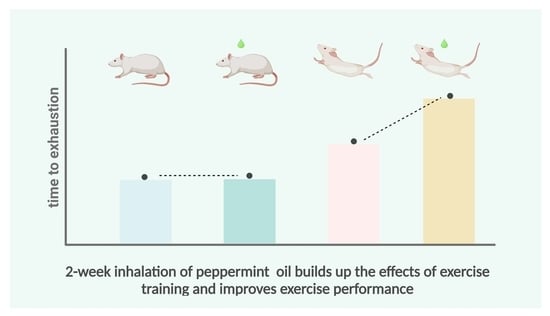
Repeated Inhalation of Peppermint Essential Oil Improves Exercise Performance in Endurance-Trained Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Materials and Chemicals
2.3. Gas Chromatography-Mass Spectrometry (GC-MS) Analysis
2.4. Determination of Peppermint Essential Oil Dosage
2.5. Weight-Loaded Swimming Training Protocol
2.6. Exhaustive Forced Swimming Test
2.7. Body Mass Measurement and Grip Strength Test
2.8. Elevated plus Maze (EPM) Test
2.9. Novel Object Recognition (NOR)
2.10. Determination of Biochemical Parameters Related to Fatigue in Serum, Muscle, and Liver Tissues
2.11. Statistical Analysis
3. Results
3.1. Composition and Content of Peppermint Essential Oil
3.2. Peppermint Essential Oil Treatment Had No Effect on Body Weight and Grip Strength in Rats
3.3. Peppermint Essential Oil Extended the Time to Exhaustion
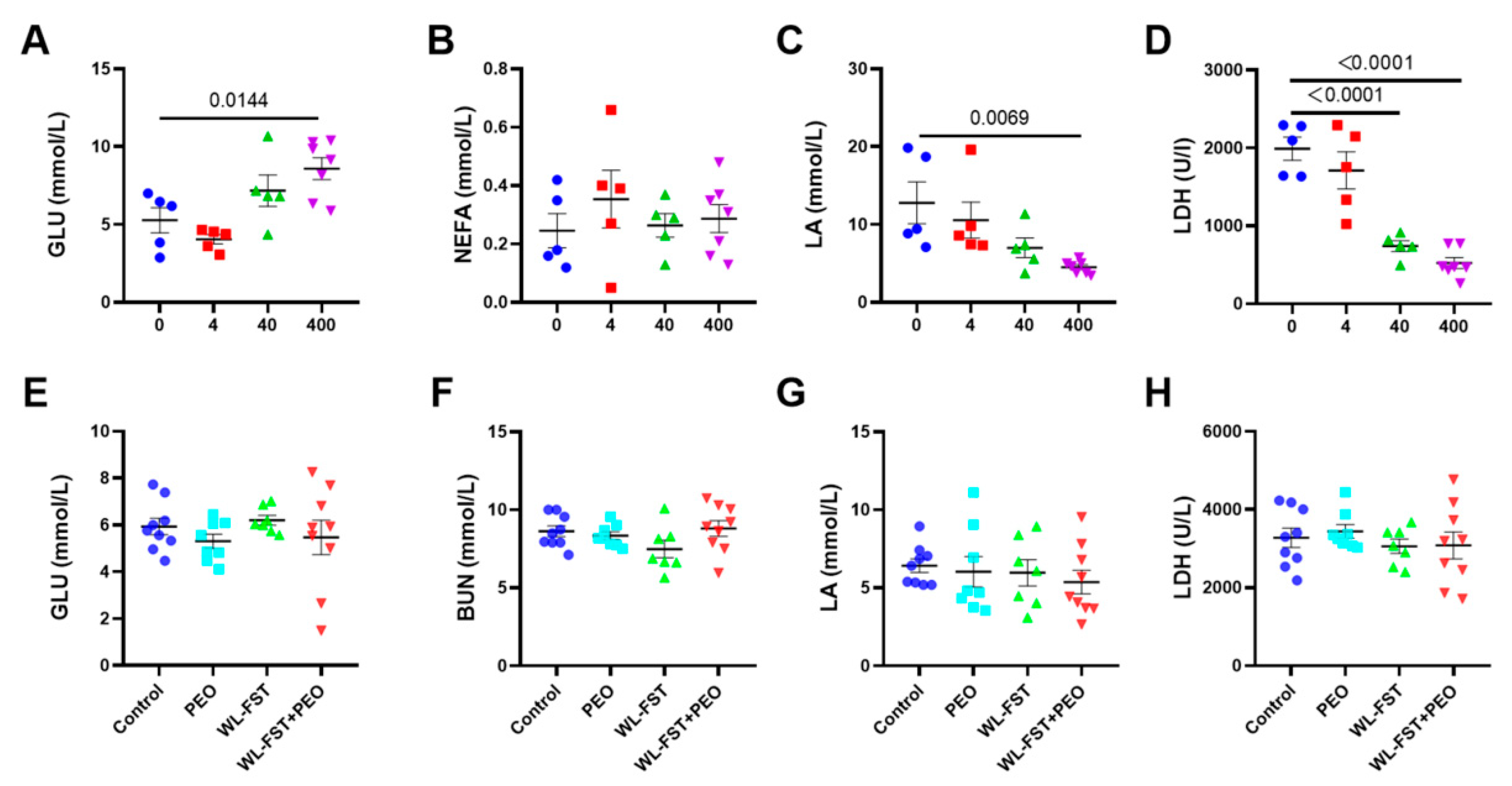
3.4. Effects of Peppermint Essential Oil on Energy Metabolites in Serum
3.5. Effects of Peppermint Essential Oil on Redox Biomarkers in Skeletal Muscle and Liver Tissues
3.6. Peppermint Essential Oil Had No Significant Effect on Cognitive Functions of Rats
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Groups | Mean (SEM) | 95% CI | Effect Size (Cohen’s d) | ||
|---|---|---|---|---|---|
| vs. PEO | vs. WL-FST | vs. WL-FST+PEO | |||
| Control | 32.77 (1.47) | (29.52, 36.01) | 0.20 | 2.61 | 1.86 |
| PEO | 31.97 (0.75) | (30.33, 33.62) | / | 3.28 | 2.18 |
| WL-FST | 20.67 (1.46) | (17.22, 24.12) | / | / | −0.63 |
| WL-FST+PEO | 23.50 (1.46) | (19.77, 27.23) | / | / | / |
| Groups | Mean (SEM) | 95% CI | Effect Size (Cohen’s d) | ||
|---|---|---|---|---|---|
| vs. PEO | vs. WL-FST | vs. WL-FST+PEO | |||
| Control | 3.46 (0.35) | (2.69, 4.22) | 0.40 | −2.14 | −2.53 |
| PEO | 3.07 (0.19) | (2.64, 3.49) | / | −2.44 | −2.60 |
| WL-FST | 8.16 (1.09) | (5.51, 10.81) | / | / | −1.74 |
| WL-FST+PEO | 20.03 (3.25) | (12.34, 27.71) | / | / | / |
| Groups | Mean (SEM) | 95% CI | Effect Size (Cohen’s d) | |||
|---|---|---|---|---|---|---|
| vs. 4 | vs. 40 | vs. 400 | ||||
| GLU | 0 | 5.28 (0.81) | (3.03, 7.52) | 0.89 | −0.93 | −1.81 |
| 4 | 4.06 (0.31) | (3.21, 4.91) | / | −1.87 | −3.25 | |
| 40 | 7.18 (1.01) | (4.38, 9.98) | / | / | −0.68 | |
| 400 | 8.60 (0.70) | (6.88, 10.31) | / | / | / | |
| LA | 0 | 12.80 (2.68) | (5.35, 20.24) | 0.40 | 1.23 | 1.94 |
| 4 | 10.57 (2.31) | (4.16, 16.98) | / | 0.85 | 1.63 | |
| 40 | 7.02 (1.26) | (3.53, 10.51) | / | / | 1.21 | |
| 400 | 4.51 (0.31) | (3.75, 5.28) | / | / | / | |
| LDH | 0 | 1990.52 (147.08) | (1582.16, 2376.98) | 0.63 | 4.85 | 5.49 |
| 4 | 1712.41 (239.36) | (1047.84, 2376.98) | / | 2.46 | 2.97 | |
| 40 | 741.86 (69.58) | (548.67, 935.05) | / | / | 1.27 | |
| 400 | 522.86 (70.47) | (350.42, 695.30) | / | / | // | |
| Groups | Mean (SEM) | 95% CI | Effect Size (Cohen’s d) | |||
|---|---|---|---|---|---|---|
| vs. PEO | vs. WL-FST | vs. WL-FST+PEO | ||||
| CAT | Control | 92.12 (5.00) | (81.12, 103.11) | 0.49 | 1.32 | 0.67 |
| PEO | 84.61 (3.80) | (76.24, 92.97) | / | 0.95 | 0.16 | |
| WL-FST | 73.08 (3.85) | (63.99, 82.18) | / | / | −0.68 | |
| WL-FST+PEO | 82.82 (3.02) | (75.85, 89.78) | / | / | / | |
| MDA | Control | 1.51 (0.16) | (0.15, 1.87) | −0.19 | −2.55 | −0.32 |
| PEO | 1.61 (0.16) | (1.25, 1.96) | / | −2.43 | −0.14 | |
| WL-FST | 3.73 (0.40) | (2.79, 4.68) | / | / | 2.28 | |
| WL-FST+PEO | 1.68 (0.59) | (1.23, 2.14) | / | / | / | |
| GSH-PX | Control | 370.76 (54.93) | (246.49, 495.03) | 1.10 | 1.55 | 1.39 |
| PEO | 201.12 (39.68) | (112.71, 289.54) | / | 0.33 | 0.16 | |
| WL-FST | 166.26 (24.73) | (108.86, 223.66) | / | / | −0.12 | |
| WL-FST+PEO | 183.76 (25.57) | (124.81, 242.72) | / | / | / | |
References
- Azizbeigi, K.; Stannard, S.R.; Atashak, S.; Haghighi, M.M. Antioxidant Enzymes and Oxidative Stress Adaptation to Exercise Training: Comparison of Endurance, Resistance, and Concurrent Training in Untrained Males. J. Exerc. Sci. Fit. 2014, 12, 1–6. [Google Scholar] [CrossRef]
- Lee, J.-S.; Kim, H.-G.; Han, J.-M.; Kim, Y.-A.; Son, C.-G. Anti-Fatigue Effect of Myelophil in a Chronic Forced Exercise Mouse Model. Eur. J. Pharmacol. 2015, 764, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Noakes, T.D. Fatigue Is a Brain-Derived Emotion That Regulates the Exercise Behavior to Ensure the Protection of Whole Body Homeostasis. Front. Physiol. 2012, 3, 82. [Google Scholar] [CrossRef] [PubMed]
- Ament, W.; Verkerke, G.J. Exercise and Fatigue. Sports Med. 2009, 39, 389–422. [Google Scholar] [CrossRef] [PubMed]
- Bowtell, J.; Kelly, V. Fruit-Derived Polyphenol Supplementation for Athlete Recovery and Performance. Sports Med. 2019, 49, 3–23. [Google Scholar] [CrossRef]
- Liu, Y.; Shen, X.; Sha, M.; Feng, Z.; Liu, Y. Natural Bioactive Flavonoids as Promising Agents in Alleviating Exercise-Induced Fatigue. Food Biosci. 2023, 51, 102360. [Google Scholar] [CrossRef]
- Luo, C.; Wei, X.; Song, J.; Xu, X.; Huang, H.; Fan, S.; Zhang, D.; Han, L.; Lin, J. Interactions between Gut Microbiota and Polyphenols: New Insights into the Treatment of Fatigue. Molecules 2022, 27, 7377. [Google Scholar] [CrossRef]
- Bastaki, S.M.; Adeghate, E.; Amir, N.; Ojha, S.; Oz, M. Menthol Inhibits Oxidative Stress and Inflammation in Acetic Acid-Induced Colitis in Rat Colonic Mucosa. Am. J. Transl. Res. 2018, 10, 4210–4222. [Google Scholar]
- Mani Badal, R.; Badal, D.; Badal, P.; Khare, A.; Shrivastava, J.; Kumar, V. Pharmacological Action of Mentha Piperita on Lipid Profile in Fructose-Fed Rats. Iran J. Pharm. Res. 2011, 10, 843–848. [Google Scholar]
- Zhao, H.; Ren, S.; Yang, H.; Tang, S.; Guo, C.; Liu, M.; Tao, Q.; Ming, T.; Xu, H. Peppermint Essential Oil: Its Phytochemistry, Biological Activity, Pharmacological Effect and Application. Biomed. Pharmacother. 2022, 154, 113559. [Google Scholar] [CrossRef]
- Lin, T.-C.; Wang, S.-H.; Huan, C.-C.; Lai, Y.-C.; Song, T.-Y.; Tsai, M.-S. Anti-Fatigue, Antioxidation, and Anti- Inflammatory Effects of Eucalyptus Oil Aromatherapy in Swimming-Exercised Rats. Chin. J. Physiol. 2018, 61, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Umezu, T.; Sakata, A.; Ito, H. Ambulation-Promoting Effect of Peppermint Oil and Identification of Its Active Constituents. Pharmacol. Biochem. Behav. 2001, 69, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Chumpitazi, B.P.; Kearns, G.; Shulman, R.J. Review Article: The Physiologic Effects and Safety of Peppermint Oil and Its Efficacy in Irritable Bowel Syndrome and Other Functional Disorders. Aliment. Pharmacol. Ther. 2018, 47, 738–752. [Google Scholar] [CrossRef]
- Orhan, C.; Gencoglu, H.; Tuzcu, M.; Sahin, N.; Ojalvo, S.P.; Sylla, S.; Komorowski, J.R.; Sahin, K. Maca Could Improve Endurance Capacity Possibly by Increasing Mitochondrial Biogenesis Pathways and Antioxidant Response in Exercised Rats. J. Food Biochem. 2022, 46, e14159. [Google Scholar] [CrossRef]
- Yan, K.; Gao, H.; Liu, X.; Zhao, Z.; Gao, B.; Zhang, L. Establishment and Identification of an Animal Model of Long-Term Exercise-Induced Fatigue. Front. Endocrinol. 2022, 13, 915937. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Nakamura, F.; Mizokawa, S.; Matsumura, A.; Nozaki, S.; Watanabe, Y. Establishment and Assessment of a Rat Model of Fatigue. Neurosci. Lett. 2003, 352, 159–162. [Google Scholar] [CrossRef] [PubMed]
- Proschinger, S.; Freese, J. Neuroimmunological and Neuroenergetic Aspects in Exercise-Induced Fatigue. Exerc. Immunol. Rev. 2019, 25, 8–19. [Google Scholar]
- Gáll, Z.; Kelemen, K.; Tolokán, A.; Zolcseak, I.; Sável, I.; Bod, R.; Ferencz, E.; Vancea, S.; Urkon, M.; Kolcsár, M. Anticonvulsant Action and Long-Term Effects of Chronic Cannabidiol Treatment in the Rat Pentylenetetrazole-Kindling Model of Epilepsy. Biomedicines 2022, 10, 1811. [Google Scholar] [CrossRef]
- Pezze, M.A.; Marshall, H.J.; Fone, K.C.F.; Cassaday, H.J. Dopamine D1 Receptor Stimulation Modulates the Formation and Retrieval of Novel Object Recognition Memory: Role of the Prelimbic Cortex. Eur. Neuropsychopharmacol. 2015, 25, 2145–2156. [Google Scholar] [CrossRef]
- Saqib, S.; Ullah, F.; Naeem, M.; Younas, M.; Ayaz, A.; Ali, S.; Zaman, W. Mentha: Nutritional and Health Attributes to Treat Various Ailments Including Cardiovascular Diseases. Molecules 2022, 27, 6728. [Google Scholar] [CrossRef]
- Golestani, M.R.; Rad, M.; Bassami, M.; Afkhami-Goli, A. Analysis and Evaluation of Antibacterial Effects of New Herbal Formulas, AP-001 and AP-002, against Escherichia Coli O157:H7. Life Sci. 2015, 135, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Engel, M.A.; Koch, E.; Reeh, P.W.; Khalil, M. Menthacarin Induces Calcium Ion Influx in Sensory Neurons, Macrophages and Colonic Organoids of Mice. Life Sci. 2021, 264, 118682. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wu, F.; Shao, H.; Zhang, Y.; Fan, A.; Li, F. Does the Fragrance of Essential Oils Alleviate the Fatigue Induced by Exercise? A Biochemical Indicator Test in Rats. Evid. Based Complement. Altern. Med. 2017, 2017, e5027372. [Google Scholar] [CrossRef] [PubMed]
- Sönmez, G.; Çolak, M.; Sönmez, S.; Schoenfeld, B. Effects of Oral Supplementation of Mint Extract on Muscle Pain and Blood Lactate. Biomed. Hum. Kinet. 2010, 2, 66–69. [Google Scholar] [CrossRef]
- Kennedy, D.; Okello, E.; Chazot, P.; Howes, M.-J.; Ohiomokhare, S.; Jackson, P.; Haskell-Ramsay, C.; Khan, J.; Forster, J.; Wightman, E. Volatile Terpenes and Brain Function: Investigation of the Cognitive and Mood Effects of Mentha × Piperita L. Essential Oil with In Vitro Properties Relevant to Central Nervous System Function. Nutrients 2018, 10, 1029. [Google Scholar] [CrossRef]
- Umezu, T. Evaluation of Central Nervous System Acting Effects of Plant-Derived Essential Oils Using Ambulatory Activity in Mice. Pharmacol. Pharm. 2013, 04, 160–170. [Google Scholar] [CrossRef]
- Meamarbashi, A.; Rajabi, A. The Effects of Peppermint on Exercise Performance. J. Int. Soc. Sport. Nutr. 2013, 10, 15. [Google Scholar] [CrossRef] [PubMed]
- Jaradat, N.A.; Al Zabadi, H.; Rahhal, B.; Hussein, A.M.A.; Mahmoud, J.S.; Mansour, B.; Khasati, A.I.; Issa, A. The Effect of Inhalation of Citrus Sinensis Flowers and Mentha Spicata Leave Essential Oils on Lung Function and Exercise Performance: A Quasi-Experimental Uncontrolled before-and-after Study. J. Int. Soc. Sports Nutr. 2016, 13, 36. [Google Scholar] [CrossRef]
- Shepherd, K.; Peart, D.J. Aerobic Capacity Is Not Improved Following 10-Day Supplementation with Peppermint Essential Oil. Appl. Physiol. Nutr. Metab. 2017, 42, 558–561. [Google Scholar] [CrossRef]
- Richmond, S.; Brammer, C.; Sosa, A. The Effects of Peppermint Inhalation on Broad Jump and Vertical Leap. Med. Sci. Sports Exerc. 2016, 48, 1046. [Google Scholar] [CrossRef]
- Pournemati, P.; Azarbayjani, M.A.; Rezaee, M.B.; Ziaee, V.; Pournemati, P. The Effect of Inhaling Peppermint Odor and Ethanol in Women Athletes. Bratisl. Med. J. 2009, 110, 782–787. [Google Scholar]
- Ohata, M.; Zhou, L.; Ando, S.; Kaneko, S.; Osada, K.; Yada, Y. Application of Integrative Physiological Approach to Evaluate Human Physiological Responses to the Inhalation of Essential Oils of Japanese Citrus Fruits Iyokan (Citrus Iyo) and Yuzu (Citrus Junos). Biosci. Biotechnol. Biochem. 2022, 86, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.; Ahn, J.; Jeon, H. Can Aromatherapy Make People Feel Better Throughout Exercise? Int. J. Environ. Res. Public Health 2020, 17, 4559. [Google Scholar] [CrossRef]
- Köteles, F.; Babulka, P.; Szemerszky, R.; Dömötör, Z.; Boros, S. Inhaled Peppermint, Rosemary and Eucalyptus Essential Oils Do Not Change Spirometry in Healthy Individuals. Physiol. Behav. 2018, 194, 319–323. [Google Scholar] [CrossRef] [PubMed]
- Barwood, M.J.; Gibson, O.R.; Gillis, D.J.; Jeffries, O.; Morris, N.B.; Pearce, J.; Ross, M.L.; Stevens, C.; Rinaldi, K.; Kounalakis, S.N.; et al. Menthol as an Ergogenic Aid for the Tokyo 2021 Olympic Games: An Expert-Led Consensus Statement Using the Modified Delphi Method. Sports Med. 2020, 50, 1709–1727. [Google Scholar] [CrossRef]






| Peak NO. | Components | CAS NO. | RT (min) | Area (%) |
|---|---|---|---|---|
| 1 | menthol | 1490-04-6 | 11.13 | 37.01 |
| 2 | menthone | 14073-97-3 | 10.55 | 21.83 |
| 3 | 1,8-cineole | 470-82-6 | 7.56 | 6.36 |
| 4 | L-menthol acetate | 16409-45-3 | 13.62 | 5.34 |
| 5 | (+)-menthone | 3391-87-5 | 10.73 | 4.71 |
| 6 | D-menthol | 15356-60-2 | 10.83 | 3.41 |
| 7 | β-Caryophyllene | 87-44-5 | 16.61 | 2.66 |
| 8 | menthofuran | 494-90-6 | 10.67 | 2.43 |
| 9 | (+)-pulegone | 89-82-7 | 12.43 | 1.94 |
| 10 | (R)-(+)-limonene | 5989-27-5 | 7.47 | 1.89 |
| 11 | β-Cyclopentene | 18252-44-3 | 17.89 | 1.62 |
| 12 | β-Pinene | 18172-67-3 | 6.34 | 1.41 |
| 90.61 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, W.; Shi, R.; Gao, T.; Hu, Y.; Zhou, J.; Li, C.; Wang, P.; Yang, H.; Xing, W.; Dong, L.; et al. Repeated Inhalation of Peppermint Essential Oil Improves Exercise Performance in Endurance-Trained Rats. Nutrients 2023, 15, 2480. https://doi.org/10.3390/nu15112480
Zhang W, Shi R, Gao T, Hu Y, Zhou J, Li C, Wang P, Yang H, Xing W, Dong L, et al. Repeated Inhalation of Peppermint Essential Oil Improves Exercise Performance in Endurance-Trained Rats. Nutrients. 2023; 15(11):2480. https://doi.org/10.3390/nu15112480
Chicago/Turabian StyleZhang, Wei, Rongpei Shi, Tian Gao, Yang Hu, Jiaheng Zhou, Chenhan Li, Panpan Wang, Hongyan Yang, Wenjuan Xing, Ling Dong, and et al. 2023. "Repeated Inhalation of Peppermint Essential Oil Improves Exercise Performance in Endurance-Trained Rats" Nutrients 15, no. 11: 2480. https://doi.org/10.3390/nu15112480
APA StyleZhang, W., Shi, R., Gao, T., Hu, Y., Zhou, J., Li, C., Wang, P., Yang, H., Xing, W., Dong, L., & Gao, F. (2023). Repeated Inhalation of Peppermint Essential Oil Improves Exercise Performance in Endurance-Trained Rats. Nutrients, 15(11), 2480. https://doi.org/10.3390/nu15112480