Energy Availability and Nutritional Intake during Different Training Phases of Wheelchair Athletes
Abstract
1. Introduction
2. Materials and Methods
2.1. Setting and Study Population
2.2. Data Collection
2.3. Data Preparation
2.4. Data Analyses
3. Results
3.1. Athlete Characteristics
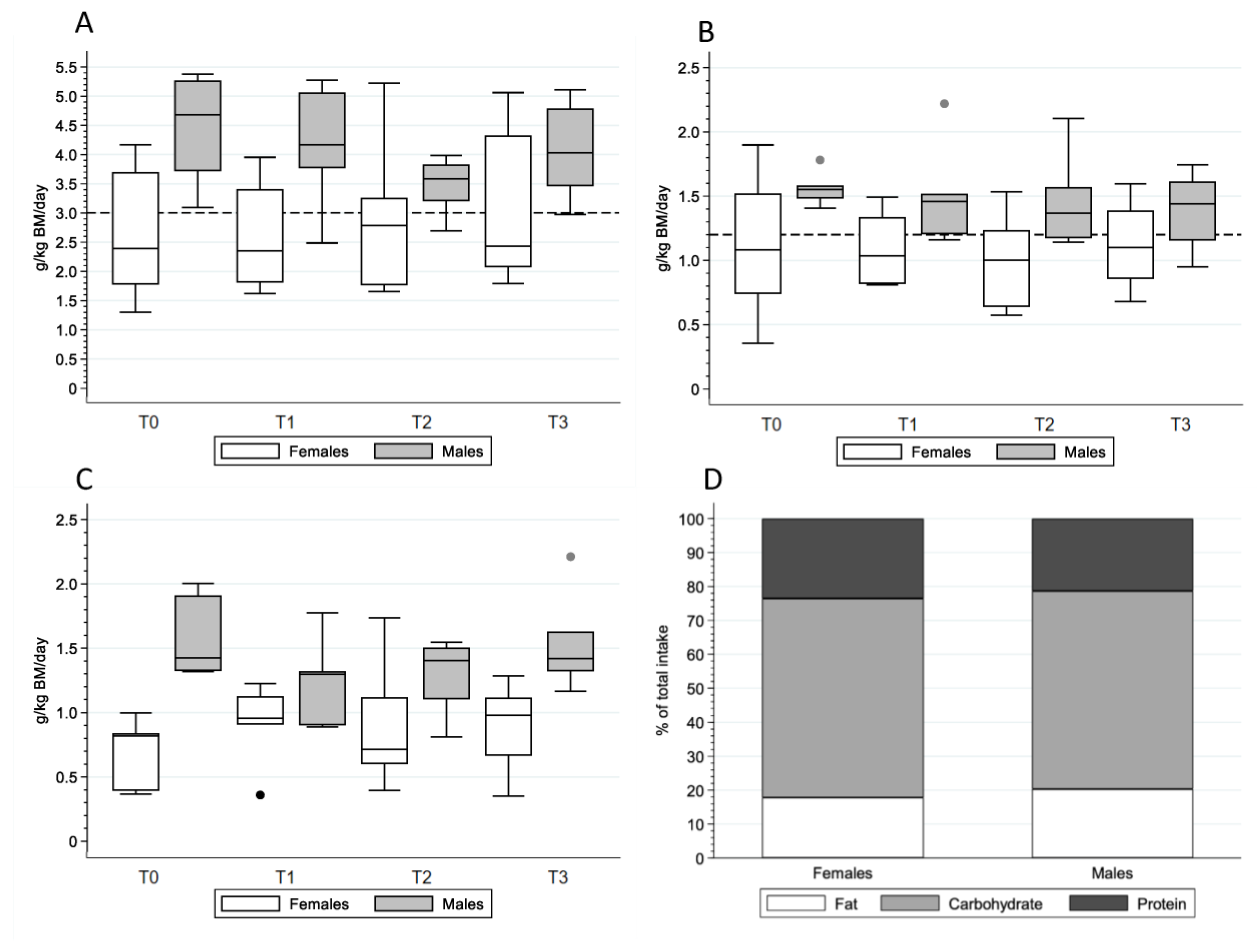
3.2. Energy Expenditure and Intake
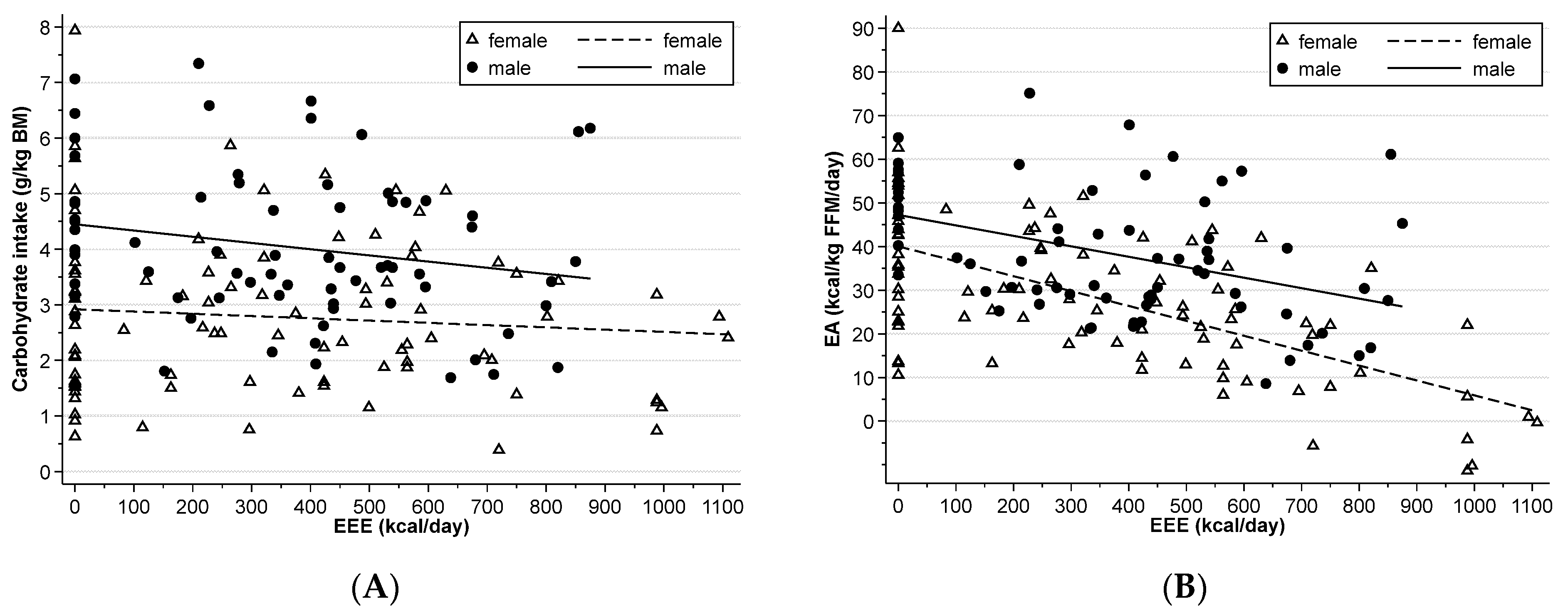
3.3. Energy Availability
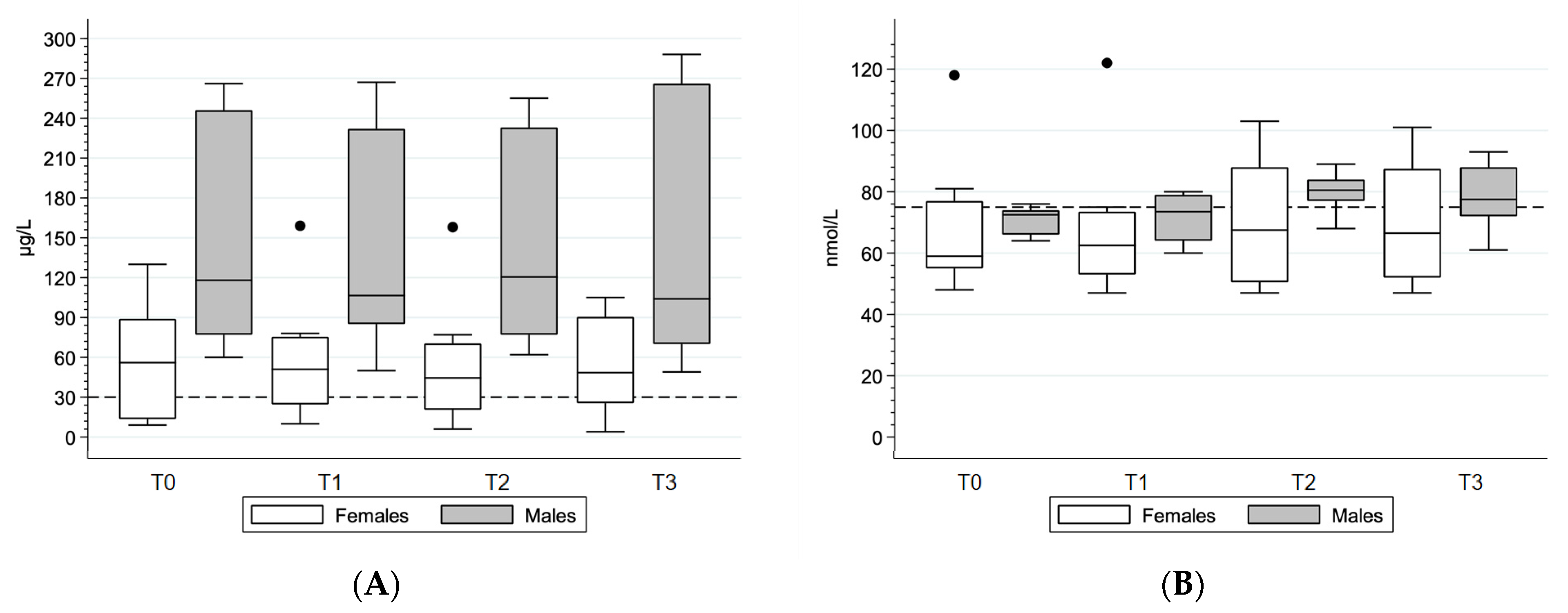
3.4. Blood Biochemical Parameters
4. Discussion
4.1. Energy Availability and Intake across Athletic Seasons
4.2. Macronutrient Intake across Athletic Seasons
4.3. Blood Biochemical Parameters
4.4. Strengths and Limitations
4.5. The Future of Nutrition in Wheelchair Athletes
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thomas, D.T.; Erdman, K.A.; Burke, L.M. American College of Sports Medicine Joint Position Statement. Nutrition and Athletic Performance. Med. Sci. Sport. Exerc. 2016, 48, 543–568. [Google Scholar]
- Mountjoy, M.; Sundgot-Borgen, J.K.; Burke, L.M.; Ackerman, K.E.; Blauwet, C.; Constantini, N.; Lebrun, C.; Lundy, B.; Melin, A.K.; Meyer, N.L.; et al. IOC consensus statement on relative energy deficiency in sport (RED-S): 2018 update. Br. J. Sports Med. 2018, 52, 687–697. [Google Scholar] [CrossRef]
- Logue, D.; Madigan, S.M.; Delahunt, E.; Heinen, M.; McDonnell, S.J.; Corish, C.A. Low Energy Availability in Athletes: A Review of Prevalence, Dietary Patterns, Physiological Health, and Sports Performance. Sports Med. 2018, 48, 73–96. [Google Scholar] [CrossRef] [PubMed]
- Logue, D.M.; Madigan, S.M.; Melin, A.; Delahunt, E.; Heinen, M.; Mc Donnell, S.-J.; Corish, C.A. Low Energy Availability in Athletes 2020: An Updated Narrative Review of Prevalence, Risk, Within-Day Energy Balance, Knowledge, and Impact on Sports Performance. Nutrients 2020, 12, 835. [Google Scholar] [CrossRef] [PubMed]
- Ackerman, K.E.; Holtzman, B.; Cooper, K.M.; Flynn, E.F.; Bruinvels, G.; Tenforde, A.S.; Popp, K.L.; Simpkin, A.J.; Parziale, A.L. Low energy availability surrogates correlate with health and performance consequences of Relative Energy Deficiency in Sport. Br. J. Sports Med. 2019, 53, 628–633. [Google Scholar] [CrossRef] [PubMed]
- Blauwet, C.A.; Brook, E.M.; Tenforde, A.S.; Broad, E.; Hu, C.; Abdu-Glass, E.; Matzkin, E.G. Low Energy Availability, Menstrual Dysfunction, and Low Bone Mineral Density in Individuals with a Disability: Implications for the Para Athlete Population. Sports Med. 2017, 47, 1697–1708. [Google Scholar] [CrossRef]
- Figel, K.; Pritchett, K.; Pritchett, R.; Broad, E. Energy and Nutrient Issues in Athletes with Spinal Cord Injury: Are They at Risk for Low Energy Availability? Nutrients 2018, 10, 1078. [Google Scholar] [CrossRef]
- Pritchett, K.; DiFolco, A.; Glasgow, S.; Pritchett, R.; Williams, K.; Stellingwerff, T.; Roney, P.; Scaroni, S.; Broad, E. Risk of Low Energy Availability in National and International Level Paralympic Athletes: An Exploratory Investigation. Nutrients 2021, 13, 979. [Google Scholar] [CrossRef]
- Egger, T.; Flueck, J.L. Energy Availability in Male and Female Elite Wheelchair Athletes over Seven Consecutive Training Days. Nutrients 2020, 12, 3262. [Google Scholar] [CrossRef]
- Price, M. Energy Expenditure and Metabolism during Exercise in Persons with a Spinal Cord Injury. Sports Med. 2010, 40, 681–696. [Google Scholar] [CrossRef]
- Scaramella, J.; Kirihennedige, N.; Broad, E. Key Nutritional Strategies to Optimize Performance in Para Athletes. Phys. Med. Rehabil. Clin. N. Am. 2018, 29, 283–298. [Google Scholar] [CrossRef] [PubMed]
- Islamoglu, A.H.; Kenger, E.B. Nutrition Considerations for Athletes with Physical Disabilities. Curr. Sports Med. Rep. 2019, 18, 270–274. [Google Scholar] [CrossRef] [PubMed]
- Flueck, J.L. Nutritional Considerations for Para-Cycling Athletes: A Narrative Review. Sports 2021, 9, 154. [Google Scholar] [CrossRef] [PubMed]
- Jonvik, K.L.; Vardardottir, B.; Broad, E. How Do We Assess Energy Availability and RED-S Risk Factors in Para Athletes? Nutrients 2022, 14, 1068. [Google Scholar] [CrossRef] [PubMed]
- Pelly, F.E.; Broad, E.M.; Stuart, N.; Holmes, M.A. Resting energy expenditure in male athletes with a spinal cord injury. J. Spinal Cord Med. 2018, 41, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Perret, C.; Flueck, J. Supplementation and Performance in Spinal Cord Injured Elite Athletes: A Systematic Review. Dtsch. Z. Sportmed. 2016, 67, 209–213. [Google Scholar] [CrossRef]
- Flueck, J.L.; Parnell, J.A. Protein Considerations for Athletes with a Spinal Cord Injury. Front. Nutr. 2021, 8, 652441. [Google Scholar] [CrossRef]
- Ruettimann, B.; Perret, C.; Parnell, J.A.; Flueck, J.L. Carbohydrate Considerations for Athletes with a Spinal Cord Injury. Nutrients 2021, 13, 2177. [Google Scholar] [CrossRef]
- Sim, M.; Garvican-Lewis, L.A.; Cox, G.R.; Govus, A.; McKay, A.K.A.; Stellingwerff, T.; Peeling, P. Iron considerations for the athlete: A narrative review. Eur. J. Appl. Physiol. 2019, 119, 1463–1478. [Google Scholar] [CrossRef]
- Moran, D.S.; McClung, J.P.; Kohen, T.; Lieberman, H.R. Vitamin D and Physical Performance. Sports Med. 2013, 43, 601–611. [Google Scholar] [CrossRef]
- Clénin, G.; Cordes, M.; Huber, A.; Schumacher, Y.O.; Noack, P.; Scales, J.; Kriemler, S. Iron deficiency in sports—Definition, influence on performance and therapy. Swiss Med. Wkly 2015, 145, w14196. [Google Scholar] [CrossRef] [PubMed]
- Dahlquist, D.T.; Dieter, B.P.; Koehle, M.S. Plausible ergogenic effects of vitamin D on athletic performance and recovery. J. Int. Soc. Sports Nutr. 2015, 12, 33. [Google Scholar] [CrossRef] [PubMed]
- Beck, K.L.; von Hurst, P.R.; O’Brien, W.J.; Badenhorst, C.E. Micronutrients and athletic performance: A review. Food Chem. Toxicol. 2021, 158, 112618. [Google Scholar] [CrossRef]
- Heydenreich, J.; Kayser, B.; Schutz, Y.; Melzer, K. Total Energy Expenditure, Energy Intake, and Body Composition in Endurance Athletes across the Training Season: A Systematic Review. Sports Med.-Open 2017, 3, 8. [Google Scholar] [CrossRef] [PubMed]
- Glisic, M.; Flueck, J.L.; Ruettimann, B.; Hertig-Godeschalk, A.; Valido, E.; Bertolo, A.; Stucki, G.; Stoyanov, J. The feasibility of a crossover, randomized controlled trial design to assess the effect of probiotics and prebiotics on health of elite Swiss para-athletes: A study protocol. Pilot Feasibility Stud. 2022, 8, 94. [Google Scholar] [CrossRef] [PubMed]
- Hertig-Godeschalk, A.; Glisic, M.; Ruettimann, B.; Valido, E.; Capossela, S.; Stoyanov, J.; Flueck, J.L. The feasibility of a randomized controlled crossover trial to assess the effect of probiotic and prebiotic supplementation on the health of elite wheelchair athletes. Pilot Feasibility Stud. 2023. under review. [Google Scholar]
- Hottenrott, K.; Neumann, G. Trainingswissenschaft. In Ein Lehrbuch in 14 Lektionen, 3rd ed.; Meyer & Meyer: Aachen, Germany, 2016. [Google Scholar]
- Eypasch, E.; Wood-Dauphinée, S.; Williams, J.I.; Ure, B.; Neugebauer, E.; Troidl, H. The Gastrointestinal Quality of Life Index. A clinical index for measuring patient status in gastroenterologic surgery. Der Chir. 1993, 64, 264–274. [Google Scholar]
- Conger, S.A.; Bassett, D.R. A Compendium of Energy Costs of Physical Activities for Individuals Who use Manual Wheelchairs. Adapt. Phys. Act. Q. 2011, 28, 310–325. [Google Scholar] [CrossRef]
- Loucks, A.B.; Kiens, B.; Wright, H.H. Energy availability in athletes. J. Sports Sci. 2011, 29 (Suppl. S1), S7–S15. [Google Scholar] [CrossRef]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M. Evaluation, Treatment, and Prevention of Vitamin D Deficiency: An Endocrine Society Clinical Practice Guideline. Med. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef]
- Stellingwerff, T. Case Study: Body Composition Periodization in an Olympic-Level Female Middle-Distance Runner over a 9-Year Career. Int. J. Sport Nutr. Exerc. Metab. 2018, 28, 428–433. [Google Scholar] [CrossRef] [PubMed]
- Krempien, J.L.; Barr, S.I. Risk of Nutrient Inadequacies in Elite Canadian Athletes with Spinal Cord Injury. Int. J. Sport Nutr. Exerc. Metab. 2011, 21, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Gerrish, H.R.; Broad, E.; Lacroix, M.; Ogan, D.; Pritchett, R.C.; Pritchett, K. Nutrient Intake of Elite Canadian and American Athletes with Spinal Cord Injury. Int. J. Exerc. Sci. 2017, 10, 1018–1028. [Google Scholar] [PubMed]
- Madden, R.F.; Shearer, J.; Parnell, J.A. Evaluation of Dietary Intakes and Supplement Use in Paralympic Athletes. Nutrients 2017, 9, 1266. [Google Scholar] [CrossRef] [PubMed]
- Casazza, G.A.; Tovar, A.; Richardson, C.; Cortez, A.; Davis, B. Energy Availability, Macronutrient Intake, and Nutritional Supplementation for Improving Exercise Per-formance in Endurance Athletes. Curr. Sports Med. Rep. 2018, 17, 215–223. [Google Scholar] [CrossRef]
- Jesus, F.; Sousa, M.; Nunes, C.L.; Francisco, R.; Rocha, P.; Minderico, C.S.; Sardinha, L.B.; Silva, A.M. Energy Availability over One Athletic Season: An Observational Study among Athletes from Different Sports. Int. J. Sport Nutr. Exerc. Metab. 2022, 32, 479–490. [Google Scholar] [CrossRef]
- Taylor, H.L.; Garabello, G.; Pugh, J.; Morton, J.; Langan-Evans, C.; Louis, J.; Borgersen, R.; Areta, J.L. Patterns of energy availability of free-living athletes display day-to-day variability that is not reflected in la-boratory-based protocols: Insights from elite male road cyclists. J. Sports Sci. 2022, 40, 1849–1856. [Google Scholar] [CrossRef]
- McHaffie, S.J.; Langan-Evans, C.; Morehen, J.C.; Strauss, J.A.; Areta, J.L.; Rosimus, C.; Evans, M.; Elliott-Sale, K.J.; Cronin, C.J.; Morton, J.P. Carbohydrate fear, skinfold targets and body image issues: A qualitative analysis of player and stakeholder perceptions of the nutrition culture within elite female soccer. Sci. Med. Footb. 2022, 6, 675–685. [Google Scholar] [CrossRef]
- McKay, A.K.A.; Pyne, D.B.; Burke, L.M.; Peeling, P. Iron Metabolism: Interactions with Energy and Carbohydrate Availability. Nutrients 2020, 12, 3692. [Google Scholar] [CrossRef]
- Shimizu, Y.; Mutsuzaki, H.; Tachibana, K.; Hotta, K.; Wadano, Y. Investigation of the Female Athlete Triad in Japanese Elite Wheelchair Basketball Players. Medicina 2019, 56, 10. [Google Scholar] [CrossRef]
- Shaw, K.A.; Zello, G.A.; Bandy, B.; Ko, J.; Bertrand, L.; Chilibeck, P.D. Dietary Supplementation for Para-Athletes: A Systematic Review. Nutrients 2021, 13, 2016. [Google Scholar] [CrossRef] [PubMed]
- Lamarche, J.; Mailhot, G. Vitamin D and spinal cord injury: Should we care? Spinal Cord 2016, 54, 1060–1075. [Google Scholar] [CrossRef] [PubMed]
- Flueck, J.L.; Perret, C. Vitamin D deficiency in individuals with a spinal cord injury: A literature review. Spinal Cord 2017, 55, 428–434. [Google Scholar] [CrossRef] [PubMed]
- Pritchett, K.; Pritchett, R.; Ogan, D.; Bishop, P.; Broad, E.; Lacroix, M. 25(OH)D Status of Elite Athletes with Spinal Cord Injury Relative to Lifestyle Factors. Nutrients 2016, 8, 374. [Google Scholar] [CrossRef]
- Flueck, J.L.; Schlaepfer, M.W.; Perret, C. Effect of 12-Week Vitamin D Supplementation on 25[OH]D Status and Performance in Athletes with a Spinal Cord Injury. Nutrients 2016, 8, 586. [Google Scholar] [CrossRef]
- Sasaki, C.A.L.; da Costa, T.H.M. Micronutrient deficiency in the diets of para-athletes participating in a sports scholarship program. Nutrition 2021, 81, 110992. [Google Scholar] [CrossRef]
- Madden, R.F.; Lalonde-Bester, S.; Manocha, R.H.; Martin, J.M.; Flueck, J.L.; Hertig-Godeschalk, A.; Shearer, J.; Parnell, J.A. Sports nutrition knowledge in athletes with a spinal cord injury and coaches of para sports. Appl. Physiol. Nutr. Metab. 2022, 47, 1075–1084. [Google Scholar] [CrossRef]
- Keil, M.; De Zepetnek, J.O.T.; Brooke-Wavell, K.; Goosey-Tolfrey, V.L. Measurement precision of body composition variables in elite wheelchair athletes, using dual-energy X-ray absorptiometry. Eur. J. Sport Sci. 2016, 16, 65–71. [Google Scholar] [CrossRef]
- van der Scheer, J.W.; de Zepetnek, J.O.T.; Blauwet, C.; Brooke-Wavell, K.; Graham-Paulson, T. Assessment of body composition in spinal cord injury: A scoping review. PLoS ONE 2021, 16, e0251142. [Google Scholar] [CrossRef]
- Burke, L.M.; Lundy, B.; Fahrenholtz, I.L.; Melin, A.K. Pitfalls of Conducting and Interpreting Estimates of Energy Availability in Free-Living Athletes. Int. J. Sport Nutr. Exerc. Metab. 2018, 28, 350–363. [Google Scholar] [CrossRef]
- Melin, A.K.; Heikura, I.A.; Tenforde, A.; Mountjoy, M. Energy Availability in Athletics: Health, Performance, and Physique. Int. J. Sport Nutr. Exerc. Metab. 2019, 29, 152–164. [Google Scholar] [CrossRef] [PubMed]
- Magkos, F.; Yannakoulia, M. Methodology of dietary assessment in athletes: Concepts and pitfalls. Curr. Opin. Clin. Nutr. Metab. Care 2003, 6, 539–549. [Google Scholar] [CrossRef]
- Capling, L.; Beck, K.L.; Gifford, J.A.; Slater, G.; Flood, V.M.; O’Connor, H. Validity of Dietary Assessment in Athletes: A Systematic Review. Nutrients 2017, 9, 1313. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, H.; Deguchi, M.; Hongu, N. The Role of Diets and Dietitians for Para-Athletes: A Pilot Study Based on Interviews. Nutrients 2022, 14, 3720. [Google Scholar] [CrossRef] [PubMed]
- Brook, E.M.; Tenforde, A.S.; Broad, E.M.; Matzkin, E.G.; Yang, H.Y.; Collins, J.E.; Blauwet, C.A. Low energy availability, menstrual dysfunction, and impaired bone health: A survey of elite para athletes. Scand. J. Med. Sci. Sports 2019, 29, 678–685. [Google Scholar] [CrossRef]

| Overall | Females (n = 8) | Males (n = 6) | |
|---|---|---|---|
| Age (years) | 34 (9) | 32 (11) | 36 (8) |
| Height (cm) | 165 (13) | 159 (14) | 172 (7) |
| Body mass (kg) | 58 (10) | 59 (12) | 58 (7) |
| BMI (kg/m²) | 22 (4) | 23 (5) | 20 (2) |
| Fat mass (kg) | 18 (8) | 22 (8) | 13 (4) |
| FFM (kg) | 40 (8) | 36 (5) | 46 (7) |
| Diagnosis (n(%)) | |||
| Traumatic SCI | 6 (43) | 2 (25) | 4 (67) |
| Meningomyelocele | 5 (36) | 3 (38) | 2 (33) |
| Multiple sclerosis | 2 (14) | 2 (25) | 0 (0) |
| Arthrogryposis | 1 (7) | 1 (13) | 0 (0) |
| Time since injury (years) | 19 (4) | 18 (6) | 20 (4) |
| NLI (n(%)) | |||
| Tetraplegia | 4 (36) | 2 (40) | 2 (33) |
| Paraplegia | 7 (64) | 3 (60) | 4 (67) |
| AIS (n(%)) | |||
| A | 5 (56) | 2 (50) | 3 (60) |
| B–C | 3 (33) | 1 (25) | 2 (40) |
| D | 1 (11) | 1 (25) | 0 |
| Mean training duration (hours/week) | 14 (5) | 14 (5) | 14 (6) |
| All (n = 14) | Females (n = 8) | Males (n = 6) | |
|---|---|---|---|
| Daily energy intake | |||
| Total energy intake (kcal) | 1674 (481) | 1343 (257) | 2116 (315) |
| Daily Carbohydrate Intake | |||
| Total intake (g) | 190 (56) | 156 (41) | 235 (38) |
| Relative intake (g/kg BM) | 3.3 (1.0) | 2.7 (0.9) | 4.0 (0.7) |
| Recommended intake 3–12 g/kg BM (% of days) | 57 (36) | 39 (34) | 80 (22) |
| Daily Protein Intake | |||
| Total intake (g) | 73 (19) | 62 (13) | 87 (17) |
| Relative intake (g/kg BM) | 1.3 (0.3) | 1.1 (0.3) | 1.5 (0.3) |
| Recommended intake >1.2 g/kg BM (% of days) | 52 (30) | 36 (24) | 74 (24) |
| Daily Fat Intake | |||
| Total intake (g) | 63 (23) | 47 (14) | 83 (15) |
| Relative intake (g/kg BM) | 1.1 (0.4) | 0.8 (0.3) | 1.4 (0.2) |
| Recommended intake 20–35% of total energy (% of days) | 42 (23) | 40 (28) | 45 (15) |
| Sport | Mean (SD) EA over Three Days | Mean (SD) EA All Time Points | Number of Days LEA | ||||
|---|---|---|---|---|---|---|---|
| T0 | T1 | T2 | T3 | ||||
| F01 | Cycling | 29.6 (11.8) | 26.8 (7.7) | 50.2 (7.6) | 43.3 (7.0) | 37.5 (11.1) | 3/12 |
| F02 | Tennis | 24.7 (2.1) | 30.1 (9.7) | 38.1 (5.5) | 30.8 (12.6) | 30.9 (5.5) | 6/12 |
| F03 | Badminton | 15.5 (5.7) | . | 9.4 (4.4) | 10.2 (10.7) | 11.7 (3.3) | 9/9 |
| F04 | Athletics | 17.7 (4.7) | 18.3 (9.4) | . | 24.9 (18.7) | 20.3 (4.0) | 8/9 |
| F05 | Shooting | 17.2 (6.8) | 41.2 (15.2) | 25.6 (5.7) | 42.0 (17.6) | 31.5 (12.2) | 7/12 |
| F06 | Tennis | 38.8 (4.2) | 33.4 (13.7) | 35.3 (6.7) | 42.4 (11.5) | 37.5 (4.0) | 3/12 |
| F07 | Tennis | 25.8 (10.7) | 14.3 (22.2) | 4.3 (5.8) | 52.6 (9.5) | 24.3 (20.8) | 6/12 |
| F08 | Basketball | 39.9 (44.3) | 21.2 (25.0) | 13.4 (21.4) | 22.2 (32.1) | 24.2 (11.2) | 9/12 |
| Total female athletes | 26.2 (9.5) | 26.5 (9.3) | 25.2 (16.9) | 33.6 (14.0) | 27.2 (8.9) | 58 (29)% | |
| M01 | Cycling | 48.1 (10.7) | 45.1 (12.1) | 25.8 (5.0) | 30.8 (5.2) | 37.5 (10.8) | 4/12 |
| M02 | Athletics | 37.3 (14.7) | 30.3 (24.9) | 30.2 (15.2) | 40.5 (12.8) | 34.6 (5.2) | 5/12 |
| M03 | Athletics | 36.0 (16.6) | 19.1 (10.0) | 25.8 (2.9) | 28.4 (6.4) | 27.3 (7.0) | 9/12 |
| M04 | Cycling | 59.1 (15.1) | 50.1 (10.2) | 48.4 (17.6) | 42.8 (19.3) | 50.1 (6.7) | 2/11 |
| M05 | Cycling | 50.2 (15.5) | 38.3 (7.2) | 42.6 (13.0) | 54.5 (2.2) | 46.4 (7.3) | 1/12 |
| M06 | Cycling | 35.8 (4.6) | 30.9 (14.9) | 30.6 (6.2) | 47.7 (16.5) | 36.3 (8.0) | 3/12 |
| Total male athletes | 44.4 (9.6) | 35.6 (11.2) | 33.9 (9.4) | 40.8 (10.0) | 38.7 (8.3) | 34 (23)% | |
| All athletes | 34.0 (13.1) | 30.7 (10.9) | 29.2 (14.2) | 36.7 (12.5) | 32.1 (10.2) | 48 (28)% | |
| T0 | T1 | T2 | T3 | |
|---|---|---|---|---|
| Hemoglobin (g/L) | 138 (11) | 135 (14) | 137 (12) | 138 (15) |
| Anemia (n (%)) | ||||
| Females (hemoglobin < 120 g/dL) | 1 (13) | 1 (13) | 2 (25) | 1 (13) |
| Males (hemoglobin < 140 g/dL) | 2 (33) | 3 (50) | 2 (33) | 1 (17) |
| Iron deficiency (<30 μg/L) (n (%)) | ||||
| Females | 3 (38) | 2 (25) | 3 (38) | 2 (25) |
| Males | 0 | 0 | 0 | 0 |
| Iron deficiency with anemia (n (%)) | ||||
| Females | 1 (13) | 1 (13) | 2 (25) | 1 (13) |
| Males | 0 | 0 | 0 | 0 |
| Insufficient vitamin D (<75 nmol/L) (n (%)) | ||||
| Females | 6 (75) | 6 (75) | 5 (63) | 4 (50) |
| Males | 5 (83) | 3 (50) | 1 (17) | 3 (50) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hertig-Godeschalk, A.; Ruettimann, B.; Valido, E.; Glisic, M.; Stoyanov, J.; Flueck, J.L. Energy Availability and Nutritional Intake during Different Training Phases of Wheelchair Athletes. Nutrients 2023, 15, 2578. https://doi.org/10.3390/nu15112578
Hertig-Godeschalk A, Ruettimann B, Valido E, Glisic M, Stoyanov J, Flueck JL. Energy Availability and Nutritional Intake during Different Training Phases of Wheelchair Athletes. Nutrients. 2023; 15(11):2578. https://doi.org/10.3390/nu15112578
Chicago/Turabian StyleHertig-Godeschalk, Anneke, Belinda Ruettimann, Ezra Valido, Marija Glisic, Jivko Stoyanov, and Joelle L. Flueck. 2023. "Energy Availability and Nutritional Intake during Different Training Phases of Wheelchair Athletes" Nutrients 15, no. 11: 2578. https://doi.org/10.3390/nu15112578
APA StyleHertig-Godeschalk, A., Ruettimann, B., Valido, E., Glisic, M., Stoyanov, J., & Flueck, J. L. (2023). Energy Availability and Nutritional Intake during Different Training Phases of Wheelchair Athletes. Nutrients, 15(11), 2578. https://doi.org/10.3390/nu15112578







