Lactobacillus casei and Its Supplement Alleviate Stress-Induced Depression and Anxiety in Mice by the Regulation of BDNF Expression and NF-κB Activation
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains
2.2. Preparation of Probiotics-Fermented Supplement (pFS)
2.3. SH-SY5Y Cell Culture and Selection of BDNF Expression-Inducing Probiotics
2.4. Animals
2.5. Behavioral Tasks
2.6. Eenzyme-Linked Immunosorbent Assay (ELISA)
2.7. Immunofluorescence Staining
2.8. Microbiota Analysis
2.9. Statistics
3. Results
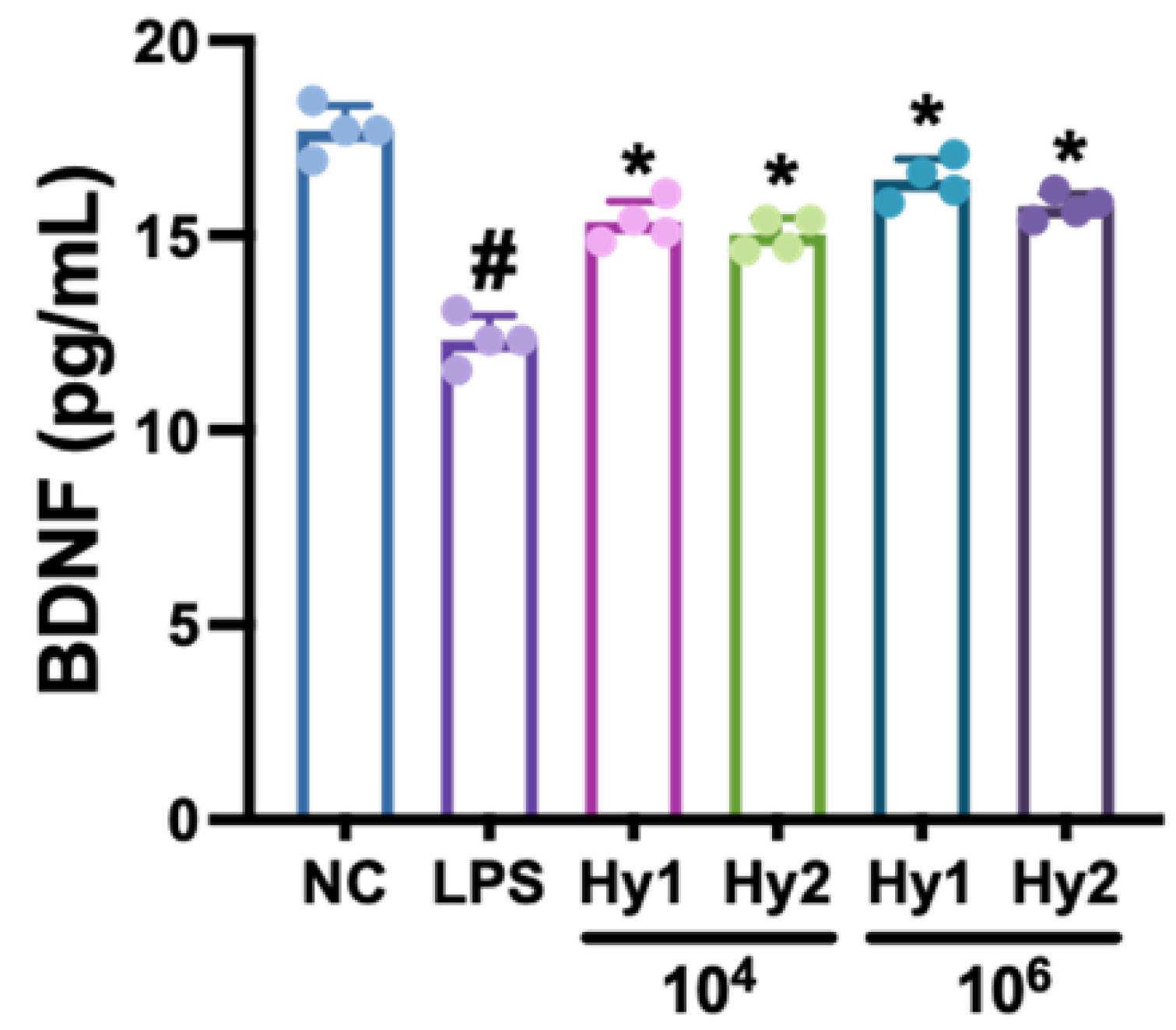
3.1. L. casei and B. lactis Up-Regulated LPS-Suppressed BDNF Expression in SH-SY5Y Cells
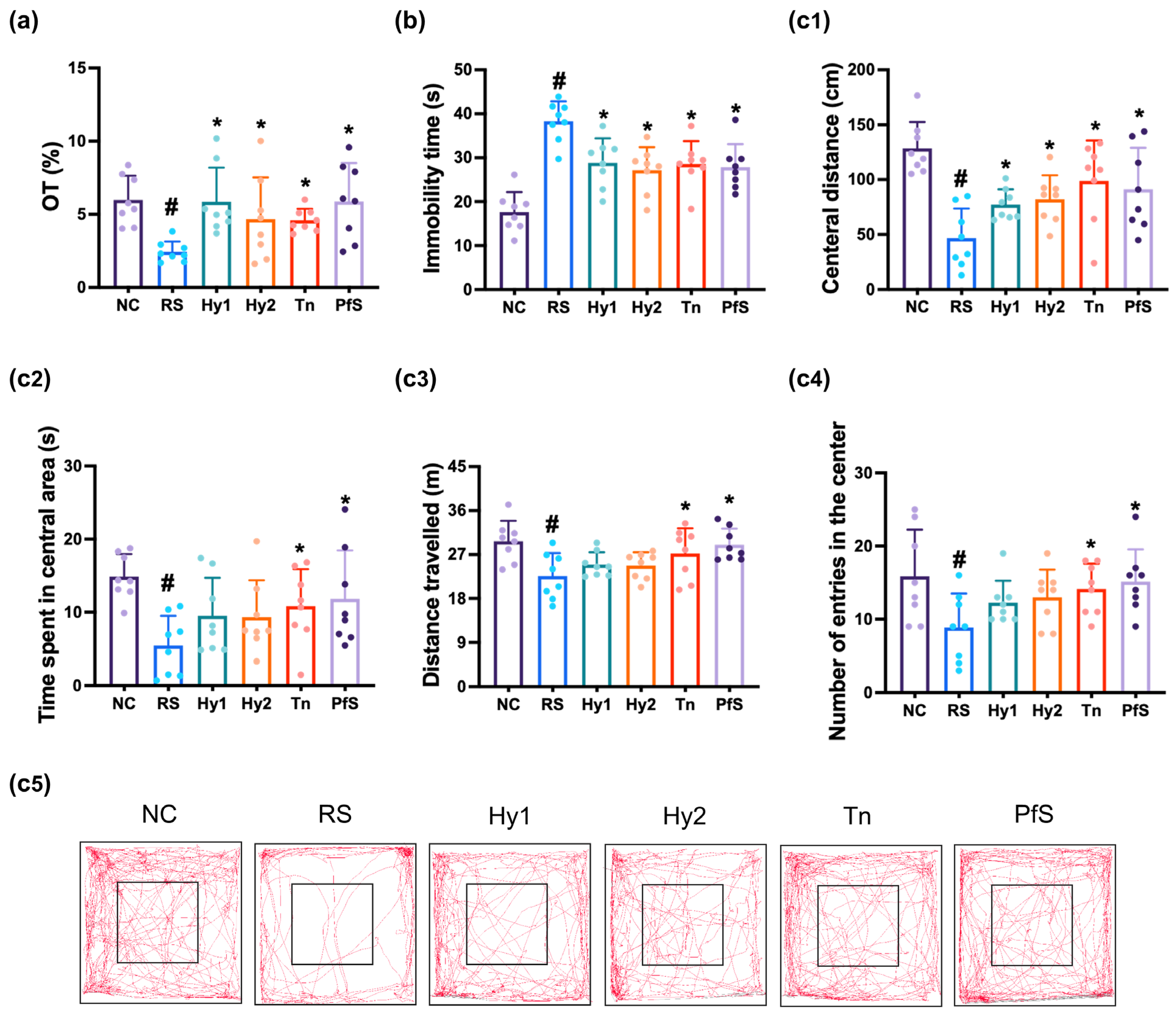
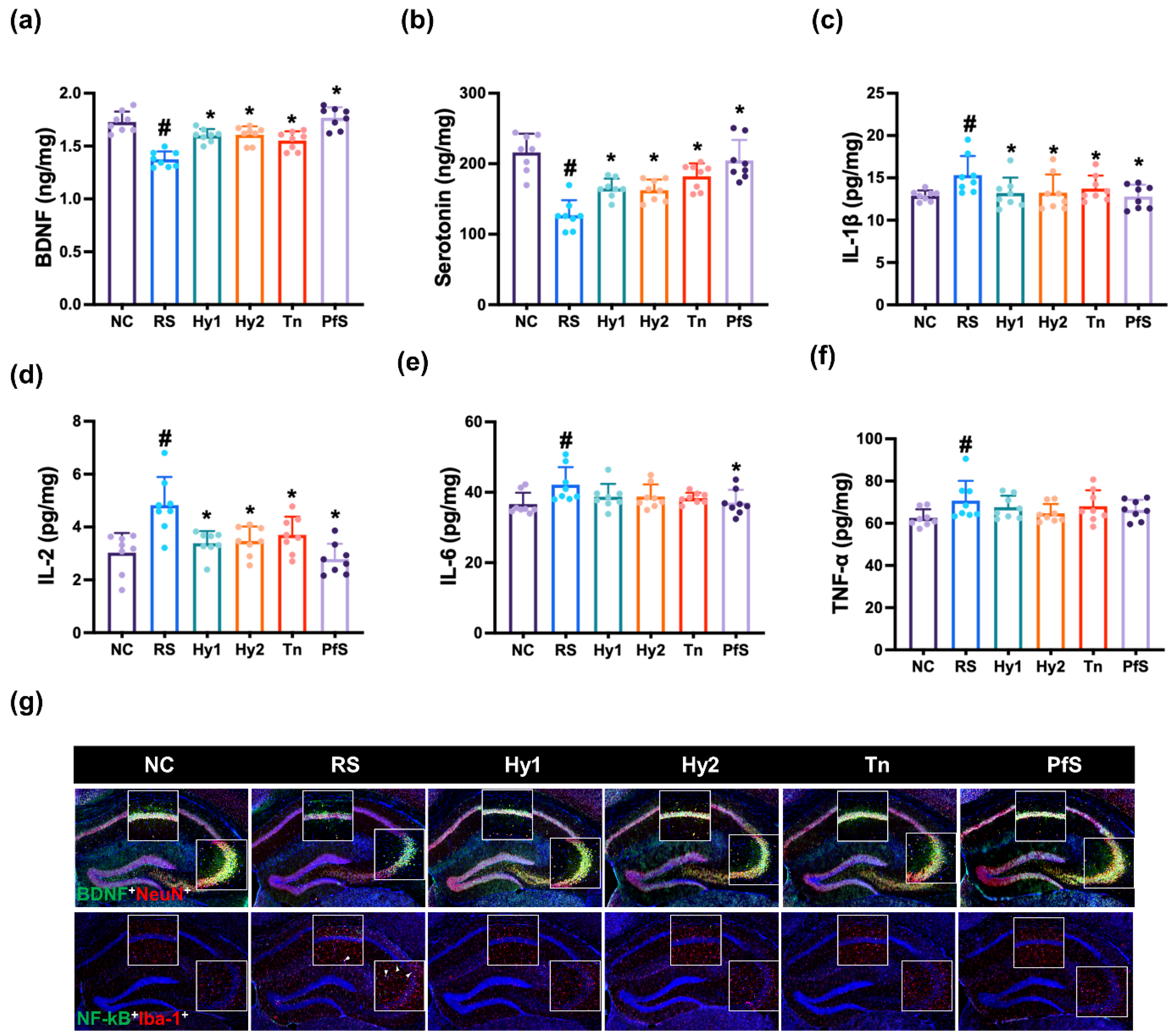
3.2. L. casei, B. lactis, L-Theanine, and Their Supplement PfS Improved RS-Induced DA-like Behaviors and Neuroinflammation in Mice
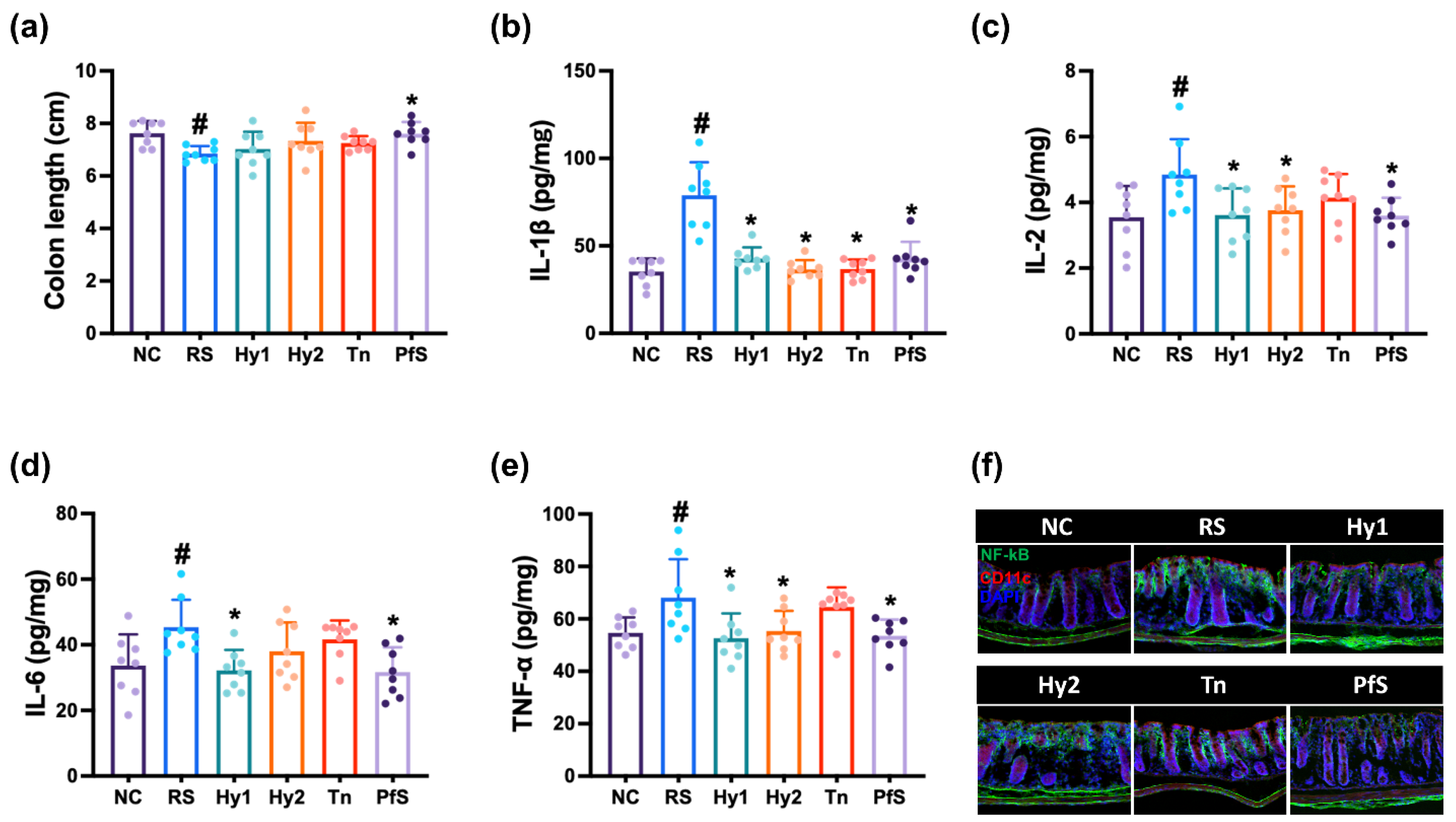
3.3. L. casei, B. lactis, L-Theanine, and Their Supplement Improved RS-Induced GI and GD in Mice
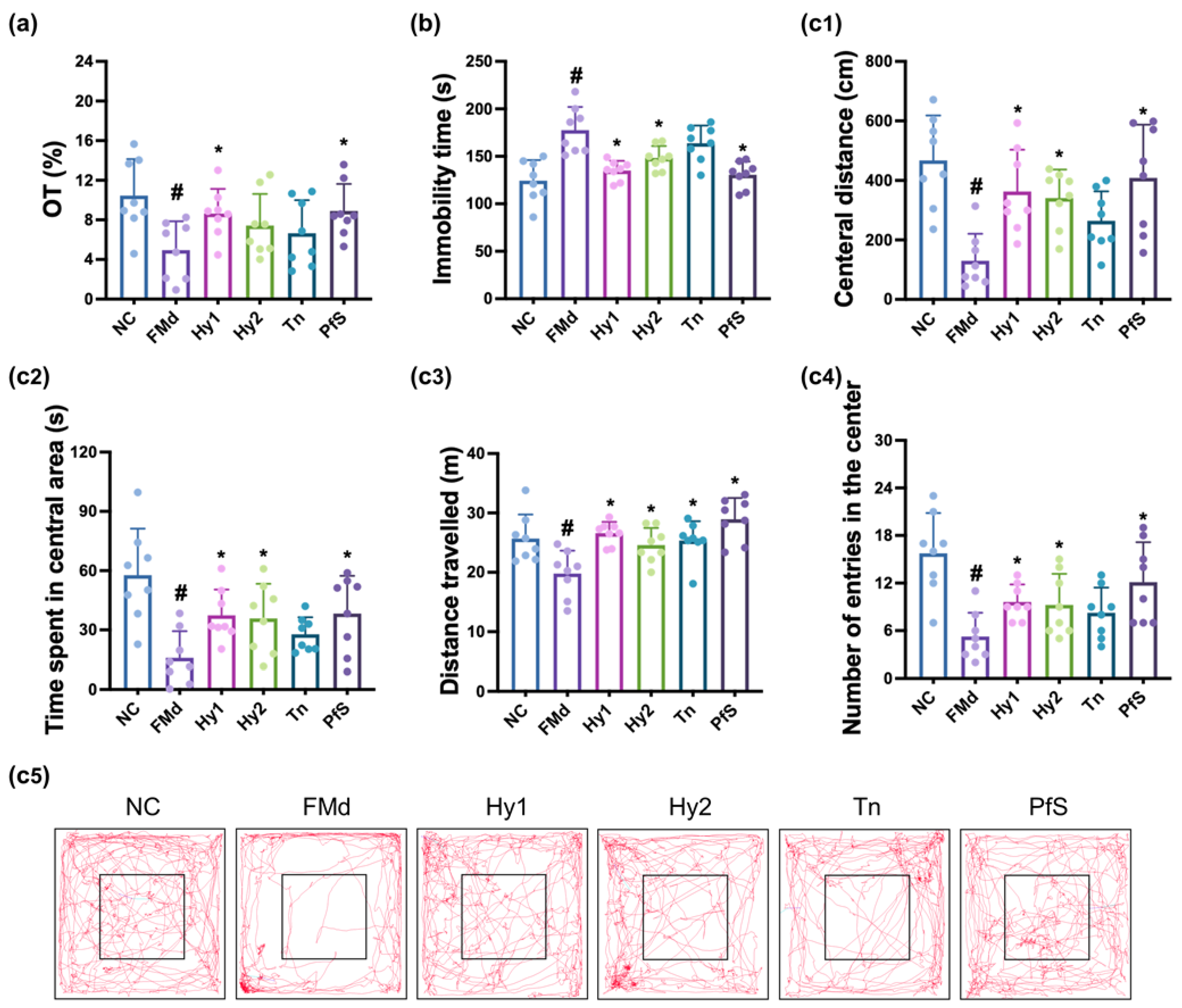
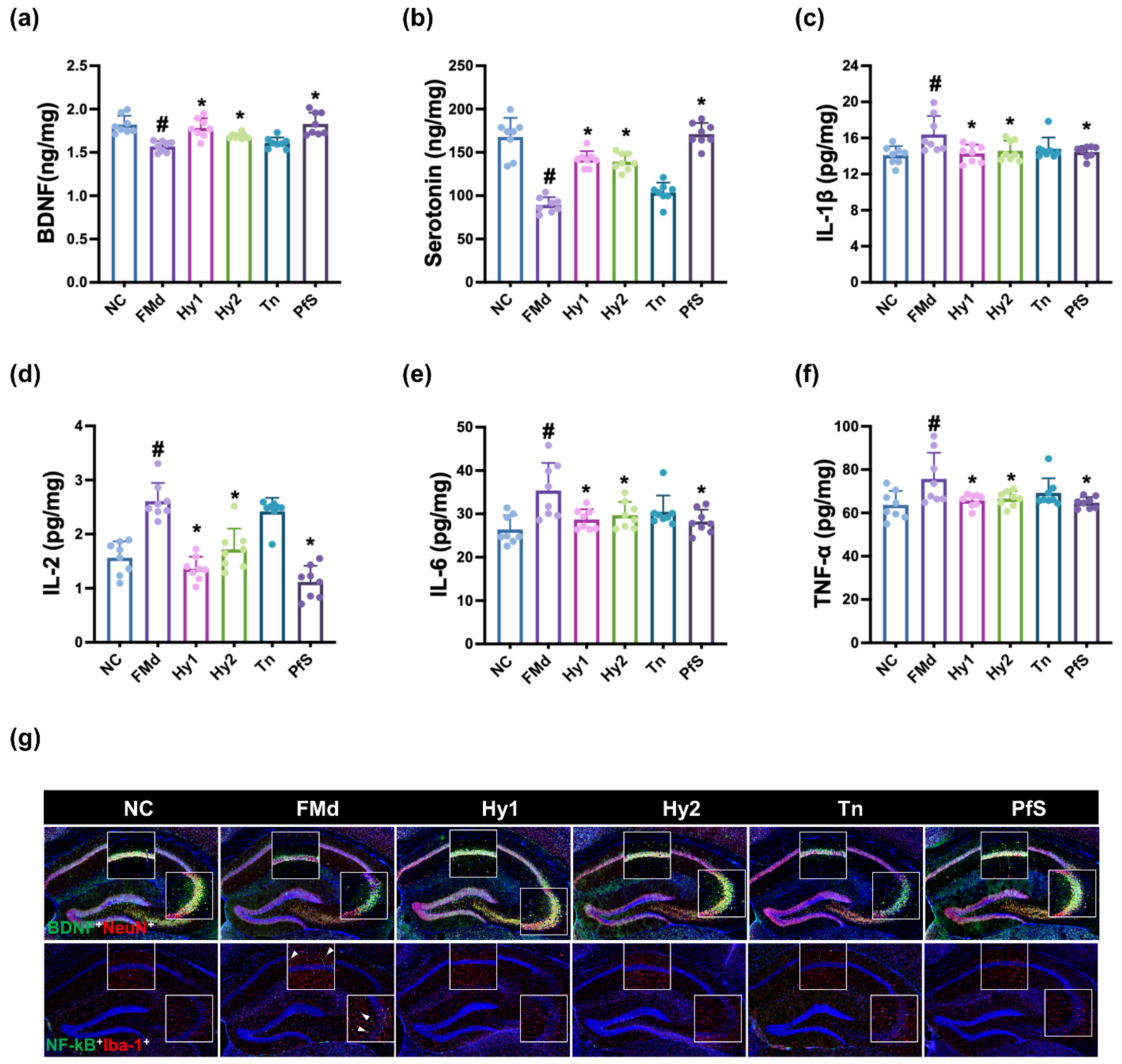
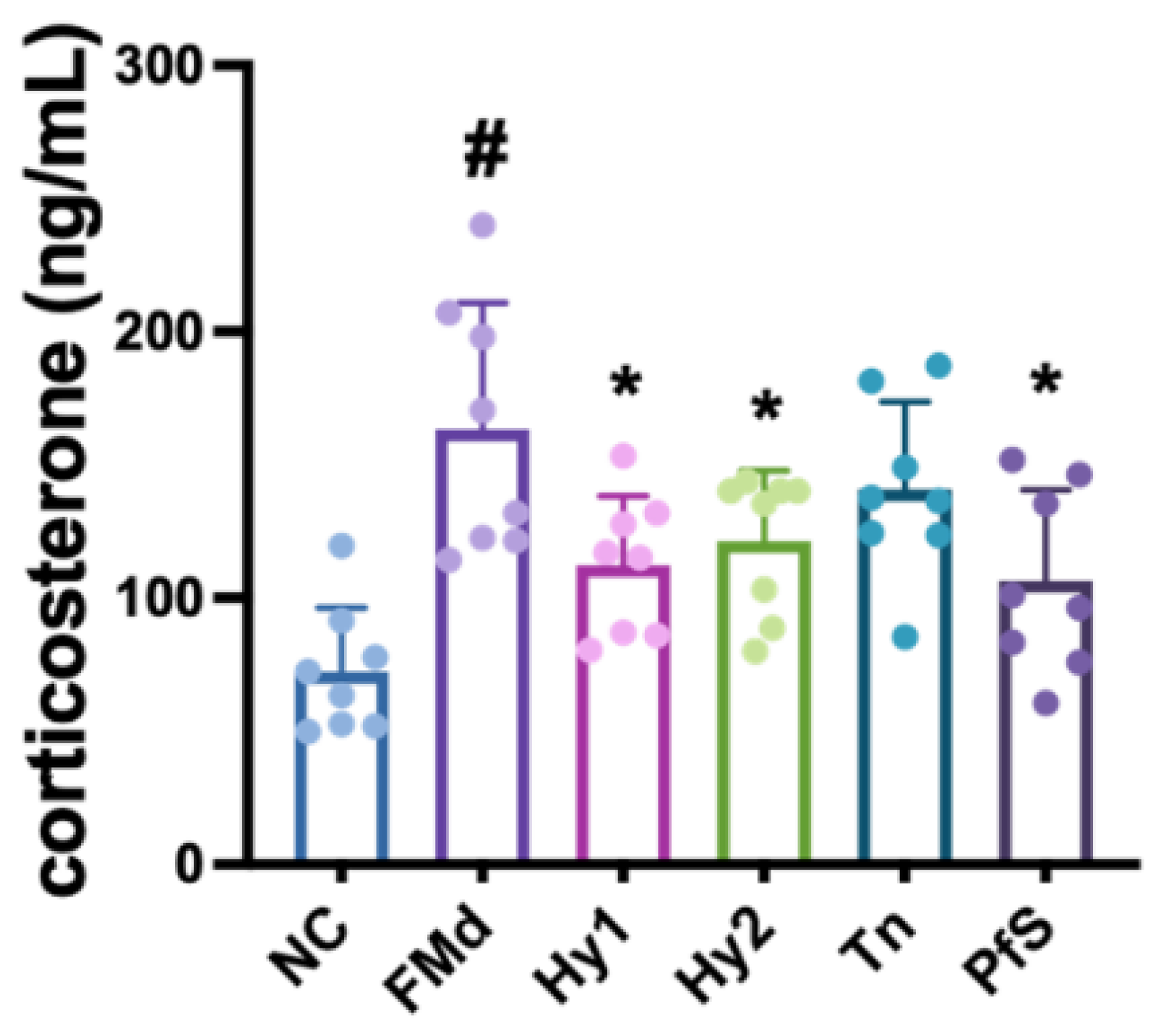
3.4. L. casei, B. lactis, and Its Supplement Improved FMd-Induced DA-like Behaviors and Neuroinflammation in Mice
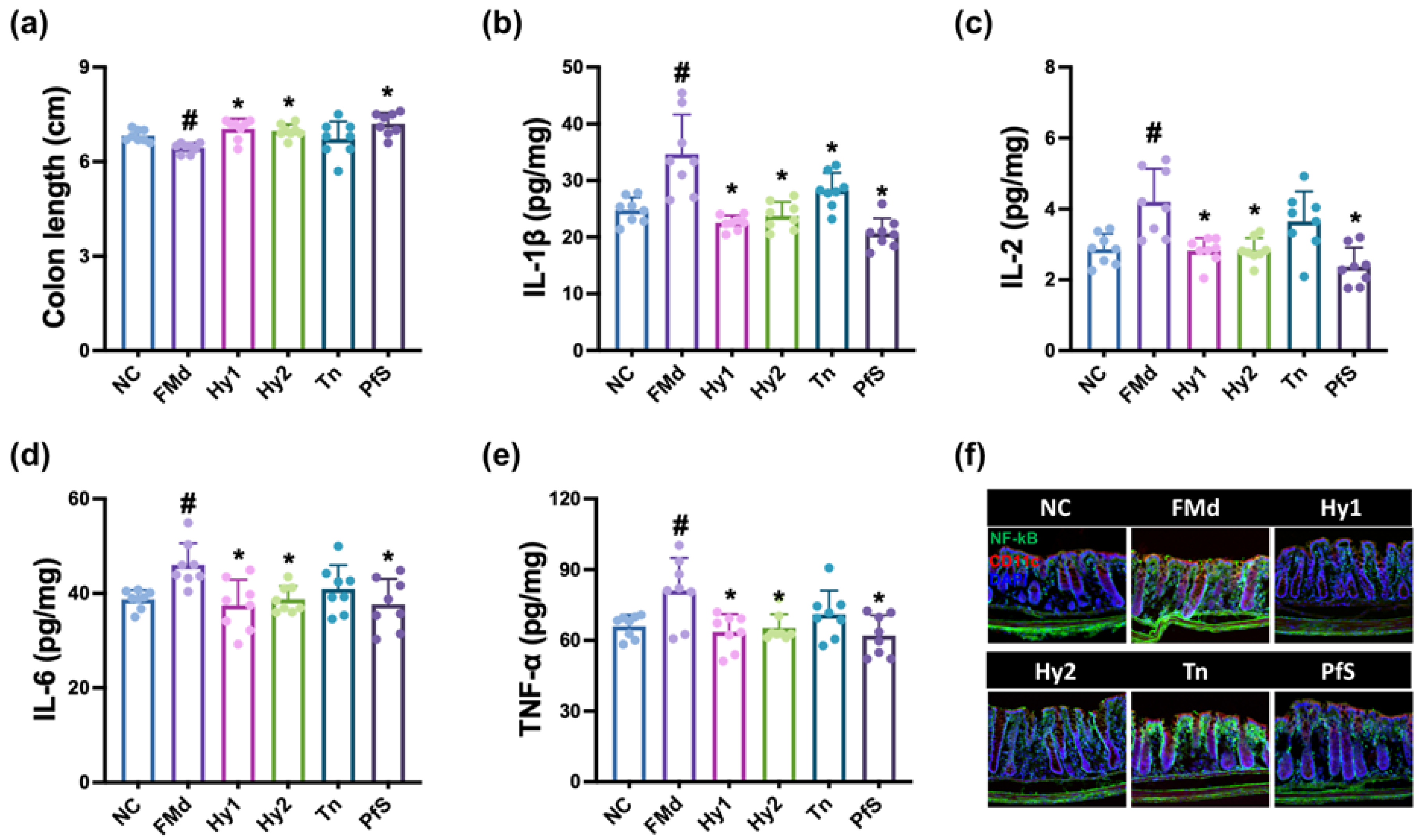
3.5. L. casei, B. lactis, L-Theanine, and Their Supplement Improved FMd Transplantation-Induced GI in Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Madison, A.; Kiecolt-Glaser, J.K. Stress, depression, diet, and the gut microbiota: Human-bacteria interactions at the core of psychoneuroimmunology and nutrition. Curr. Opin. Behav. Sci. 2019, 28, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Misiak, B.; Łoniewski, I.; Marlicz, W.; Frydecka, D.; Szulc, A.; Rudzki, L.; Samochowiec, J. The HPA axis dysregulation in severe mental illness: Can we shift the blame to gut microbiota? Prog. Neuropsychopharmacol. Biol. Psychiatry 2020, 102, 109951. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.M.; Lee, K.E.; Lee, H.J.; Kim, D.H. Immobilization stress-induced Escherichia coli causes anxiety by inducing NF-κB activation through gut microbiota disturbance. Sci. Rep. 2018, 8, 13897. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.K.; Lee, K.E.; Lee, S.A.; Jang, H.M.; Kim, D.H. Interplay Between Human Gut Bacteria Escherichia coli and Lactobacillus mucosae in the Occurrence of Neuropsychiatric Disorders in Mice. Front. Immunol. 2020, 11, 273. [Google Scholar] [CrossRef]
- Kim, K.A.; Jeong, J.J.; Yoo, S.Y.; Kim, D.H. Gut microbiota lipopolysaccharide accelerates inflamm-aging in mice. BMC Microbiol. 2016, 16, 9. [Google Scholar] [CrossRef]
- Porter, G.A.; O’Connor, J.C. Brain-derived neurotrophic factor and inflammation in depression: Pathogenic partners in crime? World J. Psychiatry 2022, 12, 77–97. [Google Scholar] [CrossRef]
- Bojović, K.; Ignjatović, Ð.I.; Soković Bajić, S.; Vojnović Milutinović, D.; Tomić, M.; Golić, N.; Tolinački, M. Gut Microbiota Dysbiosis Associated with Altered Production of Short Chain Fatty Acids in Children with Neurodevelopmental Disorders. Front. Cell Infect. Microbiol. 2020, 10, 223. [Google Scholar] [CrossRef]
- Angelucci, F.; Cechova, K.; Amlerova, J.; Hort, J. Antibiotics, gut microbiota, and Alzheimer’s disease. J. Neuroinflamm. 2019, 16, 108. [Google Scholar] [CrossRef]
- Barko, P.C.; McMichael, M.A.; Swanson, K.S.; Williams, D.A. The Gastrointestinal Microbiome: A Review. J. Vet. Intern. Med. 2018, 32, 9–25. [Google Scholar] [CrossRef]
- Carabotti, M.; Scirocco, A.; Maselli, M.A.; Severi, C. The gut-brain axis: Interactions between enteric microbiota, central and enteric nervous systems. Ann. Gastroenterol. 2015, 28, 203–209. [Google Scholar]
- Gomaa, E.Z. Human gut microbiota/microbiome in health and diseases: A review. Antonie Van Leeuwenhoek 2020, 113, 2019–2040. [Google Scholar] [CrossRef]
- Bercik, P.; Park, A.J.; Sinclair, D.; Khoshdel, A.; Lu, J.; Huang, X.; Deng, Y.; Blennerhassett, P.A.; Fahnestock, M.; Moine, D.; et al. The anxiolytic effect of Bifidobacterium longum NCC3001 involves vagal pathways for gut-brain communication. Neurogastroenterol. Motil. 2011, 23, 1132–1139. [Google Scholar] [CrossRef]
- Pinto-Sanchez, M.I.; Hall, G.B.; Ghajar, K.; Nardelli, A.; Bolino, C.; Lau, J.T.; Martin, F.P.; Cominetti, O.; Welsh, C.; Rieder, A.; et al. Probiotic Bifidobacterium longum NCC3001 Reduces Depression Scores and Alters Brain Activity: A Pilot Study in Patients with Irritable Bowel Syndrome. Gastroenterology 2017, 153, 448–459.e448. [Google Scholar] [CrossRef]
- Lee, H.J.; Hong, J.K.; Kim, J.K.; Kim, D.H.; Jang, S.W.; Han, S.W.; Yoon, I.Y. Effects of Probiotic NVP-1704 on Mental Health and Sleep in Healthy Adults: An 8-Week Randomized, Double-Blind, Placebo-Controlled Trial. Nutrients 2021, 13, 2660. [Google Scholar] [CrossRef]
- Han, S.K.; Kim, J.K.; Joo, M.K.; Lee, K.E.; Han, S.W.; Kim, D.H. Lactobacillus reuteri NK33 and Bifidobacterium adolescentis NK98 Alleviate Escherichia coli-Induced depression and Gut Dysbiosis in Mice. J. Microbiol. Biotechnol. 2020, 30, 1222–1226. [Google Scholar] [CrossRef]
- Tian, P.; O’Riordan, K.J.; Lee, Y.K.; Wang, G.; Zhao, J.; Zhang, H.; Cryan, J.F.; Chen, W. Towards a psychobiotic therapy for depression: Bifidobacterium breve CCFM1025 reverses chronic stress-induced depressive symptoms and gut microbial abnormalities in mice. Neurobiol. Stress. 2020, 12, 100216. [Google Scholar] [CrossRef]
- Tian, P.; Chen, Y.; Zhu, H.; Wang, L.; Qian, X.; Zou, R.; Zhao, J.; Zhang, H.; Qian, L.; Wang, Q.; et al. Bifidobacterium breve CCFM1025 attenuates major depression disorder via regulating gut microbiome and tryptophan metabolism: A randomized clinical trial. Brain Behav. Immun. 2022, 100, 233–241. [Google Scholar] [CrossRef]
- Huang, L.; Lv, X.; Ze, X.; Ma, Z.; Zhang, X.; He, R.; Fan, J.; Zhang, M.; Sun, B.; Wang, F.; et al. Combined probiotics attenuate chronic unpredictable mild stress-induced depressive-like and anxiety-like behaviors in rats. Front. Psychiatry 2022, 13, 990465. [Google Scholar] [CrossRef]
- Slykerman, R.F.; Hood, F.; Wickens, K.; Thompson, J.M.D.; Barthow, C.; Murphy, R.; Kang, J.; Rowden, J.; Stone, P.; Crane, J.; et al. Effect of Lactobacillus rhamnosus HN001 in Pregnancy on Postpartum Symptoms of Depression and Anxiety: A Randomised Double-blind Placebo-controlled Trial. EBioMedicine 2017, 24, 159–165. [Google Scholar] [CrossRef]
- Ma, X.; Yoo, J.W.; Shin, Y.J.; Park, H.S.; Son, Y.H.; Kim, D.H. Alleviation of Porphyromonas gingivalis or Its Extracellular Vesicles Provoked Periodontitis and Cognitive Impairment by Lactobacillus pentosus NK357 and Bifidobacterium bifidum NK391. Nutrients 2023, 15, 1068. [Google Scholar] [CrossRef]
- Yeung, K.S.; Hernandez, M.; Mao, J.J.; Haviland, I.; Gubili, J. Herbal medicine for depression and anxiety: A systematic review with assessment of potential psycho-oncologic relevance. Phytother. Res. 2018, 32, 865–891. [Google Scholar] [CrossRef] [PubMed]
- Di Lorenzo, A.; Nabavi, S.F.; Sureda, A.; Moghaddam, A.H.; Khanjani, S.; Arcidiaco, P.; Nabavi, S.M.; Daglia, M. Antidepressive-like effects and antioxidant activity of green tea and GABA green tea in a mouse model of post-stroke depression. Mol. Nutr. Food Res. 2016, 60, 566–579. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Xiao, X.; Mao, X.; Chen, D.; Yu, B.; Wang, J.; Yan, H. Tea bioactive components prevent carcinogenesis via anti-pathogen, anti-inflammation, and cell survival pathways. IUBMB Life 2021, 73, 328–340. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.; Yang, Y.; Wu, Y.; Zhang, B.; Wu, H.; Wang, L.; Tang, H.; Chen, J. L-theanine ameliorate depressive-like behavior in a chronic unpredictable mild stress rat model via modulating the monoamine levels in limbic-cortical-striatal-pallidal-thalamic-circuit related brain regions. Phytother. Res. 2019, 33, 412–421. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.W.; Shin, Y.J.; Ma, X.; Son, Y.H.; Jang, H.M.; Lee, C.K.; Kim, D.H. The Alleviation of Gut Microbiota-Induced Depression and Colitis in Mice by Anti-Inflammatory Probiotics NK151, NK173, and NK175. Nutrients 2022, 14, 2080. [Google Scholar] [CrossRef]
- Joo, M.K.; Ma, X.; Yoo, J.W.; Shin, Y.J.; Kim, H.J.; Kim, D.H. Patient-derived Enterococcus mundtii and its capsular polysaccharides cause depression through the downregulation of NF-κB-involved serotonin and BDNF expression. Microbes Infect. 2023, 105116. [Google Scholar] [CrossRef]
- Lee, H.J.; Lee, K.E.; Kim, J.K.; Kim, D.H. Suppression of gut dysbiosis by Bifidobacterium longum alleviates cognitive decline in 5XFAD transgenic and aged mice. Sci. Rep. 2019, 9, 11814. [Google Scholar] [CrossRef]
- Jang, H.M.; Lee, H.J.; Jang, S.E.; Han, M.J.; Kim, D.H. Evidence for interplay among antibacterial-induced gut microbiota disturbance, neuro-inflammation, and anxiety in mice. Mucosal Immunol. 2018, 11, 1386–1397. [Google Scholar] [CrossRef]
- Lee, K.E.; Kim, J.K.; Han, S.K.; Lee, D.Y.; Lee, H.J.; Yim, S.V.; Kim, D.H. The extracellular vesicle of gut microbial Paenalcaligenes hominis is a risk factor for vagus nerve-mediated cognitive impairment. Microbiome 2020, 8, 107. [Google Scholar] [CrossRef]
- Smith, S.M.; Vale, W.W. The role of the hypothalamic-pituitary-adrenal axis in neuroendocrine responses to stress. Dialogues Clin. Neurosci. 2006, 8, 383–395. [Google Scholar] [CrossRef]
- Lehmann, M.L.; Brachman, R.A.; Listwak, S.J.; Herkenham, M. NF-kappaB activity affects learning in aversive tasks: Possible actions via modulation of the stress axis. Brain Behav. Immun. 2010, 24, 1008–1017. [Google Scholar] [CrossRef]
- Freimer, D.; Yang, T.T.; Ho, T.C.; Tymofiyeva, O.; Leung, C. The gut microbiota, HPA axis, and brain in adolescent-onset depression: Probiotics as a novel treatment. Brain Behav. Immun. Health 2022, 26, 100541. [Google Scholar] [CrossRef]
- Gotlib, I.H.; Joormann, J.; Minor, K.L.; Hallmayer, J. HPA axis reactivity: A mechanism underlying the associations among 5-HTTLPR, stress, and depression. Biol. Psychiatry 2008, 63, 847–851. [Google Scholar] [CrossRef]
- Peirce, J.M.; Alviña, K. The role of inflammation and the gut microbiome in depression and anxiety. J. Neurosci. Res. 2019, 97, 1223–1241. [Google Scholar] [CrossRef]
- Wahlestedt, C.; Ekman, R.; Widerlöv, E. Neuropeptide Y (NPY) and the central nervous system: Distribution effects and possible relationship to neurological and psychiatric disorders. Prog. Neuropsychopharmacol. Biol. Psychiatry 1989, 13, 31–54. [Google Scholar] [CrossRef]
- Stevens, C.; Walz, G.; Singaram, C.; Lipman, M.L.; Zanker, B.; Muggia, A.; Antonioli, D.; Peppercorn, M.A.; Strom, T.B. Tumor necrosis factor-alpha, interleukin-1 beta, and interleukin-6 expression in inflammatory bowel disease. Dig. Dis. Sci. 1992, 37, 818–826. [Google Scholar] [CrossRef]
- Lee, D.H.; Lee, J.Y.; Hong, D.Y.; Lee, E.C.; Park, S.W.; Lee, M.R.; Oh, J.S. Neuroinflammation in Post-Traumatic Stress Disorder. Biomedicines 2022, 10, 953. [Google Scholar] [CrossRef]
- Zhang, L.; Yao, X.; Ma, M.; Ding, Y.; Zhang, H.; He, X.; Song, Z. Protective Effect of l-Theanine against DSS-Induced Colitis by Regulating the Lipid Metabolism and Reducing Inflammation via the NF-κB Signaling Pathway. J. Agric. Food Chem. 2021, 69, 14192–14203. [Google Scholar] [CrossRef]
- Jang, H.M.; Kim, J.K.; Joo, M.K.; Shin, Y.J.; Lee, C.K.; Kim, H.J.; Kim, D.H. Transplantation of fecal microbiota from patients with inflammatory bowel disease and depression alters immune response and behavior in recipient mice. Sci. Rep. 2021, 11, 20406. [Google Scholar] [CrossRef]
- Fukui, H. Increased Intestinal Permeability and Decreased Barrier Function: Does It Really Influence the Risk of Inflammation? Inflamm. Intest. Dis. 2016, 1, 135–145. [Google Scholar] [CrossRef]
- Cenit, M.C.; Sanz, Y.; Codoñer-Franch, P. Influence of gut microbiota on neuropsychiatric disorders. World J. Gastroenterol. 2017, 23, 5486–5498. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.E.; Lim, S.M.; Jeong, J.J.; Jang, H.M.; Lee, H.J.; Han, M.J.; Kim, D.H. Gastrointestinal inflammation by gut microbiota disturbance induces memory impairment in mice. Mucosal Immunol. 2018, 11, 369–379. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.H.; Chiou, S.H.; Chen, S.J.; Chou, Y.C.; Ku, H.H.; Cheng, C.K.; Yen, C.J.; Tsai, T.H.; Chang, Y.L.; Kao, C.L. Neuroprotection by Imipramine against lipopolysaccharide-induced apoptosis in hippocampus-derived neural stem cells mediated by activation of BDNF and the MAPK pathway. Eur. Neuropsychopharmacol. 2008, 18, 128–140. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, X.; Shin, Y.-J.; Park, H.-S.; Jeong, J.-W.; Kim, J.Y.; Shim, J.-J.; Lee, J.-L.; Kim, D.-H. Lactobacillus casei and Its Supplement Alleviate Stress-Induced Depression and Anxiety in Mice by the Regulation of BDNF Expression and NF-κB Activation. Nutrients 2023, 15, 2488. https://doi.org/10.3390/nu15112488
Ma X, Shin Y-J, Park H-S, Jeong J-W, Kim JY, Shim J-J, Lee J-L, Kim D-H. Lactobacillus casei and Its Supplement Alleviate Stress-Induced Depression and Anxiety in Mice by the Regulation of BDNF Expression and NF-κB Activation. Nutrients. 2023; 15(11):2488. https://doi.org/10.3390/nu15112488
Chicago/Turabian StyleMa, Xiaoyang, Yoon-Jung Shin, Hee-Seo Park, Ji-Woong Jeong, Joo Yun Kim, Jae-Jung Shim, Jung-Lyoul Lee, and Dong-Hyun Kim. 2023. "Lactobacillus casei and Its Supplement Alleviate Stress-Induced Depression and Anxiety in Mice by the Regulation of BDNF Expression and NF-κB Activation" Nutrients 15, no. 11: 2488. https://doi.org/10.3390/nu15112488
APA StyleMa, X., Shin, Y.-J., Park, H.-S., Jeong, J.-W., Kim, J. Y., Shim, J.-J., Lee, J.-L., & Kim, D.-H. (2023). Lactobacillus casei and Its Supplement Alleviate Stress-Induced Depression and Anxiety in Mice by the Regulation of BDNF Expression and NF-κB Activation. Nutrients, 15(11), 2488. https://doi.org/10.3390/nu15112488






