Nutrition Therapy by Nutrition Support Team: A Comparison of Multi-Chamber Bag and Customized Parenteral Nutrition in Hospitalized Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Source
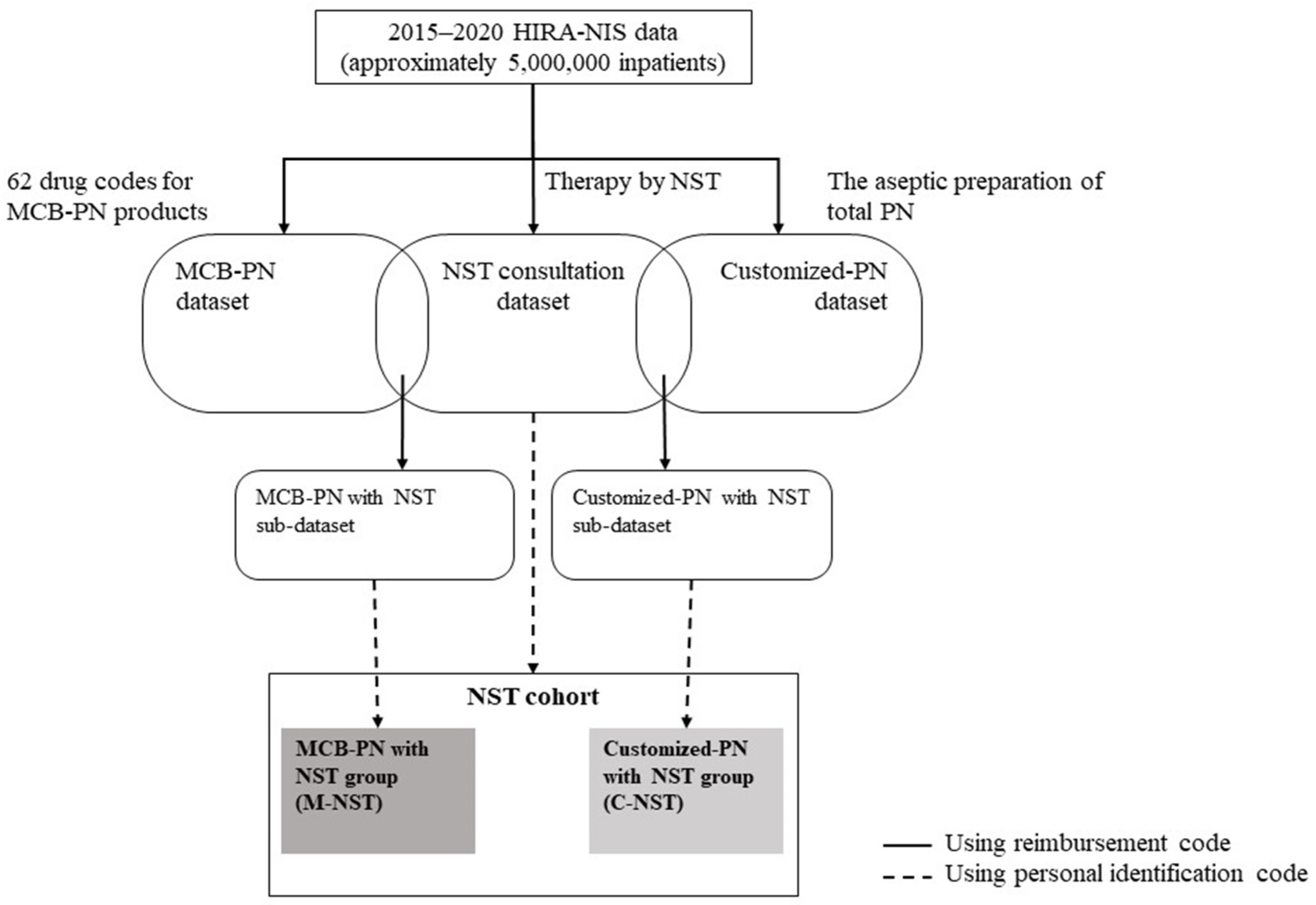
2.2. Dataset and Cohort Construction
2.3. Study Variables and Analysis
3. Results
3.1. NST Consultation Dataset and NST Cohort
3.2. MCB-PN Dataset and M-NST
3.3. Customized-PN Dataset and C-NST
3.4. Subgroup Comparison: M-NST vs. C-NST
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lin, A.; Micic, D. Nutrition Considerations in Inflammatory Bowel Disease. Nutr. Clin. Pract. 2021, 36, 298–311. [Google Scholar] [CrossRef] [PubMed]
- D’Almeida, C.A.; Peres, W.A.F.; de Pinho, N.B.; Martucci, R.B.; Rodrigues, V.D.; Ramalho, A. Prevalence of Malnutrition in Older Hospitalized Cancer Patients: A Multicenter and Multiregional Study. J. Nutr. Health Aging 2020, 24, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Ghimire, S.; Baral, B.K.; Pokhrel, B.R.; Pokhrel, A.; Acharya, A.; Amatya, D.; Amatya, P.; Mishra, S.R. Depression, malnutrition, and health-related quality of life among Nepali older patients. BMC Geriatr. 2018, 18, 191. [Google Scholar] [CrossRef]
- Dempsey, D.; Mullen, J.; Buzby, G.P. The link between nutritional status and clinical outcome: Can nutritional intervention modify it? Am. J. Clin. Nutr. 1988, 47, 352–356. [Google Scholar] [CrossRef] [PubMed]
- Alberda, C.; Gramlich, L.; Jones, N.; Jeejeebhoy, K.; Day, A.G.; Dhaliwal, R.; Heyland, D.K. The relationship between nutritional intake and clinical outcomes in critically ill patients: Results of an international multicenter observational study. J. Intensive Care Med. 2009, 35, 1728–1737. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, E.; Ferguson, M.; Banks, M.; Batterham, M.; Bauer, J.; Capra, S.; Isenring, E. Malnutrition and poor food intake are associated with prolonged hospital stay, frequent readmissions, and greater in-hospital mortality: Results from the Nutrition Care Day Survey 2010. Clin. Nutr. 2013, 32, 737–745. [Google Scholar] [CrossRef]
- Norman, K.; Pichard, C.; Lochs, H.; Pirlich, M. Prognostic impact of disease-related malnutrition. Clin. Nutr. 2008, 27, 5–15. [Google Scholar] [CrossRef]
- Braun, K.; Utech, A.; Velez, M.E.; Walker, R. Parenteral Nutrition Electrolyte Abnormalities and Associated Factors Before and After Nutrition Support Team Initiation. J. Parenter. Enter. Nutr. 2018, 42, 387–392. [Google Scholar] [CrossRef]
- Reber, E.; Strahm, R.; Bally, L.; Schuetz, P.; Stanga, Z. Efficacy and efficiency of nutritional support teams. J. Clin. Med. 2019, 8, 1281. [Google Scholar] [CrossRef]
- Gonzalez-Granda, A.; Schollenberger, A.; Thorsteinsson, R.; Haap, M.; Bischoff, S.C. Impact of an interdisciplinary nutrition support team (NST) on the clinical outcome of critically ill patients. A pre/post NST intervention study. Clin. Nutr. ESPEN 2021, 45, 486–491. [Google Scholar] [CrossRef]
- Traeger, S.M.; Williams, G.B.; Milliren, G.; Young, D.S.; Fisher, M.; Haug, M.T., III. Total Parenteral Nutrition by a Nutrition Support Team: Improved Quality of Care. J. Parenter. Enter. Nutr. 1986, 10, 408–412. [Google Scholar] [CrossRef] [PubMed]
- Vlug, L.E.; Nagelkerke, S.C.J.; Jonkers-Schuitema, C.F.; Rings, E.H.H.M.; Tabbers, M.M. The Role of a Nutrition Support Team in the Management of Intestinal Failure Patients. Nutrients 2020, 12, 172. [Google Scholar] [CrossRef] [PubMed]
- Seol, E.M.; Suh, Y.S.; Ju, D.L.; Bae, H.J.; Kim, E.; Lee, H.J. Nutrition Support Team Reconsultation During Nutrition Therapy in Korea. J. Parenter. Enter. Nutr. 2021, 45, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Ock, M.; Lee, S.; Kim, J.T. Nutrition support team service in hospitalized patients in Korea. Clin. Nutr. 2018, 37, S132. [Google Scholar] [CrossRef]
- Oh, E.; Shim, H.; Yon, H.J.; Moon, J.S.; Kang, D.R.; Jang, J.Y.; Care, C. Effectiveness of a multidisciplinary team for nutrition support in a trauma intensive care unit. Acute Crit. Care 2020, 35, 142–148. [Google Scholar] [CrossRef]
- Park, H.J.; Oh, S.-H.; Kim, J.T.; Bae, H.J.; Nam, J.H.; Sung, Y.W.; Kim, S.H.; Jung, M.J.; Chung, J.E. Report on the Current Trend of Nutrition Support Team Consultation Fee and Fee for Aseptic Preparation of Parenteral Nutrition in Korea: 2014~2020. J. Korean Soc. Health-Syst. Pharm. 2022, 39, 69–80. [Google Scholar] [CrossRef]
- Christensen, M.L.; Ayers, P.; Boullata, J.I.; Guenter, P.; Gura, K.M.; Holcombe, B.; Seres, D.S.; Sacks, G.S.; ASPEN PN Safety Committee. Lipid Injectable Emulsion Survey With Gap Analysis. Nutr. Clin. Pract. 2017, 32, 694–702. [Google Scholar] [CrossRef]
- Stidham, M.A.; Douglas, J.W. Nutrition Support Team Oversight and Appropriateness of Parenteral Nutrition in Hospitalized Adults: A Systematic Review. J. Parenter. Enter. Nutr. 2020, 44, 1447–1460. [Google Scholar] [CrossRef]
- Berger, M.M.; Pichard, C.; Bao, J. When is parenteral nutrition indicated? J. Intensive Med. 2022, 2, 22–28. [Google Scholar] [CrossRef]
- Braga, M.; Ljungqvist, O.; Soeters, P.; Fearon, K.; Weimann, A.; Bozzetti, F. ESPEN Guidelines on Parenteral Nutrition: Surgery. Clin. Nutr. 2009, 28, 378–386. [Google Scholar] [CrossRef]
- Prathik, B.H.; Aradhya, A.S.; Sahoo, T.; Saini, S.S. Neonatal Total Parenteral Nutrition: Clinical Implications From Recent NICE Guidelines. Indian Pediatr. 2021, 58, 67–70. [Google Scholar] [CrossRef] [PubMed]
- Worthington, P.; Balint, J.; Bechtold, M.; Bingham, A.; Chan, L.-N.; Durfee, S.; Jevenn, A.K.; Malone, A.; Mascarenhas, M.; Robinson, D.T.; et al. When Is Parenteral Nutrition Appropriate? J. Parenter. Enter. Nutr. 2017, 41, 324–377. [Google Scholar] [CrossRef] [PubMed]
- Jeejeebhoy, K.N. Enteral nutrition versus parenteral nutrition—The risks and benefits. Nat. Clin. Pract. Gastroenterol. Hepatol. 2007, 4, 260–265. [Google Scholar] [CrossRef] [PubMed]
- Bischoff, S.C.; Kester, L.; Meier, R.; Radziwill, R.; Schwab, D.; Thul, P.; Working group for developing the guidelines for parenteral nutrition of The German Association for Nutrition Medicine. Organisation, regulations, preparation and logistics of parenteral nutrition in hospitals and homes; the role of the nutrition support team–Guidelines on Parenteral Nutrition, Chapter 8. Ger. Med. Sci. 2009, 7. [Google Scholar] [CrossRef]
- Mistiaen, P.; Van den Heede, K. Nutrition Support Teams: A Systematic Review. J. Parenter. Enter. Nutr. 2020, 44, 1004–1020. [Google Scholar] [CrossRef]
- Eriksen, M.K.; Crooks, B.; Baunwall, S.M.D.; Rud, C.L.; Lal, S.; Hvas, C.L. Systematic review with meta-analysis: Effects of implementing a nutrition support team for in-hospital parenteral nutrition. Aliment. Pharmacol. Ther. 2021, 54, 560–570. [Google Scholar] [CrossRef]
- Lee, J.S.; Kim, S.; Sung, S.-Y.; Kim, Y.H.; Lee, H.W.; Hong, J.H.; Ko, Y.H. Treatment Outcomes of 9994 Patients with Extensive-Disease Small-Cell Lung Cancer from a Retrospective Nationwide Population-Based Cohort in the Korean HIRA Database. Front. Oncol. 2021, 11, 546672. [Google Scholar] [CrossRef]
- Kim, S.; Kim, M.S.; You, S.H.; Jung, S.Y. Conducting and Reporting a Clinical Research Using Korean Healthcare Claims Database. Korean J. Fam. Med. 2023, 41, 146–152. [Google Scholar] [CrossRef]
- Chazard, E.; Focheur, G.; Beuscart, J.-B.; Preda, C. How to Compare the Length of Stay of Two Samples of Inpatients? A Simulation Study to Compare Type I and Type II Errors of 12 Statistical Tests. Value Health 2017, 20, 992–998. [Google Scholar] [CrossRef]
- Plauth, M.; Bernal, W.; Dasarathy, S.; Merli, M.; Plank, L.D.; Schütz, T.; Bischoff, S.C. ESPEN guideline on clinical nutrition in liver disease. Clin. Nutr. 2019, 38, 485–521. [Google Scholar] [CrossRef]
- Volkert, D.; Beck, A.M.; Cederholm, T.; Cruz-Jentoft, A.; Goisser, S.; Hooper, L.; Kiesswetter, E.; Maggio, M.; Raynaud-Simon, A.; Sieber, C.C.; et al. ESPEN guideline on clinical nutrition and hydration in geriatrics. Clin. Nutr. 2019, 38, 10–47. [Google Scholar] [CrossRef] [PubMed]
- Moore, F.A.; Feliciano, D.V.; Andrassy, R.J.; McArdle, A.H.; Booth, F.V.; Morgenstein-Wagner, T.B.; Kellum, J.M., Jr.; Welling, R.E.; Moore, E.E. Early enteral feeding, compared with parenteral, reduces postoperative septic complications. The results of a meta-analysis. Ann. Surg. 1992, 216, 172–183. [Google Scholar] [CrossRef] [PubMed]
- Gramlich, L.; Kichian, K.; Pinilla, J.; Rodych, N.J.; Dhaliwal, R.; Heyland, D.K. Does enteral nutrition compared to parenteral nutrition result in better outcomes in critically ill adult patients? A systematic review of the literature. Nutrition 2004, 20, 843–848. [Google Scholar] [CrossRef] [PubMed]
- Dissanaike, S.; Shelton, M.; Warner, K.; O’Keefe, G.E. The risk for bloodstream infections is associated with increased parenteral caloric intake in patients receiving parenteral nutrition. Crit. Care 2007, 11, R114. [Google Scholar] [CrossRef]
- Elke, G.; van Zanten, A.R.H.; Lemieux, M.; McCall, M.; Jeejeebhoy, K.N.; Kott, M.; Jiang, X.; Day, A.G.; Heyland, D.K. Enteral versus parenteral nutrition in critically ill patients: An updated systematic review and meta-analysis of randomized controlled trials. Crit. Care 2016, 20, 117. [Google Scholar] [CrossRef]
- Reignier, J.; Boisramé-Helms, J.; Brisard, L.; Lascarrou, J.-B.; Ait Hssain, A.; Anguel, N.; Argaud, L.; Asehnoune, K.; Asfar, P.; Bellec, F.; et al. Enteral versus parenteral early nutrition in ventilated adults with shock: A randomised, controlled, multicentre, open-label, parallel-group study (NUTRIREA-2). Lancet 2018, 391, 133–143. [Google Scholar] [CrossRef]
- Goh, R.J.L.; Li, H.; Cheah, M.C.C.; Salazar, E. The use of standardized commercially available parenteral nutrition as a bridge to customized compounded bag in the acute hospital setting is safe and feasible. Clin. Nutr. ESPEN 2022, 47, 321–324. [Google Scholar] [CrossRef]
- Banko, D.; Rosenthal, N.; Chung, J.; Lomax, C.; Washesky, P.F. Comparing the risk of bloodstream infections by type of parenteral nutrition preparation method: A large retrospective, observational study. Clin. Nutr. ESPEN 2019, 30, 100–106. [Google Scholar] [CrossRef]
- Hall, J.W. Safety, Cost, and Clinical Considerations for the Use of Premixed Parenteral Nutrition. Nutr. Clin. Pract. 2015, 30, 325–330. [Google Scholar] [CrossRef]
- Turpin, R.S.; Canada, T.; Rosenthal, V.D.; Nitzki-George, D.; Liu, F.X.; Mercaldi, C.J.; Pontes-Arruda, A. Bloodstream Infections Associated With Parenteral Nutrition Preparation Methods in the United States. J. Parenter. Enter. Nutr. 2012, 36, 169–176. [Google Scholar] [CrossRef]
- Mundi, M.S.; Klek, S.; Martindale, R.G. Use of Lipids in Adult Patients Requiring Parenteral Nutrition in the Home Setting. J. Parenter. Enter. Nutr. 2020, 44, S39–S44. [Google Scholar] [CrossRef]
- Mundi, M.S.; Pattinson, A.; McMahon, M.T.; Davidson, J.; Hurt, R.T. Prevalence of Home Parenteral and Enteral Nutrition in the United States. Nutr. Clin. Pract. 2017, 32, 799–805. [Google Scholar] [CrossRef]
- Choe, J.H.; Baek, J.H.; Jo, Y.H.; Cho, Y.S. Infection Control in Parenteral Nutrition Preparation and Compounding. J. Clin. Nutr. 2018, 10, 31–37. [Google Scholar] [CrossRef]
- Maisonneuve, N.; Raguso, C.A.; Paoloni-Giacobino, A.; Mühlebach, S.; Corriol, O.; Saubion, J.L.; Hecq, J.D.; Bailly, A.; Berger, M.; Pichard, C. Parenteral nutrition practices in hospital pharmacies in Switzerland, France, and Belgium. Nutrition 2004, 20, 528–535. [Google Scholar] [CrossRef] [PubMed]
- Shafiekhani, M.; Nikoupour, H.; Mirjalili, M. The experience and outcomes of multidisciplinary clinical pharmacist-led parenteral nutrition service for individuals with intestinal failure in a center without home parenteral nutrition. Eur. J. Clin. Nutr. 2022, 76, 841–847. [Google Scholar] [CrossRef] [PubMed]
- Stettler, N.; Sentongo, T.A.; Carroll, M.; Schears, G.J.; Mascarenhas, M.R. Impact of Customized Parenteral Nutrition in a Pediatric Hospital. Nutr. Clin. Pract. 2001, 16, 345–348. [Google Scholar] [CrossRef]
- Smolkin, T.; Diab, G.; Shohat, I.; Jubran, H.; Blazer, S.; Rozen, G.S.; Makhoul, I.R. Standardized versus individualized parenteral nutrition in very low birth weight infants: A comparative study. Neonatology 2010, 98, 170–178. [Google Scholar] [CrossRef]
- Boscarino, G.; Conti, M.G.; Gasparini, C.; Onestà, E.; Faccioli, F.; Dito, L.; Regoli, D.; Spalice, A.; Parisi, P.; Terrin, G. Neonatal Hyperglycemia Related to Parenteral Nutrition Affects Long-Term Neurodevelopment in Preterm Newborn: A Prospective Cohort Study. Nutrients 2021, 13, 1930. [Google Scholar] [CrossRef]
- Terrin, G.; Boscarino, G.; Gasparini, C.; Di Chiara, M.; Faccioli, F.; Onestà, E.; Parisi, P.; Spalice, A.; De Nardo, M.C.; Dito, L.; et al. Energy-enhanced parenteral nutrition and neurodevelopment of preterm newborns: A cohort study. Nutrition 2021, 89, 111219. [Google Scholar] [CrossRef]
- Vos, T.; Lim, S.S.; Abbafati, C.; Abbas, K.M.; Abbasi, M.; Abbasifard, M.; Abbasi-Kangevari, M.; Abbastabar, H.; Abd-Allah, F.; Abdelalim, A.; et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1204–1222. [Google Scholar] [CrossRef]
- Ostermann, S.; Morel, P.; Chalé, J.-J.; Bucher, P.; Konrad, B.; Meier, R.P.H.; Ris, F.; Schiffer, E.R.C. Randomized Controlled Trial of Enhanced Recovery Program Dedicated to Elderly Patients After Colorectal Surgery. Dis. Colon Rectum 2019, 62, 1105–1116. [Google Scholar] [CrossRef] [PubMed]
- Guenter, P.; Blackmer, A.; Malone, A.; Mirtallo, J.M.; Phillips, W.; Tyler, R.; Barrocas, A.; Resnick, H.E.; Anthony, P.; Abdelhadi, R. Update on use of enteral and parenteral nutrition in hospitalized patients with a diagnosis of malnutrition in the United States. Nutr. Clin. Pract. 2022, 37, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Sabbouh, T.; Torbey, M.T. Malnutrition in Stroke Patients: Risk Factors, Assessment, and Management. Neurocritical Care 2018, 29, 374–384. [Google Scholar] [CrossRef]
- Chen, B.; Liu, W.; Chen, Y.; She, Q.; Li, M.; Zhao, H.; Zhao, W.; Peng, Z.; Wu, J. Effect of Poor Nutritional Status and Comorbidities on the Occurrence and Outcome of Pneumonia in Elderly Adults. Front. Med. 2021, 8, 719530. [Google Scholar] [CrossRef]
- Yeo, H.J.; Byun, K.S.; Han, J.; Kim, J.H.; Lee, S.E.; Yoon, S.H.; Jeon, D.; Kim, Y.S.; Cho, W.H. Prognostic significance of malnutrition for long-term mortality in community-acquired pneumonia: A propensity score matched analysis. Korean J. Intern. Med. 2019, 34, 841–849. [Google Scholar] [CrossRef]
- Gomes, F.; Emery, P.W.; Weekes, C.E. Risk of Malnutrition Is an Independent Predictor of Mortality, Length of Hospital Stay, and Hospitalization Costs in Stroke Patients. J. Stroke Cerebrovasc. Dis. 2016, 25, 799–806. [Google Scholar] [CrossRef] [PubMed]
- Cogle, S.V.; Martindale, R.G.; Ramos, M.; Roberti, G.J.; Roberts, P.R.; Taylor, K.; Sacks, G.S. Multicenter Prospective Evaluation of Parenteral Nutrition Preparation Time and Resource Utilization: 3-Chamber Bags Compared with Hospital Pharmacy–Compounded Bags. J. Parenter. Enter. Nutr. 2021, 45, 1552–1558. [Google Scholar] [CrossRef]
- Berlana, D.; Almendral, M.A.; Abad, M.R.; Fernández, A.; Torralba, A.; Cervera-Peris, M.; Piñeiro, G.; Romero-Jiménez, R.; Vázquez, A.; Ramírez, E.; et al. Cost, Time, and Error Assessment During Preparation of Parenteral Nutrition: Multichamber Bags versus Hospital-Compounded Bags. J. Parenter. Enter. Nutr. 2019, 43, 557–565. [Google Scholar] [CrossRef]
- Tucker, A.; Ybarra, J.; Bingham, A.; Blackmer, A.; Curtis, C.; Mattox, T.; Miller, C.; Ward, C.; Williams, N.T.; Standards of Practice for Nutrition Support Pharmacists Task Force; et al. American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.) Standards of Practice for Nutrition Support Pharmacists. Nutr. Clin. Pract. 2015, 30, 139–146. [Google Scholar] [CrossRef]
- Katoue, M.G. Role of pharmacists in providing parenteral nutrition support: Current insights and future directions. Integr. Pharm. Res. Pract. 2018, 7, 125–140. [Google Scholar] [CrossRef]


| NST Cohort n (%) | Comparative Analysis of Subgroups | ||||
|---|---|---|---|---|---|
| M-NST n (%) | C-NST n (%) | p-Value | |||
| No. of patients | 70,665 | 50,010 | 7591 | - | |
| No. of patients per 1000 inpatients | 14.13 | 10.00 | 1.52 | - | |
| Gender | Male | 40,190 (56.9) | 28,796 (57.6) | 4383 (57.7) | p < 0.001 |
| Female | 30,475 (43.1) | 21,214 (42.4) | 3208 (42.3) | ||
| Age | <5 | 4995 (7.1) | 262 (0.5) | 3208 (42.3) | p < 0.001 |
| 5–9 | 390 (0.6) | 158 (0.3) | 67 (0.9) | ||
| 10–14 | 367 (0.5) | 205 (0.4) | 47 (0.6) | ||
| 15–19 | 590 (0.8) | 419 (0.8) | 76 (1.0) | ||
| 20–24 | 636 (0.9) | 485 (1.0) | 48 (0.6) | ||
| 25–29 | 635 (0.9) | 516 (1.0) | 34 (0.4) | ||
| 30–34 | 965 (1.4) | 746 (1.5) | 60 (0.8) | ||
| 35–39 | 1235 (1.7) | 955 (1.9) | 108 (1.4) | ||
| 40–44 | 1830 (2.6) | 1397 (2.8) | 150 (2.0) | ||
| 45–49 | 3045 (4.3) | 2299 (4.6) | 222 (2.9) | ||
| 50–54 | 4228 (6.0) | 3270 (6.5) | 312 (4.1) | ||
| 55–59 | 6048 (8.6) | 4718 (9.4) | 455 (6.0) | ||
| 60–64 | 6636 (9.4) | 5167 (10.3) | 547 (7.2) | ||
| 65–69 | 6945 (9.8) | 5337 (10.7) | 560 (7.4) | ||
| 70–74 | 8323 (11.8) | 6348 (12.7) | 572 (7.5) | ||
| ≥75 | 23,797 (33.7) | 17,728 (35.4) | 1125 (14.8) | ||
| No. of reimbursements | 91,384 | 60,916 | 9343 | - | |
| No. of reimbursements per 1000 inpatients | 18.28 | 12.18 | 1.87 | - | |
| Medical department | Internal medicine | 44,978 (49.2) | 33,395 (54.8) | 2807 (30.0) | p < 0.001 |
| Gastroenterology | 11,659 (12.8) | 9205 (15.1) | 812 (8.7) | ||
| Cardiology | 3364 (3.7) | 1927 (3.2) | 165 (1.8) | ||
| Pulmonology | 11,888 (13.0) | 8627 (14.2) | 544 (5.8) | ||
| Nephrology | 4175 (4.6) | 2765 (4.5) | 170 (1.8) | ||
| Hemato-oncology | 9254 (10.1) | 7954 (13.1) | 868 (9.3) | ||
| Infection | 2439 (2.7) | 1619 (2.7) | 89 (1.0) | ||
| Other IMs | 2199 (2.4) | 1298 (2.1) | 159 (1.7) | ||
| Neuropsychiatry | 4851 (5.3) | 2462 (4.0) | 124 (1.3) | ||
| General surgery | 12,628 (13.8) | 10,699 (17.6) | 1304 (14.0) | ||
| Neurosurgery | 8998 (9.8) | 6114 (10.0) | 233 (2.5) | ||
| Chest surgery | 2455 (2.7) | 1692 (2.8) | 360 (3.9) | ||
| Pediatrics | 8076 (8.8) | 991 (1.6) | 3997 (42.8) | ||
| Others | 9398 (10.3) | 5563 (9.1) | 518 (5.5) | ||
| Admission route | Outpatients | 37,074 (40.6) | 23,477 (38.5) | 5086 (54.4) | p < 0.001 |
| Emergency | 50,847 (55.6) | 34,871 (57.2) | 3980 (42.6) | ||
| Transfer from other institutions | 3463 (3.8) | 2568 (4.2) | 277 (3.0) | ||
| Clinical outcomes | Continuation | 26,863 (29.4) | 17,163 (28.2) | 3553 (38.0) | p < 0.001 |
| Death | 9429 (10.3) | 7683 (12.6) | 891 (9.5) | ||
| Discharge | 49,059 (53.7) | 32,411 (53.2) | 4507 (48.2) | ||
| Others | 6033 (6.6) | 3659 (6.0) | 392 (4.2) | ||
| Days of hospitalization (mean ± SD) | 20.8 ± 15.0 | 21.2 ± 15.0 | 26.2 ± 17.3 | p < 0.001 | |
| Days of care (mean ± SD) | 28.7 ± 18.7 | 28.5 ± 18.0 | 32.4 ± 20.6 | p < 0.001 | |
| ICD-10 Classification | NST Cohort n (%) | M-NST * n (%) | C-NST * n (%) | |
|---|---|---|---|---|
| A00–B99 | Certain infectious and parasitic disease | 1740 (2.5) | 1287 (2.6) | 90 (1.2) |
| C00–D48 | Neoplasms | 19,149 (27.1) | 16,683 (33.4) | 2142 (28.2) |
| D50–D89 | Diseases of the blood and blood-forming organs and certain disorders involving the immune mechanism | 264 (0.4) | 142 (0.3) | 14 (0.2) |
| E00–E90 | Endocrine, nutritional, and metabolic diseases | 815 (1.2) | 426 (0.9) | 44 (0.6) |
| F00–F99 | Mental and behavioral disorders | 284 (0.4) | 178 (0.4) | 11 (0.1) |
| G00–G99 | Diseases of the nervous system | 1815 (2.6) | 960 (1.9) | 108 (1.4) |
| H00–H95 | Diseases of the eye and adnexa and diseases of the ear and mastoid process | 71 (0.1) | 34 (0.1) | 3 (0.0) |
| I00–I99 | Diseases of the circulatory system | 11,303 (16.0) | 7093 (14.2) | 433 (5.7) |
| J00–J99 | Diseases of the respiratory system | 7667 (10.8) | 5604 (11.2) | 381 (5.0) |
| K00–K93 | Diseases of the digestive system | 8174 (11.6) | 6634 (13.3) | 612 (8.1) |
| L00–L99 | Diseases of the skin and subcutaneous tissue | 268 (0.4) | 145 (0.3) | 18 (0.2) |
| M00–M99 | Diseases of the musculoskeletal system and connective tissue | 958 (1.4) | 583 (1.2) | 47 (0.6) |
| N00–N99 | Diseases of the genitourinary system | 3124 (4.4) | 2070 (4.1) | 137 (1.8) |
| O00–O99 | Pregnancy, childbirth, and the puerperium | 44 (0.1) | 26 (0.1) | 2 (0.0) |
| P00–P96 | Certain conditions originating in the perinatal period | 3144 (4.4) | 65 (0.1) | 2260 (29.8) |
| Q00–Q99 | Congenital malformations, deformations, and chromosomal abnormalities | 587 (0.8) | 65 (0.1) | 337 (4.4) |
| R00–R99 | Symptoms, signs, and abnormal clinical and laboratory findings, not elsewhere classified | 1210 (1.7) | 759 (1.5) | 73 (1.0) |
| S00–T98 | Injury, poisoning, and certain other consequences of external causes | 4416 (6.2) | 3089 (6.2) | 215 (2.8) |
| U00–U85 | Codes for special purposes | 79 (0.1) | 63 (0.1) | 8 (0.1) |
| V01–Y98 | External causes of morbidity and mortality | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Z00–Z99 | Factors influencing health status and contact with health services | 1365 (1.9) | 995 (2.0) | 111 (1.5) |
| Not specified # | 4188 (5.9) | 3109 (6.2) | 545 (7.2) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheon, S.; Oh, S.-H.; Kim, J.-T.; Choi, H.-G.; Park, H.; Chung, J.-E. Nutrition Therapy by Nutrition Support Team: A Comparison of Multi-Chamber Bag and Customized Parenteral Nutrition in Hospitalized Patients. Nutrients 2023, 15, 2531. https://doi.org/10.3390/nu15112531
Cheon S, Oh S-H, Kim J-T, Choi H-G, Park H, Chung J-E. Nutrition Therapy by Nutrition Support Team: A Comparison of Multi-Chamber Bag and Customized Parenteral Nutrition in Hospitalized Patients. Nutrients. 2023; 15(11):2531. https://doi.org/10.3390/nu15112531
Chicago/Turabian StyleCheon, Seunghyun, Sang-Hyeon Oh, Jung-Tae Kim, Han-Gon Choi, Hyojung Park, and Jee-Eun Chung. 2023. "Nutrition Therapy by Nutrition Support Team: A Comparison of Multi-Chamber Bag and Customized Parenteral Nutrition in Hospitalized Patients" Nutrients 15, no. 11: 2531. https://doi.org/10.3390/nu15112531
APA StyleCheon, S., Oh, S.-H., Kim, J.-T., Choi, H.-G., Park, H., & Chung, J.-E. (2023). Nutrition Therapy by Nutrition Support Team: A Comparison of Multi-Chamber Bag and Customized Parenteral Nutrition in Hospitalized Patients. Nutrients, 15(11), 2531. https://doi.org/10.3390/nu15112531





