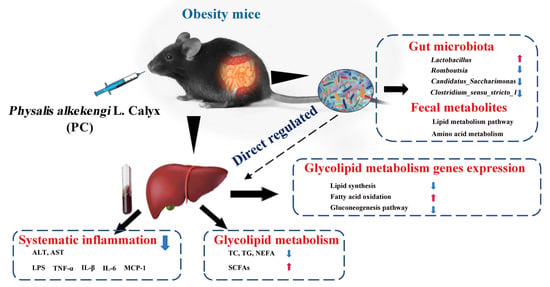
Physalis alkekengi L. Calyx Extract Alleviates Glycolipid Metabolic Disturbance and Inflammation by Modulating Gut Microbiota, Fecal Metabolites, and Glycolipid Metabolism Gene Expression in Obese Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Animals and Experimental Design
2.3. Biochemical Analysis
2.4. Histopathological Analysis
2.5. Quantitative Real-Time PCR
2.6. Analysis of Short-Chain Fatty Acids
2.7. Analysis of Gut Microbiota
2.8. Untargeted Metabolomics Analysis
2.9. Statistical Analysis
3. Results
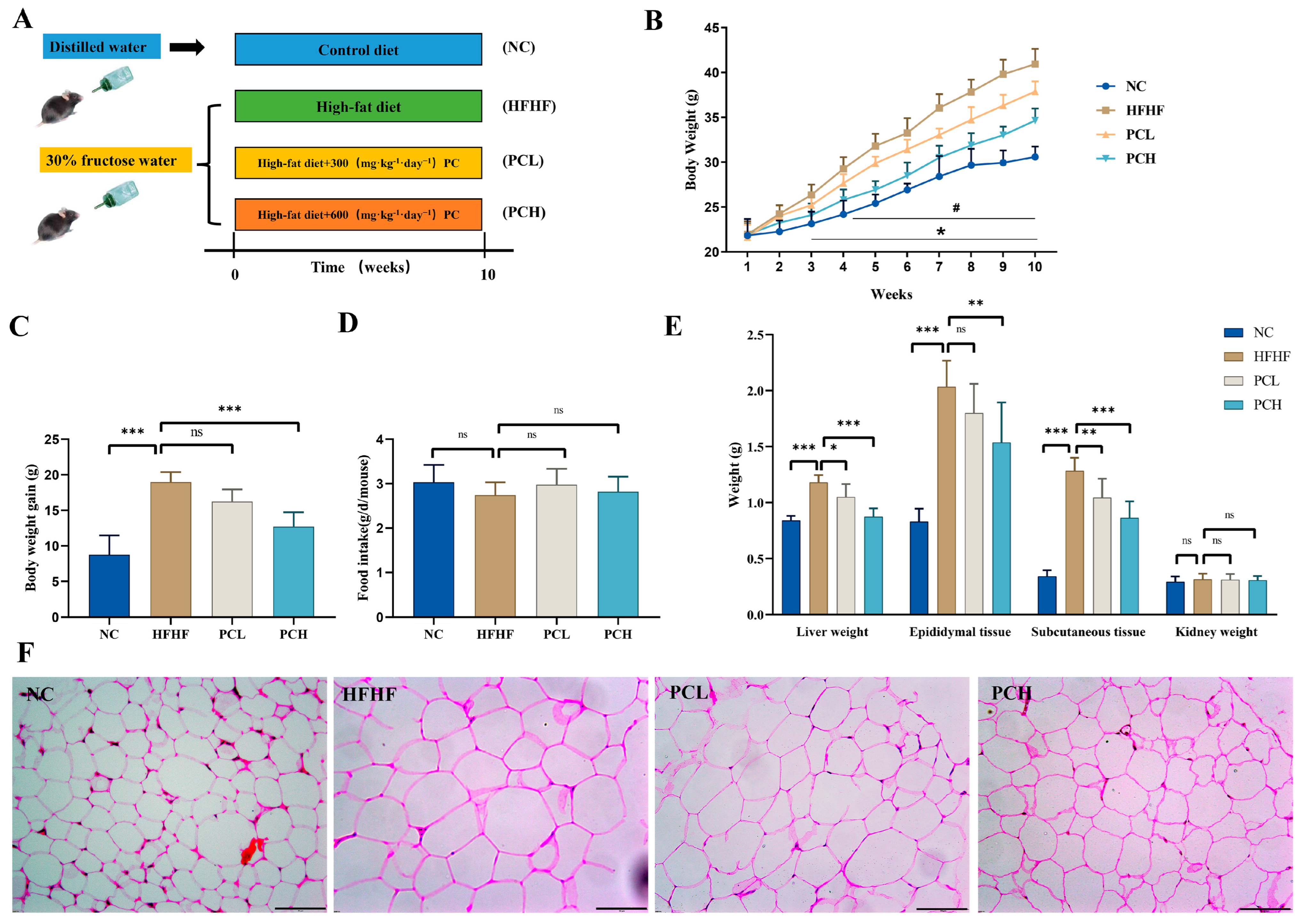
3.1. PC Prevented HFHF-Induced Obesity in Mice
3.2. PC Improved Glucose Homeostasis and Alleviated HFHF-Induced Insulin Resistance
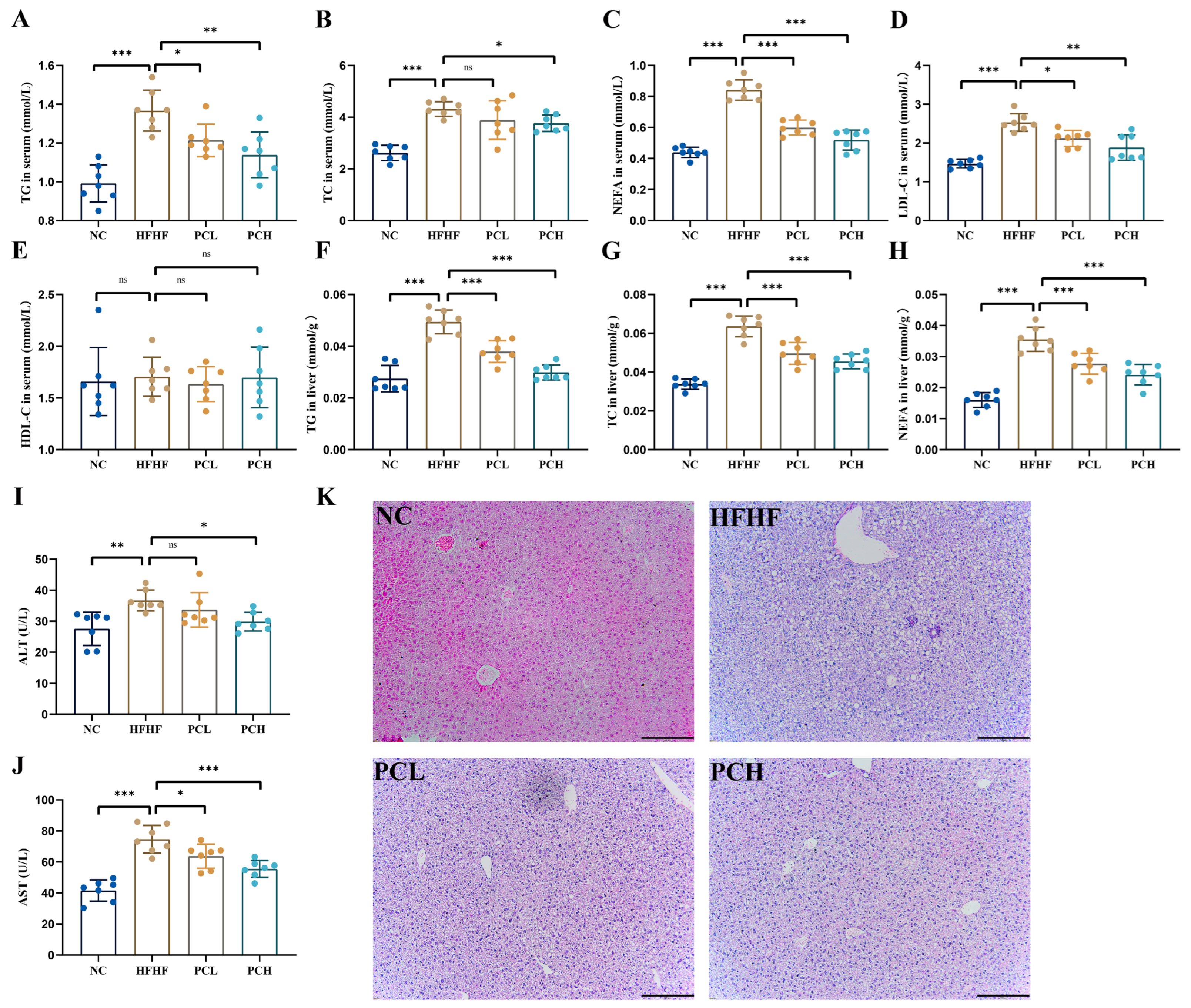
3.3. PC Attenuated the HFHF-Induced Dyslipidemia
3.4. PC Modified the Expression Profiles of Genes Involved in Glycolipid Metabolism in Livers
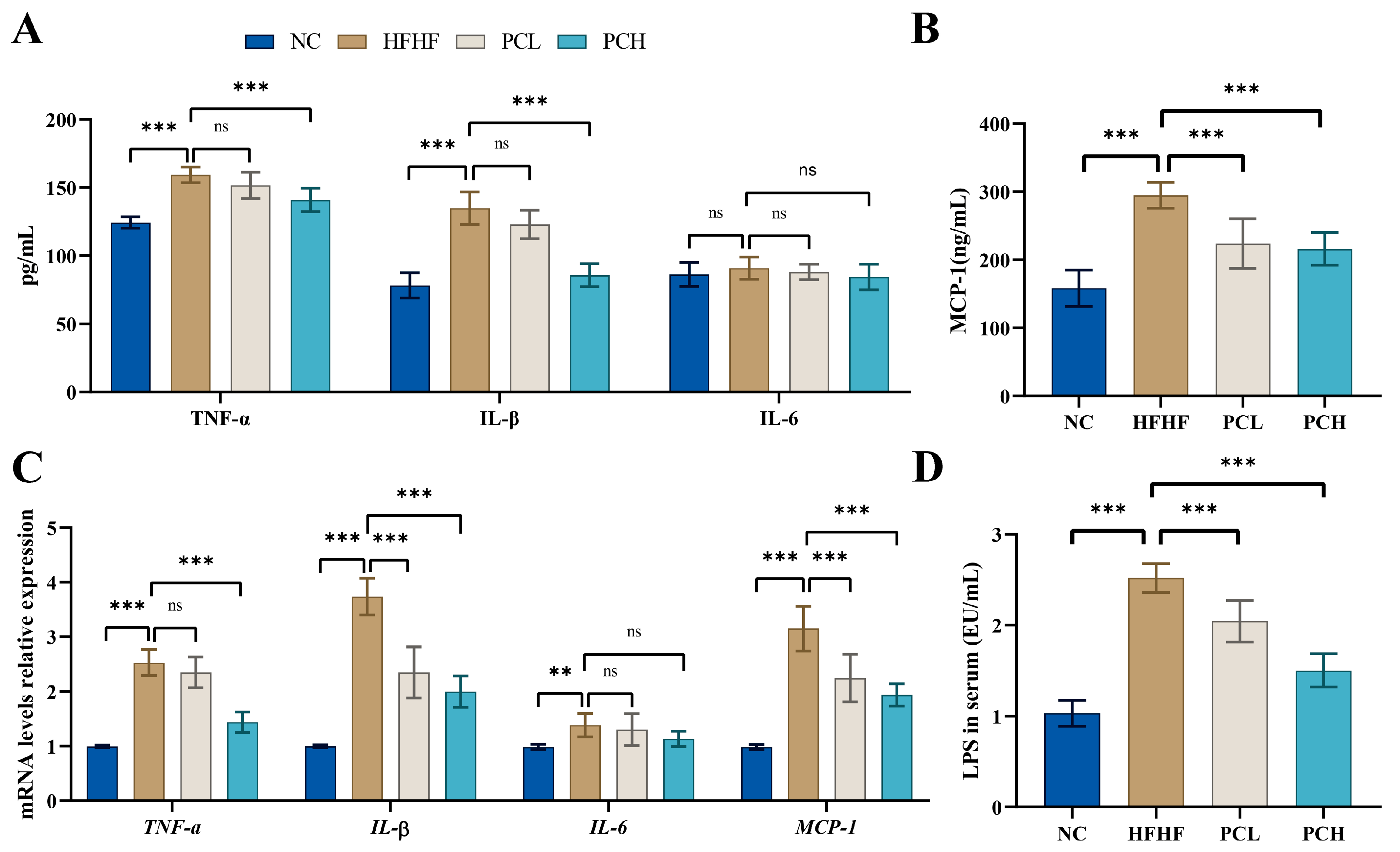
3.5. PC Inhibited the HFHF-Induced Secretion of Proinflammatory Cytokines
3.6. PC Altered the Fecal SCFAs Composition
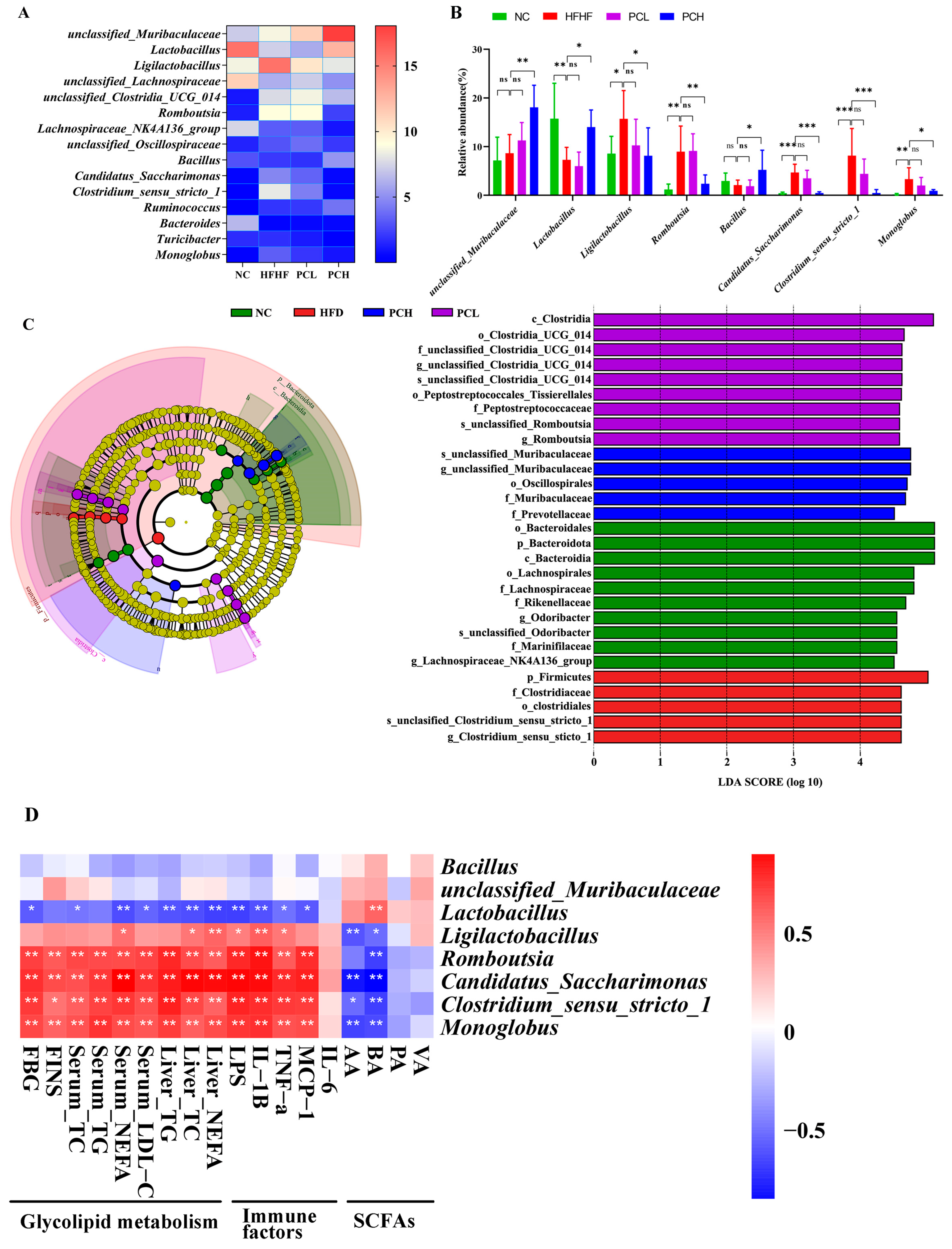
3.7. PC Modulated Gut Microbiota Composition at Different Taxonomic Levels
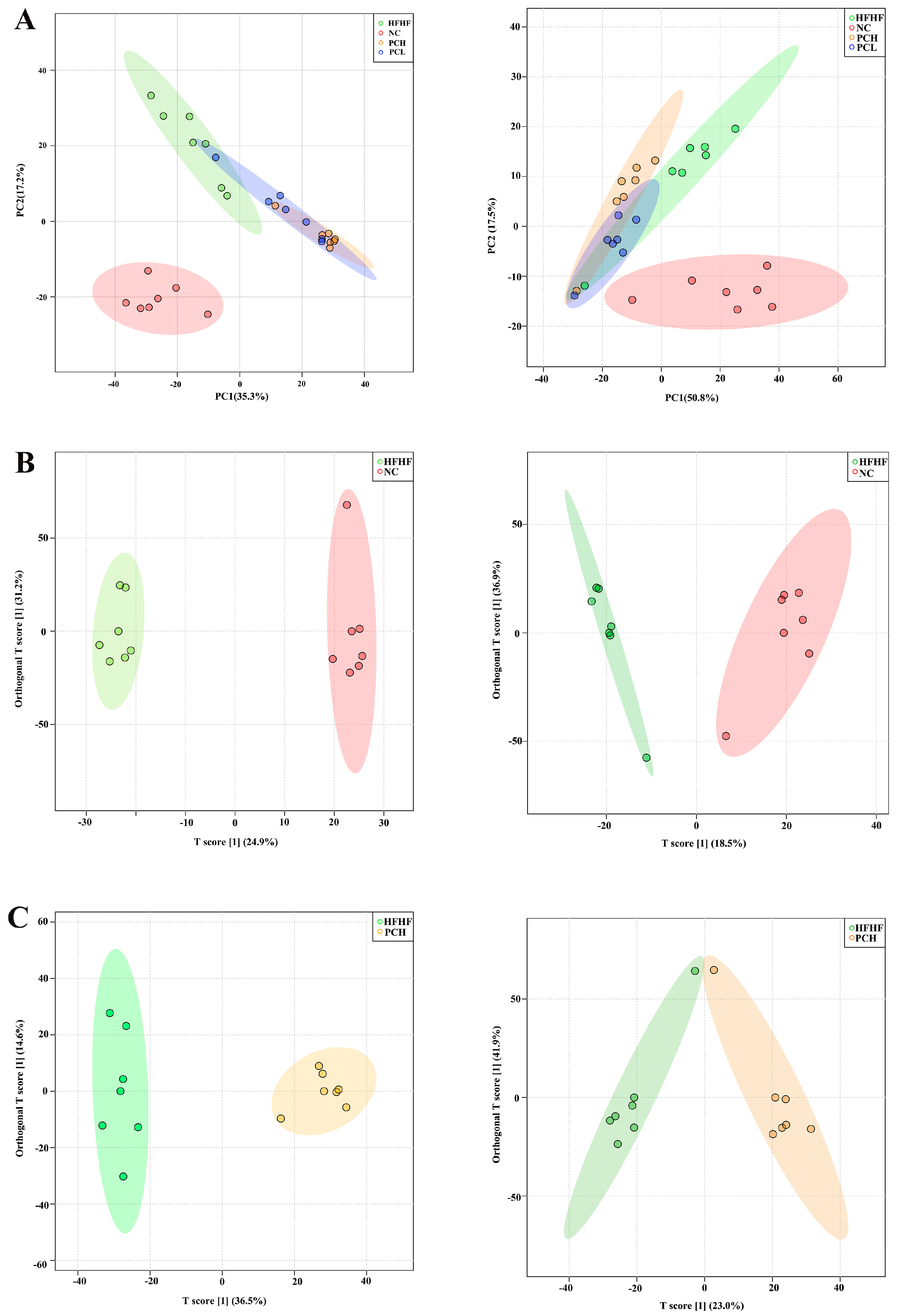
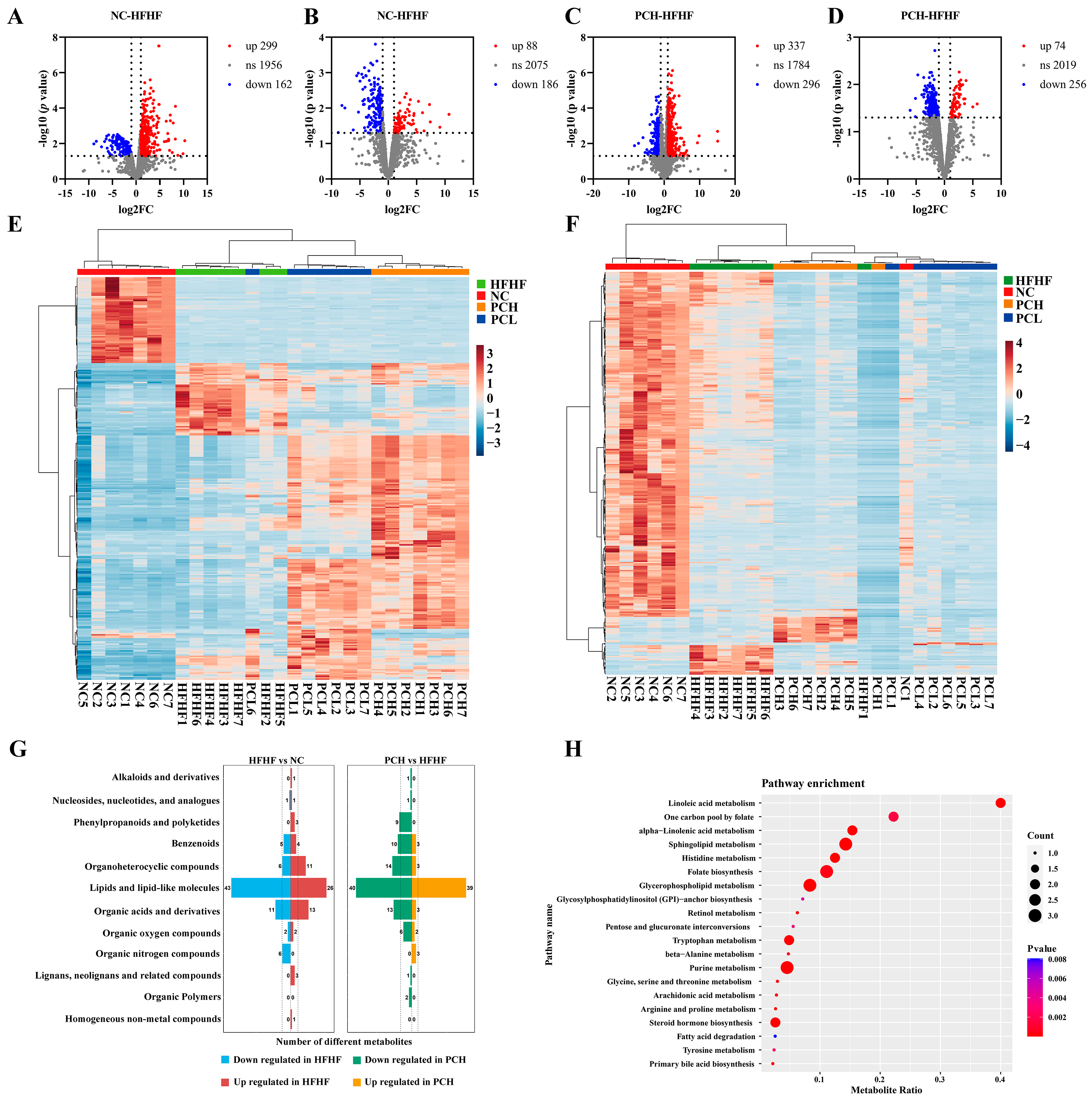
3.8. PC Altered the Gut Metabolic Profile in Mice
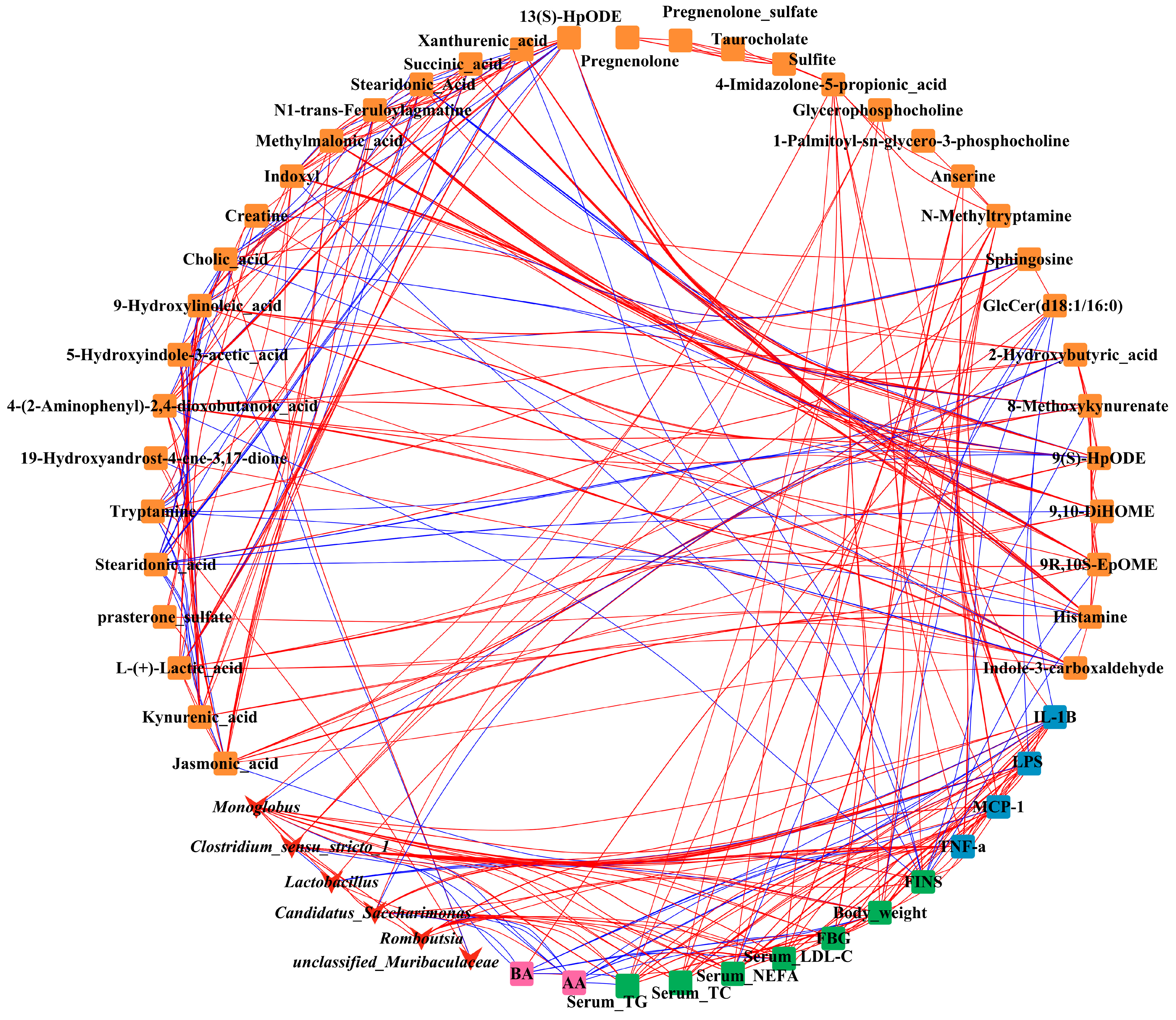
3.9. Correlations Network among Gut Microbiota, Differential Metabolites in Feces, Glycolipid Metabolism Parameters in Serum, and Inflammatory Factor in Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aron-Wisnewsky, J.; Vigliotti, C.; Witjes, J.; Le, P.; Holleboom, A.G.; Verheij, J. Gut microbiota and human NAFLD: Disentangling microbial signatures from metabolic disorders. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 279–297. [Google Scholar] [CrossRef] [PubMed]
- Miah, L.; Strafford, H.; Fonferko-Shadrach, B.; Hollinghurst, J.; Pickrell, W.O.J. Incidence, Prevalence, and Health Care Outcomes in Idiopathic Intracranial Hypertension: A Population Study. Neurology 2021, 96, 1251–1261. [Google Scholar] [CrossRef]
- Saltiel, A.R.; Olefsky, J.M. Inflammatory mechanisms linking obesity and metabolic disease. J. Clin. Investig. 2017, 127, 1–4. [Google Scholar] [CrossRef]
- Friedman, S.L.; Neuschwander-Tetri, B.A.; Mary, R.; Sanyal, A.J. Mechanisms of NAFLD development and therapeutic strategies. Nat. Med. 2018, 24, 908–922. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.S.; Yoon, M. Korean red ginseng (Panax ginseng) inhibits obesity and improves lipid metabolism in high fat diet-fed castrated mice. J. Ethnopharmacol. 2018, 210, 80–87. [Google Scholar] [CrossRef]
- Bendor, C.D.; Aya, B.; Orit, P.H.; Arnon, A.; Gilad, T. Cardiovascular morbidity, diabetes and cancer risk among children and adolescents with severe obesity. Cardiovasc. Diabetol. 2020, 19, 79. [Google Scholar] [CrossRef]
- de Sousa, A.R.; de Castro Moreira, M.E.; Toledo, R.C.L.; dos Anjos Benjamin, L.; Queiroz, V.A.V.; Veloso, M.P.; de Souza Reis, K.; Martino, H.S.D. Extruded sorghum (Sorghum bicolor L.) reduces metabolic risk of hepatic steatosis in obese rats consuming a high fat diet. Food Res. Int 2018, 112, 48–55. [Google Scholar] [CrossRef]
- Yu, K.; Huang, K.; Tang, Z.; Huang, X.; Sun, L.; Pang, L.; Mo, C. Metabolism and antioxidation regulation of total flavanones from Sedum sarmentosum Bunge against high-fat diet-induced fatty liver disease in Nile tilapia (Oreochromis niloticus). J. Fish Physiol. Biochem. 2021, 47, 1149–1164. [Google Scholar] [CrossRef]
- Shen, H.; Huang, L.; Dou, H.; Yang, Y.; Wu, H. Effect of Trilobatin from Lithocarpus polystachyus Rehd on Gut Microbiota of Obese Rats Induced by a High-Fat Diet. Nutrients 2021, 13, 891. [Google Scholar] [CrossRef] [PubMed]
- Nemati, M.; Zardooz, H.; Rostamkhani, F.; Abadi, A.; Foroughi, F. High-fat diet effects on metabolic responses to chronic stress. Arch. Physiol. Biochem. 2017, 123, 182–191. [Google Scholar] [CrossRef]
- Harris, K.F. An introductory review of resistant starch type 2 from high-amylose cereal grains and its effect on glucose and insulin homeostasis. Nutr. Rev. 2019, 77, 748–764. [Google Scholar] [CrossRef]
- Liu, B.N.; Liu, X.T.; Liang, Z.H.; Wang, J.H. Gut microbiota in obesity. World J. Gastroenterol. 2021, 27, 3850–3873. [Google Scholar] [CrossRef]
- Sang, T.; Guo, C.; Guo, D.; Wu, J.; Wang, Y.; Wang, Y.; Chen, J.; Chen, C.; Wu, K.; Na, K.; et al. Suppression of obesity and inflammation by polysaccharide from sporoderm-broken spore of Ganoderma lucidum via gut microbiota regulation. Carbohydr. Polym. 2021, 256, 117594. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Hamady, M.; Yatsunenko, T.; Cantarel, B.L.; Duncan, A.; Ley, R.E.; Sogin, M.L.; Jones, W.J.; Roe, B.A.; Affourtit, J. A core gut microbiome in obese and lean twins. Nature 2009, 457, 480. [Google Scholar] [CrossRef] [PubMed]
- Fischer, M.M.; Kessler, A.M.; Kieffer, D.A.; Knotts, T.A.; Kim, K.; Wei, A.; Ramsey, J.J.; Fascetti, A.J. Effects of obesity, energy restriction and neutering on the faecal microbiota of cats. Br. J. Nutr. 2017, 7, 513–524. [Google Scholar] [CrossRef]
- Bäckhed, F.; Fraser, C.M.; Ringel, Y.; Sanders, M.E.; Sartor, R.B.; Sherman, P.M.; Versalovic, J.; Young, V.; Finlay, B.B. Defining a healthy human gut microbiome: Current concepts, future directions, and clinical applications. Cell Host Microbe 2012, 12, 611–622. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Guo, W.L.; Zhang, W.; Xu, J.; Qian, M.; Bai, W.D.; Zhang, Y.Y.; Rao, P.; Ni, L.; Lv, X.C. Grifola frondosa polysaccharides ameliorate lipid metabolic disorders and gut microbiota dysbiosis in high-fat diet fed rats. Food Funct. 2019, 10, 2560–2572. [Google Scholar] [CrossRef] [PubMed]
- Zhi, C.; Huang, J.; Wang, J.; Cao, H.; Bai, Y.; Guo, J.; Su, Z. Connection between gut microbiome and the development of obesity. Eur. J. Clin. Microbiol. 2019, 38, 1919–1987. [Google Scholar] [CrossRef]
- Yang, C.; Xu, Z.; Deng, Q.; Huang, Q.; Huang, F. Beneficial effects of flaxseed polysaccharides on metabolic syndrome via gut microbiota in high-fat diet fed mice. Food Res. Int. 2020, 131, 108994. [Google Scholar] [CrossRef]
- Feng, L.; Zhou, J.; Zhang, L.; Liu, P.; Wan, X. Gut microbiota-mediated improvement of metabolic disorders by Qingzhuan tea in high fat diet-fed mice. J. Funct. Foods 2021, 78, 104366. [Google Scholar] [CrossRef]
- Chen, M.; Liao, Z.; Lu, B.; Wang, M.; Lin, L.; Zhang, S.; Li, Y.; Liu, D.; Liao, Q.; Xie, Z. Huang-Lian-Jie-Du-Decoction Ameliorates Hyperglycemia and Insulin Resistant in Association with Gut Microbiota Modulation. Front. Microbiol. 2018, 9, 2380. [Google Scholar] [CrossRef]
- Ding, L.; Ren, S.; Song, Y.; Zang, C.; Liu, Y.; Guo, H.; Yang, W.; Guan, H.; Liu, J. Modulation of gut microbiota and fecal metabolites by corn silk among high-fat diet-induced hypercholesterolemia mice. Front. Nutr. 2022, 9, 935612. [Google Scholar] [CrossRef]
- Wenjie, J.L.L.P.T. Bacterial fatty acid biosynt hesisenzy mesdrug targets for anti bacterial agent screen. Period. Dep. Shenyang Pharm. Univ. 2006, 12, 774–775. [Google Scholar]
- Hu, H.X.; Xu, L.T.; Gao, H.; Lv, H.; Shen, T. Chemical Constituents from Physalis Calyx seu Fructus and Their Inhibitory Effects against Oxidative Stress and Inflammatory Response. Planta Med. 2020, 86, 1191–1203. [Google Scholar] [CrossRef]
- Zhao, X.; Chen, Z.; Yin, Y.; Li, X. Effects of polysaccharide from Physalis alkekengi var. francheti on liver injury and intestinal microflora in type-2 diabetic mice. Pharm. Biol. 2017, 55, 2020–2025. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y. The Toxicology of Total Saponins from Physalis alkekengi L. Calyx and the Anti-Hyperlipidemia Activities; Shanxi Agricultural University: Taiyuan, China, 2015. [Google Scholar]
- Guo, Y.; Li, S.; Li, J.; Ren, Z.; Chen, F.; Wang, X. Anti-hyperglycemic activity of polysaccharides from calyx of Physalis alkekengi var. franchetii Makino on alloxan-induced mice. Int. J. Biol. Macromol. 2017, 99, 249–257. [Google Scholar] [CrossRef]
- Li, A.L.; Chen, B.J.; Li, G.H.; Zhou, M.X.; Li, Y.R.; Ren, D.M.; Lou, H.X.; Wang, X.N.; Shen, T. Physalis alkekengi L. var. franchetii (Mast.) Makino: An ethnomedical, phytochemical and pharmacological review. J. Ethnopharmacol. 2017, 210, 260–274. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Li, Y.; Li, S.; Guan, H.; Chen, X. Anti-aging activity of physalis calyx and its mechanism. Food Ferment. Ind. 2021, 47, 140–144. [Google Scholar] [CrossRef]
- Shang, Y.; Zhou, H.; Hu, M.; Feng, H. Effect of diet on insulin resistance in polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 2020, 105, 425. [Google Scholar] [CrossRef]
- Song, H.; Chu, Q.; Xu, D.; Xu, Y.; Zheng, X. Purified Betacyanins from Hylocereus undatus Peel Ameliorate Obesity and Insulin Resistance in High-Fat-Diet-Fed Mice. J. Agric. Food Chem. 2016, 64, 236–244. [Google Scholar] [CrossRef]
- Wang, X.L.; Li, L.; Bai, M.J.; Zhao, J.X.; Sun, X.J.; Gao, Y.; Yu, H.T.; Chen, X.; Zhang, C.J. Dietary supplementation with Tolypocladium sinense mycelium prevents dyslipidemia inflammation in high fat diet mice by modulation of gut microbiota in mice. Front. Immunol. 2022, 13, 977528. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef] [PubMed]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef]
- Wang, X.; Li, L.; Bian, C.; Bai, M.; Yu, H.; Gao, H.; Zhao, J.; Zhang, C.; Zhao, R. Alterations and correlations of gut microbiota, fecal, and serum metabolome characteristics in a rat model of alcohol use disorder. Front. Microbiol. 2023, 13, 1068825. [Google Scholar] [CrossRef]
- Smith, C.A.; Want, E.J.; O’Maille, G.; Abagyan, R.; Siuzdak, G. XCMS: Processing Mass Spectrometry Data for Metabolite Profiling Using Nonlinear Peak Alignment, Matching, and Identification. Anal. Chem. 2006, 78, 779–787. [Google Scholar] [CrossRef]
- He, N.; Ye, H. Exercise and Hyperlipidemia. Adv. Exp. Med. Biol. 2020, 1228, 79–90. [Google Scholar] [CrossRef]
- Cho, I.J.; Kim, S.E.; Choi, B.R.; Park, H.R.; Park, J.E.; Hong, S.H.; Kwon, Y.S.; Oh, W.S.; Ku, S.K. Lemon Balm and Corn Silk Extracts Mitigate High-Fat Diet-Induced Obesity in Mice. Antioxidants 2021, 10, 2015. [Google Scholar] [CrossRef]
- Zhu, Z.; Huang, R.; Liu, W.; Wang, J.; Wu, S.; Chen, M.; Xie, Y.; Chen, M.; Jiao, C.; Zhang, J. Front Cover: Whole Agrocybe cylindracea Prevented Obesity Linking with Modification of Gut Microbiota and Associated Fecal Metabolites in High-Fat Diet-Fed Mice. Mol. Nutr. Food Res. 2022, 66, 2270028. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; He, Z.; Ma, N.; Chen, Z.Y. Beneficial Effects of Dietary Polyphenols on High-Fat Diet-Induced Obesity Linking with Modulation of Gut Microbiota. J. Agric. Food Chem. 2020, 68, 33–47. [Google Scholar] [CrossRef]
- Hao, Y.; Zhou, F.; Dong, J.; Wang, Y.; Lang, Z.; Li, S.; Li, S. Study on the role of flavonoids derived extract from seed residues of hippophae rhamnoides on high-fat diet induced obese mice. J. King Saud Univ. Sci. 2020, 32, 1597–1603. [Google Scholar] [CrossRef]
- Ya, A.; Hy, B.; Em, C.; My, D.; Ik, E.; At, F.; Yn, E.; Rf, E.; Gme, F.; Ns, A. Maternal High-Fructose Corn Syrup consumption causes insulin resistance and hyperlipidemia in offspring via DNA methylation of the Pparα promoter region. J. Nutr. Biochem. 2022, 103, 108951. [Google Scholar] [CrossRef]
- Ahmed, H.M.S.; Mohamed, S.G.; Ibrahim, W.S.; Rezk, A.M.; Mahmoud, A.A.A.; Mahmoud, M.F.; Ibrahim, I. Acute and chronic metabolic effects of carvedilol in high-fructose, high-fat diet-fed mice: Implication of beta-arrestin2 pathway. Can. J. Physiol. Pharmacol. 2022, 100, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Wu Hongjie, C.D. Effects of different extracts of Physalis alkekengi L. var. franchetii (Mast.) Makino on blood glucose tolerance in mice and blood glucose level in diabetic nephropathy rats. Anhui Med. Pharm. J. 2018, 22, 1245–1247. [Google Scholar]
- Postic, C.; Girard, J. Contribution of de novo fatty acid synthesis to hepatic steatosis and insulin resistance: Lessons from genetically engineered mice. J. Clin. Investig. 2008, 118, 829–838. [Google Scholar] [CrossRef]
- Song, Z.Y.; Xiaoli, A.M.; Yang, F.J. Regulation and Metabolic Significance of De Novo Lipogenesis in Adipose Tissues. Nutrients 2018, 10, 1383. [Google Scholar] [CrossRef]
- Bódis, K.; Kahl, S.; Simon, M.-C.; Zhou, Z.; Sell, H.; Knebel, B.; Tura, A.; Strassburger, K.; Burkart, V.; Müssig, K.; et al. Reduced expression of stearoyl-CoA desaturase-1, but not free fatty acid receptor 2 or 4 in subcutaneous adipose tissue of patients with newly diagnosed type 2 diabetes mellitus. Nutr. Diabetes 2018, 8, 49. [Google Scholar] [CrossRef]
- Won, S.M.; Seo, M.J.; Kwon, M.J.; Park, K.W.; Yoon, J.H. Oral Administration of Latilactobacillus sakei ADM14 Improves Lipid Metabolism and Fecal Microbiota Profile Associated with Metabolic Dysfunction in a High-Fat Diet Mouse Model. Front. Microbiol. 2021, 12, 746601. [Google Scholar] [CrossRef] [PubMed]
- Rong, S.X.; Cortes, V.A.; Rashid, S.; Anderson, N.N.; McDonald, J.G.; Liang, G.S.; Moon, Y.A.; Hammer, R.E.; Horton, J.D. Expression of SREBP-lc Requires SREBP2-mediated Generation of a Sterol Ligand for LXR in Livers of Mice. eLife 2017, 6, e25015. [Google Scholar] [CrossRef]
- Gong, Z.Q.; Han, S.; Li, C.L.; Meng, T.X.; Huo, Y.; Liu, X.F.; Huang, Y.H.; Yang, L.F. Rhinacanthin C Ameliorates Insulin Resistance and Lipid Accumulation in NAFLD Mice via the AMPK/SIRT1 and SREBP-1c/FAS/ACC Signaling Pathways. Evid. Based Complement. Altern. Med. 2023, 2023, 6603522. [Google Scholar] [CrossRef]
- Ortega-Prieto, P.; Postic, C. Carbohydrate Sensing Through the Transcription Factor ChREBP. Front. Genet. 2019, 10, 472. [Google Scholar] [CrossRef]
- Herman, M.A.; Peroni, O.D.; Villoria, J.; Schon, M.R.; Abumrad, N.A.; Bluher, M.; Klein, S.; Kahn, B.B. A novel ChREBP isoform in adipose tissue regulates systemic glucose metabolism. Nature 2012, 484, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Ke, H.Y.; Luan, Y.; Wu, S.M.; Zhu, Y.M.; Tong, X.M. The Role of Mondo Family Transcription Factors in Nutrient-Sensing and Obesity. Front. Endocrinol. 2021, 12, 653972. [Google Scholar] [CrossRef] [PubMed]
- Jie, X.U.; Yan, T.; Zhang, K.; Gao, Y.; Liu, H. Protective Effects of Yinzhihuang Combined with Metformin on Nonalcoholic Fatty Liver Diseases Based on PPAR-α Signaling Pathway. Med. Plant 2020, 11, 66–69. [Google Scholar] [CrossRef]
- Ham, J.R.; Lee, M.J.; Lee, H.-I.; Lee, H.-J.; Kim, H.Y.; Seo, W.-D.; Son, Y.-J.; Mi-Kyung, L. Anti-Diabetic Activity of Heuksoojeongchal Bran Prethanol Extract in HFD/STZ-Induced Diabetic Mice. J. Korean Soc. Food Sci. Nutr. 2021, 50, 655–663. [Google Scholar] [CrossRef]
- Zhu, Y.X.; Hu, H.Q.; Zuo, M.L.; Mao, L.; Song, G.L.; Li, T.M.; Dong, L.C.; Yang, Z.B.; Sheikh, M.S.A. Effect of oxymatrine on liver gluconeogenesis is associated with the regulation of PEPCK and G6Pase expression and AKT phosphorylation. Biomed. Rep. 2021, 15, 56. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.F.; Lin, H. Inflammation initiates a vicious cycle between obesity and nonalcoholic fatty liver disease. Immun. Inflamm. Dis. 2021, 9, 59–73. [Google Scholar] [CrossRef]
- Li, H.; Meng, Y.; He, S.W.; Tan, X.C.; Zhang, Y.J.; Zhang, X.L.; Wang, L.L.; Zheng, W.S. Macrophages, Chronic Inflammation, and Insulin Resistance. Cells 2022, 11, 3001. [Google Scholar] [CrossRef]
- Xu, L.; Yan, X.Y.; Zhao, Y.X.; Wang, J.; Liu, B.H.; Yu, S.H.; Fu, J.Y.; Liu, Y.N.; Su, J. Macrophage Polarization Mediated by Mitochondrial Dysfunction Induces Adipose Tissue Inflammation in Obesity. Int. J. Mol. Sci. 2022, 23, 9252. [Google Scholar] [CrossRef]
- Yin, H.W.; Yang, X.J.; Liu, S.B.; Zeng, J.; Chen, S.H.; Zhang, S.L.; Liu, Y.; Zhao, Y.T. Total flavonoids from Lagerstroemia speciosa (L.) Pers inhibits TNF-alpha-induced insulin resistance and inflammatory response in 3T3-L1 adipocytes via MAPK and NF-kappa B signaling pathways. Food Sci. Technol. 2022, 42, e45222. [Google Scholar] [CrossRef]
- Chen, L.L.; Kan, J.T.; Zheng, N.N.; Li, B.B.; Hong, Y.; Yan, J.; Tao, X.; Wu, G.S.; Ma, J.L.; Zhu, W.Z.; et al. A botanical dietary supplement from white peony and licorice attenuates nonalcoholic fatty liver disease by modulating gut microbiota and reducing inflammation. Phytomedicine 2021, 91, 153693. [Google Scholar] [CrossRef]
- Li, J.M.; Yu, R.; Zhang, L.P.; Wen, S.Y.; Wang, S.J.; Zhang, X.Y.; Xu, Q.; Kong, L.D. Dietary fructose-induced gut dysbiosis promotes mouse hippocampal neuroinflammation: A benefit of short-chain fatty acids. Microbiome 2019, 7, 98. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Yin, Y.X.; Wang, F.; Zhang, H.H.; Ma, X.K.; Yin, Y.L.; Tan, B.; Chen, J.S. Supplementation with Lycium barbarum Polysaccharides Reduce Obesity in High-Fat Diet-Fed Mice by Modulation of Gut Microbiota. Front. Microbiol. 2021, 12, 719967. [Google Scholar] [CrossRef]
- Amiri, P.; Arefhosseini, S.; Bakhshimoghaddam, F.; Gurvan, H.; Hosseini, S.A. Mechanistic insights into the pleiotropic effects of butyrate as a potential therapeutic agent on NAFLD management: A systematic review. Front. Nutr. 2022, 9, 1037696. [Google Scholar] [CrossRef]
- Chen, Y.F.; Jin, L.; Li, Y.H.; Xia, G.B.; Chen, C.; Zhang, Y. Bamboo-shaving polysaccharide protects against high-diet induced obesity and modulates the gut microbiota of mice. J. Funct. Foods 2018, 49, 20–31. [Google Scholar] [CrossRef]
- Wu, Y.; Dong, L.; Song, Y.; Wu, Y.; Zhang, Y.; Wang, S. Preventive effects of polysaccharides from Physalis alkekengi L. on dietary advanced glycation end product-induced insulin resistance in mice associated with the modulation of gut microbiota. Int. J. Biol. Macromol. 2022, 204, 204–214. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Z.Y.; Zhang, F.; Cao, R.H.; Ni, X.J.; Xin, Z.Q.; Deng, J.P.; Wu, G.Y.; Ren, W.K.; Yin, Y.L.; Deng, B.C. Cecropin A Alleviates Inflammation Through Modulating the Gut Microbiota of C57BL/6 Mice with DSS-Induced IBD. Front. Microbiol. 2019, 10, 1595. [Google Scholar] [CrossRef]
- Shi, N.; Zhang, S.; Silverman, G.; Li, M.; Cai, J.; Niu, H. Protective effect of hydroxychloroquine on rheumatoid arthritis-associated atherosclerosis. Anim. Model. Exp. Med. 2019, 2, 98–106. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, S.X.; Chang, M.J.; Qiao, J.; Wang, C.H.; Li, X.F.; Yu, Q.; He, P.F. Characteristics of the Gut Microbiome and Its Relationship with Peripheral CD4(+) T Cell Subpopulations and Cytokines in Rheumatoid Arthritis. Front. Microbiol. 2022, 13, 799602. [Google Scholar] [CrossRef]
- Tain, Y.-L.; Chang, C.-I.; Hou, C.-Y.; Chang-Chien, G.-P.; Lin, S.; Hsu, C.-N. Dietary Resveratrol Butyrate Monoester Supplement Improves Hypertension and Kidney Dysfunction in a Young Rat Chronic Kidney Disease Model. Nutrients 2023, 15, 635. [Google Scholar] [CrossRef] [PubMed]
- DiNicolantonio, J.J.; McCarty, M.F.; Okeefe, J.H. Role of dietary histidine in the prevention of obesity and metabolic syndrome. Open Heart 2018, 5, e000676. [Google Scholar] [CrossRef] [PubMed]
- An, D.; Na, C.; Bielawski, J.; Hannun, Y.A.; Kasper, D.L. Membrane sphingolipids as essential molecular signals for Bacteroides survival in the intestine. Proc. Natl. Acad. Sci. USA 2011, 108, 4666–4671. [Google Scholar] [CrossRef] [PubMed]
- Snider, S.A.; Margison, K.D.; Ghorbani, P.; LeBlond, N.D.; O’Dwyer, C.; Nunes, J.R.C.; Thao, N.; Xu, H.; Bennett, S.A.L.; Fullerton, M.D. Choline transport links macrophage phospholipid metabolism and inflammation. J. Biol. Chem. 2018, 293, 11600–11611. [Google Scholar] [CrossRef]
- Singer, P.; Jaeger, W.; Berger, I.; Barleben, H.; Wirth, M.; Richter-Heinrich, E.; Voigt, S.; Godicke, W. Effects of dietary oleic, linoleic and alpha-linolenic acids on blood pressure, serum lipids, lipoproteins and the formation of eicosanoid precursors in patients with mild essential hypertension. J. Hum. Hypertens. 1990, 4, 227–233. [Google Scholar]
- Xia, J.; Zheng, M.; Li, L.; Hou, X.; Zeng, W. Conjugated linoleic acid improves glucose and lipid metabolism in diabetic mice. J. S. Med. Univ. 2019, 39, 740–746. [Google Scholar] [CrossRef]
- Xu, Q.; Liu, Y.; Zhang, Q.; Ma, B.; Yang, Z.; Liu, L.; Yao, D.; Cui, G.; Sun, J.; Wu, Z. Metabolomic analysis of simvastatin and fenofibrate intervention in high-lipid diet-induced hyperlipidemia rats. Acta Pharmacol. Sin. 2014, 35, 1265–1273. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, A.L.; Stephens, J.W.; Harris, D.A. Intestinal microbiota and their metabolic contribution to type 2 diabetes and obesity. J. Diabetes Metab. Disord. 2021, 20, 1855–1870. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, L.; Wang, X.; Zhou, Y.; Yan, N.; Gao, H.; Sun, X.; Zhang, C. Physalis alkekengi L. Calyx Extract Alleviates Glycolipid Metabolic Disturbance and Inflammation by Modulating Gut Microbiota, Fecal Metabolites, and Glycolipid Metabolism Gene Expression in Obese Mice. Nutrients 2023, 15, 2507. https://doi.org/10.3390/nu15112507
Li L, Wang X, Zhou Y, Yan N, Gao H, Sun X, Zhang C. Physalis alkekengi L. Calyx Extract Alleviates Glycolipid Metabolic Disturbance and Inflammation by Modulating Gut Microbiota, Fecal Metabolites, and Glycolipid Metabolism Gene Expression in Obese Mice. Nutrients. 2023; 15(11):2507. https://doi.org/10.3390/nu15112507
Chicago/Turabian StyleLi, Lin, Xiaolong Wang, Ying Zhou, Na Yan, Han Gao, Xiaojie Sun, and Chunjing Zhang. 2023. "Physalis alkekengi L. Calyx Extract Alleviates Glycolipid Metabolic Disturbance and Inflammation by Modulating Gut Microbiota, Fecal Metabolites, and Glycolipid Metabolism Gene Expression in Obese Mice" Nutrients 15, no. 11: 2507. https://doi.org/10.3390/nu15112507
APA StyleLi, L., Wang, X., Zhou, Y., Yan, N., Gao, H., Sun, X., & Zhang, C. (2023). Physalis alkekengi L. Calyx Extract Alleviates Glycolipid Metabolic Disturbance and Inflammation by Modulating Gut Microbiota, Fecal Metabolites, and Glycolipid Metabolism Gene Expression in Obese Mice. Nutrients, 15(11), 2507. https://doi.org/10.3390/nu15112507