Maternal Dietary Zinc Intake during Pregnancy and Childhood Allergic Diseases up to Four Years: The Japan Environment and Children’s Study
Abstract
:1. Introduction
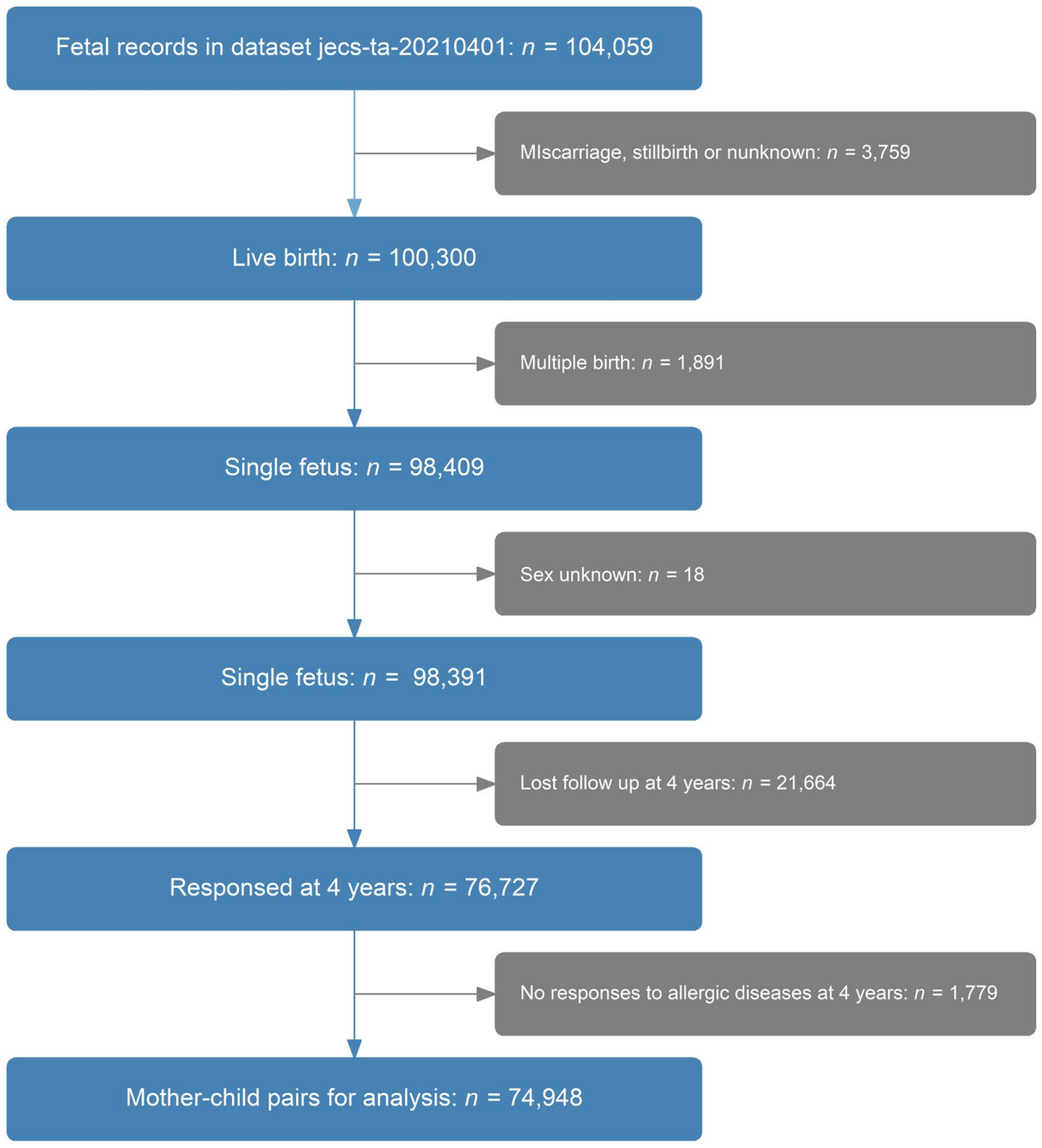
2. Materials and Methods
2.1. Study Design
2.2. Maternal Dietary Zinc Intake
2.3. Allergic Diseases in Children
2.4. Other Variables Used in the Models
2.5. Statistical Methods
3. Results
3.1. Baseline Characteristics of the Participants
3.2. Association between Maternal Dietary Zinc during Pregnancy and Children’s Allergic Diseases
3.3. Sensitivity Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Venter, C.; Agostoni, C.; Arshad, S.H.; Ben-Abdallah, M.; Du Toit, G.; Fleischer, D.M.; Greenhawt, M.; Glueck, D.H.; Groetch, M.; Lunjani, N.; et al. Dietary factors during pregnancy and atopic outcomes in childhood: A systematic review from the European Academy of Allergy and Clinical Immunology. Pediatr. Allergy Immunol. 2020, 31, 889–912. [Google Scholar] [CrossRef] [PubMed]
- Mitre, E.; Susi, A.; Kropp, L.E.; Schwartz, D.J.; Gorman, G.H.; Nylund, C.M. Association Between Use of Acid-Suppressive Medications and Antibiotics During Infancy and Allergic Diseases in Early Childhood. JAMA Pediatr. 2018, 172, e180315. [Google Scholar] [CrossRef]
- Martindale, S.; McNeill, G.; Devereux, G.; Campbell, D.; Russell, G.; Seaton, A. Antioxidant intake in pregnancy in relation to wheeze and eczema in the first two years of life. Am. J. Respir. Crit. Care Med. 2005, 171, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Prasad, A.S. Zinc is an Antioxidant and Anti-Inflammatory Agent: Its Role in Human Health. Front. Nutr. 2014, 1, 14. [Google Scholar] [CrossRef]
- Bonaventura, P.; Benedetti, G.; Albarede, F.; Miossec, P. Zinc and its role in immunity and inflammation. Autoimmun. Rev. 2015, 14, 277–285. [Google Scholar] [CrossRef]
- Litonjua, A.A.; Rifas-Shiman, S.L.; Ly, N.P.; Tantisira, K.G.; Rich-Edwards, J.W.; Camargo, C.A., Jr.; Weiss, S.T.; Gillman, M.W.; Gold, D.R. Maternal antioxidant intake in pregnancy and wheezing illnesses in children at 2 y of age. Am. J. Clin. Nutr. 2006, 84, 903–911. [Google Scholar] [CrossRef] [PubMed]
- Zalewski, P.D.; Truong-Tran, A.Q.; Grosser, D.; Jayaram, L.; Murgia, C.; Ruffin, R.E. Zinc metabolism in airway epithelium and airway inflammation: Basic mechanisms and clinical targets. A review. Pharmacol. Ther. 2005, 105, 127–149. [Google Scholar] [CrossRef]
- Japan Environment and Children’s Study (JECS); Study Protocol (Ver. 1.4). Available online: https://www.env.go.jp/chemi/ceh/en/about/advanced/material/jecs-study_protocol_14_en.pdf (accessed on 21 November 2022).
- Matsumura, K.; Morozumi, R.; Hamazaki, K.; Tsuchida, A.; Inadera, H.; Japan, E.; Children’s Study Group. Effect estimate of time-varying social support and trust on the physical and mental health of mothers at 2.5 years postpartum: The Japan Environment and Children’s Study (JECS). J. Epidemiol. 2021, 33, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Iwai-Shimada, M.; Nakayama, S.F.; Isobe, T.; Michikawa, T.; Yamazaki, S.; Nitta, H.; Takeuchi, A.; Kobayashi, Y.; Tamura, K.; Suda, E.; et al. Questionnaire results on exposure characteristics of pregnant women participating in the Japan Environment and Children Study (JECS). Environ. Health Prev. Med. 2018, 23, 45. [Google Scholar] [CrossRef]
- Suzumori, N.; Ebara, T.; Matsuki, T.; Yamada, Y.; Kato, S.; Omori, T.; Saitoh, S.; Kamijima, M.; Sugiura-Ogasawara, M.; Japan, E.; et al. Effects of long working hours and shift work during pregnancy on obstetric and perinatal outcomes: A large prospective cohort study-Japan Environment and Children’s Study. Birth 2020, 47, 67–79. [Google Scholar] [CrossRef]
- Kawamoto, T.; Nitta, H.; Murata, K.; Toda, E.; Tsukamoto, N.; Hasegawa, M.; Yamagata, Z.; Kayama, F.; Kishi, R.; Ohya, Y.; et al. Rationale and study design of the Japan environment and children’s study (JECS). BMC Public Health 2014, 14, 25. [Google Scholar] [CrossRef] [PubMed]
- Michikawa, T.; Nitta, H.; Nakayama, S.F.; Yamazaki, S.; Isobe, T.; Tamura, K.; Suda, E.; Ono, M.; Yonemoto, J.; Iwai-Shimada, M.; et al. Baseline Profile of Participants in the Japan Environment and Children’s Study (JECS). J. Epidemiol. 2018, 28, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, M.; Takami, M.; Misumi, T.; Kawakami, C.; Miyagi, E.; Ito, S.; Aoki, S.; Japan, E.; Children’s Study Group. Effects of breastfeeding on postpartum weight change in Japanese women: The Japan Environment and Children’s Study (JECS). PLoS ONE 2022, 17, e0268046. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, Y.; Takachi, R.; Ishihara, J.; Ishii, Y.; Sasazuki, S.; Sawada, N.; Shinozawa, Y.; Tanaka, J.; Kato, E.; Kitamura, K.; et al. Validity of Short and Long Self-Administered Food Frequency Questionnaires in Ranking Dietary Intake in Middle-Aged and Elderly Japanese in the Japan Public Health Center-Based Prospective Study for the Next Generation (JPHC-NEXT) Protocol Area. J. Epidemiol. 2016, 26, 420–432. [Google Scholar] [CrossRef]
- Kyozuka, H.; Murata, T.; Fukuda, T.; Yamaguchi, A.; Kanno, A.; Yasuda, S.; Sato, A.; Ogata, Y.; Kuse, M.; Hosoya, M.; et al. Association between pre-pregnancy calcium intake and hypertensive disorders during the first pregnancy: The Japan environment and children’s study. BMC Pregnancy Childbirth 2020, 20, 424. [Google Scholar] [CrossRef]
- Yang, L.; Sato, M.; Saito-Abe, M.; Irahara, M.; Nishizato, M.; Sasaki, H.; Konishi, M.; Ishitsuka, K.; Mezawa, H.; Yamamoto-Hanada, K.; et al. Association of Hemoglobin and Hematocrit Levels during Pregnancy and Maternal Dietary Iron Intake with Allergic Diseases in Children: The Japan Environment and Children’s Study (JECS). Nutrients 2021, 13, 810. [Google Scholar] [CrossRef]
- Minami, M.; Awn J.-P., N.; Noguchi, S.; Eitoku, M.; Muchanga, S.M.J.; Mitsuda, N.; Komori, K.; Yasumitsu-Lovell, K.; Maeda, N.; Fujieda, M.; et al. Gestational weight gain mediates the effects of energy intake on birth weight among singleton pregnancies in the Japan Environment and Children’s Study. BMC Pregnancy Childbirth 2022, 22, 568. [Google Scholar] [CrossRef]
- Science and Technology Agency. Standard Table of Food Composition in Japan. The Fifth Revised Edition Tokyo: Printing Bureau. Ministry of Finance. 2000. Available online: https://www.ishiyaku.co.jp/download/kanei-khp/data/info_pdf/shokuji_kijun_2010.pdf (accessed on 1 April 2023). (In Japanese).
- Willett, W.C. Implications of total energy intake for epidemiologic studies of breast and large-bowel cancer. Am. J. Clin. Nutr. 1987, 45, 354–360. [Google Scholar] [CrossRef]
- Asher, M.I.; Keil, U.; Anderson, H.R.; Beasley, R.; Crane, J.; Martinez, F.; Mitchell, E.A.; Pearce, N.; Sibbald, B.; Stewart, A.W.; et al. International Study of Asthma and Allergies in Childhood (ISAAC): Rationale and methods. Eur. Respir. J. 1995, 8, 483–491. [Google Scholar] [CrossRef]
- Weiland, S.K.; Bjorksten, B.; Brunekreef, B.; Cookson, W.O.; von Mutius, E.; Strachan, D.P.; The International Study of Asthma and Allergies in Childhood Phase II Study Group. Phase II of the International Study of Asthma and Allergies in Childhood (ISAAC II): Rationale and methods. Eur. Respir. J. 2004, 24, 406–412. [Google Scholar] [CrossRef]
- Ellwood, P.; Asher, M.I.; Beasley, R.; Clayton, T.O.; Stewart, A.W.; Committee, I.S. The international study of asthma and allergies in childhood (ISAAC): Phase three rationale and methods. Int. J. Tuberc. Lung Dis. 2005, 9, 10–16. [Google Scholar] [PubMed]
- Yang, L.; Sato, M.; Saito-Abe, M.; Irahara, M.; Nishizato, M.; Sasaki, H.; Konishi, M.; Ishitsuka, K.; Mezawa, H.; Yamamoto-Hanada, K.; et al. Hypertensive disorders of pregnancy and risk of allergic conditions in children: Findings from the Japan Environment and Children’s study (JECS). World Allergy Organ. J. 2021, 14, 100581. [Google Scholar] [CrossRef]
- West, C.E.; Dunstan, J.; McCarthy, S.; Metcalfe, J.; D’Vaz, N.; Meldrum, S.; Oddy, W.H.; Tulic, M.K.; Prescott, S.L. Associations between maternal antioxidant intakes in pregnancy and infant allergic outcomes. Nutrients 2012, 4, 1747–1758. [Google Scholar] [CrossRef] [PubMed]
- Miyake, Y.; Sasaki, S.; Tanaka, K.; Hirota, Y. Consumption of vegetables, fruit, and antioxidants during pregnancy and wheeze and eczema in infants. Allergy 2010, 65, 758–765. [Google Scholar] [CrossRef]
- Shaheen, S.O.; Newson, R.B.; Henderson, A.J.; Emmett, P.M.; Sherriff, A.; Cooke, M.; Team, A.S. Umbilical cord trace elements and minerals and risk of early childhood wheezing and eczema. Eur. Respir. J. 2004, 24, 292–297. [Google Scholar] [CrossRef]
- Beckhaus, A.A.; Garcia-Marcos, L.; Forno, E.; Pacheco-Gonzalez, R.M.; Celedon, J.C.; Castro-Rodriguez, J.A. Maternal nutrition during pregnancy and risk of asthma, wheeze, and atopic diseases during childhood: A systematic review and meta-analysis. Allergy 2015, 70, 1588–1604. [Google Scholar] [CrossRef]
- Zalewski, P.D. Zinc metabolism in the airway: Basic mechanisms and drug targets. Curr. Opin. Pharmacol. 2006, 6, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Vojnik, C.; Hurley, L.S. Abnormal prenatal lung development resulting from maternal zinc deficiency in rats. J. Nutr. 1977, 107, 862–872. [Google Scholar] [CrossRef]
- Yang, L.; Sato, M.; Saito-Abe, M.; Miyaji, Y.; Shimada, M.; Sato, C.; Nishizato, M.; Kumasaka, N.; Mezawa, H.; Yamamoto-Hanada, K.; et al. Allergic Disorders and Risk of Anemia in Japanese Children: Findings from the Japan Environment and Children’s Study. Nutrients 2022, 14, 4335. [Google Scholar] [CrossRef]
- Van Oeffelen, A.A.; Bekkers, M.B.; Smit, H.A.; Kerkhof, M.; Koppelman, G.H.; Haveman-Nies, A.; van der, A.D.; Jansen, E.H.; Wijga, A.H. Serum micronutrient concentrations and childhood asthma: The PIAMA birth cohort study. Pediatr. Allergy Immunol. 2011, 22, 784–793. [Google Scholar] [CrossRef]
- Harding, J.E. The nutritional basis of the fetal origins of adult disease. Int. J. Epidemiol. 2001, 30, 15–23. [Google Scholar] [CrossRef]
- Kuehni, C.E.; Davis, A.; Brooke, A.M.; Silverman, M. Are all wheezing disorders in very young (preschool) children increasing in prevalence? Lancet 2001, 357, 1821–1825. [Google Scholar] [CrossRef] [PubMed]
- Luyt, D.K.; Burton, P.; Brooke, A.M.; Simpson, H. Wheeze in preschool children and its relation with doctor diagnosed asthma. Arch. Dis. Child. 1994, 71, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Martinez, F.D.; Wright, A.L.; Taussig, L.M.; Holberg, C.J.; Halonen, M.; Morgan, W.J. Asthma and wheezing in the first six years of life. The Group Health Medical Associates. N. Engl. J. Med. 1995, 332, 133–138. [Google Scholar] [CrossRef] [PubMed]
| Outcome Events | Question | Answer |
|---|---|---|
| Current wheeze at 4 years | “Has your child ever had wheezing or whistling in the past 12 months?” | Yes |
| Ever wheeze at 4 years | “Has your child ever had wheezing or whistling in the chest at any time in the past?” | Yes |
| Ever asthma at 4 years | “Has your children ever been diagnosed as asthma by a doctor?” | Yes |
| Ever rhinitis at 4 years | “Has your child ever had a problem with sneezing, or a runny, or blocked nose when he/she DID NOT have a cold or the flu?” | Yes |
| Rhinitis at 4 years | “In the past 12 months, has your child had a problem with sneezing, or a runny or blocked nose when he/she did not have a cold or the flu?” | Yes |
| Ever AD at 4 years | “Has your child ever had atopic dermatitis?” | Yes |
| Current AD at 4 years | (1) “Has your child had itchy rash at any time in the past 12 months?” (2) “Has this itchy rash at any time affected any of the following places: the folds of the elbows, behind the knees, in front of the ankles, under the buttocks, or around the neck, ears or eyes?” | Yes |
| Current FA at 4 years | “Has your child ever been diagnosed by a physician as having food allergy in the past 6 months?” | Yes |
| 95% CI | 95% CI | ||||||
|---|---|---|---|---|---|---|---|
| OR # | Lower | Upper | OR & | Lower | Upper | ||
| Ever wheeze | Q1 | 1.001 | 0.952 | 1.053 | 1.001 | 0.952 | 1.053 |
| Q2 | 0.972 | 0.924 | 1.022 | 0.971 | 0.923 | 1.022 | |
| Q3 | 1.000 | 1.000 | |||||
| Q4 | 0.962 | 0.915 | 1.013 | 0.961 | 0.914 | 1.011 | |
| Q5 | 0.994 | 0.945 | 1.045 | 0.991 | 0.942 | 1.043 | |
| Current wheeze | Q1 | 0.967 | 0.906 | 1.032 | 0.967 | 0.906 | 1.032 |
| Q2 | 0.952 | 0.892 | 1.016 | 0.951 | 0.891 | 1.015 | |
| Q3 | 1.000 | 1.000 | |||||
| Q4 | 0.914 | 0.855 | 0.976 | 0.912 | 0.854 | 0.974 | |
| Q5 | 1.001 | 0.939 | 1.069 | 0.999 | 0.936 | 1.066 | |
| Ever asthma | Q1 | 1.021 | 0.951 | 1.095 | 1.021 | 0.951 | 1.095 |
| Q2 | 0.971 | 0.904 | 1.042 | 0.970 | 0.903 | 1.042 | |
| Q3 | 1.000 | 1.000 | |||||
| Q4 | 0.950 | 0.884 | 1.021 | 0.949 | 0.883 | 1.020 | |
| Q5 | 1.057 | 0.985 | 1.134 | 1.055 | 0.983 | 1.132 | |
| Ever AD | Q1 | 1.044 | 0.974 | 1.118 | 1.043 | 0.974 | 1.118 |
| Q2 | 0.989 | 0.923 | 1.060 | 0.989 | 0.923 | 1.060 | |
| Q3 | 1.000 | 1.000 | |||||
| Q4 | 0.931 | 0.868 | 0.998 | 0.931 | 0.868 | 0.999 | |
| Q5 | 1.002 | 0.935 | 1.075 | 1.003 | 0.936 | 1.075 | |
| Current AD | Q1 | 1.013 | 0.948 | 1.082 | 1.013 | 0.948 | 1.082 |
| Q2 | 1.018 | 0.953 | 1.088 | 1.018 | 0.953 | 1.088 | |
| Q3 | 1.000 | 1.000 | |||||
| Q4 | 0.954 | 0.892 | 1.020 | 0.955 | 0.893 | 1.021 | |
| Q5 | 0.961 | 0.899 | 1.028 | 0.962 | 0.900 | 1.029 | |
| Ever rhinitis | Q1 | 1.046 | 0.996 | 1.097 | 1.045 | 0.996 | 1.097 |
| Q2 | 0.972 | 0.927 | 1.020 | 0.972 | 0.927 | 1.020 | |
| Q3 | 1.000 | 1.000 | |||||
| Q4 | 0.979 | 0.933 | 1.027 | 0.979 | 0.933 | 1.028 | |
| Q5 | 1.010 | 0.962 | 1.060 | 1.010 | 0.963 | 1.060 | |
| Current rhinitis | Q1 | 1.035 | 0.985 | 1.087 | 1.035 | 0.985 | 1.087 |
| Q2 | 0.978 | 0.931 | 1.027 | 0.978 | 0.931 | 1.027 | |
| Q3 | 1.000 | 1.000 | |||||
| Q4 | 0.977 | 0.930 | 1.026 | 0.977 | 0.930 | 1.027 | |
| Q5 | 0.994 | 0.946 | 1.044 | 0.994 | 0.946 | 1.044 | |
| Current FA | Q1 | 1.059 | 0.958 | 1.169 | 1.058 | 0.958 | 1.169 |
| Q2 | 1.012 | 0.915 | 1.119 | 1.012 | 0.915 | 1.119 | |
| Q3 | 1.000 | 1.000 | |||||
| Q4 | 0.987 | 0.892 | 1.091 | 0.988 | 0.893 | 1.092 | |
| Q5 | 1.069 | 0.968 | 1.180 | 1.070 | 0.969 | 1.181 | |
| Any allergy | Q1 | 1.024 | 0.977 | 1.072 | 1.024 | 0.977 | 1.072 |
| Q2 | 0.982 | 0.938 | 1.028 | 0.981 | 0.937 | 1.028 | |
| Q3 | 1.000 | 1.000 | |||||
| Q4 | 0.953 | 0.911 | 0.998 | 0.954 | 0.911 | 0.998 | |
| Q5 | 0.973 | 0.929 | 1.019 | 0.973 | 0.929 | 1.019 | |
| 95% CI | 95% CI | ||||||
|---|---|---|---|---|---|---|---|
| OR # | Lower | Upper | OR & | Lower | Upper | ||
| Current wheeze | Q1 | 1.027 | 0.986 | 1.071 | 1.028 | 0.986 | 1.071 |
| Q2 | 0.969 | 0.929 | 1.010 | 0.968 | 0.928 | 1.009 | |
| Q3 | 1.000 | 1.000 | |||||
| Q4 | 0.981 | 0.941 | 1.023 | 0.980 | 0.940 | 1.022 | |
| Q5 | 1.013 | 0.972 | 1.056 | 1.011 | 0.970 | 1.054 | |
| Current AD | Q1 | 1.014 | 0.965 | 1.067 | 1.014 | 0.965 | 1.066 |
| Q2 | 0.991 | 0.942 | 1.042 | 0.991 | 0.942 | 1.042 | |
| Q3 | 1.000 | 1.000 | |||||
| Q4 | 0.921 | 0.875 | 0.969 | 0.921 | 0.876 | 0.969 | |
| Q5 | 0.943 | 0.896 | 0.992 | 0.944 | 0.897 | 0.994 | |
| Current FA | Q1 | 1.080 | 1.007 | 1.158 | 1.080 | 1.007 | 1.158 |
| Q2 | 1.051 | 0.980 | 1.127 | 1.051 | 0.980 | 1.127 | |
| Q3 | 1.000 | 1.000 | |||||
| Q4 | 0.968 | 0.902 | 1.039 | 0.969 | 0.903 | 1.041 | |
| Q5 | 0.971 | 0.904 | 1.042 | 0.972 | 0.906 | 1.043 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, L.; Sato, M.; Saito-Abe, M.; Miyaji, Y.; Shimada, M.; Sato, C.; Nishizato, M.; Kumasaka, N.; Mezawa, H.; Yamamoto-Hanada, K.; et al. Maternal Dietary Zinc Intake during Pregnancy and Childhood Allergic Diseases up to Four Years: The Japan Environment and Children’s Study. Nutrients 2023, 15, 2568. https://doi.org/10.3390/nu15112568
Yang L, Sato M, Saito-Abe M, Miyaji Y, Shimada M, Sato C, Nishizato M, Kumasaka N, Mezawa H, Yamamoto-Hanada K, et al. Maternal Dietary Zinc Intake during Pregnancy and Childhood Allergic Diseases up to Four Years: The Japan Environment and Children’s Study. Nutrients. 2023; 15(11):2568. https://doi.org/10.3390/nu15112568
Chicago/Turabian StyleYang, Limin, Miori Sato, Mayako Saito-Abe, Yumiko Miyaji, Mami Shimada, Chikako Sato, Minaho Nishizato, Natsuhiko Kumasaka, Hidetoshi Mezawa, Kiwako Yamamoto-Hanada, and et al. 2023. "Maternal Dietary Zinc Intake during Pregnancy and Childhood Allergic Diseases up to Four Years: The Japan Environment and Children’s Study" Nutrients 15, no. 11: 2568. https://doi.org/10.3390/nu15112568





