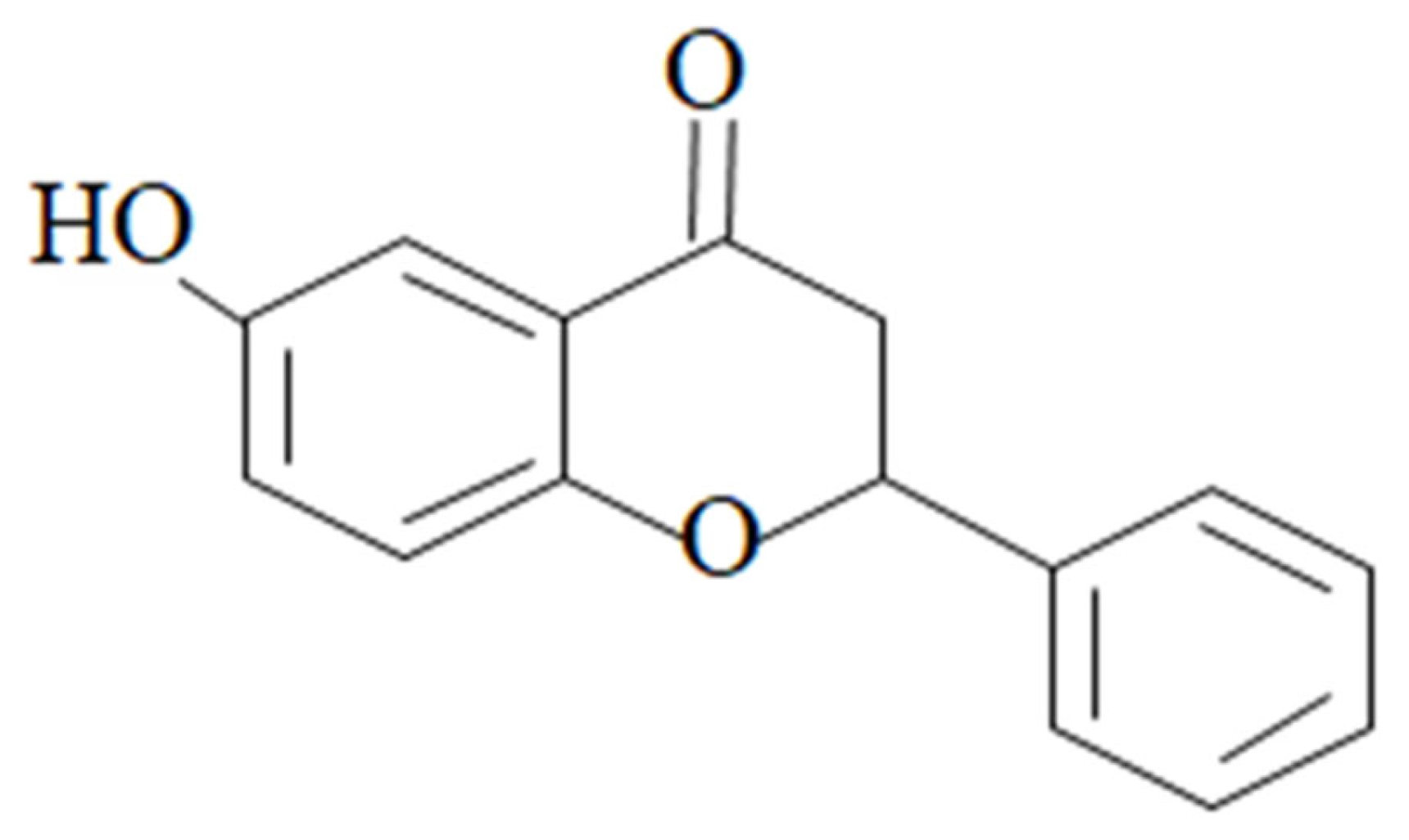
Targeting Anti-Inflammatory Pathways to Treat Diabetes-Induced Neuropathy by 6-Hydroxyflavanone
Abstract
1. Introduction
2. Material and Methods
2.1. Chemicals and Reagents
2.2. Animals for Behavioral Studies
2.3. In Silico Molecular Simulation Studies
2.4. In Vitro Cycloxygenase (COX-2) Inhibitory Assay
2.5. In Vitro 5-LOX Inhibition Assay
2.6. In Vivo Hot Plate Analgesiometer Study
2.7. Carrageenan-Induced Inflammation
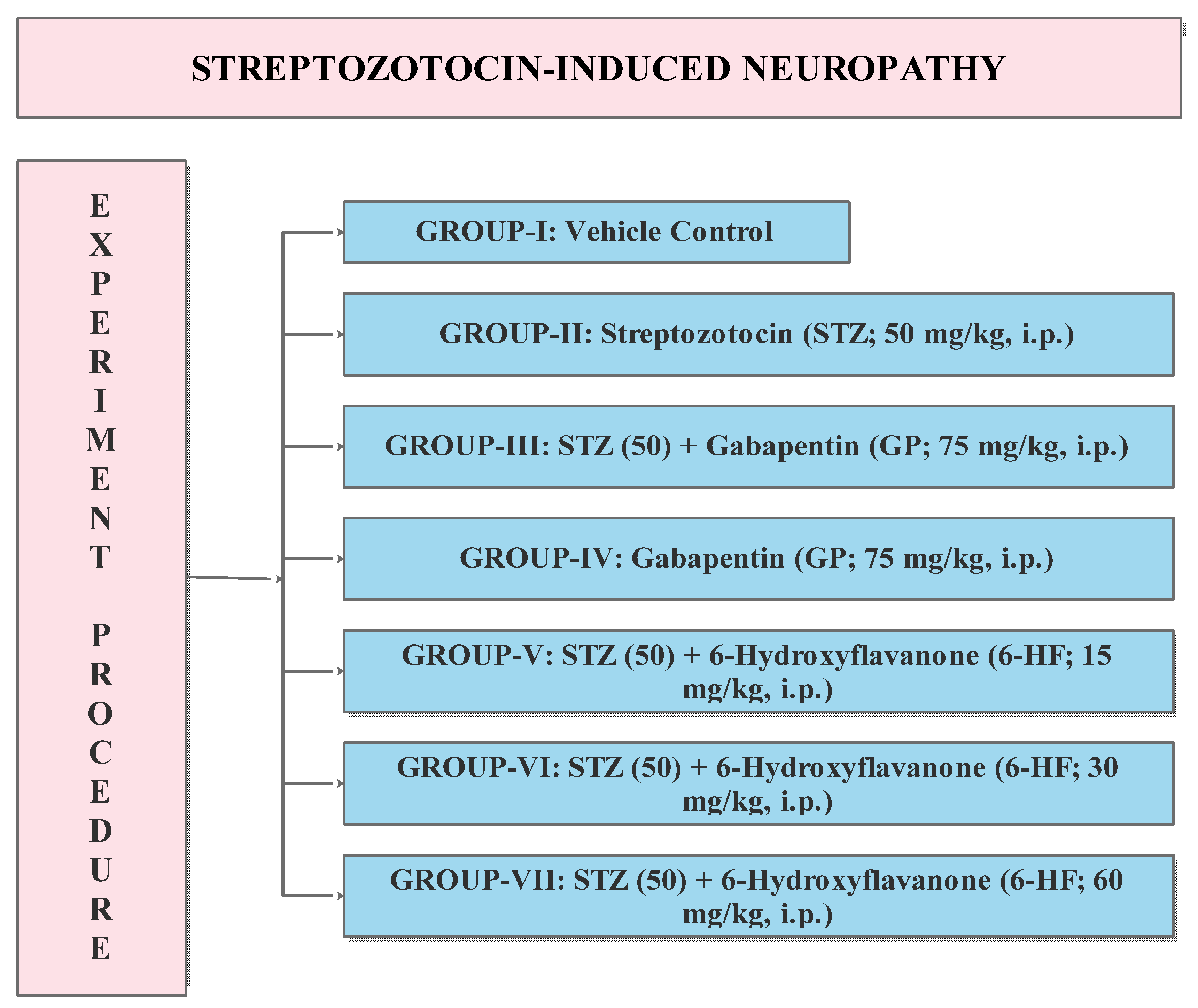
2.8. Diabetes-Induced Neuropathic Pain (DINP); Development of Diabetes and Neuropathic Pain
2.9. Experimental Protocol
2.10. Estimation of Static/Dynamic Allodynia and Static/Dynamic Mechanical Vulvodynia
2.11. Statistical Analysis
3. Results
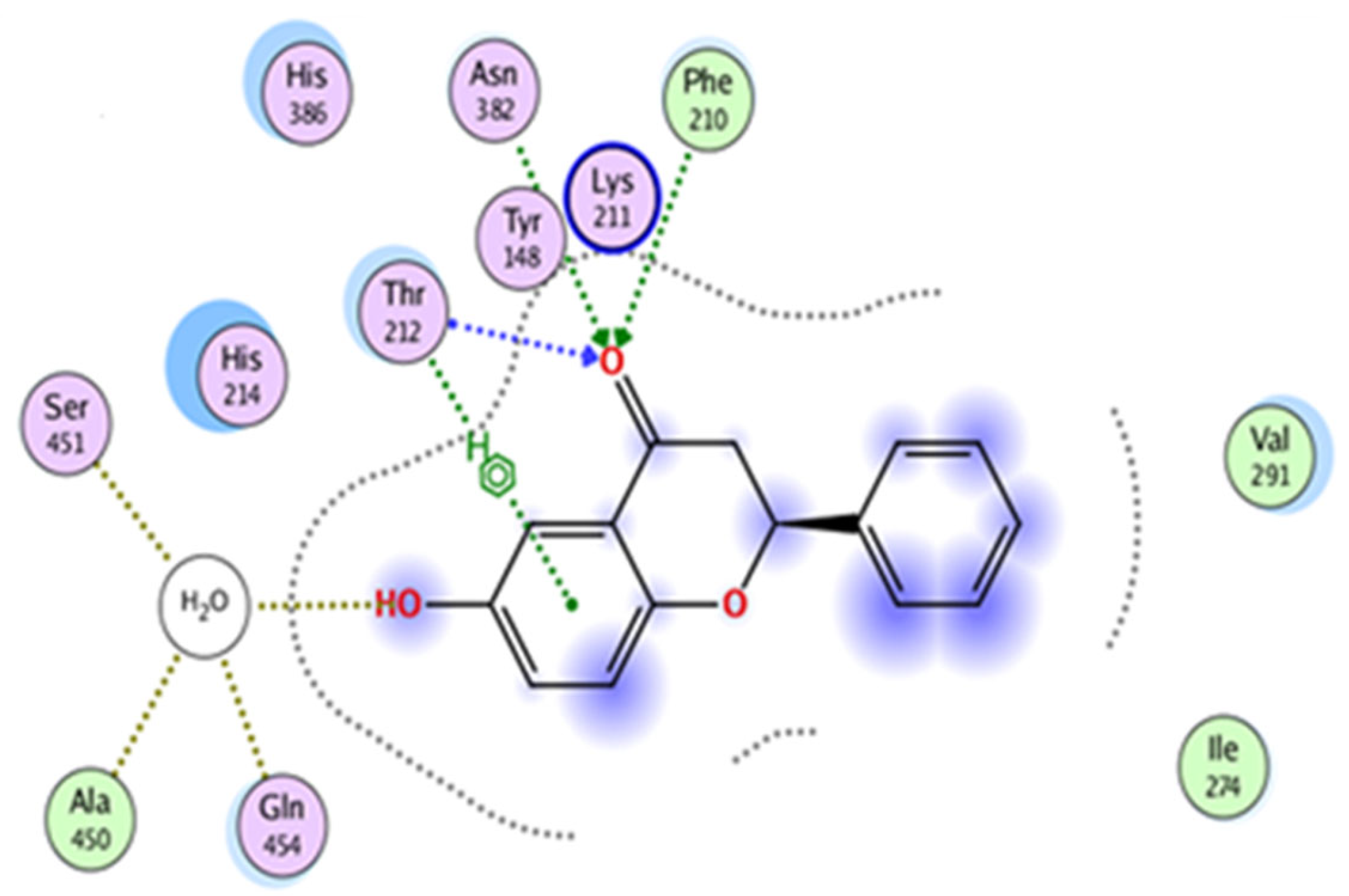
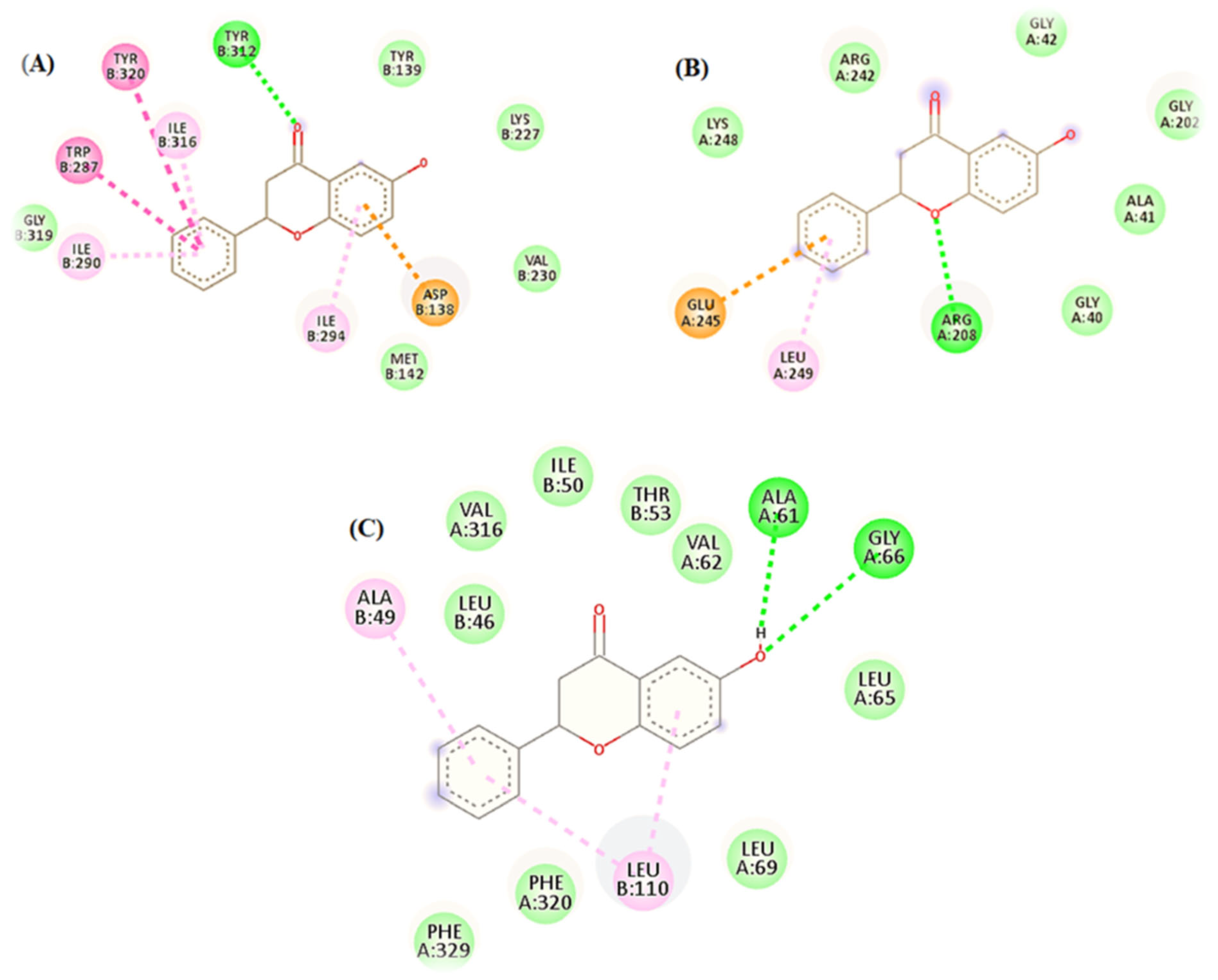
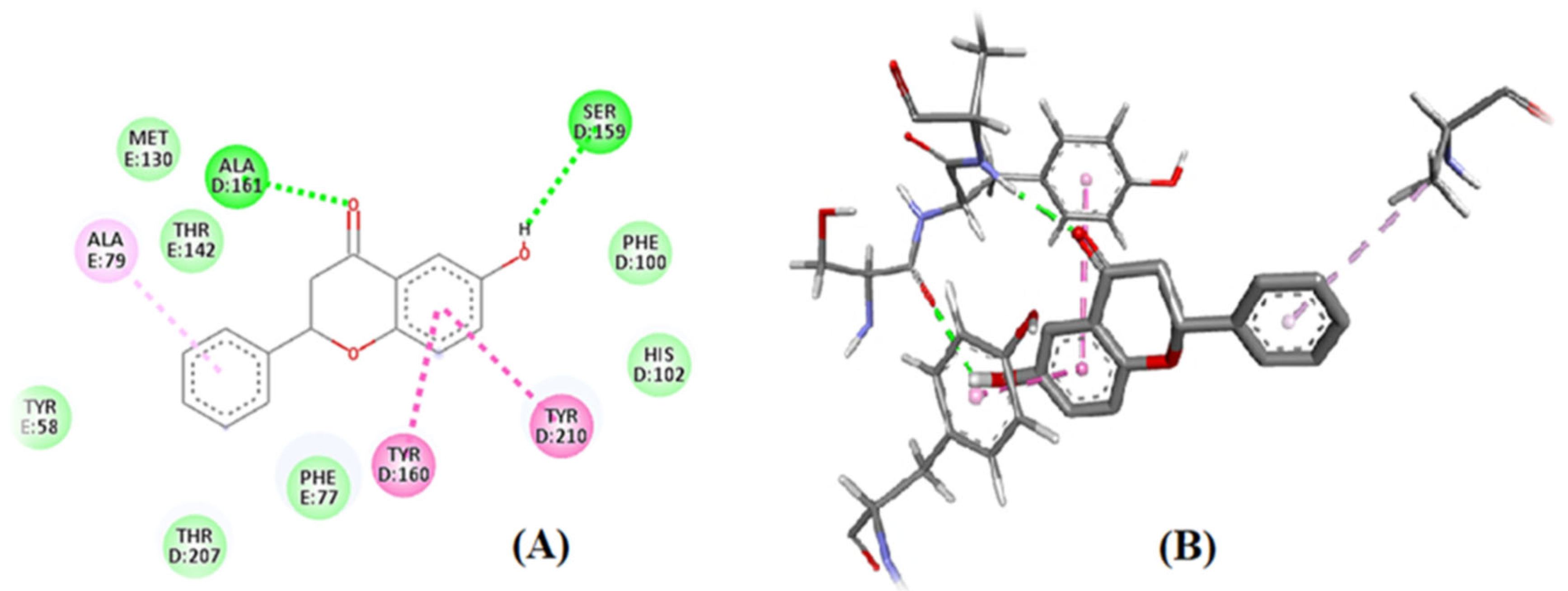
3.1. In Silico: Interaction of 6-HF with the Cyclooxygenase-2 enzymes (COX-2), Opioid and GABA-A Receptors
3.2. In Vitro Studies: Cycloxygenase (COX-2) and Lipoxygenase (5-LOX) Inhibitory Assay
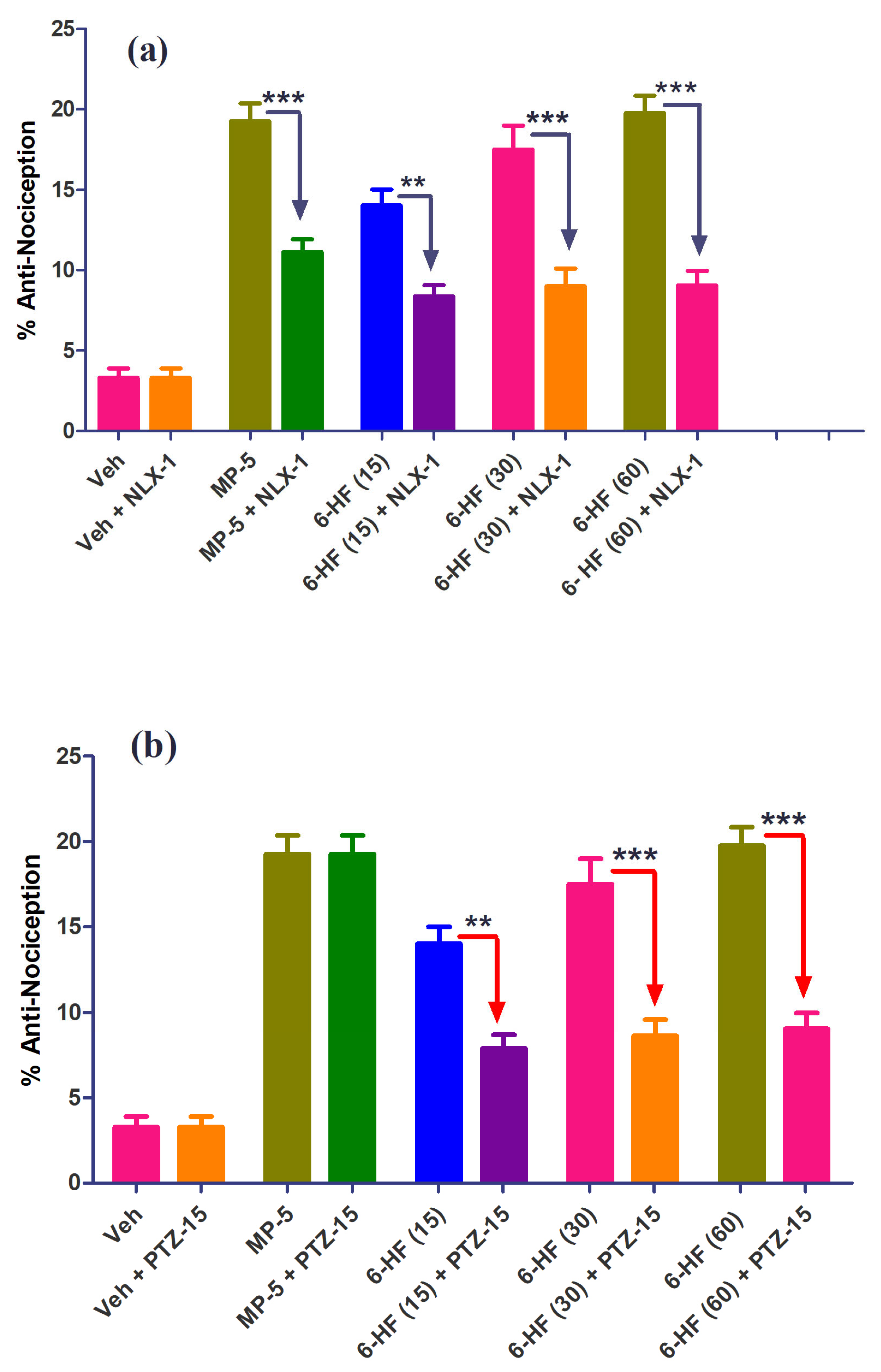
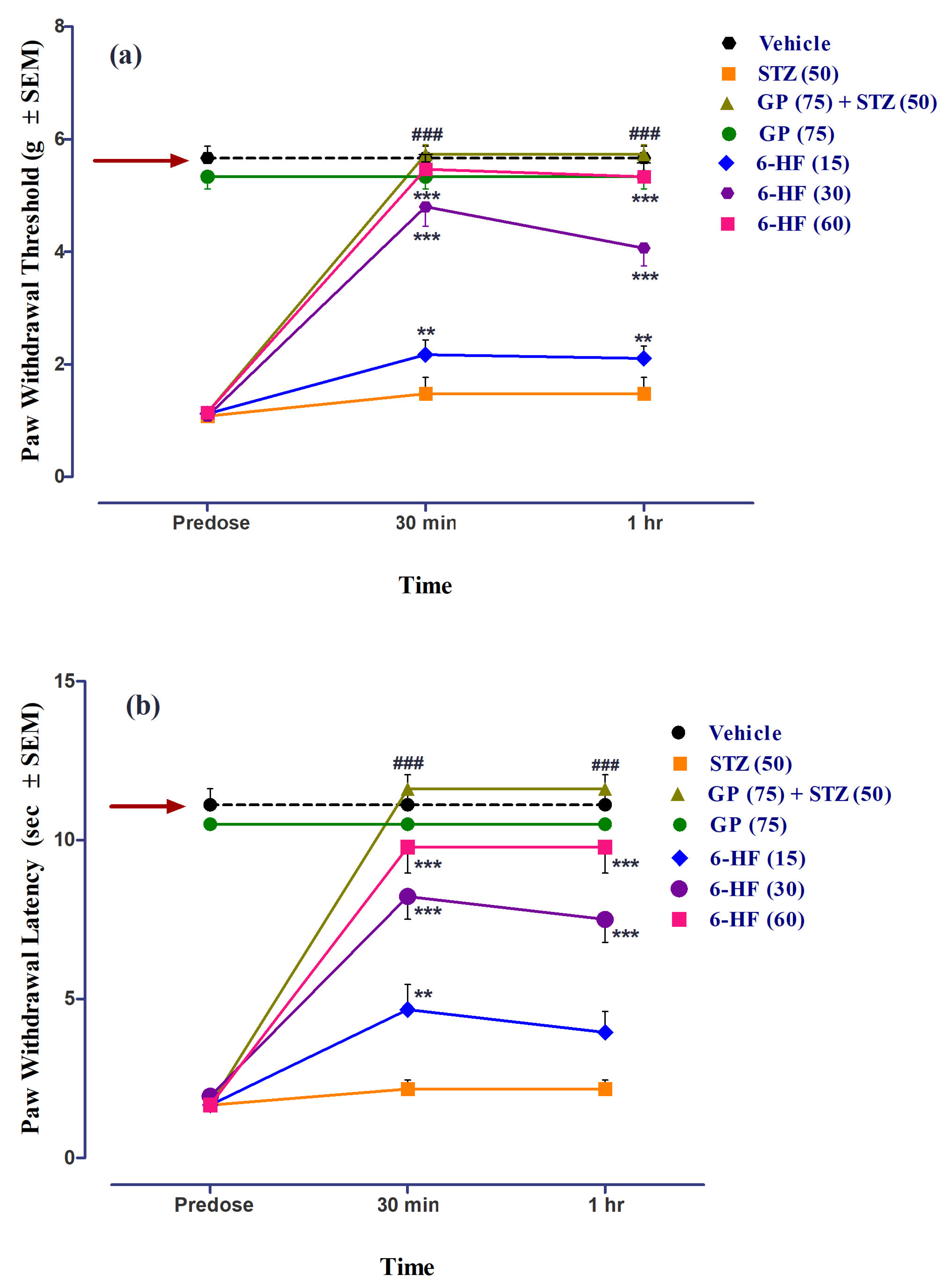
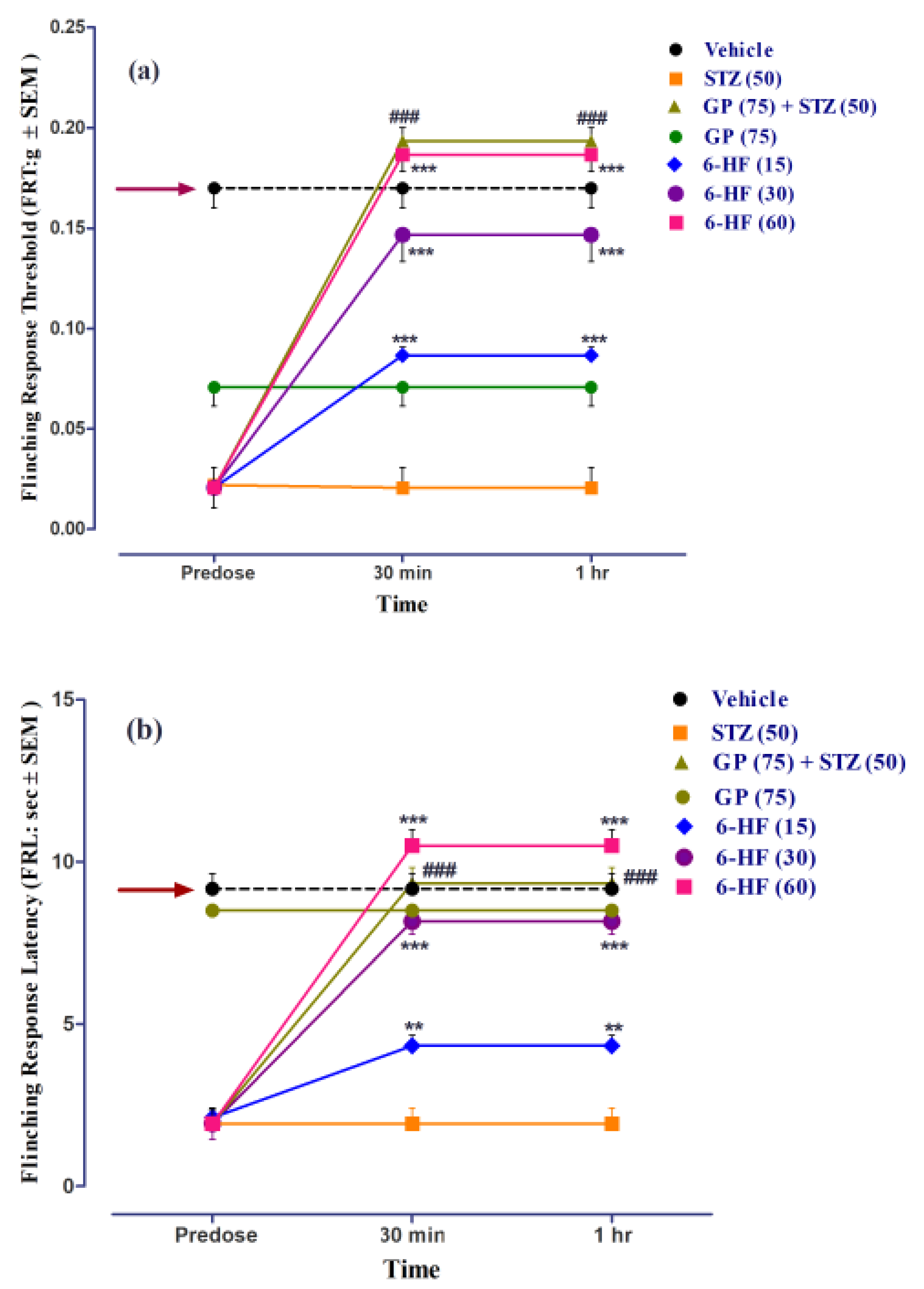
3.3. In Vivo Studies: Evaluation of 6-HF for Thermal Anti-Nociception in the Hot Plate Analgesiometer
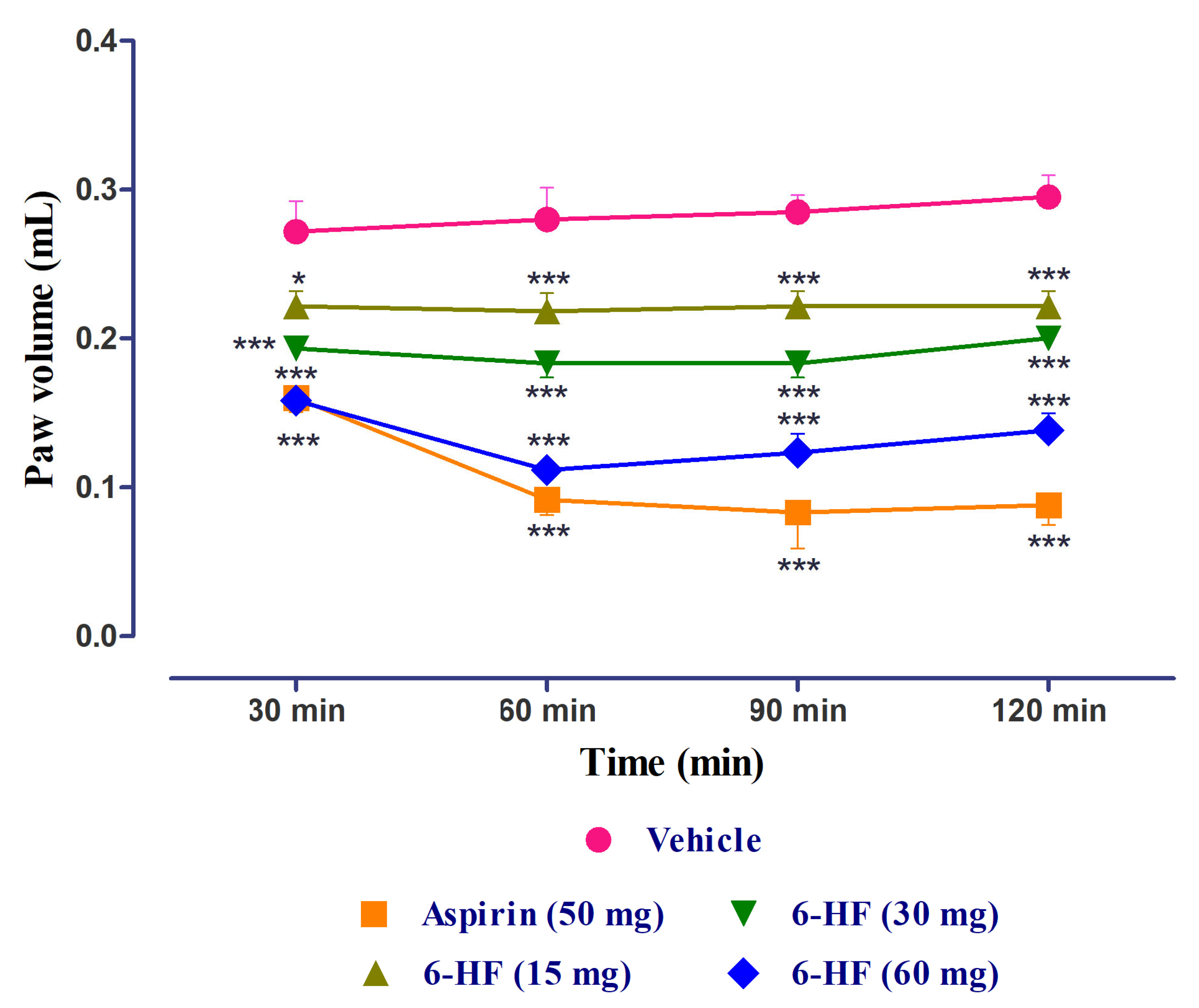
3.4. Anti-Inflammatory Activity of 6-HF
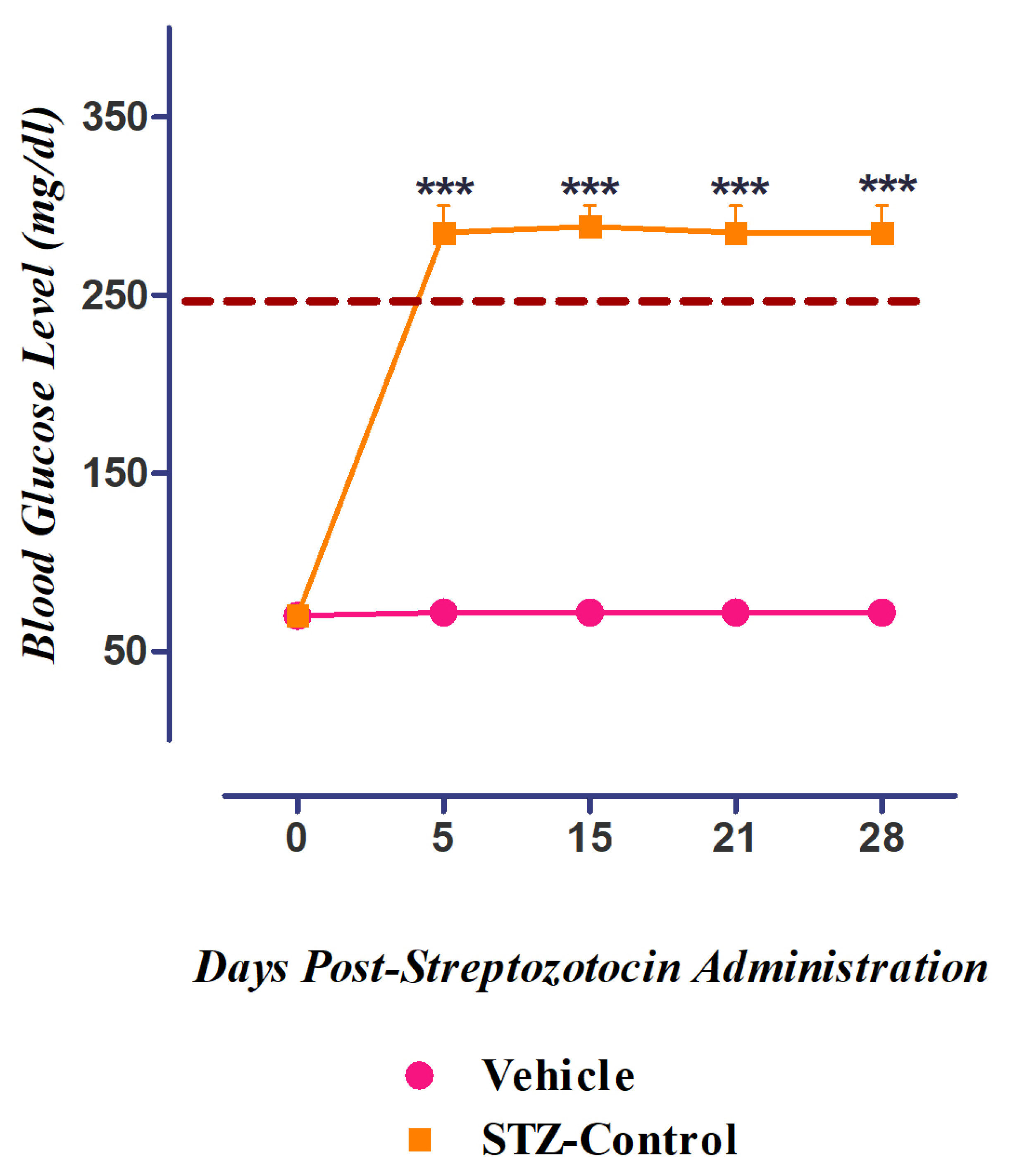
3.5. Development of Diabetes Mellitus (DM) by Streptozotocin
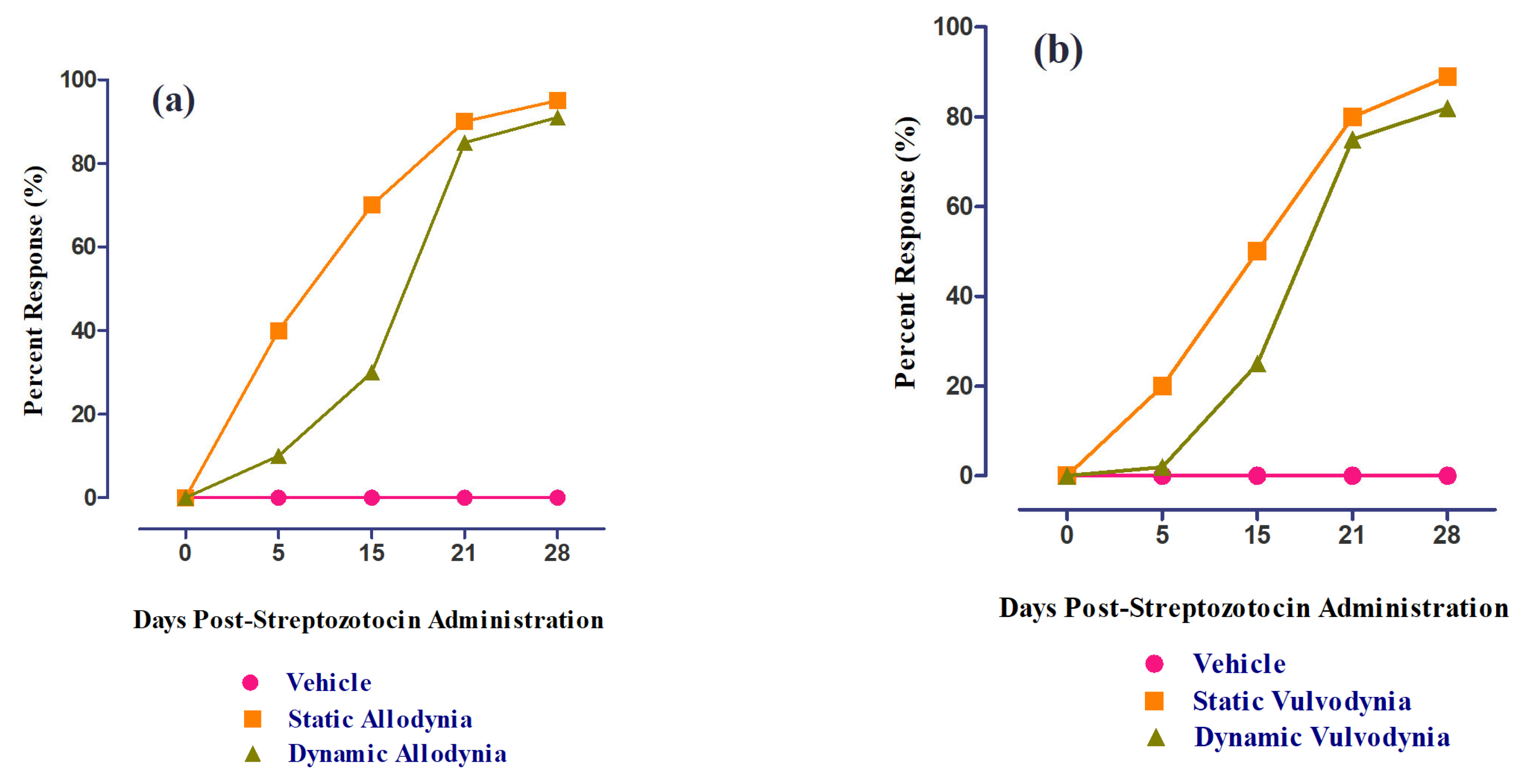
3.6. Role of 6-HF in Ameliorating Static/Dynamic Allodynia
3.7. Role of 6-HF in Ameliorating Static/Dynamic Vulvodynia
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kazamel, M.; Stino, A.M.; Smith, A.G. Metabolic syndrome and peripheral neuropathy. Muscle Nerve 2021, 63, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Stavniichuk, R.; Shevalye, H.; Lupachyk, S.; Obrosov, A.; Groves, J.T.; Obrosova, I.G.; Yorek, M.A. Peroxynitrite and protein nitration in the pathogenesis of diabetic peripheral neuropathy. Diabetes Metab. Res. Rev. 2014, 30, 669–678. [Google Scholar] [CrossRef] [PubMed]
- Kuffler, D.P. Mechanisms for reducing neuropathic pain. Mol. Neurobiol. 2020, 57, 67–87. [Google Scholar] [CrossRef] [PubMed]
- Akbar, S.; Subhan, F.; Karim, N.; Shahid, M.; Ahmad, N.; Ali, G.; Mahmood, W.; Fawad, K. 6-Methoxyflavanone attenuates mechanical allodynia and vulvodynia in the streptozotocin-induced diabetic neuropathic pain. Biomed. Pharmacother. 2016, 84, 962–971. [Google Scholar] [CrossRef] [PubMed]
- Narayana, K.R.; Reddy, M.S.; Chaluvadi, M.; Krishna, D. Bioflavonoids classification, pharmacological, biochemical effects and therapeutic potential. Indian J. Pharmacol. 2001, 33, 2–16. [Google Scholar]
- Andrew, A.; Brian, K.; Catherine, E. Prediction of Protein−Ligand Interactions. Docking and Scoring: Successes and Gaps. J. Med. Chem. 2006, 49, 5851–5855. [Google Scholar]
- Bergmann, R.; Kongsbak, K.; Sørensen, P.L.; Sander, T.; Balle, T. A unified model of the GABAA receptor comprising agonist and benzodiazepine binding sites. PLoS ONE 2013, 8, e52323. [Google Scholar] [CrossRef]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4, Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef]
- Burnett, B.; Jia, Q.; Zhao, Y.; Levy, R. A medicinal extract of Scutellaria baicalensis and Acacia catechu acts as a dual inhibitor of cyclooxygenase and 5-lipoxygenase to reduce inflammation. J. Med. Food 2007, 10, 442–451. [Google Scholar] [CrossRef]
- Wisastra, R.; Kok, P.A.M.; Eleftheriadis, N.; Baumgartner, M.P.; Camacho, C.J.; Haisma, H.J.; Dekker, F.J. Discovery of a novel activator of 5-lipoxygenase from an anacardic acid derived compound collection. Bioorganic Med. Chem. 2013, 21, 7763–7778. [Google Scholar] [CrossRef]
- Woolfe, G.; Macdonald, A.D. The evaluation of the analgesic actions of pethidine hydrochlodide (Demerol). J. Pharmacol. Exp. Ther. March 1944, 80, 300–307. [Google Scholar]
- Winter, C.A.; Risley, E.A.; Nuss, G.W. Carrageenin-induced edema in hind paw of the rat as an assay for antiinflammatory drugs. Exp. Biol. Med. 1962, 111, 544–547. [Google Scholar] [CrossRef]
- Field, M.J.; Bramwell, S.; Hughes, J.; Singh, L. Detection of static and dynamic components of mechanical allodynia in rat models of neuropathic pain: Are they signalled by distinct primary sensory neurones? Pain 1999, 83, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Chaplan, S.R.; Bach, F.W.; Pogrel, J.; Chung, J.; Yaksh, T. Quantitative assessment of tactile allodynia in the rat paw. J. Neurosci. Methods 1994, 53, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Ali, G.; Subhan, F.; Abbas, M.; Zeb, J.; Shahid, M.; Sewell, R.D. A streptozotocin-induced diabetic neuropathic pain model for static or dynamic mechanical allodynia and vulvodynia: Validation using topical and systemic gabapentin. Naunyn Schmiedeberg’s Arch. Pharmacol. 2015, 388, 1129–1140. [Google Scholar] [CrossRef] [PubMed]
- Befort, K.; Tabbara, L.; Kling, D.; Maigret, B.; Kieffer, B.L. Role of Aromatic Transmembrane Residues of the δ-Opioid Receptor in Ligand Recognition (∗). J. Biol. Chem. 1996, 271, 10161–10168. [Google Scholar] [CrossRef]
- Sloan, G.; Selvarajah, D.; Tesfaye, S. Pathogenesis, diagnosis and clinical management of diabetic sensorimotor peripheral neuropathy. Nat. Rev. Endocrinol. 2021, 17, 400–420. [Google Scholar] [CrossRef]
- Bán, E.G.; Brassai, A.; Vizi, E.S. The role of the endogenous neurotransmitters associated with neuropathic pain and in the opioid crisis: The innate pain-relieving system. Brain Res. Bull. 2020, 155, 129–136. [Google Scholar] [CrossRef]
- Kaur, S.; Pandhi, P.; Dutta, P. Painful diabetic neuropathy: An update. Ann. Neurosci. 2011, 18, 168. [Google Scholar] [CrossRef]
- Evcimen, N.D.; King, G.L. The role of protein kinase C activation and the vascular complications of diabetes. Pharmacol. Res. 2007, 55, 498–510. [Google Scholar]
- Tsatsanis, C.; Androulidaki, A.; Venihaki, M.; Margioris, A.N. Signalling networks regulating cyclooxygenase-2. Int. J. Biochem. Cell Biol. 2006, 38, 1654–1661. [Google Scholar] [CrossRef]
- Aksh, T.L.; Dirig, D.M.; Conway, C.M.; Svensson, C.; Luo, Z.D.; Isakson, P.C. The acute antihyperalgesic action of nonsteroidal, anti-inflammatory drugs and release of spinal prostaglandin E2 is mediated by the inhibition of constitutive spinal cyclooxygenase-2 (COX-2) but not COX-1. J. Neurosci. 2001, 21, 5847–5853. [Google Scholar] [CrossRef]
- Lee, H.; Selvaraj, B.; Yoo, K.Y.; Ko, S.-H. Flavonoids as anti-inflammatory and neuroprotective agents. Int. J. Oral Biol. 2020, 45, 33–41. [Google Scholar] [CrossRef]
- Natarajan, A.; Manikandan, S.; Chellappa, S.; Kulandaivel, S. Molecular modelling, synthesis and evaluation of flavone and flavanone scaffolds as anti-inflammatory agents. Anti Inflamm. Anti Allergy Agents Med. Chem. 2021, 20, 20–38. [Google Scholar]
- Basu, A.D.; Anindhya, S.S.; Manoj, P.; Manash, P.C.; Pronobesh, B.; Kaushik, M.R. STAT3 and NF-κB are common targets for kaempferol-mediated attenuation of COX-2 expression in IL-6-induced macrophages and carrageenan-induced mouse paw edema. Biochem. Biophys. Rep. 2017, 12, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.J.; Spencer, J.P.; Rice-Evans, C. Flavonoids: Antioxidants or signalling molecules? Free Radic. Biol. Med. 2004, 36, 838–849. [Google Scholar] [CrossRef] [PubMed]
- Rudolph, U.; Knoflach, F. Beyond classical benzodiazepines: Novel therapeutic potential of GABAA receptor subtypes. Nat. Rev. Drug Discov. 2011, 10, 685–697. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chou, X.-L.; Zhang, L.I.; Tao, H.W. Zona incerta: An integrative node for global behavioral modulation. Trends Neurosci. 2020, 43, 82–87. [Google Scholar] [CrossRef]
- Chen, S.-R.; Zhu, L.; Chen, H.; Wen, L.; Laumet, G.; Pan, H.-L. Increased spinal cord Na+-K+-2Cl− cotransporter-1 (NKCC1) activity contributes to impairment of synaptic inhibition in paclitaxel-induced neuropathic pain. J. Biol. Chem. 2014, 289, 31111–31120. [Google Scholar] [CrossRef]
- Zhou, H.-Y.; Chen, S.-R.; Byun, H.-S.; Chen, H.; Li, L.; Han, H.-D.; Lopez-Berestein, G.; Sood, A.K.; Pan, H.-L. N-methyl-D-aspartate receptor-and calpain-mediated proteolytic cleavage of K+-Cl− cotransporter-2 impairs spinal chloride homeostasis in neuropathic pain. J. Biol. Chem. 2012, 287, 33853–33864. [Google Scholar] [CrossRef]
- Hwang, J.H.; Yaksh, T.L. The effect of spinal GABA receptor agonists on tactile allodynia in a surgically-induced neuropathic pain model in the rat. Pain 1997, 70, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Vaysse, L.; Sol, J.; Lazorthes, Y.; Courtade-Saidi, M.; Eaton, M.; Jozan, S. GABAergic pathway in a rat model of chronic neuropathic pain: Modulation after intrathecal transplantation of a human neuronal cell line. Neurosci. Res. 2011, 69, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Burgess, S.E.; Gardell, L.R.; Ossipov, M.H.; Malan, T.P.; Vanderah, T.W.; Lai, J.; Porreca, F. Time-dependent descending facilitation from the rostral ventromedial medulla maintains, but does not initiate, neuropathic pain. J. Neurosci. 2002, 22, 5129–5136. [Google Scholar] [CrossRef]
- Stubley, L.A.; Martinez, M.A.; Karmally, S.; Lopez, T.; Cejas, P.; Eaton, M.J. Only early intervention with gamma-aminobutyric acid cell therapy is able to reverse neuropathic pain after partial nerve injury. J. Neurotrauma 2001, 18, 471–477. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Yaksh, T.L. Effects of intrathecal strychnine and bicuculline on nerve compression-induced thermal hyperalgesia and selective antagonism by MK-801. Pain 1993, 54, 79–84. [Google Scholar] [CrossRef]
- Kabli, N.; Cahill, C.M. Anti-allodynic effects of peripheral delta opioid receptors in neuropathic pain. Pain 2007, 127, 84–93. [Google Scholar] [CrossRef]
- Vicario, N.; Pasquinucci, L.; Spitale, F.M.; Chiechio, S.; Turnaturi, R.; Caraci, F.; Tibullo, D.; Avola, R.; Gulino, R.; Parenti, R. Simultaneous activation of mu and delta opioid receptors reduces allodynia and astrocytic connexin 43 in an animal model of neuropathic pain. Mol. Neurobiol. 2019, 56, 7338–7354. [Google Scholar] [CrossRef]
- Tegeder, I.; Geisslinger, G. Opioids as modulators of cell death and survival—Unraveling mechanisms and revealing new indications. Pharmacol. Rev. 2004, 56, 351–369. [Google Scholar] [CrossRef]
- Machelska, H. Targeting of opioid-producing leukocytes for pain control. Neuropeptides 2007, 41, 355–363. [Google Scholar] [CrossRef]
- Rittner, H.; Brack, A.; Stein, C. Pain and the immune system. Br. J. Anaesth. 2008, 101, 40–44. [Google Scholar] [CrossRef]
- Ren, L.; Wang, F.; Xu, Z.; Chan, W.M.; Zhao, C.; Xue, H. GABAA receptor subtype selectivity underlying anxiolytic effect of 6-hydroxyflavone. Biochem. Pharmacol. 2010, 79, 1337–1344. [Google Scholar] [CrossRef] [PubMed]
- Hall, B.J.; Chebib, M.; Hanrahan, J.R.; Johnston, G.A. 6-Methylflavanone, a more efficacious positive allosteric modulator of γ-aminobutyric acid (GABA) action at human recombinant α2β2γ2L than at α1β2γ2L and α1β2 GABAA receptors expressed in Xenopus oocytes. Eur. J. Pharmacol. 2005, 512, 97–104. [Google Scholar] [CrossRef] [PubMed]
- D’Mello, R.; Dickenson, A.H. Spinal cord mechanisms of pain. Br. J. Anaesth. 2008, 101, 8–16. [Google Scholar] [CrossRef]
- Enna, S.; McCarson, K.E. The role of GABA in the mediation and perception of pain. Adv. Pharmacol. 2006, 54, 1–27. [Google Scholar]

| Concentration (µg/mL) | COX-2 % Inhibition (Mean ± S.E.M.) | |
|---|---|---|
| Celecoxib | 6-HF | |
| 1000 | 98.13 ± 0.70 | 82.64 ± 0.75 *** |
| 500 | 95.17 ± 0.53 | 77.58 ± 0.77 *** |
| 250 | 90.39 ± 0.49 | 74.75 ± 0.63 *** |
| 125 | 85.13 ± 0.20 | 68.58 ± 0.70 *** |
| 62.5 | 81.80 ± 0.37 | 63.61 ± 0.53 *** |
| 31.25 | 78.13 ± 0.20 | 57.79 ± 0.62 *** |
| IC50 (µM) | 0.28 | 21.86 |
| Concentration (µg/mL) | 5-LOX % Inhibition (Mean ± S.E.M.) | |
|---|---|---|
| Nor-Dihydro Guaiaretic Acid (NDGA) | 6-HF | |
| 1000 | 97.87 ± 0.26 | 78.37 ± 0.52 *** |
| 500 | 94.37 ± 1.65 | 75.90 ± 1.16 *** |
| 250 | 89.85 ± 0.97 | 71.48 ± 0.54 *** |
| 125 | 85.65 ± 1.47 | 65.56 ± 0.69 *** |
| 62.5 | 83.17 ± 0.72 | 61.83 ± 1.07 *** |
| 31.25 | 71.93 ± 1.13 | 56.38 ± 0.76 *** |
| IC50 (µM) | 0.63 | 36.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akbar, S.; Subhan, F.; Akbar, A.; Habib, F.; Shahbaz, N.; Ahmad, A.; Wadood, A.; Salman, S. Targeting Anti-Inflammatory Pathways to Treat Diabetes-Induced Neuropathy by 6-Hydroxyflavanone. Nutrients 2023, 15, 2552. https://doi.org/10.3390/nu15112552
Akbar S, Subhan F, Akbar A, Habib F, Shahbaz N, Ahmad A, Wadood A, Salman S. Targeting Anti-Inflammatory Pathways to Treat Diabetes-Induced Neuropathy by 6-Hydroxyflavanone. Nutrients. 2023; 15(11):2552. https://doi.org/10.3390/nu15112552
Chicago/Turabian StyleAkbar, Shehla, Fazal Subhan, Aroosha Akbar, Faiza Habib, Naila Shahbaz, Ashfaq Ahmad, Abdul Wadood, and Saad Salman. 2023. "Targeting Anti-Inflammatory Pathways to Treat Diabetes-Induced Neuropathy by 6-Hydroxyflavanone" Nutrients 15, no. 11: 2552. https://doi.org/10.3390/nu15112552
APA StyleAkbar, S., Subhan, F., Akbar, A., Habib, F., Shahbaz, N., Ahmad, A., Wadood, A., & Salman, S. (2023). Targeting Anti-Inflammatory Pathways to Treat Diabetes-Induced Neuropathy by 6-Hydroxyflavanone. Nutrients, 15(11), 2552. https://doi.org/10.3390/nu15112552








