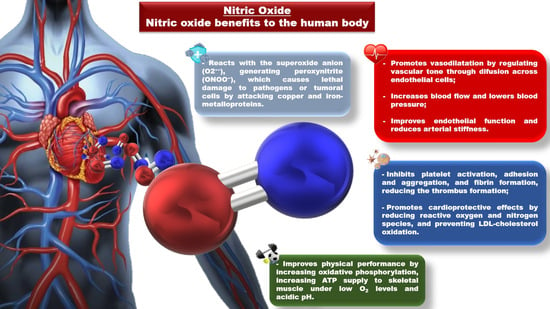
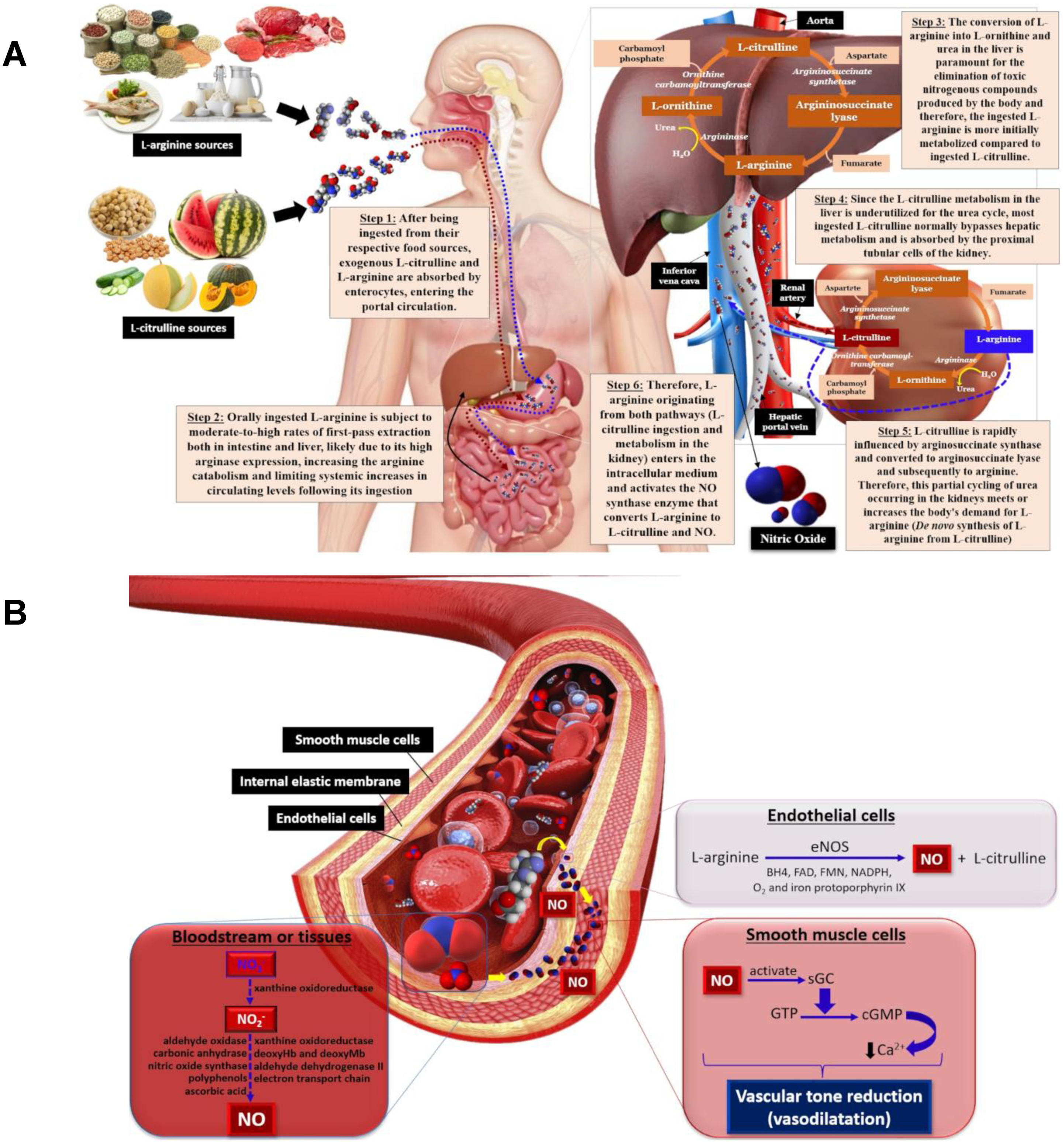
A Critical Review on Vasoactive Nutrients for the Management of Endothelial Dysfunction and Arterial Stiffness in Individuals under Cardiovascular Risk
Abstract
:1. Introduction
2. Nitrate
3. L-Arginine
4. L-Citrulline
5. Potassium
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Rahimi, N. Defenders and Challengers of Endothelial Barrier Function. Front. Immunol. 2017, 8, 1847. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, Y.; Liu, F.; Zhang, Y.; Lu, Y.; Su, Z.; Ji, H.; Cheng, Y.; Song, W.; Hidru, T.H.; Yang, X.; et al. The Relationship of Endothelial Function and Arterial Stiffness with Subclinical Target Organ Damage in Essential Hypertension. J. Clin. Hypertens. 2022, 24, 418–429. [Google Scholar] [CrossRef] [PubMed]
- dos Santos Baião, D.; Vieira Teixeira da Silva, D.; Margaret Flosi Paschoalin, V. A Narrative Review on Dietary Strategies to Provide Nitric Oxide as a Non-Drug Cardiovascular Disease Therapy: Beetroot Formulations—A Smart Nutritional Intervention. Foods 2021, 10, 859. [Google Scholar] [CrossRef] [PubMed]
- Lai, W.K.; Kan, M.Y. Homocysteine-Induced Endothelial Dysfunction. Ann. Nutr. Metab. 2015, 67, 1–12. [Google Scholar] [CrossRef]
- Ghosh, A.; Gao, L.; Thakur, A.; Siu, P.M.; Lai, C.W.K. Role of Free Fatty Acids in Endothelial Dysfunction. J. Biomed. 2017, 24, 50. [Google Scholar] [CrossRef] [Green Version]
- Ohsugi, M.; Adachi, K.; Horai, R.; Kakuta, S.; Sudo, K.; Kotaki, H.; Tokai-Nishizumi, N.; Sagara, H.; Iwakura, Y.; Yamamoto, T. Kid-mediated Chromosome Compaction Ensures Proper Nuclear Envelope Formation. Cell 2008, 132, 771–782. [Google Scholar] [CrossRef] [Green Version]
- Blumenthal, J.A.; Hinderliter, A.L.; Smith, P.J.; Mabe, S.; Watkins, L.L.; Craighead, L.; Ingle, K.; Tyson, C.; Lin, P.H.; Kraus, W.E.; et al. Effects of Lifestyle Modification on Patients with Resistant Hypertension: Results of the TRIUMPH Randomized Clinical Trial. Circulation 2021, 144, 1212–1226. [Google Scholar] [CrossRef]
- Ozkor, M.A.; Hayek, S.S.; Rahman, A.M.; Murrow, J.R.; Kavtaradze, N.; Lin, J.; Manatunga, A.; Quyyumi, A.A. Contribution of Endothelium-Derived Hyperpolarizing Factor to Exercise-induced Vasodilation in Health and Hypercholesterolemia. Vasc. Med. 2015, 20, 14–22. [Google Scholar] [CrossRef] [Green Version]
- Seals, D.R.; Jablonski, K.L.; Donato, A.J. Aging and Vascular Endothelial Function in Humans. Clin. Sci. 2011, 120, 357–375. [Google Scholar] [CrossRef] [Green Version]
- Virdis, A. Endothelial Dysfunction in Obesity: Role of Inflammation. High Blood Press. Cardiovasc. Prev. 2016, 23, 83–85. [Google Scholar] [CrossRef]
- Messner, B.; Bernhard, D. Smoking and Cardiovascular Disease: Mechanisms of Endothelial Dysfunction and Early Atherogenesis. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 509–515. [Google Scholar] [CrossRef] [Green Version]
- Pober, J.S.; Min, W.; Bradley, J.R. Mechanisms of Endothelial Dysfunction, Injury, and Death. Annu. Rev. Pathol. 2009, 4, 71–95. [Google Scholar] [CrossRef]
- Daiber, A.; Steven, S.; Weber, A.; Shuvaev, V.V.; Muzykantov, V.R.; Laher, I.; Li, H.; Lamas, S.; Münzel, T. Targeting vascular (endothelial) dysfunction. Br. J. Pharmacol. 2017, 174, 1591–1619. [Google Scholar] [CrossRef]
- Oliver, J.J.; Webb, D.J. Noninvasive Assessment of Arterial Stiffness and Risk of Atherosclerotic Events. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 554–566. [Google Scholar] [CrossRef] [Green Version]
- Cavalcante, J.L.; Lima, J.A.; Redheuil, A.; Al-Mallah, M.H. Aortic Stiffness: Current Understanding and Future Directions. J. Am. Coll. Cardiol. 2011, 57, 1511–1522. [Google Scholar] [CrossRef] [Green Version]
- Zieman, S.J.; Melenovsky, V.; Kass, D.A. Mechanisms, Pathophysiology, and Therapy of Arterial Stiffness. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 932–943. [Google Scholar] [CrossRef] [Green Version]
- Kohn, J.C.; Lampi, M.C.; Reinhart-King, A. Age-Related Vascular Stiffening: Causes and Consequences. Front. Genet. 2015, 6, 112. [Google Scholar] [CrossRef] [Green Version]
- Schram, M.T.; Schalkwijk, C.G.; Bootsma, H.A.; Fuller, J.H.; Chaturvedi, N.; Stehouwer, C.D.A. Advanced Glycation end Products are Associated with Pulse Pressure in Type 1 Diabetes. Hypertension 2005, 46, 232–237. [Google Scholar] [CrossRef] [Green Version]
- Laurent, S.; Boutouyrie, P. The Structural Factor of Hypertension: Large and Small Artery Alterations. Circ. Res. 2015, 116, 1007–1021. [Google Scholar] [CrossRef]
- Zhang, Y.; Murugesan, P.; Huang, K.; Cai, H. NADPH Oxidases and Oxidase Crosstalk in Cardiovascular Diseases: Novel Therapeutic Targets. Nat. Rev. Cardiol. 2020, 17, 170–194. [Google Scholar] [CrossRef]
- Lacolley, P.; Regnault, V.; Laurent, S. Mechanisms of Arterial Stiffening: From Mechanotransduction to Epigenetics. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 1055–1062. [Google Scholar] [CrossRef] [PubMed]
- Segers, P.; Rietzschel, E.R.; Chirinos, J.A. How to Measure Arterial Stiffness in Humans. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 1034–1043. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tršan, J.; Košuta, D.; Rajkovič, U.; Fras, Z.; Jug, B.; Novaković, M. Vascular Function in Patients after Myocardial Infarction: The Importance of Physical Activity. Front. Physiol. 2021, 12, 763043. [Google Scholar] [CrossRef] [PubMed]
- Maruhashi, T.; Soga, J.; Fujimura, N.; Idei, N.; Mikami, S.; Iwamoto, Y.; Iwamoto, A.; Kajikawa, M.; Matsumoto, T.; Oda, N.; et al. Endothelial Dysfunction, Increased Arterial Stiffness, and Cardiovascular Risk Prediction in Patients with Coronary Artery Disease: FMD-J (Flow-Mediated Dilation Japan) Study A. J. Am. Heart Assoc. 2018, 7, e008588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chia, P.Y.; Teo, A.; Yeo, T.W. Overview of the Assessment of Endothelial Function in Humans. Front. Med. 2020, 7, 542567. [Google Scholar] [CrossRef] [PubMed]
- Yubero-Serrano, E.M.; Fernandez-Gandara, C.; Garcia-Rios, A.; Rangel-Zuñiga, O.A.; Gutierrez-Mariscal, F.M.; Torres-Peña, J.D.; Marin, C.; Lopez-Moreno, J.; Castaño, J.P.; Delgado-Lista, J.; et al. Mediterranean Diet and Endothelial Function in Patients with Coronary Heart Disease: An Analysis of the CORDIOPREV Randomized Controlled Trial. PLoS Med. 2020, 17, e1003282. [Google Scholar] [CrossRef]
- Baião, D.D.S.; de Freitas, C.S.; Gomes, L.P.; da Silva, D.; Correa, A.C.N.T.F.; Pereira, P.R.; Aguila, E.M.D.; Paschoalin, V.M.F. Polyphenols from Root, Tubercles and Grains Cropped in Brazil: Chemical and Nutritional Characterization and Their Effects on Human Health and Diseases. Nutrients 2017, 9, 1044. [Google Scholar] [CrossRef] [Green Version]
- Yu, E.; Malik, V.S.; Hu, F.B. Cardiovascular Disease Prevention by Diet Modification: JACC Health Promotion Series. J. Am. Coll. Cardiol. 2018, 72, 914–926. [Google Scholar] [CrossRef]
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990-2019: Update from the GBD 2019 Study. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef]
- Carlström, M.; Larsen, F.J.; Nyström, T.; Hezel, M.; Borniquel, S.; Weitzberg, E.; Lundberg, J.O. Dietary Inorganic Nitrate Reverses Features of Metabolic Syndrome in Endothelial Nitric Oxide Synthase-Deficient Mice. Proc. Natl. Acad. Sci. USA 2010, 107, 17716–17720. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.X.; Tong, L.; Zhang, G.M.; Zhao, X.H.; Sa, Y.P.; Liu, Y.; Lu, D.X.; Ga, Q.; Wu, P. L-Arginine Supplementation Improves Vascular Endothelial Dysfunction Induced by High-Fat Diet in Rats Exposed to Hypoxia. Wilderness Environ. Med. 2020, 31, 400–406. [Google Scholar] [CrossRef]
- Tsuboi, T.; Maeda, M.; Hayashi, T. Administration of L-Arginine Plus L-Citrulline or L-Citrulline Alone Successfully Retarded Endothelial Senescence. PLoS ONE 2018, 13, e0192252. [Google Scholar] [CrossRef] [Green Version]
- Zhou, M.S.; Kosaka, H.; Yoneyama, H. Potassium Augments Vascular Relaxation Mediated by Nitric Oxide in The Carotid Arteries of Hypertensive Dahl Rats. Am. J. Hypertens. 2000, 13, 666–672. [Google Scholar] [CrossRef] [Green Version]
- Bode-Böger, S.M.; Muke, J.; Surdacki, A.; Brabant, G.; Böger, R.H.; Frölich, J.C. Oral L-Arginine Improves Endothelial Function in Healthy Individuals Older than 70 Years. Vasc. Med. 2003, 8, 77–81. [Google Scholar] [CrossRef] [Green Version]
- Allerton, T.D.; Proctor, D.N.; Stephens, J.M.; Dugas, T.R.; Spielmann, G.; Irving, B.A. L-Citrulline Supplementation: Impact on Cardiometabolic Health. Nutrients 2018, 10, 921. [Google Scholar] [CrossRef] [Green Version]
- Raubenheimer, K.; Hickey, D.; Leveritt, M.; Fassett, R.; Ortiz de Zevallos Munoz, J.; Allen, J.D.; Briskey, D.; Parker, T.J.; Kerr, G.; Peake, J.M.; et al. Acute Effects of Nitrate-Rich Beetroot Juice on Blood Pressure, Hemostasis and Vascular Inflammation Markers in Healthy Older Adults: A Randomized, Placebo-Controlled Crossover Study. Nutrients 2017, 22, 1270. [Google Scholar] [CrossRef] [Green Version]
- Blanch, N.; Clifton, P.M.; Petersen, K.S.; Keogh, J.B. Effect of Sodium and Potassium Supplementation on Vascular and Endothelial Function: A Randomized Controlled Trial. Am. J. Clin. Nutr. 2015, 101, 939–946. [Google Scholar] [CrossRef] [Green Version]
- Lundberg, J.O.; Weitzberg, E.; Gladwin, M.T. The Nitrate-Nitrite-Nitric Oxide Pathway in Physiology and Therapeutics. Nat. Rev. Drug Discov. 2008, 7, 156–167. [Google Scholar] [CrossRef]
- Nyawose, S.; Naidoo, R.; Naumovski, N.; McKune, A.J. The Effects of Consuming Amino Acids L-Arginine, L-Citrulline (and Their Combination) as a Beverage or Powder, on Athletic and Physical Performance: A Systematic Review. Beverages 2022, 8, 48. [Google Scholar] [CrossRef]
- Nomura, N.; Shoda, W.; Uchida, S. Clinical Importance of Potassium Intake and Molecular Mechanism of Potassium Regulation. Clin. Exp. Nephrol. 2019, 23, 1175–1180. [Google Scholar] [CrossRef] [Green Version]
- Ekmekcioglu, C.; Elmadfa, I.; Meyer, A.L.; Moeslinger, T. The Role of Dietary Potassium in Hypertension and Diabetes. J. Physiol. Biochem. 2016, 72, 93–106. [Google Scholar] [CrossRef] [PubMed]
- Omar, S.A.; Artime, E.; Webb, A.J. A Comparison of Organic and Inorganic Nitrates/Nitrites. Nitric Oxide 2012, 26, 229–240. [Google Scholar] [CrossRef] [PubMed]
- Alzahrani, H.S.; Jackson, K.G.; Hobbs, D.A.; Lovegrove, J.A. The Role of Dietary Nitrate and the Oral Microbiome on Blood Pressure and Vascular Tone. Nutr. Res. Rev. 2020, 34, 222–239. [Google Scholar] [CrossRef] [PubMed]
- Reina-Torres, E.; De Leso, M.L.; Pasquale, L.R.; Madekurozwa, M.; van Batenburg-Sherwood, J.; Overby, D.R.; Daniel Stamer, W. The Vital Role for Nitric Oxide in Intraocular Pressure Homeostasis. Prog. Retin. Eye Res. 2021, 83, 100922. [Google Scholar] [CrossRef] [PubMed]
- Gilchrist, M.; Winyard, P.G.; Benjamin, N. Dietary Nitrate—Good or Bad? Nitric Oxide 2010, 22, 104–109. [Google Scholar] [CrossRef]
- Baião, D.S.; da Silva, D.V.T.; Del Aguila, E.M.; Paschoalin, V.M.F. Nutritional, Bioactive and Physicochemical Characteristics of Different Beetroot Formulations. In Food Additives; Intech Open: London, UK, 2017; Volume 2, pp. 20–44. [Google Scholar] [CrossRef] [Green Version]
- González-Soltero, R.; Bailén, M.; de Lucas, B.; Ramírez-Goercke, M.I.; Pareja-Galeano, H.; Larrosa, M. Role of Oral and Gut Microbiota in Dietary Nitrate Metabolism and Its Impact on Sports Performance. Nutrients 2020, 12, 3611. [Google Scholar] [CrossRef]
- Lidder, S.; Webb, A.J. Vascular Effects of Dietary Nitrate (as Found in Green Leafy Vegetables and Beetroot) via the Nitrate-Nitrite-Nitric Oxide Pathway. Br. J. Clin. Pharmacol. 2013, 75, 677–696. [Google Scholar] [CrossRef] [Green Version]
- Blekkenhorst, L.C.; Bondonno, N.P.; Liu, A.H.; Ward, N.C.; Prince, R.L.; Lewis, J.R.; Devine, A.; Croft, K.D.; Hodgson, J.M.; Bondonno, C.P. Nitrate, the Oral Microbiome, and Cardiovascular Health: A Systematic Literature Review of Human and Animal Studies. Am. J. Clin. Nutr. 2018, 107, 504–522. [Google Scholar] [CrossRef] [Green Version]
- Burleigh, M.; Liddle, L.; Muggeridge, D.J.; Monaghan, C.; Sculthorpe, N.; Butcher, J.; Henriquez, F.; Easton, C. Dietary Nitrate Supplementation Alters the Oral Microbiome but Does Not Improve the Vascular Responses to an Acute Nitrate Dose. Nitric Oxide 2019, 89, 54–63. [Google Scholar] [CrossRef]
- Karwowska, M.; Kononiuk, A. Nitrates/Nitrites in Food-Risk for Nitrosative Stress and Benefits. Antioxidants 2020, 9, 241. [Google Scholar] [CrossRef] [Green Version]
- McDonagh, S.T.J.; Wylie, L.J.; Webster, J.M.A.; Vanhatalo, A.; Jones, A.M. Influence of Dietary Nitrate Food Forms on Nitrate Metabolism and Blood Pressure in Healthy Normotensive Adults. Nitric Oxide 2018, 72, 66–74. [Google Scholar] [CrossRef]
- Weitzberg, E.; Lundberg, J.O. Novel Aspects of Dietary Nitrate and Human Health. Annu. Rev. Nutr. 2013, 33, 129–159. [Google Scholar] [CrossRef]
- Lundberg, J.O.; Gladwin, M.T.; Weitzberg, E. Strategies to Increase Nitric Oxide Signalling in Cardiovascular Disease. Nat. Rev. Drug Discov. 2015, 14, 623–641. [Google Scholar] [CrossRef]
- Woessner, M.N.; Levinger, I.; Neil, C.; Smith, C.; Allen, J.D. Effects of Dietary Inorganic Nitrate Supplementation on Exercise Performance in Patients with Heart Failure: Protocol for a Randomized, Placebo-Controlled, Cross-Over Trial. JMIR Res. Protoc. 2018, 7, e86. [Google Scholar] [CrossRef]
- Koch, C.D.; Gladwin, M.T.; Freeman, B.A.; Lundberg, J.O.; Weitzberg, E.; Morris, A. Enterosalivary nitrate metabolism and the microbiome: Intersection of microbial metabolism, nitric oxide and diet in cardiac and pulmonary vascular health. Free Radic Biol Med. 2017, 105, 48–67. [Google Scholar] [CrossRef] [Green Version]
- Tamme, T.; Reinik, M.; Püssa, T.; Roasto, M.; Meremäe, K.; Kiis, A. Dynamics of Nitrate and Nitrite Content During Storage of Home-Made and Small-Scale Industrially Produced Raw Vegetable Juices and Their Dietary Intake. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2010, 27, 487–495. [Google Scholar] [CrossRef] [Green Version]
- Baião, D.S.; da Silva, D.V.T.; Paschoalin, V.M.F. Beetroot, a Remarkable Vegetable: Its Nitrate and Phytochemical Contents Can Be Adjusted in Novel Formulations to Benefit Health and Support Cardiovascular Disease Therapies. Antioxidants 2020, 9, 960. [Google Scholar] [CrossRef]
- Spiegelhalder, B.; Eisenbrand, G.; Preussman, R. Influence of Dietary Nitrate on Nitrite Content of Human Saliva: Possible Relevance to In-Vivo Formation of N-Nitroso Compounds. Food Cosmet. Toxicol. 1976, 14, 545–548. [Google Scholar] [CrossRef]
- Tannenbaum, S.R.; Weisman, M.; Fett, D. The Effect of Nitrate Intake on Nitrite Formation in Human Saliva. Food Cosmet. Toxicol. 1976, 14, 549–552. [Google Scholar] [CrossRef]
- Powlson, D.S.; Addiscott, T.M.; Benjamin, N.; Cassman, K.G.; de Kok, T.M.; van Grinsven, H.; L’Hirondel, J.L.; Avery, A.A.; van Kessel, C. When Does Nitrate Become a Risk For Humans? J. Environ. Qual. 2008, 37, 291–295. [Google Scholar] [CrossRef] [Green Version]
- Food and Agriculture Organization of the United Nations and World Health Organization (FAO/WHO). Evaluation of Certain Food Additives and Contaminants; WHO: Geneva, Switzerland, 1995; Available online: https://apps.who.int/iris/bitstream/handle/10665/42849/WHO_TRS_922.pdf;sequence=1 (accessed on 30 January 2023).
- European Food Safety Authority. Opinion of the Scientific Panel on Contaminants in the Food Chain on a Request from the European Commission to Perform a Scientific Risk Assessment on Nitrate in Vegetables. EFSA J. 2008, 6, 689. Available online: https://www.efsa.europa.eu/en/efsajournal/pub/689 (accessed on 30 January 2023). [CrossRef]
- Katan, M.B. Nitrate in Foods: Harmful or Healthy? Am. J. Clin. Nutr. 2009, 90, 11–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dejam, A.; Hunter, C.J.; Tremonti, C.; Pluta, R.M.; Hon, Y.Y.; Grimes, G.; Partovi, K.; Pelletier, M.M.; Oldfield, E.H.; Cannon, R.O., III; et al. Nitrite Infusion in Humans and Nonhuman Primates: Endocrine Effects, Pharmacokinetics, and Tolerance Formation. Circulation 2007, 116, 1821–1831. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hord, N.G.; Tang, Y.; Bryan, N.S. Food Sources of Nitrates and Nitrites: The Physiologic Context for Potential Health Benefits. Am. J. Clin. Nutr. 2009, 90, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Chazelas, E.; Pierre, F.; Druesne-Pecollo, N.; Esseddik, Y.; Szabo de Edelenyi, F.; Agaesse, C.; De Sa, A.; Lutchia, R.; Gigandet, S.; Srour, B.; et al. Nitrites and Nitrates from Food Additives and Natural Sources and Cancer Risk: Results from the Nutrinet-Santé Cohort. Int. J. Epidemiol. 2022, 51, 1106–1119. [Google Scholar] [CrossRef]
- Van Velzen, A.G.; Sips, A.J.; Schothorst, R.C.; Lambers, A.C.; Meulenbelt, J. The Oral Bioavailability of Nitrate from Nitrate-Rich Vegetables in Humans. Toxicol. Lett. 2008, 181, 177–181. [Google Scholar] [CrossRef]
- Gilchrist, M.; Winyard, P.G.; Aizawa, K.; Anning, C.; Shore, A.; Benjamin, N. Effect of Dietary Nitrate on Blood Pressure, Endothelial Function, and Insulin Sensitivity in Type 2 Diabetes. Free Radic. Biol. Med. 2013, 60, 89–97. [Google Scholar] [CrossRef] [Green Version]
- Bondonno, C.P.; Liu, A.H.; Croft, K.D.; Ward, N.C.; Yang, X.; Considine, M.J.; Puddey, I.B.; Woodman, R.J.; Hodgson, J.M. Short-Term Effects of Nitrate-Rich Green Leafy Vegetables on Blood Pressure and Arterial Stiffness in Individuals with High-Normal Blood Pressure. Free Radic. Biol. Med. 2014, 77, 353–362. [Google Scholar] [CrossRef] [Green Version]
- Bondonno, C.P.; Liu, A.H.; Croft, K.D.; Ward, N.C.; Puddey, I.B.; Woodman, R.J.; Hodgson, J.M. Short-Term Effects of a High Nitrate Diet on Nitrate Metabolism in Healthy Individuals. Nutrients 2015, 7, 1906–1915. [Google Scholar] [CrossRef] [Green Version]
- Kapil, V.; Khambata, R.S.; Robertson, A.; Caulfield, M.J.; Ahluwalia, A. Dietary Nitrate Provides Sustained Blood Pressure Lowering in Hypertensive Patients: A Randomized, Phase 2, Double-Blind, Placebo Controlled Study. Hypertension 2015, 65, 320–327. [Google Scholar] [CrossRef] [Green Version]
- Rammos, C.; Hendgen-Cotta, U.B.; Sobierajski, J.; Bernard, A.; Kelm, M.; Rassaf, T. Dietary Nitrate Reverses Vascular Dysfunction in Older Adults with Moderately Increased Cardiovascular Risk. J. Am. Coll. Cardiol 2014, 63, 1584–1585. [Google Scholar] [CrossRef] [Green Version]
- Bahadoran, Z.; Mirmiran, P.; Kabir, A.; Azizi, F.; Ghasemi, A. The Nitrate-Independent Blood Pressure-Lowering Effect of Beetroot Juice: A Systematic Review and Meta-Analysis. Hypertension 2015, 65, 320–327. [Google Scholar] [CrossRef] [Green Version]
- Lara, J.; Ashor, A.W.; Oggioni, C.; Ahluwalia, A.; Mathers, J.C.; Siervo, M. Effects of Inorganic Nitrate and Beetroot Supplementation on Endothelial Function: A Systematic Review and Meta-Analysis. Eur. J. Nutr. 2016, 55, 451–459. [Google Scholar] [CrossRef]
- Da Silva, D.V.T.; Silva, F.O.; Perrone, D.; Pierucci, A.P.T.R.; Conte-Junior, C.A.; Alvares, T.S.; Del Aguila, E.M.; Paschoalin, V.M.F. Physicochemical, Nutritional, and Sensory Analyses of a Nitrate-Enriched Beetroot Gel and its Effects on Plasmatic Nitric Oxide and Blood Pressure. Food Nutr. Res. 2016, 60, 29909. [Google Scholar] [CrossRef] [Green Version]
- Webb, A.J.; Patel, N.; Loukogeorgakis, S.; Okorie, M.; Aboud, Z.; Misra, S.; Rashid, R.; Miall, P.; Deanfield, J.; Benjamin, N.; et al. Acute Blood Pressure Lowering, Vasoprotective, and Antiplatelet Properties of Dietary Nitrate via Bioconversion to Nitrite. Hypertension 2008, 51, 784–790. [Google Scholar] [CrossRef] [Green Version]
- Joris, P.J.; Mensink, R.P. Beetroot Juice Improves in Overweight and Slightly Obese Men Postprandial Endothelial Function after Consumption of a Mixed Meal. Atherosclerosis 2013, 231, 78–83. [Google Scholar] [CrossRef]
- Liu, A.H.; Bondonno, C.P.; Croft, K.D.; Puddey, I.B.; Woodman, R.J.; Rich, L.; Ward, N.C.; Vita, J.A.; Hodgson, J.M. Effects of a Nitrate-Rich Meal on Arterial Stiffness and Blood Pressure in Healthy Volunteers. Nitric Oxide Biol. Chem. 2013, 35, 123–130. [Google Scholar] [CrossRef]
- Hughes, W.E.; Ueda, K.; Treichler, D.P.; Casey, D.P. Effects of Acute Dietary Nitrate Supplementation on Aortic Blood Pressure and Aortic Augmentation Index in Young and Older Adults. Nitric Oxide 2016, 59, 21–27. [Google Scholar] [CrossRef]
- Kim, D.J.K.; Roe, C.A.; Somani, Y.B.; Moore, D.J.; Barrett, M.A.; Flanagan, M.; Kim-Shapiro, D.B.; Basu, S.; Muller, M.D.; Proctor, D.N. Effects of Acute Dietary Nitrate Supplementation on Aortic Blood Pressures and Pulse Wave Characteristics in Post-Menopausal Women. Nitric Oxide 2019, 85, 10–16. [Google Scholar] [CrossRef]
- Walker, M.A.; Bailey, T.G.; Mcllvenna, L.; Allen, J.D.; Green, D.J.; Askew, C.D. Acute Dietary Nitrate Supplementation Improves Flow Mediated Dilatation of the Superficial Femoral Artery in Healthy Older Males. Nutrients 2019, 11, 954. [Google Scholar] [CrossRef] [Green Version]
- Pavitt, M.J.; Tanner, R.J.; Lewis, A.; Buttery, S.; Mehta, B.; Jefford, H.; Curtis, K.J.; Banya, W.A.S.; Husain, S.; Satkunam, K.; et al. Oral Nitrate Supplementation to Enhance Pulmonar Rehabilitation in COPD: ON-EPIC a Multicentre, Double-Blind, Placebo-Controlled, Randomised Parallel Group Study. Thorax 2020, 75, 547–555. [Google Scholar] [CrossRef] [PubMed]
- Broxterman, R.M.; Taylor La Salle, D.; Zhao, J.; Reese, V.R.; Richardson, R.S.; Trinity, J.D. Influence of Dietary Inorganic Nitrate on Blood Pressure and Vascular Function in Hypertension: Prospective Implications for Adjunctive Treatment. J. Appl. Physiol. 2019, 127, 1085–1094. [Google Scholar] [CrossRef] [PubMed]
- Asgary, S.; Afshani, M.R.; Sahebkar, A.; Keshvari, M.; Taheri, M.; Jahanian, E.; Rafieian-Kopaei, M.; Malekian, F.; Sarrafzadegan, N. Improvement of Hypertension, Endothelial Function and Systemic Inflammation Following Short-Term Supplementation with Red Beet (Beta vulgaris L.) Juice: A Randomized Crossover Pilot Study. J. Hum. Hypertens. 2016, 30, 627–632. [Google Scholar] [CrossRef] [PubMed]
- Jones, T.; Dunn, E.L.; Macdonald, J.H.; Kubis, H.P.; McMahon, N.; Sandoo, A. The Effects of Beetroot Juice on Blood Pressure, Microvascular Function and Large-Vessel Endothelial Function: A Randomized, Doubleblind, Placebo-Controlled Pilot Study in Healthy Older Adults. Nutrients 2019, 11, 1792. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baião, D.S.; d’El-Rei, J.; Alves, G.; Fritsch, M.F.; Perrone, D.; Del Aguila, E.D.; Paschoalin, V.M.F. Chronic Effects of Nitrate Supplementation with a Newly Designed Beetroot Formulation on Biochemical and Hemodynamic Parameters of Individuals Presenting Risk Factors for Cardiovascular Diseases: A Pilot Study. J. Funct. Foods 2019, 58, 85–94. [Google Scholar] [CrossRef]
- Velmurugan, S.; Gan, J.M.; Rathod, K.S.; Khambata, R.S.; Ghosh, S.M.; Hartley, A.; Eijl, S.V.; Sagi-Kiss, V.; Chowdhury, T.A.; Curtis, M.; et al. Dietary Nitrate Improves Vascular Function in Patients with Hypercholesterolemia: A Randomized, Double-Blind, Placebo-Controlled Study. Am. J. Clin. Nutr. 2016, 103, 25–38. [Google Scholar] [CrossRef] [Green Version]
- Bock, J.M.; Hughes, W.E.; Ueda, K.; Feider, A.J.; Hanada, S.; Casey, D.P. Dietary Inorganic Nitrate/Nitrite Supplementation Reduces Central and Peripheral Blood Pressure in Patients with Type 2 Diabetes Mellitus. Am. J. Hypertens. 2022, 35, 803–809. [Google Scholar] [CrossRef]
- Wijnands, K.A.; Meesters, D.M.; van Barneveld, K.W.; Visschers, R.G.; Briedé, J.J.; Vandendriessche, B.; Van Eijk, H.M.; Bessems, B.A.; van den Hoven, N.; Von Wintersdorff, C.J.; et al. Citrulline Supplementation Improves Organ Perfusion and Arginine Availability Under Conditions with Enhanced Arginase Activity. Nutrients 2015, 7, 5217–5238. [Google Scholar] [CrossRef] [Green Version]
- Alderton, W.K.; Cooper, C.E.; Knowles, R.G. Nitric Oxide Synthases: Structure, Function and Inhibition. Biochem. J. 2001, 357, 593–615. [Google Scholar] [CrossRef]
- Ataabadi, A.E.; Golshiri, K.; Jüttner, A.; Krenning, G.; Danser, A.H.J.; Roks, A.J. Nitric Oxide-cGMP Signaling in Hypertension: Current and Future Options for Pharmacotherapy. Hypertension 2020, 76, 1055–1068. [Google Scholar] [CrossRef]
- Blatter, L.A.; Wier, W.G. Nitric Oxide Decreases [Ca2+] in Vascular Smooth Muscle by Inhibition of the Calcium Current. Cell Calcium 1994, 15, 122–131. [Google Scholar] [CrossRef]
- Vtolix, M.L.; Raeymaeken, F.; Wuytack, F.; Hofmann, F.; Casteels, R. Cyclic GMP-dependent protein kinase stimulates the plasmalemmal Ca2+ pump of smooth muscle via phosphorylation of phosphatidylinositol. Biochem. J. 1988, 255, 855–863. [Google Scholar] [CrossRef] [Green Version]
- Münzel, T.; Daiber, A.; Ullrich, V.; Mülsch, A. Vascular consequences of endothelial nitric oxide synthase uncoupling for the activity and expression of the soluble guanylyl cyclase and the cGMP-dependent protein kinase. Arterioscler Thromb Vasc Biol. 2005, 25, 1551–1557. [Google Scholar] [CrossRef]
- King, D.E.; Mainous, A.G., 3rd; Geesey, M.E. Variation in L-Arginine Intake Follow Demographics and Lifestyle Factors That May Impact Cardiovascular Disease Risk. Nutr. Res. 2008, 28, 21–24. [Google Scholar] [CrossRef] [Green Version]
- Siasos, G.; Tousoulis, D.; Vlachopoulos, C.; Antoniades, C.; Stefanadi, E.; Ioakeimidis, N.; Andreou, I.; Zisimos, K.; Papavassiliou, A.G.; Stefanadis, C. Short-Term Treatment with L-Arginine Prevents the Smoking-Induced Impairment of Endothelial Function and Vascular Elastic Properties in Young Individuals. Int. J. Cardiol. 2008, 126, 394–399. [Google Scholar] [CrossRef]
- Siasos, G.; Tousoulis, D.; Vlachopoulos, C.; Antoniades, C.; Stefanadi, E.; Ioakeimidis, N.; Zisimos, K.; Siasou, Z.; Papavassiliou, A.G.; Stefanadis, C. The impact of oral L-arginine supplementation on acute smoking-induced endothelial injury and arterial performance. Am. J. Hypertens. 2009, 22, 586–592. [Google Scholar] [CrossRef] [Green Version]
- Adams, M.R.; McCredie, R.; Jessup, W.; Robinson, J.; Sullivan, D.; Celermajer, D.S. Oral L-Arginine Improves Endothelium-Dependent Dilatation and Reduces Monocyte Adhesion to Endothelial Cells in Young Men with Coronary Artery Disease. Atherosclerosis 1997, 129, 261–269. [Google Scholar] [CrossRef]
- Yin, W.H.; Chen, J.W.; Tsai, C.; Chiang, M.C.; Young, M.S.; Lin, S.J. L-Arginine Improves Endothelial Function and Reduces LDL Oxidation in Patients with Stable Coronary Artery Disease. Clin. Nutr. 2005, 24, 988–997. [Google Scholar] [CrossRef]
- Lucotti, P.; Monti, L.; Setola, E.; La Canna, G.; Castiglioni, A.; Rossodivita, A.; Pala, M.G.; Formica, F.; Paolini, G.; Catapano, A.L.; et al. Oral L-Arginine Supplementation Improves Endothelial Function and Ameliorates Insulin Sensitivity and Inflammation in Cardiopathic Nondiabetic Patients after an Aortocoronary Bypass. Metabolism 2009, 58, 1270–1276. [Google Scholar] [CrossRef]
- Schächinger, V.; Britten, M.B.; Zeiher, A.M. Prognostic Impact of Coronary Vasodilator Dysfunction on Adverse Long-Term Outcome of Coronary Heart Disease. Circulation 2000, 101, 1899–1906. [Google Scholar] [CrossRef] [Green Version]
- Osibogun, O.; Ogunmoroti, O.; Michos, E.D. Polycystic Ovary Syndrome and Cardiometabolic Risk: Opportunities for Cardiovascular Disease Prevention. Trends Cardiovasc. Med. 2020, 30, 399–404. [Google Scholar] [CrossRef] [PubMed]
- Battaglia, C.; Mancini, F.; Battaglia, B.; Facchinetti, F.; Artini, P.G.; Venturoli, S. L-Arginine Plus Drospirenone-Ethinyl Estradiol in the Treatment of Patients with PCOS: A Prospective, Placebo Controlled, Randomized, Pilot Study. Gynecol. Endocrinol. 2010, 26, 861–868. [Google Scholar] [CrossRef] [PubMed]
- Orea-Tejeda, A.; Orozco-Gutiérrez, J.J.; Castillo-Martínez, L.; Keirns-Davies, C.; Montano-Hernández, P.; Vázquez-Díaz, O.; Valdespino-Trejo, A.; Infante, O.; Martínez-Memije, R. The Effect of L-Arginine and Citrulline on Endothelial Function in Patients in Heart Failure with Preserved Ejection Fraction. Cardiol. J. 2010, 17, 464–470. [Google Scholar] [PubMed]
- Hamburg, N.M.; Keyes, M.J.; Larson, M.G.; Vasan, R.S.; Schnabel, R.; Pryde, M.M.; Mitchell, G.F.; Sheffy, J.; Vita, J.A.; Benjamin, E.J. Cross-sectional relations of digital vascular function to cardiovascular risk factors in the Framingham Heart Study. Circulation 2008, 117, 2467–2474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jankowski, J.; Floege, J.; Fliser, D.; Böhm, M.; Marx, N. Cardiovascular Disease in Chronic Kidney Disease: Pathophysiological Insights and Therapeutic Options. Circulation 2021, 143, 1157–1172. [Google Scholar] [CrossRef]
- Annavarajula, S.K.; Dakshinamurty, K.V.; Naidu, M.U.; Reddy, C.P. The Effect of L-Arginine on Arterial Stiffness and Oxidative Stress in Chronic Kidney Disease. Indian J. Nephrol. 2012, 22, 340–346. [Google Scholar] [CrossRef]
- Gaenzer, H.; Sturm, W.; Neumayr, G.; Kirchmair, R.; Ebenbichler, C.; Ritsch, A.; Föger, B.; Weiss, G.; Patsch, J.R. Pronounced postprandial lipemia impairs endothelium-dependent dilation of the brachial artery in men. Cardiovasc. Res. 2001, 52, 509–516. [Google Scholar] [CrossRef] [Green Version]
- Ceriello, A.; Taboga, C.; Tonutti, L.; Quagliaro, L.; Piconi, L.; Bais, B.; Da Ros, R.; Motz, E. Evidence for an Independent and Cumulative Effect of Postprandial Hypertriglyceridemia and Hyperglycemia on Endothelial Dysfunction and Oxidative Stress Generation—Effects of Short- and Long-Term Simvastatin Treatment. Circulation 2002, 106, 1211–1218. [Google Scholar] [CrossRef] [Green Version]
- Fewkes, J.J.; Kellow, N.J.; Cowan, S.F.; Williamson, G.; Dordevic, A.L. A single, high-fat meal adversely affects postprandial endothelial function: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2022, 116, 699–729. [Google Scholar] [CrossRef]
- Deveaux, A.; Pham, I.; West, S.G.; André, E.; Lantoine-Adam, F.; Bunouf, P.; Sadi, S.; Hermier, D.; Mathé, V.; Fouillet, H.; et al. L-Arginine Supplementation Alleviates Postprandial Endothelial Dysfunction When Baseline Fasting Plasma Arginine Concentration is Low: A Randomized Controlled Trial in Healthy Overweight Adults with Cardiometabolic Risk Factors. J. Nutr. 2016, 146, 1330–1340. [Google Scholar] [CrossRef] [Green Version]
- Lin, C.C.; Tsai, W.C.; Chen, J.Y.; Li, Y.H.; Lin, L.J.; Chen, J.H. Supplements of L-arginine attenuate the effects of high-fat meal on endothelial function and oxidative stress. Int. J. Cardiol. 2008, 127, 337–341. [Google Scholar] [CrossRef]
- Gambardella, J.; Khondkar, W.; Morelli, M.B.; Wang, X.; Santulli, G.; Trimarco, V. Arginine and Endothelial Function. Biomedicines 2020, 8, 277. [Google Scholar] [CrossRef]
- Sudar-Milovanovic, E.; Obradovic, M.; Jovanovic, A.; Zaric, B.; Zafirovic, S.; Panic, A.; Radak, D.; Isenovic, E.R. Benefits of L-Arginine on Cardiovascular System. Mini Rev. Med. Chem. 2016, 16, 94–103. [Google Scholar] [CrossRef]
- Romero, M.J.; Platt, D.H.; Caldwell, R.B.; Caldwell, R.W. Therapeutic Use of Citrulline in Cardiovascular Disease. Cardiovasc. Drug Rev. 2006, 24, 275–290. [Google Scholar] [CrossRef]
- Aguayo, E.; Martínez-Sánchez, A.; Fernández-Lobato, B.; Alacid, F. L-Citrulline: A Non-Essential Amino Acid with Important Roles in Human Health. Appl. Sci. 2021, 11, 3293. [Google Scholar] [CrossRef]
- Orozco-Gutiérrez, J.J.; Castillo-Martínez, L.; Orea-Tejeda, A.; Vázquez-Díaz, O.; Valdespino-Trejo, A.; Narváez-David, R.; Keirns-Davis, C.; Carrasco-Ortiz, O.; Navarro-Navarro, A.; Sánchez-Santillán, R. Effect of L-arginine or L-Citrulline Oral Supplementation on Blood Pressure and Right Ventricular Function in Heart Failure Patients with Preserved Ejection Fraction. Cardiol. J. 2010, 17, 612–618. [Google Scholar]
- Figueroa, A.; Sanchez-Gonzalez, M.A.; Perkins-Veazie, P.M.; Arjmandi, B.H. Effects of Watermelon Supplementation on Aortic Blood Pressure and Wave Reflection in Individuals with Prehypertension: A Pilot Study. Am. J. Hypertens. 2011, 24, 40–44. [Google Scholar] [CrossRef] [Green Version]
- Akashi, K.; Mifune, Y.; Morita, K.; Ishitsuka, S.; Tsujimoto, H.; Ishihara, T. Spatial Accumulation Pattern of Citrulline and Other Nutrients in Immature and Mature Watermelon Fruits. J. Sci. Food Agric. 2017, 97, 479–487. [Google Scholar] [CrossRef]
- Tarazona-Díaz, M.P.; Viegas, J.; Moldao-Martins, M.; Aguayo, E. Bioactive Compounds from Flesh and By-Product of Fresh-Cut Watermelon Cultivars. J. Sci. Food Agric. 2011, 91, 805–812. [Google Scholar] [CrossRef]
- Bailey, S.J.; Blackwell, J.R.; Williams, E.; Vanhatalo, A.; Wylie, L.J.; Winyard, P.G.; Jones, A.M. Two Weeks of Watermelon Juice Supplementation Improves Nitric Oxide Bioavailability but Not Endurance Exercise Performance in Humans. Nitric Oxide-Biol. Chem. 2016, 59, 10–20. [Google Scholar] [CrossRef] [Green Version]
- Figueroa, A.; Wong, A.; Jaime, S.J.; Gonzales, J.U. Influence of L-Citrulline and Watermelon Supplementation on Vascular Function and Exercise Performance. Curr. Opin. Clin. Nutr. Metab. 2017, 20, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Bahri, S.; Zerrouk, N.; Aussel, C.; Moinard, C.; Crenn, P.; Curis, E.; Chaumeil, J.C.; Cynober, L.; Sfar, S. Citrulline: From Metabolism to Therapeutic Use. Nutrition 2013, 29, 479–484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, J.; Park, E.; Zhang, L.; Edirisinghe, I.; Burton-Freeman, B.M.; Sandhu, A.K. Pharmacokinetic Parameters of Watermelon (Rind, Flesh and Seeds) Bioactive Components in Human Plasma: A Pilot Study to Investigate the Relationship to Endothelial Function. J. Agric. Food Chem. 2020, 68, 7393–7403. [Google Scholar] [CrossRef] [PubMed]
- Volino-Souza, M.; de Oliveira, G.V.; Conte-Junior, C.A.; Figueroa, A.; Alvares, S.T. Current Evidence of Watermelon (Citrullus lanatus) Ingestion on Vascular Health: A Food Science and Technology Perspective. Nutrients 2022, 14, 2913. [Google Scholar] [CrossRef] [PubMed]
- Vincellette, C.M.; Losso, J.; Early, K.; Spielmann, G.; Irving, B.A.; Allerton, T.D. Supplemental Watermelon Juice Attenuates Acute Hyperglycemia-Induced Macro-and Microvascular Dysfunction in Healthy Adults. J. Nutr. 2021, 151, 3450–3458. [Google Scholar] [CrossRef]
- Ellis, A.C.; Mehta, T.; Nagabooshanam, V.A.; Dudenbostel, T.; Locher, J.L.; Crowe-White, K.M. Daily 100% Watermelon Juice Consumption and Vascular Function among Postmenopausal Women: A Randomized Controlled Trial. Nutr. Metab. Cardiovasc. Dis. NMCD 2021, 31, 2959–2968. [Google Scholar] [CrossRef]
- Figueroa, A.; Wong, A.; Hooshmand, S.; Sanchez-Gonzalez, M.A. Effects Of Watermelon Supplementation on Arterial Stiffness and Wave Reflection Amplitude in Postmenopausal Women. Menopause 2013, 20, 573–577. [Google Scholar] [CrossRef]
- Figueroa, A.; Sanchez-Gonzalez, M.A.; Wong, A.; Arjmandi, B.H. Watermelon Extract Supplementation Reduces Ankle Blood Pressure and Carotid Augmentation Index in Obese Adults with Prehypertension or Hypertension. Am. J. Hypertens. 2012, 25, 640–643. [Google Scholar] [CrossRef] [Green Version]
- Kim, I.Y.; Schutzler, S.E.; Schrader, A.; Spencer, H.J.; Azhar, G.; Deutz, N.E.; Wolfe, R.R. Acute Ingestion of Citrulline Stimulates Nitric Oxide Synthesis but Does Not Increase Blood Flow in Healthy Young and Older Adults with Heart Failure. Am. J. Physiol. Endocrinol. Metab. 2015, 309, E915–E924. [Google Scholar] [CrossRef] [Green Version]
- Maharaj, A.; Fischer, S.M.; Dillon, K.N.; Kang, Y.; Martinez, M.A.; Figueroa, A. Effects of L-Citrulline Supplementation on Endothelial Function and Blood Pressure in Hypertensive Postmenopausal Women. Nutrients 2022, 14, 4396. [Google Scholar] [CrossRef]
- Ochiai, M.; Hayashi, T.; Morita, M.; Ina, K.; Maeda, M.; Watanabe, F.; Morishita, K. Short-Term Effects of L-Citrulline Supplementation on Arterial Stiffness in Middle-Aged Men. Int. J. Cardiol. 2012, 155, 257–261. [Google Scholar] [CrossRef]
- Safi, M.; Mahjoob, M.P.; Nateghi, S.; Khaheshi, I.; Akbarzadeh, M.A.; Naderian, M. The Assessment of Short-Term Effect of L-Citrulline on Endothelial Function via FMD to NMD Ratio in Known Cad Patients: A Randomized, Cross-Over Clinical Trial (Clinical trial number: NCT02638727). Rom. J. Intern. Med. 2017, 55, 23–27. [Google Scholar] [CrossRef] [Green Version]
- Le Roux-Mallouf, T.; Pelen, F.; Vallejo, A.; Halimaoui, I.; Doutreleau, S.; Verges, S. Effect of Chronic Nitrate and Citrulline Supplementation on Vascular Function and Exercise Performance in Older Individuals. Aging 2019, 11, 3315–3332. [Google Scholar] [CrossRef]
- Morita, M.; Hayashi, T.; Ochiai, M.; Maeda, M.; Yamaguchi, T.; Ina, K.; Kuzuya, M. Oral Supplementation with a Combination of L-Citrulline and L-Arginine Rapidly Increases Plasma L-Arginine Concentration and Enhances NO Bioavailability. Biochem. Biophys. Res. Commun. 2013, 454, 53–57. [Google Scholar] [CrossRef] [Green Version]
- Aburto, N.J.; Hanson, S.; Gutierrez, H.; Hooper, L.; Elliott, P.; Cappuccio, F.P. Effect of Increased Potassium Intake on Cardiovascular Risk Factors and Disease: Systematic Review and Meta-Analyses. Br. Med. J. 2013, 346, f1378. [Google Scholar] [CrossRef] [Green Version]
- Lanham-New, S.A.; Lambert, H.; Frassetto, L. Potassium. Adv. Nutr. 2012, 3, 820–821. [Google Scholar] [CrossRef] [Green Version]
- Mohammadifard, N.; Gotay, C.; Humphries, K.H.; Ignaszewski, A.; Esmaillzadeh, A.; Sarrafzadegan, N. Electrolyte Minerals Intake and Cardiovascular Health. Crit. Rev. Food Sci. Nutr. 2019, 59, 2375–2385. [Google Scholar] [CrossRef]
- Gritter, M.; Rotmans, J.I.; Hoorn, E.J. Role of Dietary K+ in Natriuresis, Blood Pressure Reduction, Cardiovascular Protection, and Renoprotection. Hypertension 2019, 73, 15–23. [Google Scholar] [CrossRef]
- Institute of Medicine. Dietary Reference Intakes for Water, Potassium, Sodium, Chloride, and Sulfate; National Academics Press: Washington, DC, USA, 2004; Available online: www.nap.edu/catalog/10925.html (accessed on 22 April 2023).
- Stone, M.S.; Martyn, L.; Weaver, C.M. Potassium Intake, Bioavailability, Hypertension, and Glucose Control. Nutrients 2016, 8, 444. [Google Scholar] [CrossRef] [Green Version]
- Malavolti, M.; Naska, A.; Fairweather-Tait, S.J.; Malagoli, C.; Vescovi, L.; Marchesi, C.; Vinceti, M.; Filippini, T. Sodium and Potassium Content of Foods Consumed in an Italian Population and the Impact of Adherence to a Mediterranean Diet on Their Intake. Nutrients 2021, 13, 2681. [Google Scholar] [CrossRef]
- Kumssa, D.B.; Joy, E.J.M.; Broadley, M.R. Global Trends (1961–2017) in Human Dietary Potassium Supplies. Nutrients 2021, 13, 1369. [Google Scholar] [CrossRef] [PubMed]
- Haddy, F.J.; Vanhoutte, P.M.; Feletou, M. Role of Potassium in Regulating Blood Flow and Blood Pressure. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006, 290, R546–R552. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Meng, X.; Xuan, B.; Zhou, T.; Gao, H.; Dong, H.; Wang, Y. Na+/Ca2+ Exchanger 1 in Airway Smooth Muscle of Allergic Inflammation Mouse Model. Front. Pharmacol. 2018, 9, 1471. [Google Scholar] [CrossRef] [PubMed]
- Edwards, G.; Weston, A.H. Potassium and Potassium Clouds in Endothelium-Dependent Hyperpolarizations. Pharmacol. Res. 2004, 49, 535–541. [Google Scholar] [CrossRef] [PubMed]
- Su, X.T.; Yang, C.L.; Ellison, D.H. Kidney Is Essential for Blood Pressure Modulation by Dietary Potassium. Curr. Cardiol. Rep. 2020, 22, 124. [Google Scholar] [CrossRef]
- Oberleithner, H.; Callies, C.; Kusche-Vihrog, K.; Schillers, H.; Shahin, V.; Riethmüller, C.; Macgregor, G.A.; de Wardener, H.E. Potassium Softens Vascular Endothelium and Increases Nitric Oxide Release. Proc. Natl. Acad. Sci. USA 2009, 106, 2829–2834. [Google Scholar] [CrossRef] [Green Version]
- Chang, Y.Y.; Chen, A.; Chen, Y.H.; Hung, C.S.; Wu, V.C.; Wu, X.M.; Lin, Y.H.; Ho, Y.L.; Wu, K.D.; TAIPAI Study Group. Hypokalemia Correlated with Arterial Stiffness but Not Microvascular Endothelial Function in Patients with Primary Aldosteronism. J. Renin Angiotensin Aldosterone Syst. 2015, 16, 353–359. [Google Scholar] [CrossRef] [Green Version]
- Dickinson, K.M.; Clifton, P.M.; Keogh, J.B. Endothelial Function is Impaired after a High-Salt Meal in Healthy Subjects. Am. J. Clin. Nutr. 2011, 93, 500–505. [Google Scholar] [CrossRef] [Green Version]
- Patik, J.C.; Lennon, S.L.; Farquhar, W.B.; Edwards, D.G. Mechanisms of Dietary Sodium-Induced Impairments in Endothelial Function and Potential Countermeasures. Nutrients 2021, 13, 270. [Google Scholar] [CrossRef]
- Liu, Z.; Peng, J.; Lu, F.; Zhao, Y.; Wang, S.; Sun, S.; Zhang, H.; Diao, Y. Salt Loading and Potassium Supplementation: Effects on Ambulatory Arterial Stiffness Index and Endothelin-1 Levels in Normotensive and Mild Hypertensive Patients. J. Clin. Hypertens. 2013, 15, 485–496. [Google Scholar] [CrossRef] [Green Version]
- Smiljanec, K.; Mbakwe, A.; Ramos Gonzalez, M.; Farquhar, W.B.; Lennon, S.L. Dietary Potassium Attenuates the Effects of Dietary Sodium on Vascular Function in Salt-Resistant Adults. Nutrients 2020, 12, 1206. [Google Scholar] [CrossRef]
- Blanch, N.; Clifton, P.M.; Petersen, K.S.; Willoughby, S.R.; Keogh, J.B. Effect of High Potassium Diet on Endothelial Function. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 983–989. [Google Scholar] [CrossRef]
- Gijsbers, L.; Dower, J.I.; Schalkwijk, C.G.; Kusters, Y.H.; Bakker, S.J.; Hollman, P.C.; Geleijnse, J.M. Effects of Sodium and Potassium Supplementation on Endothelial Function: A Fully Controlled Dietary Intervention Study. Br. J. Nutr. 2015, 114, 19–26. [Google Scholar] [CrossRef] [Green Version]
- Inaba, Y.; Chen, J.A.; Bergmann, S.R. Prediction of Future Cardiovascular Outcomes by Flow-Mediated Vasodilatation of Brachial Artery: A Meta-Analysis. Int. J. Cardiovasc. Imaging 2010, 26, 631–640. [Google Scholar] [CrossRef]
- Ras, R.T.; Streppel, M.T.; Draijer, R.; Zock, P.L. Flow-Mediated Dilation and Cardiovascular Risk Prediction: A Systematic Review with Meta-Analysis. Int. J. Cardiol. 2013, 168, 344–351. [Google Scholar] [CrossRef]
- Lennon-Edwards, S.; Allman, B.R.; Schellhardt, T.A.; Ferreira, C.R.; Farquhar, W.B.; Edwards, D.G. Lower Potassium Intake is Associated with Increased Wave Reflection in Young Healthy Adults. Nutr. J. 2014, 13, 39. [Google Scholar] [CrossRef] [Green Version]
- Berry, S.E.; Mulla, U.Z.; Chowienczyk, P.J.; Sanders, T.A. Increased Potassium Intake from Fruit and Vegetables or Supplements Does Not Lower Blood Pressure or Improve Vascular Function in UK Men and Women with Early Hypertension: A Randomised Controlled Trial. Br. J. Nutr. 2010, 104, 1839–1847. [Google Scholar] [CrossRef] [Green Version]
- Matthesen, S.K.; Larsen, T.; Vase, H.; Lauridsen, T.G.; Pedersen, E.B. Effect of Potassium Supplementation on Renal Tubular Function, Ambulatory Blood Pressure and Pulse Wave Velocity in Healthy Humans. Scand. J. Clin. Lab. Investig. Suppl. 2012, 72, 78–86. [Google Scholar] [CrossRef]
- Graham, U.M.; McCance, D.R.; Young, I.S.; Mullan, K.R. A Randomised Controlled Trial Evaluating the Effect of Potassium Supplementation on Vascular Function and the Renin-Angiotensin-Aldosterone System. J. Hum. Hypertens. 2014, 28, 333–339. [Google Scholar] [CrossRef]
- He, F.J.; Marciniak, M.; Carney, C.; Markandu, N.D.; Anand, V.; Fraser, W.D.; Dalton, R.N.; Kaski, J.C.; MacGregor, G.A. Effects of Potassium Chloride and Potassium Bicarbonate on Endothelial Function, Cardiovascular Risk Factors, and Bone Turnover in Mild Hypertensives. Hypertension 2010, 55, 681–688. [Google Scholar] [CrossRef]
- Tang, X.; Wu, B.; Luo, Y.; Peng, L.; Chen, Y.; Zhu, J.; Peng, C.; Li, S.; Liu, J. Effect of Potassium Supplementation on Vascular Function: A Meta-Analysis of Randomized Controlled Trials. Int. J. Cardiol. Heart Vasc. 2017, 228, 225–232. [Google Scholar] [CrossRef] [PubMed]



| NO3− Content/Vehicle | Subjects/Age | Intervention Period | Experimental Design | Main Outcomes | Study |
|---|---|---|---|---|---|
| ≈8.0 mmol/140 mL of beetroot juice | 20 healthy overweight and slightly obese individuals (61.0 ± 7.0 y) | Single dose | Double-blind Randomized Placebo-controlled Crossover | ↑ NOx ↓ DBP ↑ FMD PWV, AIx and central AIx—no effects | Joris et al. [78] |
| 3.55 mmol/250 g of cooked spinach | 26 healthy subjects (58.8 ± 7.6 y) | Single dose | Double-blind Randomized Placebo-controlled Crossover | ↑ salivary NOx ↑ large artery elasticity index ↓ PP, SBP, cardiac ejection time, cardiac output, stroke volume and total vascular impedance | Liu et al. [79] |
| 9.4 mmol/500 mL of beetroot juice | 26 healthy adults (young: 25 ± 4 y, n = 14; older: 64 ± 5 y, n = 12) | Single dose | Double-blind Randomized Placebo-controlled Crossover | ↑ plasma NO2− ↓ Peripheral and aortic SBP, DBP, MAP and aPP in young and older subjects to a similar degree. ↓ AIx in young adults AIx older adults no—effect | Hughes et al. [80] |
| 9.7 mmol/140 mL of beetroot juice | 13 healthy postmenopausal women (22.0 ± 1.0 y) | Single dose | Double-blind Randomized Placebo-controlled Crossover | ↑ plasma NOx ↓ brachial and aortic SBP, DBP and MAP Vascular functions—no effects | Kim et al. [81] |
| 13 mmol/140 mL of beetroot juice | 15 healthy older men (69 ± 4 y) | Single dose | Double-blind Randomized Crossover Placebo-controlled Acute ingestion | ↑ plasma NOx BP and PWV—no effect ↑ FMD ↓ AIx75 | Walker et al. [82] |
| 12.9 mmol/140 mL of beetroot juice | 165 subjects COPD grade II–IV. 78 in nitrate-rich group (70 ± 14 y) and 87 in placebo group (68 ± 13 y). | Single dose | Double-blind Randomized Placebo-controlled Parallel group | ↑ Exercise capacity ↓ SBP, DBP and MAP ↑ FMD | Pavitt et al. [83] |
| 6.20 mmol/70 mL of beetroot juice | Study 1: 13 hypertensive subjects taking antihypertensive medications (age: 53 ± 12 y) Study 2: 14 hypertensive subjects not taking antihypertensive medications (49 ± 13 y) | 3 days | Double-blinded Randomized Placebo-controlled Acute-ingestion | Study 1: ↑ plasma NOx ↓ plasma L-arginine Blood pressure and vascular function—no effects Study 2: ↑ plasma NOx ↑ plasma L-arginine ↓ SBP, DBP and MAP ↑ FMD | Broxterman et al. [84] |
| 6.2 mmol/70 mL of beetroot juice | 11 healthy male subjects (age 30 ± 7 y) | 1 week | Single-blinded Randomized Crossover Placebo-controlled | ↑ plasma and salivary NOx Blood pressure and vascular function—no effect | Burleigh et al. [51] |
| 6.4 mmol/250 g of green leafy vegetables (spinach, lettuce, spinach, rocket, and other leafy greens) | 38 healthy subjects | 1 week | Randomized Placebo-controlled Crossover | ↑ plasma NOx SBP and DBP—no effects PWV and AIx—no effects | Bondono et al. [70] |
| NO3−: ≈7.0 mmol/140 mL of beetroot juice | 27 hypertensive and older subjects (63.2 ± 4.4 y) | 1 week | Double-blind Randomized Placebo-controlled Crossover | ↑ urinary, salivary and plasmatic NOx Home BP, SBP and DBP 24 h ambulatory—no effects | Bondono et al., [71] |
| 7.5 mmol/250 mL of beetroot juice | 27 subjects with type 2 diabetes (67.2 ± 4.9 y) | 2 weeks | Double-blind Randomized Placebo-controlled Crossover | ↑ NOx FMD—no effect Insulin sensitivity—no effect SBP, DBP and MAP—no effects Microvascular endothelial function—Perfusion response—Laser Doppler—no effect | Gilchrist et al. [69] |
| 250 mL of beetroot juice (NO3− contents not specified) 250 g cooked beetroot (NO3− contents not measured) | 24 hypertensive subjects without medication (55.2 ± 11.4 y beetroot juice group, 53.3 ± 10.3 y cooked beetroot group) | 2 weeks | Assessor-blind Randomized Crossover | ↓ ICAM-1, VCAM-1 and E-selectin by both treatments ↓ hs-CRP, TNF-α and IL-6 by both treatments ↓ TAC after beetroot juice ↓ SBP and DBP by both treatments ↑ FMD (beetroot juice > cooked beetroot) | Asgary et al. [85] |
| 6.45 mmol/70 mL of beetroot juice | 20 older subjects (65 ± 8 y) | 2 weeks | Double-blinded Randomized Placebo-controlled | ↑ plasma NO3− ↓ SBP and DBP ↑ FMD | Jones et al. [86] |
| 9.57 mmol/60 g of beetroot-cereal bar | 5 patients displaying three risk factors for CVD (54.25 ± 4.64 y) | 3 weeks | Double-blind Randomized Placebo-controlled Crossover | ↑ plasma NOx ↓ SBP and DBP ↓ PWV, AP, AIx, aoSP, aoPP and arterial age ↓ endothelial dysfunction by improvements in CVC peaks and AUC | Baião et al. [87] |
| ≈6.4 mmol/250 mL of beetroot juice | 34 drug-naive and 34 treated hypertensive subjects (57.6 ± 13.9 y) | 4 weeks | Double-blind Randomized Placebo-controlled | ↑ plasma NOx ↑ plasma cGMP ↓ 24 h BP ↓ PWV and AIx ↑ FMD and time to peak | Kapil et al. [72] |
| NaNO3−: 10.5 mmol/supplement dissolved in drinking water | 11 healthy older subjects (63.0 ± 5.0 y) | 4 weeks | Double-blind Randomized Placebo-controlled | ↑ NO synthesis (through plasma NO3− and NO2−) ↓ SBP, DBP, PWV and AIx ↑ FMD | Rammos et al. [73] |
| 6.0 mmol/250 mL of beetroot juice | 65 hypercholesterolemic subjects (53.3 ± 10.1 y) | 6 weeks | Double-blind Randomized Placebo-controlled Parallel group | ↑ plasma, salivary and urinary NOx ↓ platelet-monocyte aggregates ↓ SBP DBP and HR—no effects ↓ Aix ↓ aPWV ↑ FMD | Velmurugan et al. [88] |
| 4.03 mmol/beetroot juice | 37 subjects with type 2 diabetes mellitus | 8 weeks | Double-blinded Randomized Placebo-controlled parallel group Chronic ingestion | ↑ plasma NO2− ↓ Peripheral SBP ↓ Central SBP and AP ↓ MAP ↓ AIx | Bock et al. [89] |
| L-Arginine Content/Vehicle | Subjects/Age | Intervention Period | Experimental Design | Main Outcomes | Study |
|---|---|---|---|---|---|
| 16 g/in capsules | 12 healthy older (8 males, 4 females) (73.8 ± 2.7 y) | 2 weeks | Double-blind Randomized Placebo-controlled Crossover | ↑ plasma L-arginine ↑ FMD | Bode-Boger et al. [34] |
| 21 g/in capsules | 10 CAD males (41 ± 2 y) | 10 days | Double-blind Randomized Placebo-controlled Crossover | ↑ plasma l-arginine ↑ FMD BP and HR—no effects | Adams and Celermajer [99] |
| 6 g/in capsules | 10 smokers (3 males, 7 females) (24.4 ± 0.95 y) | 3 occasions after acute smoking | Double-blind Randomized Placebo-controlled Crossover | ↑ FMD ↓ cfPWV and Alx | Siasos et al. [97] |
| 21 g/in capsules | 12 smokers (5 males, 7 females) (24.4 ± 0.95 y) | three occasions after acute smoking | Double-blind Randomized Placebo-controlled Crossover | FMD—no effects ↓ cfPWV and Alx | Siasos et al. [98] |
| 10 g/in capsules | 33 CAD subjects (21 males, 12 females) (58 ± 7 y) | 4 weeks | Open-label Randomized Crossover | ↑ FMD ↓LDL oxidation ICAM-1, VCAM-1 and P-seletin—no effects | Yin et al. [101] |
| 6.4 g/in capsules | 64 CAD subjects (65 ± 10 y) | 6 months | Double-blind Placebo-controlled | ↑ plasma l-arginine ↑ c-GMP ↓ ADMA ↑ reactive hyperemia | Lucotti and Piatti [102] |
| 8 g/in capsules | 28 PCOS women (24.3 ± 3.5 y) | 4 weeks | Double-blind Randomized Placebo-controlled | ↑ plasma NOx ↑ reactive hyperemia Blunted ↑ BP-drospirenone induced | Battaglia et al. [104] |
| 3 g/in capsules | 30 CHF subjects (17 male, 13 female) (63 ± 14.5 y) | 8 weeks | Double-blind Randomized | ↓ MAT/TT ratio after forearm occlusion | Orea-Tejeda et al. [105] |
| 9 g/in capsules | 30 CKD and high PWV subjects (24 males, 6 females) (49.4 ± 11) | 12 weeks | Randomized Open-label | ↑ plasma Nox ↓ cfPWV, crPWV, Alx and AP | Annavarajula et al. [107] |
| 4.5 g/in capsules | 36 overweight (22 males, 13 females) (45 ± 8.9 y) | 4 weeks | Double-blind Randomized Placebo-controlled Crossover | ↑ plasma L-arginine Blunted ↓FMD high fat-induced ↑ reactive hyperemia | Deveaux et al. [108] |
| L-Citrulline Content/Vehicle | Subjects/Age | Intervention Period | Experimental Design | Main Outcomes | Study |
|---|---|---|---|---|---|
| watermelon rind (5 g) watermelon flesh (5 g) watermelon rind (0.1 g) | 6 overweight/obese subjects (32.2 ± 7.6 y) | 1 week | Randomized Placebo-controlled Crossover | FMD—no effect | Fan et al. [125] |
| 4 g/30 g of micro-encapsulated watermelon | 11 healthy adults | 3 occasions with a 1 week interval | Randomized Single-blind Crossover Placebo-controlled | ↑ FMD ↑ plasma L-citrulline and L-arginine | Volino-Souza et al. [126] |
| 500 mL watermelon juice | 17 healthy young adults (21–25 y) (6 males/11 females) | 2 weeks | Randomized Placebo-controlled Double-blind Crossover | ↑ FMD ↑ plasma L-arginine | Vincellette et al. [127] |
| 1.63 g/Two servings of 360 mL of 100% watermelon juice | 21 healthy postmenopausal women (55–70 y) | 4 weeks | Randomized Double-blind Placebo-controlled Crossover | FMD, PWV, MAP—no effects SBP and DBP—no effects | Ellis et al. [128] |
| watermelon (6 g/d L-citrulline/L-arginine) | 12 postmenopausal women (57 ± 1 y) | 6 weeks | Randomized Placebo-controlled Crossover | ↓ baPWV ↓ SBP and DBP ↓ aortic SBP2 radial SBP2—no effect aortic and radial—no effects | Figueroa et al. [129] |
| 2.7 g/in watermelon | 9 pre-hypertensives subjects (4 male/5 female) (54 ± 3 y) | 6 weeks | Randomized Placebo-controlled | ↓ AIx cfPWV—no effect | Figueroa et al. [130] |
| watermelon (containing L-citrulline 1.3 g plus L-arginine 2.7 g) | 14 adults (11 female/3 male) (58 ± 1 y) prehypertensive or stage 1 hypertension | 6 weeks | Randomized Placebo-controlled Two-period Crossover | ↓ cAIx ↓ SBP, DBP, MAP HR and ABI—no effects | Figueroa et al. [119] |
| 10 g/L-citrulline capsules | 7 older HF adults (>60 y) and 7 healthy young subjects (21–40 y) | 2 days | Kinetic study Placebo-controlled | ↑ plasma L-arginine (in older adults) ↑ NO synthesis rate (in older adults) ↑ NO synthesis rate (in older adults) RH-FBF—unaffected | Kim et al. [131] |
| 6 g/citrulline capsules | 25 sedentary hypertensive postmenopausal women (50–4 y) | 4 weeks | Double-blind, Randomized Placebo-controlled | ↑ plasma L-arginine ↑ FMD ↑ aortic DBP and MAP cfPWV—no effects brachial BP—no effects | Maharaj et al. [132] |
| 5.6 g/L-citrulline capsules | 15 healthy subjects (58.3 ± 4.4 y) | 1 week | Double-blind Randomized Placebo-controlled Parallel-group | ↓ baPWV DBP and SBP—no effects ↑ NO ↑ NOx ↑ plasma L-citrulline ↑ plasma L-arginine ↑ plasma ratio of arginine/ADMA ↑ endogenous inhibitor of NO synthase | Ochiai et al. [133] |
| 100 mg/kg body weight in capsules | 30 CAD and FMD/NMD (<1) subjects | 2 weeks | Randomized Crossover placebo-controlled | ↑ FMD/NMD ↑ FMD | Safi et al. [134] |
| 6 g/citrulline drink | 24 healthy subjects (12 males/12 females) (64 ± 2 y) | 4 weeks | Double-blind Randomized | ↓ SBP and DBP PWV—no effect NO—no effect | Roux-Mallouf et al. [135] |
| 800 mg/L-citrulline capsules | 22 diagnosed vasospastic angina patients (41–46 y) | 8 weeks | Open label | ↑ plasma NOx ↓ ADMA ↑ FMD ↑ plasma L-arginine/ADMA ratio | Morita et al. [136] |
| K+ Content/Vehicle | Subjects/Age | Intervention Period | Experimental Design | Main Outcomes | Study |
|---|---|---|---|---|---|
| Diet containing: (1) K+—3 mmol + Na+—3 mmol (2) K+—3 mmol + Na+—65 mmol (3) K+—38 mmol + Na+—65 mmol | 34 healthy subjects (16 males, 18 females) (37 ± 15 y) | 1 day | Double-blind Randomized Crossover | ↑ FMD (3) ↓ AIx (1) (2) (3) cfPWV—no effect BP—no effect | Blanch et al. [37] |
| Diet containing: (1) K+ 80 mmol (2) K+ 150 mmol | 35 healthy subjects (26 males, 9 females) (31 ± 11 y) | 6 days | Single-blind Randomized Crossover | ↑ FMD at 150 mmol cfPWV and AIx—no effect SBP and DBP—no effect ADMA, ICAM-1 and Endothelin-1—no effect | Blanch et al. [155] |
| K+—60 mmol + Na+—308 mmol in meals | 155 healthy subjects (89 males, 66 females) (52.7 ± 10 y) | 1 week | Randomized Open-label | ↓ AASI ↓ Endothelin-1 ↓ SBP and DBP | Liu et al. [153] |
| Diet containing: (1) K+—65 mmol + Na+—50 mmol (2) K+—65 mmol + Na+—300 mmol (3) K+ 120 mmol + Na+ 300 mmol | 33 healthy subjects (16 males, 17 females) (27 ± 1 y) | 1 week | Randomized Open-label | ↑ K+ excretion (1) ↑ FMD (1)(3) > (2) cfPWV and AIx—no effect SBP and DBP—no effect | Smiljanec et al. [154] |
| 71.6 mmol/2.8 g K+ capsules | 36 untreated (pre) hypertensive subjects (24 males, 12 females (65.8 ± 8.8 y) | 4 weeks | Double-blind Randomized Placebo-controlled Crossover | ↑ FMD ↓ IL-8 | Gijsbers et al. [156] |
| 100 mmol/K+ capsules | 21 healthy subjects (9 males, 12 females) (26 ± 15.5 y) | 4 weeks | Randomized Placebo-controlled Crossover | ↑ cfPWV AIx no effect 24 h-BP—no effect CBP—no effect | Matthesen et al. [161] |
| Diet containing: (1) placebo + 20 mmol K+ from foods (2) K+—20 mmol in meals (3) K+—40 mmol in meals (4) K+—40 mmol/capsules alone | 48 early hypertensive subjects (16 males, 13 females) (45 ± 0.49 y) | 6 weeks | Single-blind Randomized Placebo-controlled Crossover | K+ excretion—no effect FMD, cfPWV—no effect SBP and DBP—no effect | Berry et al. [160] |
| 60 mmol/K+ capsules | 40 high CVD risk subjects (32 males, 8 females) (54.8 ± 1.1 y) | 6 weeks | Double-blind Randomized Placebo-controlled Crossover | crPWV and AIx—no effects ↓ SBP and DBP ↑ plasma renin and aldosterone | Graham et al. [162] |
| (1) KCl—64 mmol/capsules (2) KHCO3—64 mmol/capsules | Untreated mildly hypertensive subjects (30 males, 12 females) (51 ± 10 y) | 12 weeks | Double-blind Randomized Placebo-controlled Crossover | ↑ K+ excretion (1) (2) ↑ FMD (1) (2) ↑ cfPWV (1) (2) ↓ SBP (1) | He et al. [163] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
da Silva, D.V.T.; Baião, D.d.S.; Almeida, C.C.; Paschoalin, V.M.F. A Critical Review on Vasoactive Nutrients for the Management of Endothelial Dysfunction and Arterial Stiffness in Individuals under Cardiovascular Risk. Nutrients 2023, 15, 2618. https://doi.org/10.3390/nu15112618
da Silva DVT, Baião DdS, Almeida CC, Paschoalin VMF. A Critical Review on Vasoactive Nutrients for the Management of Endothelial Dysfunction and Arterial Stiffness in Individuals under Cardiovascular Risk. Nutrients. 2023; 15(11):2618. https://doi.org/10.3390/nu15112618
Chicago/Turabian Styleda Silva, Davi Vieira Teixeira, Diego dos Santos Baião, Cristine Couto Almeida, and Vania Margaret Flosi Paschoalin. 2023. "A Critical Review on Vasoactive Nutrients for the Management of Endothelial Dysfunction and Arterial Stiffness in Individuals under Cardiovascular Risk" Nutrients 15, no. 11: 2618. https://doi.org/10.3390/nu15112618
APA Styleda Silva, D. V. T., Baião, D. d. S., Almeida, C. C., & Paschoalin, V. M. F. (2023). A Critical Review on Vasoactive Nutrients for the Management of Endothelial Dysfunction and Arterial Stiffness in Individuals under Cardiovascular Risk. Nutrients, 15(11), 2618. https://doi.org/10.3390/nu15112618