3. Antioxidant Ability Detection In Vitro
3.4. Antiaging Activity on C. elegans
3.4.1. Lifespan and Oviposition Ability of C. elegans
3.4.2. Stress Resistance of C. elegans
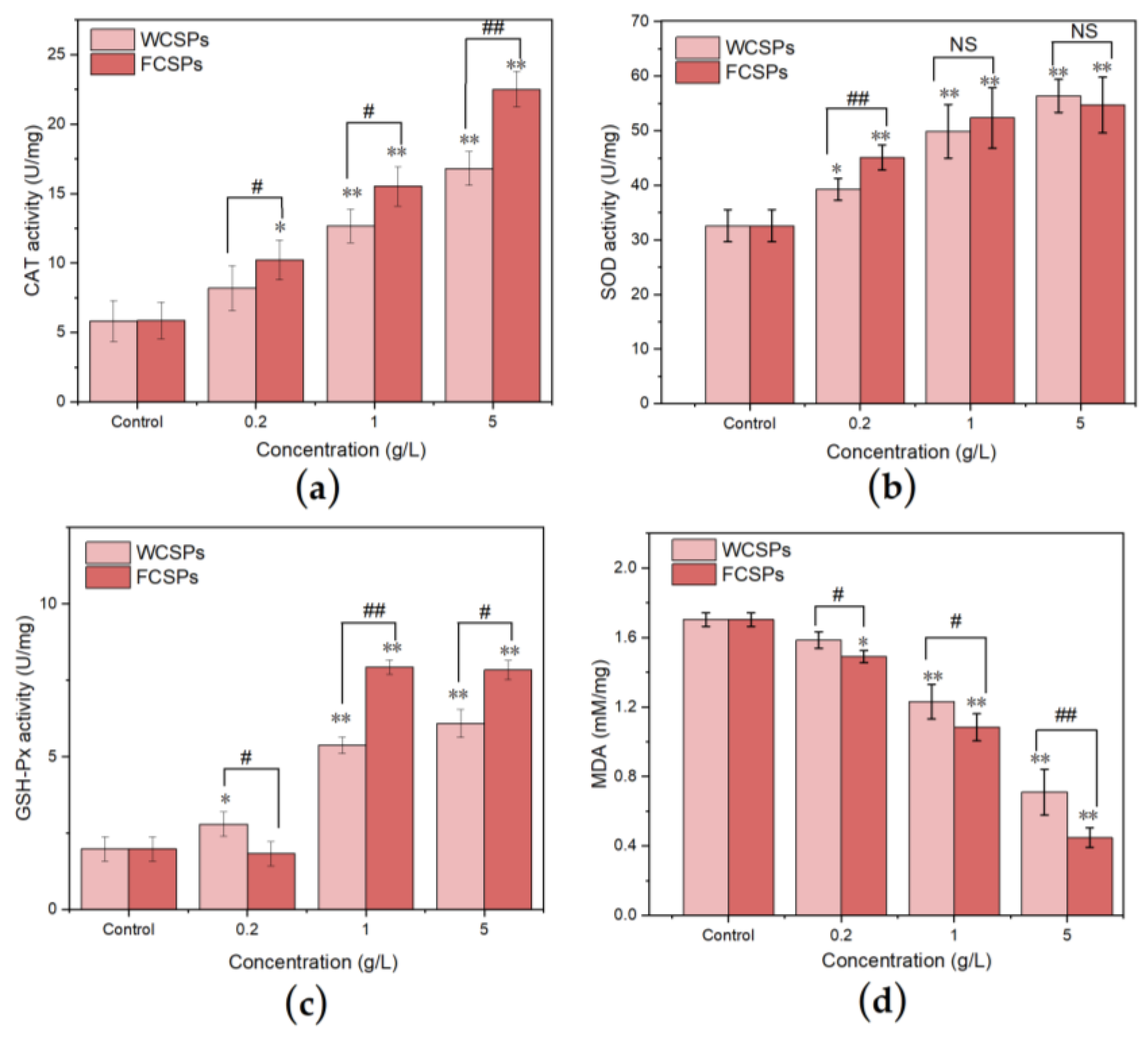
3.4.3. Antioxidant Enzyme Activities and MDA Content Detection in C. elegans
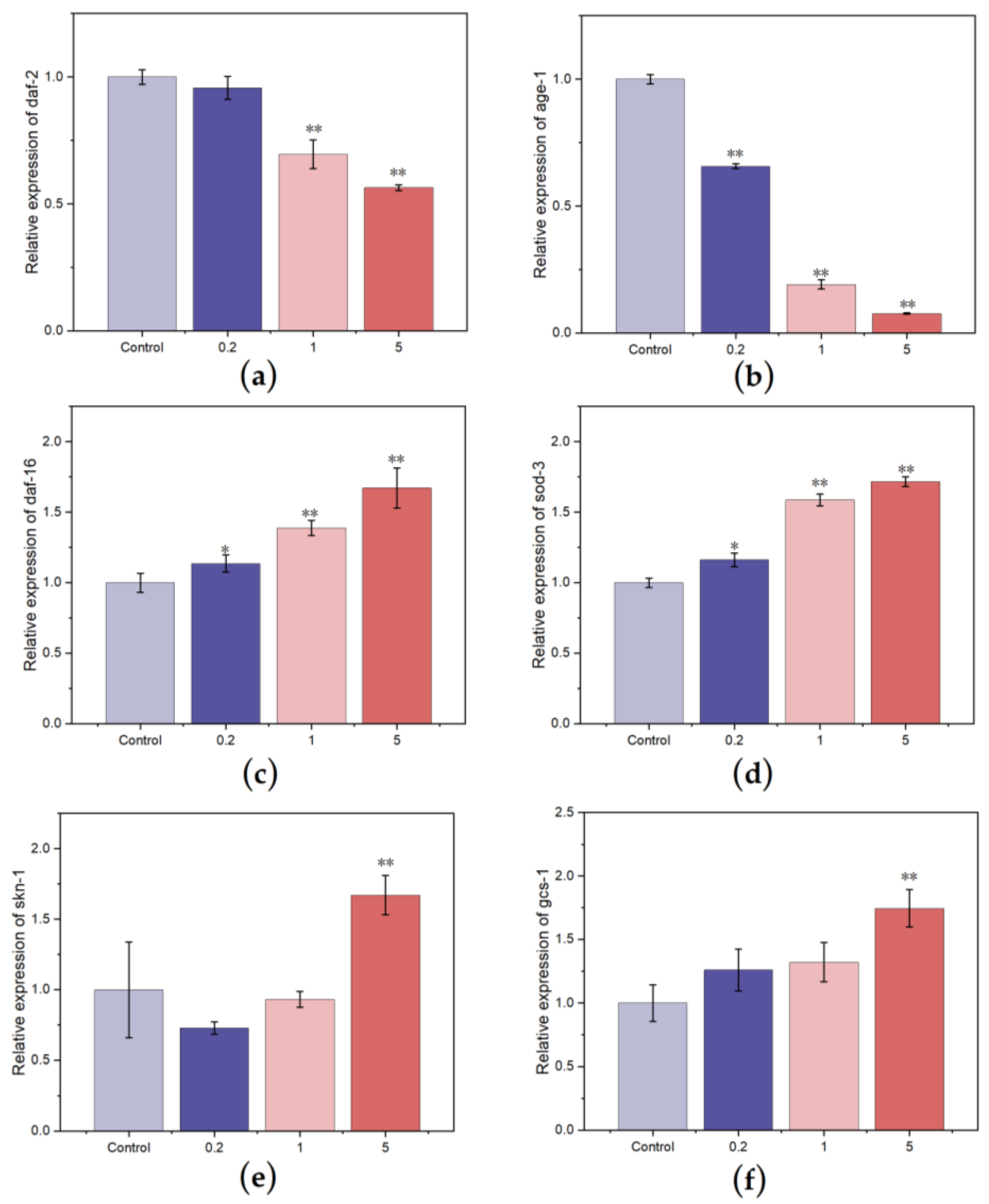
3.4.4. Effect of FCSPs on the Gene Expression of C. elegans
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della, M.D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative stress, aging, and diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef]
- Barja, G. Free radicals and aging. Trends Neurosci. 2004, 27, 595–600. [Google Scholar] [CrossRef]
- Bektas, A.; Schurman, S.H.; Sen, R.; Ferrucci, L. Aging, inflammation and the environment. Exp. Gerontol. 2018, 105, 10–18. [Google Scholar] [CrossRef]
- Warraich, U.E.; Hussain, F.; Kayani, H.U.R. Aging-Oxidative stress, antioxidants and computational modeling. Heliyon 2020, 6, e04107. [Google Scholar] [CrossRef]
- Vaiserman, A.; Koliada, A.; Zayachkivska, A.; Lushchak, O. Nanodelivery of natural antioxidants: An anti-aging perspective. Front. Bioeng. Biotechnol. 2019, 7, 447. [Google Scholar] [CrossRef]
- Chen, L.; Xue, S.; Dai, B.; Zhao, H. Effects of Coix Seed Oil on High Fat Diet-Induced Obesity and Dyslipidemia. Foods 2022, 11, 3267. [Google Scholar] [CrossRef]
- Zhou, Q.; Yu, R.; Liu, T.; Li, Y.; Zhong, J.; Zhang, T.; Liu, Z.; Hu, Y. Coix Seed Diet Ameliorates Immune Function Disorders in Experimental Colitis Mice. Nutrients 2021, 14, 123. [Google Scholar] [CrossRef]
- Chen, C.; Zhang, Y.; Gao, Y.; Xu, Q.; Ju, X.; Wang, L. Identification and anti-tumour activities of phenolic compounds isolated from defatted adlay (Coix lachryma-jobi L. var. ma-yuen Stapf) seed meal. J. Funct. Food. 2016, 26, 394–405. [Google Scholar] [CrossRef]
- Huda, M.N.; Li, X.; Jahan, T.; He, Y.; Guan, C.; Zhang, K.; Gao, A.; Georgiev, M.I.; Zhou, M. Acceleration of the genetic gain for nutraceutical improvement of adlay (Coix L.) through genomic approaches: Current status and future prospects. Food Rev. Int. 2022, 38, 1–25. [Google Scholar] [CrossRef]
- Liu, B.; Shang, Z.; Li, Q.; Zha, X.; Wu, D.; Yu, N.; Han, L.; Peng, D.; Luo, J. Structural features and anti-gastric cancer activity of polysaccharides from stem, root, leaf and flower of cultivated Dendrobium huoshanense. Int. J. Biol. Macromol. 2020, 143, 651–664. [Google Scholar] [CrossRef]
- Sui, Y.; Xu, D. Isolation and identification of anti-inflammatory and analgesic polysaccharides from Coix seed (Coix lacryma-jobi L.var. Ma-yuen (Roman.) Stapf). Nat. Prod. Res. 2022, 36, 1–10. [Google Scholar] [CrossRef]
- Wang, H.; Yin, H.; Zhong, Y.; Hu, J.; Xia, S.; Wang, Z.; Nie, S.; Xie, M. Polysaccharides from fermented coix seed modulates circulating nitrogen and immune function by altering gut microbiota. Curr. Res. Food Sci. 2022, 5, 1994–2003. [Google Scholar] [CrossRef]
- Chen, F.; Huang, G.; Huang, H. Preparation, analysis, antioxidant activities in vivo of phosphorylated polysaccharide from Momordica charantia. Carbohydr. Polym. 2021, 252, 117179. [Google Scholar] [CrossRef]
- Hu, X.; Xu, F.; Li, J.; Li, J.; Mo, C.; Zhao, M.; Wang, L. Ultrasonic-assisted extraction of polysaccharides from coix seeds: Optimization, purification, and in vitro digestibility. Food Chem. 2022, 374, 131636. [Google Scholar] [CrossRef]
- Huang, G.; Chen, F.; Yang, W.; Huang, H. Preparation, deproteinization and comparison of bioactive polysaccharides. Trends Food Sci. Technol. 2021, 109, 564–568. [Google Scholar] [CrossRef]
- Huang, H.; Huang, G. Extraction, separation, modification, structural characterization, and antioxidant activity of plant polysaccharides. Chem. Biol. Drug Des. 2020, 96, 1209–1222. [Google Scholar] [CrossRef]
- Hadj, S.J.; Bertani, G.; Levante, A.; Vezzosi, F.; Ricci, A.; Bernini, V.; Lazzi, C. Fermentation of agri-food waste: A promising route for the production of aroma compounds. Foods 2021, 10, 707. [Google Scholar] [CrossRef]
- Pessoa, M.G.; Vespermann, K.A.C.; Paulino, B.N.; Barcelos, M.C.S.; Pastore, G.M.; Molina, G. Newly isolated microorganisms with potential application in biotechnology. Biotechnol. Adv. 2019, 37, 319–339. [Google Scholar] [CrossRef]
- Jha, A.K.; Sit, N. Extraction of bioactive compounds from plant materials using combination of various novel methods: A review. Trends Food Sci. Technol. 2022, 119, 579–591. [Google Scholar] [CrossRef]
- Shen, P.; Yue, Y.; Park, Y. A living model for obesity and aging research: Caenorhabditis elegans. Crit. Rev. Food Sci. Nutr. 2018, 58, 741–754. [Google Scholar] [CrossRef]
- Yu, S.; Zheng, C.; Zhou, F.; Baillie, D.L.; Rose, A.M.; Deng, Z.; Chu, J.S. Genomic identification and functional analysis of essential genes in Caenorhabditis elegans. BMC Genom. 2018, 19, 871. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Z.; Zhou, H.; Sun, X.; Chen, X.; Xu, N. The anti-aging effects of Gracilaria lemaneiformis polysaccharide in Caenorhabditis elegans. Int. J. Biol. Macromol. 2019, 140, 600–604. [Google Scholar] [CrossRef]
- Rodriguez, M.; Snoek, L.B.; De Bono, M.; Kammenga, J.E. Worms under stress: C. elegans stress response and its relevance to complex human disease and aging. Trends Neurosci. 2013, 29, 367–374. [Google Scholar] [CrossRef]
- Son, H.G.; Altintas, O.; Kim, E.J.E.; Kwon, S.; Lee, S.V. Age-dependent changes and biomarkers of aging in Caenorhabditis elegans. Aging Cell 2019, 18, e12853. [Google Scholar] [CrossRef]
- Katic, M.; Kahn, C.R. The role of insulin and IGF-1 signaling in longevity. Cell. Mol. Life Sci. 2005, 62, 320–343. [Google Scholar] [CrossRef] [PubMed]
- Kenyon, C. The plasticity of aging: Insights from long-lived mutants. Cell 2005, 120, 449–460. [Google Scholar] [CrossRef]
- Jiang, S.; Deng, N.; Zheng, B.; Li, T.; Liu, R.H. Rhodiola extract promotes longevity and stress resistance of Caenorhabditis elegans via DAF-16 and SKN-1. Food Funct. 2021, 12, 4471–4483. [Google Scholar] [CrossRef]
- Duangjan, C.; Rangsinth, P.; Gu, X.; Zhang, S.; Wink, M.; Tencomnao, T. Glochidion zeylanicum leaf extracts exhibit lifespan extending and oxidative stress resistance properties in Caenorhabditis elegans via DAF-16/FoxO and SKN-1/Nrf-2 signaling pathways. Phytomedicine 2019, 64, 153061. [Google Scholar] [CrossRef]
- Li, H.; Su, L.; Su, X.; Liu, X.; Wang, D.; Li, H.; Ba, X.; Zhang, Y.; Lu, J.; Huang, B.; et al. Arginine methylation of SKN-1 promotes oxidative stress resistance in Caenorhabditis elegans. Redox Biol. 2019, 21, 101111. [Google Scholar] [CrossRef]
- Zeng, H.; Qin, L.; Liu, X.; Miao, S. Increases of lipophilic antioxidants and anticancer activity of coix seed fermented by Monascus purpureus. Foods 2021, 10, 566. [Google Scholar] [CrossRef]
- Yin, H.; Zhong, Y.; Xia, S.; Hu, J.; Nie, S.; Xiong, T.; Xie, M. Effects of fermentation with Lactobacillus plantarum NCU137 on nutritional, sensory and stability properties of Coix (Coix lachryma-jobi L.) seed. Food Chem. 2020, 314, 126037. [Google Scholar] [CrossRef] [PubMed]
- Song, T.; Zhang, Z.; Jin, Q.; Feng, W.; Shen, Y.; Fan, L.; Cai, W. Nutrient profiles, functional compositions, and antioxidant activities of seven types of grain fermented with Sanghuangporus sanghuang fungus. J. Food Sci. Technol. 2021, 58, 4091–4101. [Google Scholar] [CrossRef] [PubMed]
- Bharti, B.; Li, H.; Ren, Z.; Zhu, R.; Zhu, Z. Recent advances in sterilization and disinfection technology: A review. Chemosphere 2022, 308, 136404. [Google Scholar] [CrossRef] [PubMed]
- Brand, W.W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Wen, A.; Qin, L.; Zeng, H.; Zhu, Y. Comprehensive evaluation of physicochemical properties and antioxidant activity of B. subtilis-fermented polished adlay subjected to different drying methods. Food Sci. Nutr. 2020, 8, 2124–2133. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.H.; Fan, S.T.; Huang, D.F.; Yu, Q.; Liu, X.Z.; Li, C.; Wang, S.; Xiong, T.; Nie, S.P.; Xie, M.Y. Effect of Lactobacillus plantarum NCU116 Fermentation on Asparagus officinalis Polysaccharide: Characterization, Antioxidative, and Immunoregulatory Activities. J. Agric. Food Chem. 2018, 66, 10703–10711. [Google Scholar] [CrossRef]
- Wang, Z.; Sun, Q.; Fang, J.; Wang, C.; Wang, D.; Li, M. The anti-aging activity of Lycium barbarum polysaccharide extracted by yeast fermentation: In vivo and in vitro studies. Int. J. Biol. Macromol. 2022, 209, 2032–2041. [Google Scholar] [CrossRef]
- Zhang, X.; Hu, Y.; Jin, C.; Wu, W. Extraction and hypolipidemic activity of low molecular weight polysaccharides Isolated from Rosa Laevigata fruits. BioMed Res. Int. 2020, 2020, 2043785. [Google Scholar] [CrossRef]
- Li, Q.; Dou, Z.; Chen, C.; Jiang, Y.; Yang, B.; Fu, X. Study on the effect of molecular weight on the gut microbiota fermentation properties of blackberry polysaccharides in vitro. J. Agric. Food Chem. 2022, 70, 11245–11257. [Google Scholar] [CrossRef]
- Wu, Z.; Ming, J.; Gao, R.; Wang, Y.; Liang, Q.; Yu, H.; Zhao, G. Characterization and antioxidant activity of the complex of tea polyphenols and oat beta-glucan. J. Agric. Food Chem. 2011, 59, 10737–10746. [Google Scholar] [CrossRef]
- Shen, T.; Duan, C.; Chen, B.; Li, M.; Ruan, Y.; Xu, D.; Shi, D.; Yu, D.; Li, J.; Wang, C. Tremella fuciformis polysaccharide suppresses hydrogen peroxide-triggered injury of human skin fibroblasts via upregulation of SIRT1. Mol. Med. Rep. 2017, 16, 1340–1346. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Shen, M.; Song, Q.; Xie, J. Biological activities and pharmaceutical applications of polysaccharide from natural resources: A review. Carbohydr. Polym. 2018, 183, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, A.; Tissenbaum, H.A. Reproduction and longevity: Secrets revealed by C. elegans. Trends Cell Biol. 2007, 17, 65–71. [Google Scholar] [CrossRef]
- Muñoz, M.J.; Riddle, D.L. Positive selection of Caenorhabditis elegans mutants with increased stress resistance and longevity. Genetics 2003, 163, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.; Chen, Y.; Wang, G.; Feng, T.; Shen, M.; Xiao, B.; Gu, J.; Wang, W.; Li, J.; Zhang, Y. Evaluation of the antioxidant effects of acid hydrolysates from Auricularia auricular polysaccharides using a Caenorhabditis elegans model. Food Funct. 2019, 10, 5531–5543. [Google Scholar] [CrossRef] [PubMed]
- Zhan, J.; Li, G.; Dang, Y.; Pan, D. Study on the antioxidant activity of peptide isolated from porcine plasma during in vitro digestion. Food Biosci. 2021, 42, 101069. [Google Scholar] [CrossRef]
- Tissenbaum, H.A. DAF-16: FOXO in the context of C. elegans. In Current Topics in Developmental Biology; Ghaffari, S., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; Volume 127, pp. 1–21. [Google Scholar]
- Honda, Y.; Honda, S. The daf-2gene network for longevity regulates oxidative stress resistance and Mn-superoxide dismutase gene expression in Caenorhabditis elegans. FASEB J. 1999, 13, 1385–1393. [Google Scholar] [CrossRef]
- Lee, S.S.; Kennedy, S.; Tolonen, A.C.; Ruvkun, G. DAF-16 target genes that control C. elegans life-span and metabolism. Science 2003, 300, 644–647. [Google Scholar] [CrossRef]
- Uno, M.; Nishida, E. Lifespan-regulating genes in C. elegans. NPJ Aging Mech. Dis. 2016, 2, 16010. [Google Scholar] [CrossRef]
- Blackwell, T.K.; Steinbaugh, M.J.; Hourihan, J.M.; Ewald, C.Y.; Isik, M. SKN-1/Nrf, stress responses, and aging in Caenorhabditis elegans. Free Radic. Biol. Med. 2015, 88, 290–301. [Google Scholar] [CrossRef]
- Hernandez, J.M.; Podbilewicz, B. The hallmarks of cell-cell fusion. Development 2017, 144, 4481–4495. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Liao, D.; Yu, Y.; Wei, C.; Xuan, L.; Li, S.; Wang, H. Coix seed oil prolongs lifespan and enhances stress resistance in Caenorhabditis elegans. Biogerontology 2020, 21, 245–256. [Google Scholar] [CrossRef] [PubMed]
- Jinnouchi, M.; Miyahara, T.; Suzuki, Y. Coix seed consumption affects the gut microbiota and the peripheral lymphocyte subset profiles of healthy male adults. Nutrients 2021, 13, 4079. [Google Scholar] [CrossRef] [PubMed]
- Cockburn, D.W.; Koropatkin, N.M. Polysaccharide degradation by the intestinal microbiota and its influence on human health and disease. J. Mol. Biol. 2016, 428, 3230–3252. [Google Scholar] [CrossRef] [PubMed]
- Pietsch, K.; Saul, N.; Chakrabarti, S.; Sturzenbaum, S.R.; Menzel, R.; Steinberg, C.E. Hormetins, antioxidants and prooxidants: Defining quercetin-, caffeic acid- and rosmarinic acid-mediated life extension in C. elegans. Biogerontology 2011, 12, 329–347. [Google Scholar] [CrossRef]
- Van Raamsdonk, J.M.; Hekimi, S. Reactive oxygen species and aging in Caenorhabditis elegans: Causal or casual relationship? Antioxid. Redox Signal. 2010, 13, 1911–1953. [Google Scholar] [CrossRef]
- Hui, H.; Xin, A.; Cui, H.; Jin, H.; Yang, X.; Liu, H.; Qin, B. Anti-aging effects on Caenorhabditis elegans of a polysaccharide, O-acetyl glucomannan, from roots of Lilium davidii var. unicolor Cotton. Int. J. Biol. Macromol. 2020, 155, 846–852. [Google Scholar] [CrossRef]
- Shao, X.; He, T.; Lai, Y.; Chen, M.; Tong, Z. Water-soluble polysaccharides extracted from Pueraria lobata delay aging of Caenorhabditis elegans under heat stress. Plant Foods Hum. Nutr. 2022, 77, 220–225. [Google Scholar] [CrossRef]
- Zhang, Y.; You, S.; Wang, D.; Zhao, D.; Zhang, J.; An, Q.; Li, M.; Wang, C. Fermented Dendrobium officinale polysaccharides protect UVA-induced photoaging of human skin fibroblasts. Food Sci. Nutr. 2022, 10, 1275–1288. [Google Scholar] [CrossRef]
- Ayuda, D.B.; Gonzalez, M.S.; Miranda, V.A.; Duenas, M.; Santos, B.C.; Gonzalez, A.M. Epicatechin modulates stress-resistance in C. elegans via insulin/IGF-1 signaling pathway. PLoS ONE 2019, 14, e0199483. [Google Scholar]
- Sun, X.; Chen, W.; Wang, Y. DAF-16/FOXO transcription factor in aging and longevity. Front. Pharmacol. 2017, 8, 548. [Google Scholar] [CrossRef] [PubMed]
- Tullet, J.M.; Hertweck, M.; An, J.H.; Baker, J.; Hwang, J.Y.; Liu, S.; Oliveira, R.P.; Baumeister, R.; Blackwell, T.K. Direct inhibition of the longevity-promoting factor SKN-1 by insulin-like signaling in C. elegans. Cell 2008, 132, 1025–1038. [Google Scholar] [CrossRef] [PubMed]
- Miao, J.; Shi, W.; Zhang, J.; Zhang, X.; Zhang, H.; Wang, Z.; Qiu, J. Response surface methodology for the fermentation of polysaccharides from Auricularia auricula using Trichoderma viride and their antioxidant activities. Int. J. Biol. Macromol. 2020, 155, 393–402. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, R.; Deng, Y.; Zhang, Y.; Xiao, J.; Huang, F.; Wen, W.; Zhang, M. Fermentation and complex enzyme hydrolysis enhance total phenolics and antioxidant activity of aqueous solution from rice bran pretreated by steaming with alpha-amylase. Food Chem. 2017, 221, 636–643. [Google Scholar] [CrossRef] [PubMed]



| Gene | Direction | Primer (5′-3′) |
|---|---|---|
| β-actin | F | CTGAAGCCCCACTCAATCCA |
| R | GCCAAGTCAAGACGGAGGAT | |
| sod-3 | F | GACGATCAACCCCTGTCGAA |
| R | TACTGTTCT TCGGGGAACGC | |
| daf-16 | F | AAGCCAGGAAGGAATCCACG |
| R | TTGAGTTCGGGGACGGAAAG | |
| daf-2 | F | GTAATTGGAGGCCGTTCGCT |
| R | CGTGGG CACATCAATCCAGT | |
| age-1 | F | TGTGGGGACACTGACGCTG |
| R | TTGGCAGTCGGTTCAGGAG | |
| Skn-1 | F | AGTGTCGGCGTTCCAGATTTC |
| R | GTCGACGAATTGCGAATCA | |
| Gcs-1 | F | GTCGATGAAGCCAGATGGTTGT |
| R | CGATCGTCGACACTTGCACTAA |
| Molar Percentage (%) | Xylose | Arabinose | Mannose | Galactose | Glucose |
|---|---|---|---|---|---|
| WCSPs | 5.49 | 12.33 | 4.76 | 9.33 | 68.06 |
| FCSPs | 0.44 | 1.56 | 2.08 | 2.86 | 93.04 |
| Groups | Number of C. elegans | Average Lifespan (d) | Maximum Lifespan (d) |
|---|---|---|---|
| Control | 90 | 18.02 ± 1.71 | 23.31 ± 0.92 |
| WCSPs (0.2 g/L) | 90 | 18.17 ± 0.85 | 24.02 ± 1.19 |
| WCSPs (1 g/L) | 90 | 20.61 ± 0.92 | 24.95 ± 1.24 |
| WCSPs (5 g/L) | 90 | 21.32 ± 1.08 * | 25.87 ± 1.01 * |
| FCSPs (0.2 g/L) | 90 | 19.76 ± 1.15 | 25.22 ± 1.19 |
| FCSPs (1 g/L) | 90 | 21.53 ± 0.98 ** | 26.47. ± 1.58 * |
| FCSPs (5 g/L) | 90 | 22.58 ± 1.21 ** | 27.09 ± 1.03 ** |
| Groups | Average Heat Resistance Time (h) | Average Lifespan Increase (%) | The Longest Heat Resistance Time (h) |
|---|---|---|---|
| Control | 8.10 ± 0.37 | — | 12.00 ± 0.81 |
| WCSPs (0.2 g/L) | 8.60 ± 0.29 | 6.17 | 12.67 ± 0.94 |
| WCSPs (1 g/L) | 9.19 ± 0.27 ** | 13.45 | 13.67 ± 0.47 * |
| WCSPs (5 g/L) | 10.93 ± 0.69 ** | 34.93 | 14.33 ± 0.47 ** |
| FCSPs (0.2 g/L) | 9.35 ± 0.36 ** | 15.43 | 14.67 ± 0.94 ** |
| FCSPs (1 g/L) | 10.41 ± 0.58 ** | 28.51 | 18.33 ± 0.47 ** |
| FCSPs (5 g/L) | 11.21 ± 0.71 ** | 38.39 | 18.67 ± 0.94 ** |
| Groups | Average Oxidative Resistance Time (h) | Average Lifespan Increase (%) | The Longest Oxidative Resistance Time (h) |
|---|---|---|---|
| Control | 7.04 ± 0.17 | — | 11.67 ± 0.47 |
| WCSPs (0.2 g/L) | 7.42 ± 0.41 | 5.39 | 12.33 ± 0.47 |
| WCSPs (1 g/L) | 8.59 ± 0.22 ** | 22.02 | 13.67 ± 0.94 * |
| WCSPs (5 g/L) | 9.38 ± 0.59 ** | 33.23 | 14 ± 0.81 ** |
| FCSPs (0.2 g/L) | 7.67 ± 0.28 * | 8.94 | 12.67 ± 0.47 |
| FCSPs (1 g/L) | 9.51 ± 0.45 ** | 35.08 | 14.33 ± 0.47 ** |
| FCSPs (5 g/L) | 9.84 ± 0.24 ** | 39.77 | 15.67 ± 0.94 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, D.; Yan, M.; Xu, H.; Liang, H.; Zhang, J.; Li, M.; Wang, C. Antioxidant and Antiaging Activity of Fermented Coix Seed Polysaccharides on Caenorhabditis elegans. Nutrients 2023, 15, 2474. https://doi.org/10.3390/nu15112474
Zhao D, Yan M, Xu H, Liang H, Zhang J, Li M, Wang C. Antioxidant and Antiaging Activity of Fermented Coix Seed Polysaccharides on Caenorhabditis elegans. Nutrients. 2023; 15(11):2474. https://doi.org/10.3390/nu15112474
Chicago/Turabian StyleZhao, Dan, Meng Yan, Hualei Xu, Haiyan Liang, Jiachan Zhang, Meng Li, and Changtao Wang. 2023. "Antioxidant and Antiaging Activity of Fermented Coix Seed Polysaccharides on Caenorhabditis elegans" Nutrients 15, no. 11: 2474. https://doi.org/10.3390/nu15112474
APA StyleZhao, D., Yan, M., Xu, H., Liang, H., Zhang, J., Li, M., & Wang, C. (2023). Antioxidant and Antiaging Activity of Fermented Coix Seed Polysaccharides on Caenorhabditis elegans. Nutrients, 15(11), 2474. https://doi.org/10.3390/nu15112474






