A Systematic Ex-Vivo Study of the Anti-Proliferative/Cytotoxic Bioactivity of Major Olive Secoiridoids’ Double Combinations and of Total Olive Oil Phenolic Extracts on Multiple Cell-Culture Based Cancer Models Highlights Synergistic Effects
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Preparation and Analysis of Olive Oil Total Phenolic Extracts (TPEs)
2.3. Cell Lines, Culture Conditions and Treatment Protocols with OOPs
2.4. Treatment of Cell Lines with TPE and Double OOP Combinations
2.5. Cell Viability Assay
2.6. Evaluation of the Effect of Combinations of OOPs by Modified CDI
2.7. Statistical Analysis
3. Results
3.1. Synergistic Effect of OOPs’ Double Combinations on the Cell Numbers/Viability of Cancer and Non-Cancer Cell Lines; Determination of CDI Values
3.2. Anti-Proliferative/Cytotoxic Effect of TPEs on Several Cancer and Non-Tumor Derived Cell Lines
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fabiani, R. Anti-cancer properties of olive oil secoiridoid phenols: A systematic review of in vivo studies. Food Funct. 2016, 7, 4145–4159. [Google Scholar] [CrossRef] [PubMed]
- Han, X.Z.; Shen, T.; Lou, H.X. Dietary polyphenols and their biological significance. Int. J. Mol. Sci. 2007, 8, 950–988. [Google Scholar] [CrossRef]
- Emma, M.R.; Augello, G.; Di Stefano, V.; Azzolina, A.; Giannitrapani, L.; Montalto, G.; Cervello, M.; Cusimano, A. Potential Uses of Olive Oil Secoiridoids for the Prevention and Treatment of Cancer: A Narrative Review of Preclinical Studies. Int. J. Mol. Sci. 2021, 22, 1234. [Google Scholar] [CrossRef] [PubMed]
- Moral, R.; Escrich, E. Influence of Olive Oil and Its Components on Breast Cancer: Molecular Mechanisms. Molecules 2022, 27, 477. [Google Scholar] [CrossRef] [PubMed]
- Casaburi, I.; Puoci, F.; Chimento, A.; Sirianni, R.; Ruggiero, C.; Avena, P.; Pezzi, V. Potential of olive oil phenols as chemopreventive and therapeutic agents against cancer: A review of in vitro studies. Mol. Nutr. Food Res. 2013, 57, 71–83. [Google Scholar] [CrossRef]
- El Haouari, M.; Quintero, J.E.; Rosado, J.A. Anticancer molecular mechanisms of oleocanthal. Phytother. Res. PTR 2020, 34, 2820–2834. [Google Scholar] [CrossRef]
- Toric, J.; Brozovic, A.; Baus Loncar, M.; Jakobusic Brala, C.; Karkovic Markovic, A.; Bencic, D.; Barbaric, M. Biological Activity of Phenolic Compounds in Extra Virgin Olive Oils through Their Phenolic Profile and Their Combination with Anticancer Drugs Observed in Human Cervical Carcinoma and Colon Adenocarcinoma Cells. Antioxidants 2020, 9, 453. [Google Scholar] [CrossRef]
- Schwingshackl, L.; Schwedhelm, C.; Galbete, C.; Hoffmann, G. Adherence to Mediterranean Diet and Risk of Cancer: An Updated Systematic Review and Meta-Analysis. Nutrients 2017, 9, 1063. [Google Scholar] [CrossRef]
- Cicerale, S.; Conlan, X.A.; Sinclair, A.J.; Keast, R.S. Chemistry and health of olive oil phenolics. Crit. Rev. Food Sci. Nutr. 2009, 49, 218–236. [Google Scholar] [CrossRef]
- Cicerale, S.; Lucas, L.; Keast, R. Biological activities of phenolic compounds present in virgin olive oil. Int. J. Mol. Sci. 2010, 11, 458–479. [Google Scholar] [CrossRef]
- Boss, A.; Bishop, K.S.; Marlow, G.; Barnett, M.P.; Ferguson, L.R. Evidence to Support the Anti-Cancer Effect of Olive Leaf Extract and Future Directions. Nutrients 2016, 8, 513. [Google Scholar] [CrossRef] [PubMed]
- Servili, M.; Esposto, S.; Fabiani, R.; Urbani, S.; Taticchi, A.; Mariucci, F.; Selvaggini, R.; Montedoro, G.F. Phenolic compounds in olive oil: Antioxidant, health and organoleptic activities according to their chemical structure. Inflammopharmacology 2009, 17, 76–84. [Google Scholar] [CrossRef]
- Huang, Y.L.; Oppong, M.B.; Guo, Y.; Wang, L.Z.; Fang, S.M.; Deng, Y.R.; Gao, X.M. The Oleaceae family: A source of secoiridoids with multiple biological activities. Fitoterapia 2019, 136, 104155. [Google Scholar] [CrossRef]
- Boussahel, S.; Di Stefano, V.; Muscara, C.; Cristani, M.; Melilli, M.G. Phenolic Compounds Characterization and Antioxidant Properties of Monocultivar Olive Oils from Northeast Algeria. Agriculture 2020, 10, 494. [Google Scholar] [CrossRef]
- Grilo, F.; Novara, M.E.; D’Oca, M.C.; Rubino, S.; Lo Bianco, R.; Di Stefano, V. Quality evaluation of extra-virgin olive oils from Sicilian genotypes grown in a high-density system. Int. J. Food Sci. Nutr. 2020, 71, 397–409. [Google Scholar] [CrossRef] [PubMed]
- Pérez, J.A.; Hernández, J.M.; Trujillo, J.M.; López, H. Iridoids and secoiridoids from Oleaceae. Stud. Nat. Prod. Chem. 2005, 32, 303–363. [Google Scholar] [CrossRef]
- Di Stefano, V.; Melilli, M.G. Effect of storage on quality parameters and phenolic content of Italian extra-virgin olive oils. Nat. Prod. Res. 2020, 34, 78–86. [Google Scholar] [CrossRef]
- Diamantakos, P.; Velkou, A.; Killday, B.K.; Gimisis, T.; Melliou, E.; Magiatis, P. Oleokoronal and oleomissional: New major phenolic ingredients of extra virgin olive oil. OLIVAE 2015, 122, 22–33. [Google Scholar]
- Tsolakou, A.; Diamantakos, I.P.; Kalaboki, I.; Mena-Bravo, A.; Priego-Capote, F.; Abdallah, I.M.; Kaddoumi, A.; Melliou, E.; Magiatis, P. Oleocanthalic Acid, a Chemical Marker of Olive Oil Aging and Exposure to a High Storage Temperature with Potential Neuroprotective Activity. J. Agric. Food Chem. 2018, 66, 7337–7346. [Google Scholar] [CrossRef] [PubMed]
- Papakonstantinou, A.; Koumarianou, P.; Rigakou, A.; Diamantakos, P.; Frakolaki, E.; Vassilaki, N.; Chavdoula, E.; Melliou, E.; Magiatis, P.; Boleti, H. New Affordable Methods for Large-Scale Isolation of Major Olive Secoiridoids and Systematic Comparative Study of Their Antiproliferative/Cytotoxic Effect on Multiple Cancer Cell Lines of Different Cancer Origins. Int. J. Mol. Sci. 2022, 24, 3. [Google Scholar] [CrossRef]
- Fantini, M.; Benvenuto, M.; Masuelli, L.; Frajese, G.V.; Tresoldi, I.; Modesti, A.; Bei, R. In vitro and in vivo antitumoral effects of combinations of polyphenols, or polyphenols and anticancer drugs: Perspectives on cancer treatment. Int. J. Mol. Sci. 2015, 16, 9236–9282. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Williamson, G.; Morand, C.; Scalbert, A.; Remesy, C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am. J. Clin. Nutr. 2005, 81, 230S–242S. [Google Scholar] [CrossRef] [PubMed]
- Williamson, G.; Manach, C. Bioavailability and bioefficacy of polyphenols in humans. II. Review of 93 intervention studies. Am. J. Clin. Nutr. 2005, 81 (Suppl. 1), 243S–255S. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Mateos, A.; Vauzour, D.; Krueger, C.G.; Shanmuganayagam, D.; Reed, J.; Calani, L.; Mena, P.; Del Rio, D.; Crozier, A. Bioavailability, bioactivity and impact on health of dietary flavonoids and related compounds: An update. Arch. Toxicol. 2014, 88, 1803–1853. [Google Scholar] [CrossRef]
- Lewandowska, U.; Gorlach, S.; Owczarek, K.; Hrabec, E.; Szewczyk, K. Synergistic interactions between anticancer chemotherapeutics and phenolic compounds and anticancer synergy between polyphenols. Postep. Hig. I Med. Dosw. 2014, 68, 528–540. [Google Scholar] [CrossRef]
- de Kok, T.M.; van Breda, S.G.; Manson, M.M. Mechanisms of combined action of different chemopreventive dietary compounds: A review. Eur. J. Nutr. 2008, 47 (Suppl. 2), 51–59. [Google Scholar] [CrossRef]
- Niedzwiecki, A.; Roomi, M.W.; Kalinovsky, T.; Rath, M. Anticancer Efficacy of Polyphenols and Their Combinations. Nutrients 2016, 8, 552. [Google Scholar] [CrossRef]
- Karkoula, E.; Skantzari, A.; Melliou, E.; Magiatis, P. Direct Measurement of Oleocanthal and Oleacein Levels in Olive Oil by Quantitative H-1 NMR. Establishment of a New Index for the Characterization of Extra Virgin Olive Oils. J. Agric. Food Chem. 2012, 60, 11696–11703. [Google Scholar] [CrossRef]
- Karkoula, E.; Skantzari, A.; Melliou, E.; Magiatis, P. Quantitative measurement of major secoiridoid derivatives in olive oil using qNMR. Proof of the artificial formation of aldehydic oleuropein and ligstroside aglycon isomers. J. Agric. Food Chem. 2014, 62, 600–607. [Google Scholar] [CrossRef]
- Diamantakos, P.; Giannara, T.; Skarkou, M.; Melliou, E.; Magiatis, P. Influence of Harvest Time and Malaxation Conditions on the Concentration of Individual Phenols in Extra Virgin Olive Oil Related to Its Healthy Properties. Molecules 2020, 25, 2449. [Google Scholar] [CrossRef]
- Bahuguna, A.; Khan, I.; Bajpai, V.K.; Kang, S.C. MTT assay to evaluate the cytotoxic potential of a drug. Bangladesh J. Pharmacol. 2017, 12, 115–118. [Google Scholar] [CrossRef]
- Ronald, J.T. Drug Synergism: Its Detection and Applications. J. Pharmacol. Exp. Ther. 2001, 298, 865. [Google Scholar]
- Ting-Chao, C. Theoretical Basis, Experimental Design, and Computerized Simulation of Synergism and Antagonism in Drug Combination Studies. Pharmacol. Rev. 2006, 58, 621. [Google Scholar] [CrossRef]
- Indrayanto, G.; Putra, G.S.; Suhud, F. Validation of in-vitro bioassay methods: Application in herbal drug research. Profiles Drug Subst. Excip. Relat. Methodol. 2021, 46, 273–307. [Google Scholar] [CrossRef] [PubMed]
- De Stefanis, D.; Scime, S.; Accomazzo, S.; Catti, A.; Occhipinti, A.; Bertea, C.M.; Costelli, P. Anti-Proliferative Effects of an Extra-Virgin Olive Oil Extract Enriched in Ligstroside Aglycone and Oleocanthal on Human Liver Cancer Cell Lines. Cancers 2019, 11, 1640. [Google Scholar] [CrossRef] [PubMed]
- Reboredo-Rodriguez, P.; Gonzalez-Barreiro, C.; Cancho-Grande, B.; Forbes-Hernandez, T.Y.; Gasparrini, M.; Afrin, S.; Cianciosi, D.; Carrasco-Pancorbo, A.; Simal-Gandara, J.; Giampieri, F.; et al. Characterization of phenolic extracts from Brava extra virgin olive oils and their cytotoxic effects on MCF-7 breast cancer cells. Food Chem. Toxicol. 2018, 119, 73–85. [Google Scholar] [CrossRef] [PubMed]
- Polini, B.; Digiacomo, M.; Carpi, S.; Bertini, S.; Gado, F.; Saccomanni, G.; Macchia, M.; Nieri, P.; Manera, C.; Fogli, S. Oleocanthal and oleacein contribute to the in vitro therapeutic potential of extra virgin oil-derived extracts in non-melanoma skin cancer. Toxicol In Vitro 2018, 52, 243–250. [Google Scholar] [CrossRef]
- Gill, C.I.; Boyd, A.; McDermott, E.; McCann, M.; Servili, M.; Selvaggini, R.; Taticchi, A.; Esposto, S.; Montedoro, G.; McGlynn, H.; et al. Potential anti-cancer effects of virgin olive oil phenols on colorectal carcinogenesis models in vitro. Int. J. Cancer 2005, 117, 1–7. [Google Scholar] [CrossRef]
- Hashim, Y.Z.; Worthington, J.; Allsopp, P.; Ternan, N.G.; Brown, E.M.; McCann, M.J.; Rowland, I.R.; Esposto, S.; Servili, M.; Gill, C.I. Virgin olive oil phenolics extract inhibit invasion of HT115 human colon cancer cells in vitro and in vivo. Food Funct. 2014, 5, 1513–1519. [Google Scholar] [CrossRef] [PubMed]
- Pampaloni, B.; Mavilia, C.; Fabbri, S.; Romani, A.; Ieri, F.; Tanini, A.; Tonelli, F.; Brandi, M.L. In vitro effects of extracts of extra virgin olive oil on human colon cancer cells. Nutr. Cancer 2014, 66, 1228–1236. [Google Scholar] [CrossRef]
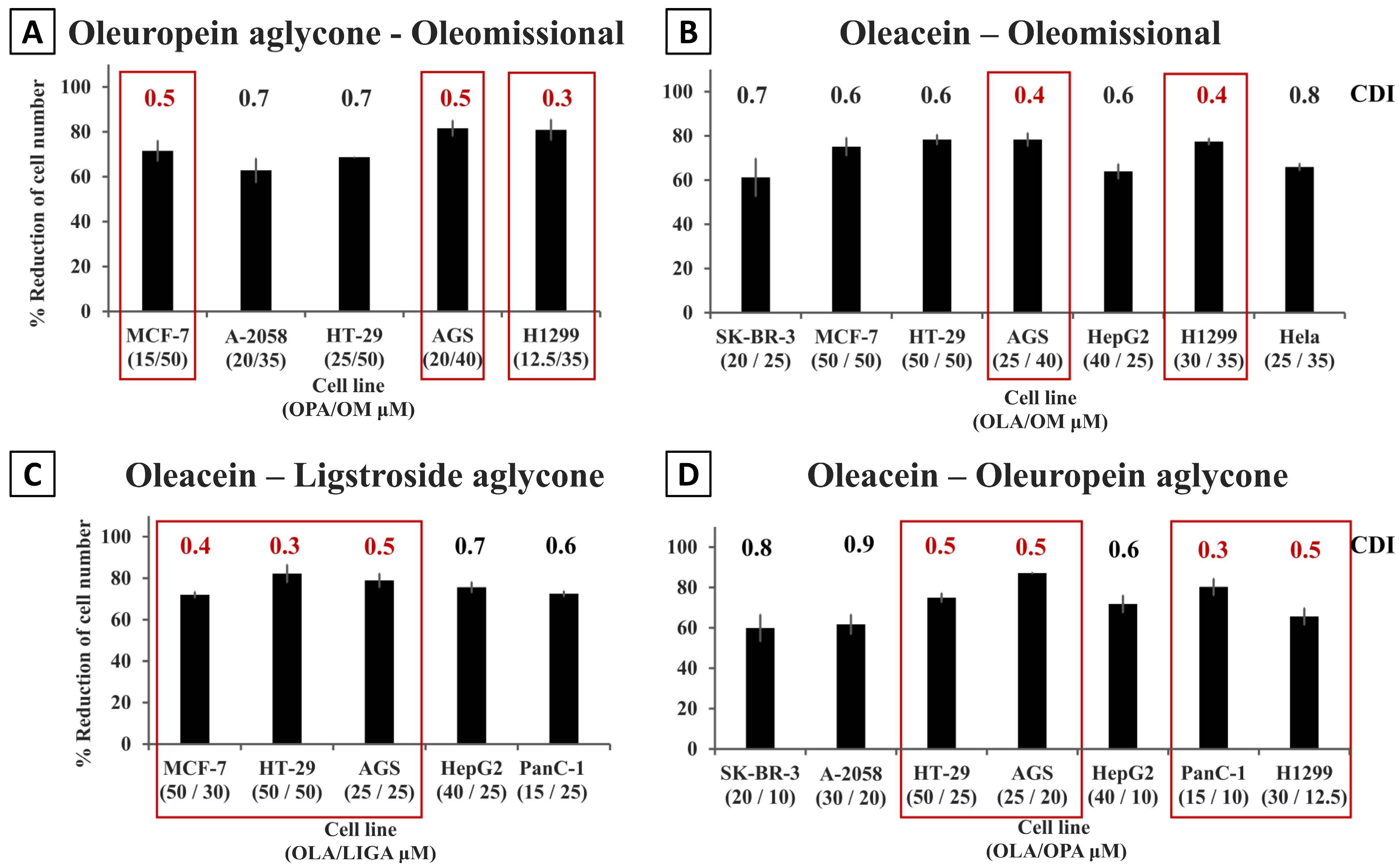
| A. | |||||
| Cell Origin | Cancer Cell Line | Combination | Concentration (OOP1/OOP2 μΜ) | % Inhibition of Viability | CDI |
| Human breast cancer cell lines | MDA-MB 231 | OLC/OLA | 5/15 | 20.3 ± 2.4 | 1.1 |
| OLC/LIGA | 5/15 | 30.4 ± 4.2 | 0.8 | ||
| OLC/OPA | 5/15 | 42.4 ± 1.7 | 0.9 | ||
| OLC/OM | 5/35 | 42.9 ± 2.4 | 1.0 | ||
| OLA/LIGA | 15/15 | 36.0 ± 3.2 | 0.8 | ||
| OLA/OPA | 15/15 | 35.2 ± 4.7 | 1.1 | ||
| OLA/OM | 15/35 | 41.9 ± 5.4 | 1.1 | ||
| LIGA/OPA | 15/15 | 39.4 ± 5.4 | 0.9 | ||
| LIGA/OM | 15/35 | 35.5 ± 2.5 | 1.0 | ||
| OPA/OM | 15/35 | 56.9 ± 4.8 | 0.9 | ||
| SK-BR-3 | OLC/OLA | 6/20 | 44.8 ± 1.2 | 1.1 | |
| OLC/LIGA | 6/10 | 38.3 ± 1.5 | 0.9 | ||
| OLC/OPA | 6/10 | 53.6 ± 3.9 | 0.9 | ||
| OLC/OM | 6/25 | 48.7 ± 1.3 | 0.9 | ||
| OLA/LIGA | 20/10 | 38.1 ± 4.4 | 1.0 | ||
| OLA/OPA | 20/10 | 59.9 ± 6.7 | 0.8 | ||
| OLA/OM | 20/25 | 61.2 ± 8.6 | 0.7 | ||
| LIGA/OPA | 10/10 | 46.5 ± 0.9 | 0.8 | ||
| LIGA/OM | 10/25 | 38.2 ± 6.1 | 0.9 | ||
| OPA/OM | 10/25 | 57.3 ± 4.5 | 0.7 | ||
| MCF-7 | OLC/OLA | 10/50 | 51.5 ± 4.1 | 1.0 | |
| OLC/LIGA | 10/30 | 46.5 ± 6.5 | 0.7 | ||
| OLC/OPA | 10/15 | 69.1 ± 0.8 | 0.5 | ||
| OLC/OM | 10/50 | 54.2 ± 3.0 | 1.0 | ||
| OLA/LIGA | 50/30 | 72.0 ± 1.5 | 0.4 | ||
| OLA/OPA | 50/15 | 43.2 ± 5.0 | 1.0 | ||
| OLA/OM | 50/50 | 75.1 ± 4.1 | 0.6 | ||
| LIGA/OPA | 30/15 | 48.9 ± 7.4 | 0.6 | ||
| LIGA/OM | 30/50 | 69.8 ± 2.8 | 0.5 | ||
| OPA/OM | 15/50 | 71.5 ± 4.6 | 0.5 | ||
| Skin melanoma cell lines | SK-MEL-28 | OLC/OLA | 5/15 | 43.2 ± 2.2 | 0.8 |
| OLC/LIGA | 5/10 | 25.5 ± 3.5 | 0.8 | ||
| OLC/OPA | 5/8 | 35.7 ± 2.4 | 0.8 | ||
| OLC/OM | 5/20 | 37.4 ± 3.6 | 0.8 | ||
| OLA/LIGA | 15/10 | 32.7 ± 3.5 | 0.8 | ||
| OLA/OPA | 15/8 | 54.0 ± 0.9 | 0.6 | ||
| OLA/OM | 15/20 | 50.7 ± 1.3 | 0.7 | ||
| LIGA/OPA | 10/8 | 29.9 ± 3.1 | 0.8 | ||
| LIGA/OM | 10/20 | 21.9 ± 4.8 | 0.8 | ||
| OPA/OM | 8/20 | 50.5 ± 2.3 | 0.6 | ||
| A2058 | OLC/OLA | 10/30 | 55.5 ± 4.3 | 1.0 | |
| OLC/LIGA | 10/35 | 58.2 ± 4.3 | 0.8 | ||
| OLC/OPA | 10/20 | 63.8 ± 5.1 | 0.8 | ||
| OLC/OM | 10/35 | 48.2 ± 2.9 | 0.9 | ||
| OLA/LIGA | 30/35 | 59.4 ± 4.7 | 0.8 | ||
| OLA/OPA | 30/20 | 61.7 ± 4.9 | 0.9 | ||
| OLA/OM | 30/35 | 53.3 ± 4.7 | 0.9 | ||
| LIGA/OPA | 35/20 | 78.2 ± 2.7 | 0.5 | ||
| LIGA/OM | 35/35 | 59.2 ± 2.7 | 0.7 | ||
| OPA/OM | 20/35 | 62.8 ± 5.4 | 0.7 | ||
| Colon and gastric cancer cell lines | HT-29 | OLC/OLA | 15/50 | 47.7 ± 0.8 | 0.9 |
| OLC/LIGA | 15/50 | 55.9 ± 5.1 | 0.6 | ||
| OLC/OPA | 15/25 | 49.2 ± 3.7 | 0.7 | ||
| OLC/OM | 15/50 | 60.8 ± 3.8 | 0.7 | ||
| OLA/LIGA | 50/50 | 82.2 ± 4.3 | 0.3 | ||
| OLA/OPA | 50/25 | 74.9 ± 2.3 | 0.5 | ||
| OLA/OM | 50/50 | 78.3 ± 2.3 | 0.6 | ||
| LIGA/OPA | 50/25 | 71.4 ± 0.2 | 0.5 | ||
| LIGA/OM | 50/50 | 71.2 ± 2.3 | 0.6 | ||
| OPA/OM | 25/50 | 68.7 ± 0.1 | 0.7 | ||
| AGS | OLC/OLA | 10/25 | 64.3 ± 3.4 | 0.8 | |
| OLC/LIGA | 10/25 | 67.2 ± 0.9 | 0.5 | ||
| OLC/OPA | 10/20 | 78.9 ± 2.4 | 0.6 | ||
| OLC/OM | 10/40 | 54.9 ± 2.3 | 0.7 | ||
| OLA/LIGA | 25/25 | 78.9 ± 3.4 | 0.5 | ||
| OLA/OPA | 25/20 | 87.1 ± 0.3 | 0.5 | ||
| OLA/OM | 25/40 | 78.3 ± 3.0 | 0.4 | ||
| LIGA/OPA | 25/20 | 83.3 ± 1.3 | 0.5 | ||
| LIGA/OM | 25/40 | 71.3 ± 4.6 | 0.4 | ||
| OPA/OM | 20/40 | 81.6 ± 3.5 | 0.5 | ||
| Liver cancer cell line | HepG2 | OLC/OLA | 20/40 | 67.8 ± 3.8 | 0.8 |
| OLC/LIGA | 20/25 | 80.2 ± 3.7 | 0.6 | ||
| OLC/OPA | 20/10 | 62.8 ± 3.9 | 0.8 | ||
| OLC/OM | 20/25 | 57.5 ± 2.4 | 0.7 | ||
| OLA/LIGA | 40/25 | 75.6 ± 2.6 | 0.7 | ||
| OLA/OPA | 40/10 | 71.8 ± 4.3 | 0.6 | ||
| OLA/OM | 40/25 | 63.9 ± 3.4 | 0.6 | ||
| LIGA/OPA | 25/10 | 75.3 ± 4.8 | 0.7 | ||
| LIGA/OM | 25/25 | 62.3 ± 0.3 | 0.7 | ||
| OPA/OM | 10/25 | 44.4 ± 1.9 | 0.8 | ||
| Pancreas cancer cell line | PANC-1 | OLC/OLA | 8/15 | 67.4 ± 3.8 | 0.5 |
| OLC/LIGA | 8/25 | 81.8 ± 2.9 | 0.4 | ||
| OLC/OPA | 8/10 | 71.6 ± 1.6 | 0.4 | ||
| OLC/OM | 8/17.5 | 59.2 ± 2.7 | 0.7 | ||
| OLA/LIGA | 15/25 | 72.5 ± 1.3 | 0.6 | ||
| OLA/OPA | 15/10 | 80.2 ± 4.2 | 0.3 | ||
| OLA/OM | 15/17.5 | 43.8 ± 1.4 | 0.8 | ||
| LIGA/OPA | 25/10 | 77.6 ± 0.6 | 0.5 | ||
| LIGA/OM | 25/17.5 | 68.5 ± 5.0 | 0.7 | ||
| OPA/OM | 10/17.5 | 48.7 ± 2.5 | 0.7 | ||
| Lung cancer cell line | H1299 | OLC/OLA | 10/30 | 63.5 ± 4.1 | 0.6 |
| OLC/LIGA | 10/40 | 77.9 ± 1.7 | 0.4 | ||
| OLC/OPA | 10/12.5 | 68.6 ± 4.5 | 0.5 | ||
| OLC/OM | 10/35 | 60.4 ± 3.5 | 0.8 | ||
| OLA/LIGA | 30/40 | 56.3 ± 4.8 | 0.7 | ||
| OLA/OPA | 30/12.5 | 65.6 ± 4.2 | 0.5 | ||
| OLA/OM | 30/35 | 77.4 ± 1.5 | 0.4 | ||
| LIGA/OPA | 40/12.5 | 64.6 ± 4.1 | 0.6 | ||
| LIGA/OM | 40/35 | 77.9 ± 4.1 | 0.4 | ||
| OPA/OM | 12.5/35 | 80.9 ± 4.6 | 0.3 | ||
| Cervix cancer cell line | Hela | OLC/OLA | 20/25 | 73.2 ± 1.3 | 0.8 |
| OLC/LIGA | 20/25 | 76.2 ± 2.5 | 0.7 | ||
| OLC/OPA | 20/15 | 62.0 ± 3.8 | 0.8 | ||
| OLC/OM | 20/35 | 78.2 ± 1.9 | 0.7 | ||
| OLA/LIGA | 25/25 | 49.1 ± 5.7 | 1.1 | ||
| OLA/OPA | 25/15 | 39.4 ± 1.6 | 0.9 | ||
| OLA/OM | 25/35 | 65.9 ± 1.6 | 0.8 | ||
| LIGA/OPA | 25/15 | 54.9 ± 3.9 | 0.7 | ||
| LIGA/OM | 25/35 | 60.3 ± 4.2 | 0.8 | ||
| OPA/OM | 15/35 | 55.0 ± 0.1 | 0.7 | ||
| B. | |||||
| Cell Origin | Cell Line | Combination | Concentration (OOP1/OOP2 μΜ) | % Inhibition of Viability | CDI |
| Spontaneously transformed aneuploidy immortal keratinocytes | HaCaT | OLC/OLA | 10/30 | 42.0 ± 6.0 | 0.8 |
| OLC/LIGA | 10/35 | 58.6 ± 3.7 | 0.7 | ||
| OLC/OPA | 10/20 | 51.9 ± 3.3 | 0.9 | ||
| OLC/OM | 10/35 | 50.3 ± 9.2 | 0.7 | ||
| OLA/LIGA | 30/35 | 53.7 ± 2.8 | 0.6 | ||
| OLA/OPA | 30/20 | 62.0 ± 7.4 | 0.5 | ||
| OLA/OM | 30/35 | 42.5 ± 2.0 | 0.6 | ||
| LIGA/OPA | 35/20 | 60.9 ± 8.5 | 0.6 | ||
| LIGA/OM | 35/35 | 55.4 ± 9.4 | 0.5 | ||
| OPA/OM | 20/35 | 56.6 ± 3.9 | 0.6 | ||
| Human non-tumorigenic epithelial cell | MCF 10A | OLC/OPA | 5/15 | 75.4 ± 4.5 | 0.5 |
| OLC/LIGA | 5/10 | 25.6 ± 4.2 | 0.9 | ||
| OLC/OPA | 5/10 | 55.3 ± 3.4 | 0.7 | ||
| OLC/OM | 5/25 | 71.9 ± 0.0 | 0.6 | ||
| LIGA/OPA | 10/10 | 41.0 ± 5.7 | 0.9 | ||
| Selectivity Indices (SI) | |
|---|---|
| RATIO HaCaT/A2058 | |
| OLC 10/OLA 30 | 1.3 |
| OLC 10/LIG 35 | 1.0 |
| OLC 10/OP 20 | 1.3 |
| OLC 10/OM 35 | 1.0 |
| OLA 30/LIG 35 | 1.1 |
| OLA 30/OP 20 | 1.0 |
| OLA 30/OM 35 | 1.2 |
| LIG 35/OP 20 | 1.8 |
| LIG 35/OM 35 | 1.1 |
| OP 20/OM 35 | 1.2 |
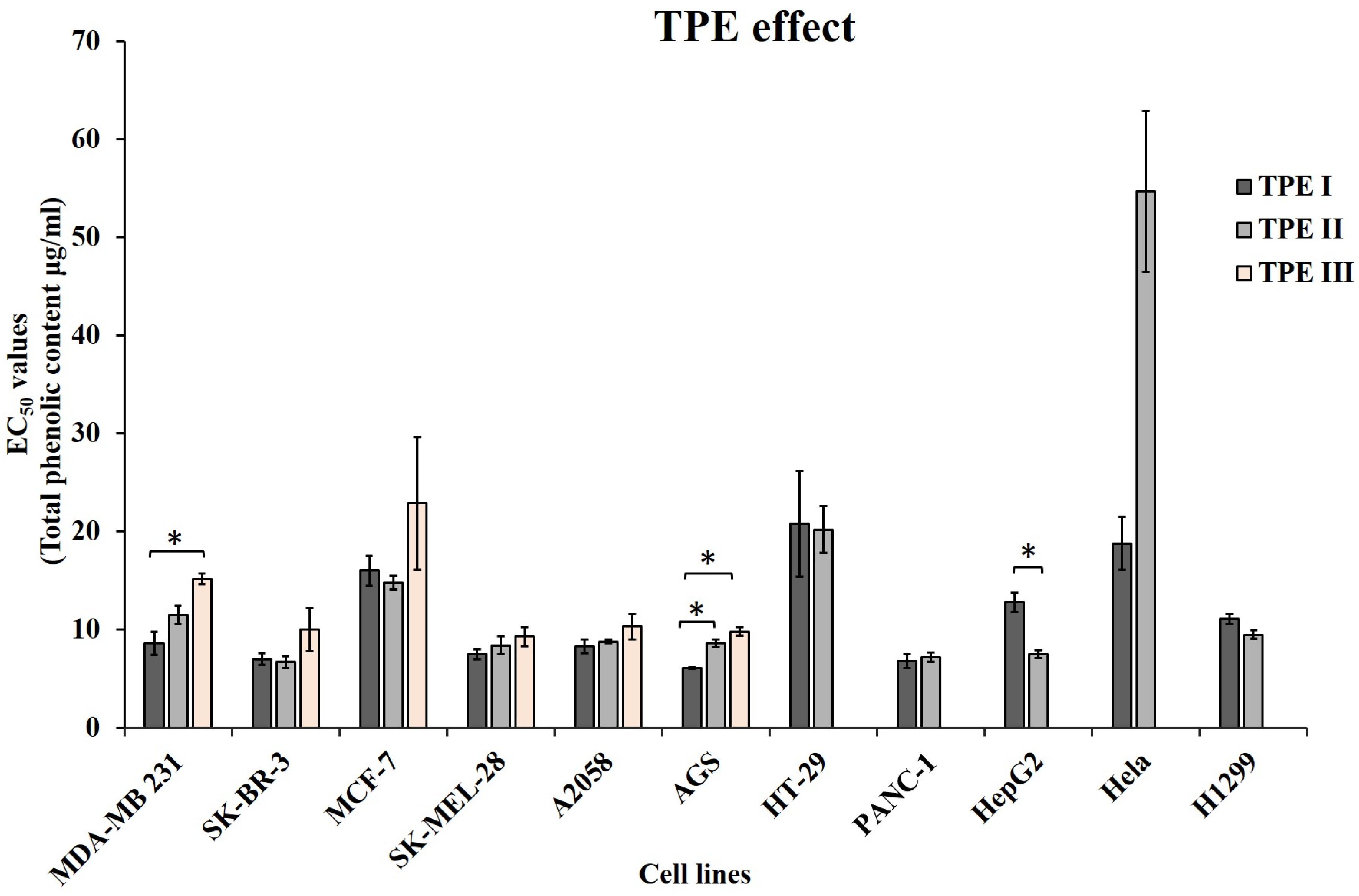
| A. | EC50 Values | |||||||
| Cell Lines | OOPs | Single OOP [20] | TPE I | TPE II | TPE III | |||
| μg/mL (±S.E.) | μΜ | μg/mL (±S.E.) | μΜ | μg/mL (±S.E.) | μΜ | μg/mL (±S.E.) | ||
| MDA-MB 231 | OLC | 3.2 ± 0.2 | 12.5 | 3.8 | 13.1 | 4.0 | 0 | 0 |
| OLA | 12.1 ± 0.7 | 5.2 | 1.7 | 5.4 | 1.7 | 0 | 0 | |
| LIGA | 11.5 ± 1.0 | 1.9 | 0.7 | 6.4 | 2.2 | 12.0 | 4.3 | |
| OPA | 9.6 ± 0.3 | 1.9 | 0.7 | 2.5 | 0.9 | 11.5 | 4.3 | |
| OM | 52.0 ± 7.6 | 0.0 | 0.0 | 1.1 | 0.4 | 1.4 | 0.5 | |
| OLK | - | 2.4 | 0.9 | 7.0 | 2.4 | 11.7 | 4.2 | |
| TY | - | 6.3 | 0.9 | 0.0 | 0.0 | 12.4 | 1.7 | |
| Total Phenolic content | 8.6 ± 1.2 | 11.5 ± 0.9 | 15.2 ± 0.5 | |||||
| SK-BR-3 | OLC | 3.9 ± 0.2 | 9.7 | 3.0 | 7.6 | 2.3 | 0 | 0 |
| OLA | 14.6 ± 0.7 | 4.1 | 1.3 | 3.2 | 1.0 | 0 | 0 | |
| LIGA | 7.8 ± 0.9 | 1.5 | 0.5 | 3.7 | 1.3 | 7.9 | 2.9 | |
| OPA | 6.3 ± 0.6 | 1.5 | 0.5 | 1.5 | 0.5 | 2.9 | 2.9 | |
| OM | 20.2 ± 0.8 | 0.0 | 0.0 | 0.6 | 0.2 | 0.4 | 0.4 | |
| OLK | - | 1.9 | 0.6 | 4.1 | 1.4 | 2.8 | 2.8 | |
| TY | - | 4.9 | 1.2 | 0.0 | 0.0 | 1.1 | 1.1 | |
| Total Phenolic content | 7.0 ± 0.6 | 6.7 ± 0.6 | 10 ± 2.2 | |||||
| MCF-7 | OLC | 7.5 ± 0.8 | 22.3 | 6.8 | 16.9 | 5.1 | 0 | 0 |
| OLA | > 30 | 9.4 | 2.9 | 7.0 | 2.2 | 0 | 0 | |
| LIGA | 22.4 ± 0.4 | 3.3 | 1.1 | 8.3 | 2.8 | 18.0 | 6.5 | |
| OPA | 12.2 ± 0.4 | 3.3 | 1.1 | 3.2 | 1.1 | 17.3 | 6.5 | |
| OM | > 30 | 0.0 | 0.0 | 1.4 | 0.5 | 2.2 | 0.8 | |
| OLK | - | 4.3 | 1.4 | 9.0 | 3.1 | 17.6 | 6.4 | |
| TY | - | 11.3 | 2.7 | 0.0 | 0.0 | 18.7 | 2.6 | |
| Total Phenolic Content | 16.0 ± 1.5 | 14.8 ± 0.7 | 22.9 ± 6.7 | |||||
| SK-MEL-28 | OLC | 3.2 ± 0.3 | 10.5 | 3.2 | 9.2 | 2.8 | 0 | 0 |
| OLA | 10.7 ± 0.8 | 4.4 | 1.4 | 3.8 | 1.2 | 0 | 0 | |
| LIGA | 8.1 ± 0.4 | 1.6 | 0.5 | 4.5 | 1.6 | 7.3 | 2.7 | |
| OPA | 5.7 ± 0.3 | 1.6 | 0.5 | 1.8 | 0.7 | 7.0 | 2.7 | |
| OM | 17.1 ± 0.2 | 0.0 | 0.0 | 0.8 | 0.3 | 0.9 | 0.3 | |
| OLK | - | 2.0 | 0.7 | 4.9 | 1.8 | 7.2 | 2.6 | |
| TY | - | 5.3 | 1.2 | 0.0 | 0.0 | 7.6 | 1.1 | |
| Total Phenolic content | 7.5 ± 0.5 | 8.4 ± 0.9 | 9.3 ± 1.0 | |||||
| A2058 | OLC | 5.6 ± 0.1 | 11.6 | 3.5 | 10.0 | 3.0 | 0 | 0 |
| OLA | 17.8 ± 1.1 | 4.9 | 1.5 | 4.2 | 1.3 | 0 | 0 | |
| LIGA | 23.1 ± 0.6 | 1.7 | 0.6 | 4.9 | 1.7 | 7.6 | 2.8 | |
| OPA | 14.1 ± 0.3 | 1.7 | 0.6 | 1.9 | 0.7 | 7.3 | 2.7 | |
| OM | 28.3 ± 0.5 | 0.0 | 0.0 | 0.8 | 0.3 | 0.9 | 0.3 | |
| OLK | - | 2.2 | 0.7 | 5.3 | 1.8 | 7.5 | 2.7 | |
| TY | - | 5.8 | 1.4 | 0.0 | 0.0 | 7.9 | 1.7 | |
| Total Phenolic content | 8.3 ± 0.7 | 8.8 ± 0.2 | 10.3 ± 1.3 | |||||
| AGS | OLC | 5.6 ± 0.3 | 8.6 | 2.6 | 9.5 | 2.9 | 0 | 0 |
| OLA | 14.8 ± 0.7 | 3.6 | 1.1 | 3.9 | 1.3 | 0 | 0 | |
| LIGA | 17.6 ± 0.9 | 1.3 | 0.4 | 4.6 | 1.7 | 7.7 | 2.8 | |
| OPA | 13.6 ± 0.5 | 1.3 | 0.4 | 1.8 | 0.7 | 7.4 | 2.8 | |
| OM | 28.5 ± 1.9 | 0.0 | 0.0 | 0.8 | 0.3 | 0.9 | 0.4 | |
| OLK | - | 1.6 | 0.6 | 5.0 | 1.8 | 7.6 | 2.7 | |
| TY | - | 4.3 | 0.9 | 0.0 | 0.0 | 8.0 | 1.1 | |
| Total Phenolic content | 6.1 ± 0.1 | 8.6 ± 0.4 | 9.8 ± 0.4 | |||||
| HT-29 | OLC | 8.0 ± 0.6 | 29.0 | 8.8 | 22.2 | 6.7 | ||
| OLA | >30 | 12.2 | 3.8 | 9.2 | 3.0 | |||
| LIGA | 35.6 ± 3.1 | 4.3 | 1.4 | 10.9 | 3.9 | |||
| OPA | 18.9 ± 0.6 | 4.3 | 1.4 | 4.3 | 1.6 | |||
| OM | >30 | 0.0 | 0.0 | 1.8 | 0.7 | |||
| OLK | - | 5.5 | 1.8 | 11.8 | 4.3 | |||
| TY | - | 14.6 | 3.5 | 0.0 | 0.0 | |||
| Total Phenolic content | 20.8 ± 5.4 | 20.2 ± 2.4 | ||||||
| PANC-1 | OLC | 4.5 ± 0.3 | 9.5 | 2.9 | 7.9 | 2.4 | ||
| OLA | 9.9 ± 0.1 | 4.0 | 1.3 | 3.3 | 1.0 | |||
| LIGA | 16.6 ± 0.5 | 1.4 | 0.5 | 3.9 | 1.4 | |||
| OPA | 7.2 ± 0.2 | 1.4 | 0.5 | 1.5 | 0.6 | |||
| OM | 13.2 ± 0.0 | 0.0 | 0.0 | 0.6 | 0.2 | |||
| OLK | - | 1.8 | 0.6 | 4.2 | 1.5 | |||
| TY | - | 4.8 | 1.1 | 0.0 | 0.0 | |||
| Total Phenolic content | 6.8 ± 0.7 | 7.2 ± 0.5 | ||||||
| HepG2 | OLC | 12.2 ± 1.6 | 18.0 | 5.5 | 8.2 | 2.5 | ||
| OLA | 26.5 ± 0.2 | 7.6 | 2.4 | 3.4 | 1.1 | |||
| LIGA | 16.6 ± 1.2 | 2.7 | 0.9 | 4.0 | 1.5 | |||
| OPA | 6.7 ± 0.2 | 2.7 | 0.9 | 1.6 | 0.6 | |||
| OM | 17.2 ± 1.3 | 0.0 | 0.0 | 0.7 | 0.3 | |||
| OLK | - | 3.4 | 1.1 | 4.4 | 1.6 | |||
| TY | - | 9.1 | 2.0 | 0.0 | 0.0 | |||
| Total Phenolic content | 12.8 ± 1.0 | 7.5 ± 0.4 | ||||||
| Hela | OLC | 13.6 ± 0.1 | 26.5 | 8.1 | 60.0 | 18.2 | ||
| OLA | 14.8 ± 1.6 | 11.2 | 3.5 | 24.9 | 8.0 | |||
| LIGA | 10.5 ± 0.1 | 4.0 | 1.3 | 29.4 | 10.7 | |||
| OPA | 17.3 ± 0.6 | 4.0 | 1.4 | 11.5 | 4.4 | |||
| OM | 26.1 ± 0.6 | 0.0 | 0.0 | 4.9 | 1.9 | |||
| OLK | - | 5.0 | 1.7 | 31.9 | 11.6 | |||
| TY | - | 13.4 | 2.8 | 0.0 | 0.0 | |||
| Total Phenolic content | 18.8 ± 2.7 | 54.7 ± 8.2 | ||||||
| H1299 | OLC | 5.5 ± 0.3 | 15.7 | 4.8 | 10.4 | 3.2 | ||
| OLA | 19.8 ± 0.4 | 6.6 | 2.1 | 4.3 | 1.4 | |||
| LIGA | 30.2 ± 0.7 | 2.3 | 0.8 | 5.1 | 1.8 | |||
| OPA | 9.1 ± 0.3 | 2.3 | 0.8 | 2.0 | 0.8 | |||
| OM | 27.8 ± 1.7 | 0.0 | 0.0 | 0.9 | 0.3 | |||
| OLK | - | 3.0 | 1.0 | 5.5 | 2.0 | |||
| TY | - | 7.9 | 1.7 | 0.0 | 0.0 | |||
| Total Phenolic Content | 11.1 ± 0.5 | 9.5 ± 0.4 | ||||||
| B. | Non—Cancer Cell Lines | |||||||
| HaCaT | OLC | 5.9 ± 0.1 | 14.7 | 4.5 | 19.1 | 5.8 | ||
| OLA | 16.6 ± 1.4 | 6.2 | 1.9 | 7.9 | 2.5 | |||
| LIGA | 20.2 ± 0.4 | 2.2 | 0.7 | 9.4 | 3.4 | |||
| OPA | 9.2 ± 4.6 | 2.2 | 0.7 | 3.7 | 1.4 | |||
| OM | 16.1 ± 8.1 | 0.0 | 0.0 | 1.6 | 0.6 | |||
| OLK | - | 2.8 | 0.9 | 10.2 | 3.7 | |||
| TY | - | 7.4 | 1.8 | 0.0 | 0.0 | |||
| Total Phenolic content | 10.5 ± 2.5 | 17.4 ± 1.2 | ||||||
| NHDF | OLC | 7.5 ± 0.1 | 18.1 | 5.5 | 16.4 | 5.0 | ||
| OLA | 15.7 ± 0.4 | 7.6 | 2.4 | 6.8 | 2.2 | |||
| LIGA | 16.9 ± 1.8 | 2.7 | 0.9 | 8.0 | 2.9 | |||
| OPA | 16.1 ± 0.2 | 2.7 | 0.9 | 3.1 | 1.2 | |||
| OM | 24.3 ± 0.7 | 0.0 | 0.0 | 1.3 | 0.5 | |||
| OLK | - | 3.4 | 1.1 | 8.7 | 3.2 | |||
| TY | - | 9.1 | 2.0 | 0.0 | 0.0 | |||
| Total Phenolic content | 12.9 ± 0.3 | 14.9 ± 0.6 | ||||||
| MCF 10A | OLC | 2.1 ± 0.1 | 7.3 | 2.2 | 14.1 | 4.3 | ||
| OLA | 7.9 ± 0.9 | 3.1 | 1.0 | 5.8 | 1.9 | |||
| LIGA | 20.2 ± 1.0 | 1.1 | 0.4 | 6.9 | 2.5 | |||
| OPA | 3.6 ± 0.2 | 1.1 | 0.4 | 2.7 | 1.0 | |||
| OM | 13.4 ± 0.7 | 0.0 | 0.0 | 1.2 | 0.4 | |||
| OLK | - | 1.4 | 0.5 | 7.5 | 2.7 | |||
| TY | - | 3.7 | 0.8 | 0.0 | 0.0 | |||
| Total Phenolic content | 5.2 ± 0.5 | 12.8 ± 0.8 | ||||||
| Cell Line/TPE | Selectivity Index (SI) (Control Cell Line) | |
|---|---|---|
| MDA-MB 231 | (MCF 10A) | |
| TPE Ι | 0.6 | |
| TPE ΙΙ | 1.1 | |
| SK-BR-3 | (MCF 10A) | |
| TPE Ι | 0.7 | |
| TPE ΙΙ | 1.9 | |
| MCF-7 | (MCF 10A) | |
| TPE Ι | 0.3 | |
| TPE ΙΙ | 0.9 | |
| SK-MEL-28 | (NHDF) | (HaCaT) |
| TPE Ι | 1.5 | 1.2 |
| TPE ΙΙ | 1.8 | 2.1 |
| A2058 | (NHDF) | (HaCaT) |
| TPE Ι | 1.5 | 1.3 |
| TPE ΙΙ | 1.7 | 2.0 |
| MDA-MB 231 | SK-BR-3 | MCF-7 | SK-MEL-28 | A-2058 | AGS | HT-29 | PANC-1 | HepG2 | Hela | H1299 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| OOPs | EC50 TPE I/EC50 OOP | ||||||||||
| OLC | 2.7 | 1.8 | 2.1 | 2.4 | 1.5 | 1.1 | 2.6 | 1.5 | 1.1 | 1.4 | 2.0 |
| OLA | 0.7 | 0.5 | 0.5 | 0.7 | 0.5 | 0.4 | 0.6 | 0.7 | 0.5 | 1.3 | 0.6 |
| LIGA | 0.8 | 0.9 | 0.7 | 0.9 | 0.4 | 0.3 | 0.6 | 0.4 | 0.8 | 1.1 | 0.4 |
| OPA | 0.9 | 1.1 | 1.3 | 1.3 | 0.6 | 0.4 | 1.1 | 0.9 | 1.9 | 1.8 | 1.2 |
| OM | 0.4 | 0.3 | 0.3 | 0.4 | 0.3 | 0.2 | 0.5 | 0.5 | 0.7 | 0.7 | 0.4 |
| EC50 TPE II/EC50 OOP | |||||||||||
| OLC | 3.6 | 1.7 | 2.0 | 2.7 | 1.6 | 1.5 | 2.5 | 1.6 | 0.6 | 4.0 | 1.7 |
| OLA | 1.0 | 0.5 | 0.5 | 0.8 | 0.5 | 0.6 | 0.6 | 0.7 | 0.3 | 3.7 | 0.5 |
| LIGA | 1.1 | 0.9 | 0.7 | 1.0 | 0.4 | 0.5 | 0.6 | 0.4 | 0.4 | 3.2 | 0.3 |
| OPA | 1.2 | 1.1 | 1.2 | 1.5 | 0.6 | 0.6 | 1.1 | 1.0 | 1.1 | 5.2 | 1.0 |
| OM | 0.6 | 0.3 | 0.3 | 0.5 | 0.3 | 0.3 | 0.5 | 0.5 | 0.4 | 2.1 | 0.3 |
| EC50 TPE III/EC50 OOP | |||||||||||
| OLC | 4.8 | 2.5 | 3.1 | 2.9 | 1.8 | 1.7 | |||||
| OLA | 1.3 | 0.7 | 0.7 | 0.9 | 0.6 | 0.7 | |||||
| LIGA | 1.4 | 1.3 | 1.0 | 1.2 | 0.4 | 0.6 | |||||
| OPA | 1.6 | 1.6 | 1.9 | 1.6 | 0.7 | 0.7 | |||||
| OM | 0.8 | 0.5 | 0.5 | 0.5 | 0.4 | 0.4 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papakonstantinou, A.; Koumarianou, P.; Diamantakos, P.; Melliou, E.; Magiatis, P.; Boleti, H. A Systematic Ex-Vivo Study of the Anti-Proliferative/Cytotoxic Bioactivity of Major Olive Secoiridoids’ Double Combinations and of Total Olive Oil Phenolic Extracts on Multiple Cell-Culture Based Cancer Models Highlights Synergistic Effects. Nutrients 2023, 15, 2538. https://doi.org/10.3390/nu15112538
Papakonstantinou A, Koumarianou P, Diamantakos P, Melliou E, Magiatis P, Boleti H. A Systematic Ex-Vivo Study of the Anti-Proliferative/Cytotoxic Bioactivity of Major Olive Secoiridoids’ Double Combinations and of Total Olive Oil Phenolic Extracts on Multiple Cell-Culture Based Cancer Models Highlights Synergistic Effects. Nutrients. 2023; 15(11):2538. https://doi.org/10.3390/nu15112538
Chicago/Turabian StylePapakonstantinou, Aikaterini, Petrina Koumarianou, Panagiotis Diamantakos, Eleni Melliou, Prokopios Magiatis, and Haralabia Boleti. 2023. "A Systematic Ex-Vivo Study of the Anti-Proliferative/Cytotoxic Bioactivity of Major Olive Secoiridoids’ Double Combinations and of Total Olive Oil Phenolic Extracts on Multiple Cell-Culture Based Cancer Models Highlights Synergistic Effects" Nutrients 15, no. 11: 2538. https://doi.org/10.3390/nu15112538
APA StylePapakonstantinou, A., Koumarianou, P., Diamantakos, P., Melliou, E., Magiatis, P., & Boleti, H. (2023). A Systematic Ex-Vivo Study of the Anti-Proliferative/Cytotoxic Bioactivity of Major Olive Secoiridoids’ Double Combinations and of Total Olive Oil Phenolic Extracts on Multiple Cell-Culture Based Cancer Models Highlights Synergistic Effects. Nutrients, 15(11), 2538. https://doi.org/10.3390/nu15112538










