Post-Recovery Relapse of Children Treated with a Simplified, Combined Nutrition Treatment Protocol in Mali: A Prospective Cohort Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Study Setting and Treatment in Place
2.3. Data Collection and Procedures
2.4. Outcomes and Sample Size
2.5. Predictors
2.6. Data Management and Analysis
2.7. Ethics
3. Results
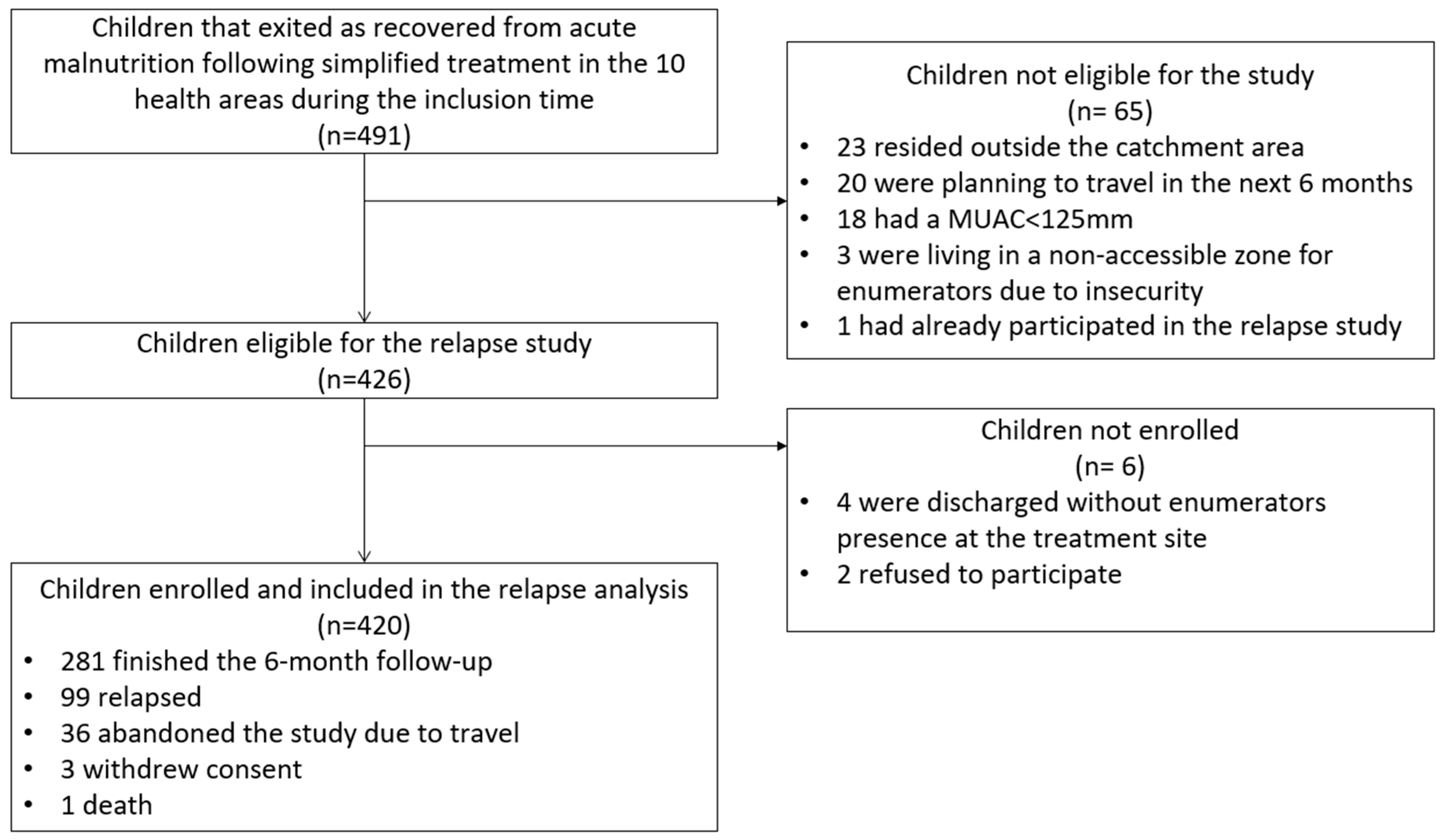
3.1. Inclusion
3.2. Data Quality
3.3. Participant Characteristics
3.4. Cumulative Relapse Incidence
3.5. Incidence Rate of Relapse
3.6. Predictors of Relapse
4. Discussion
4.1. Incidence of Relapse
4.2. Treatment Related Predictors of Relapse
4.3. Contextual Predictors of Relapse
4.4. Recommendations, Strengths, and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- United Nations Children’s Fund (UNICEF); World Health Organization; International Bank for Reconstruction and Development/The World Bank. Levels and Trends in Child Malnutrition: Key Findings of the 2020 Edition of the Joint Child Malnutrition Estimates; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- WHO. Guideline: Updates on the Management of Severe Acute Malnutrition in Infant and Children; World Health Organization: Geneva, Switzerland, 2013. [Google Scholar]
- WHO; WFP; UNSCN; UNICEF. Community-Based Management of Severe Acute Malnutrition: A Joint Statement by the World Health Organization, the World Food Programme, the United Nations System Standing Committee on Nutrition and the United Nations Children’s Fund; UNICEF: Geneva, Switzerland, 2007. [Google Scholar]
- World Health Organization. Management of Severe Malnutrition: A Manual for Physicians and Other Senior Health Workers; World Health Organization: Geneva, Switzerland, 1999; 60p. [Google Scholar]
- WFP. Technical Specifications for Ready-to-Use Supplementary Food (RUSF); Specification Reference: MIXRSF000; WFP: Rome, Italy, 2016. [Google Scholar]
- World Health Organization, UNICEF. WHO Child Growth Standards and the Identification of Severe Acute Malnutrition in Infants and Children: A Joint Statement by the World Health Organization and the United Nations Children’s Fund. 2009. Available online: http://www.ncbi.nlm.nih.gov/books/NBK200775/ (accessed on 12 March 2018).
- Sphere Project. The Sphere Handbook: Humanitarian Charter and Minimum Standards in Humanitarian Response, 4th ed.; Sphere Association: Geneva, Switzerland, 2018; 406p. [Google Scholar]
- Stobaugh, H.C.; Mayberry, A.; McGrath, M.; Bahwere, P.; Zagre, N.M.; Manary, M.J.; Black, R.; Lelijveld, N. Relapse after severe acute malnutrition: A systematic literature review and secondary data analysis. Matern. Child Nutr. 2018, 15, e12702. [Google Scholar] [CrossRef] [PubMed]
- Council of Research & Technical Advice on Acute Malnutrition (CORTASAM). Guidance to Improve the Collecting and Reporting of Data on Relapse in Children Following Treatment in Wasting Programs. 2020. Available online: https://www.ennonline.net/attachments/3636/Guidance-To-Improve-The-Collecting-And-Reporting-Of-Data-On-Relapse-In-Children-Following-Treatment-In-Wasting-Programmes.-A-statement-from-CORTASAM.pdf (accessed on 16 May 2023).
- Schaefer, R.; Mayberry, A.; Briend, A.; Manary, M.; Walker, P.; Stobaugh, H.; Hanson, K.; McGrath, M.; Black, R. Relapse and regression to severe wasting in children under 5 years: A theoretical framework. Matern. Child Nutr. 2021, 17, e13107. [Google Scholar] [CrossRef] [PubMed]
- UNICEF. Global Annual Results Report 2020: Goal Area 1: Every Child Survives and Thrives. 2021. Available online: https://www.unicef.org/media/102426/file/Global-annual-results-report-2020-goal-area-1.pdf (accessed on 31 May 2023).
- Becart, E. Meta-Analysis of Barriers and Boosters from 78 Coverage Assessments Supported by the CMN. 2014. Available online: https://www.coverage-monitoring.org/wp-content/uploads/2015/07/Meta-Analysis-of-Barriers-and-Boosters-from-78-Coverage-Assessments-supported-by-the-CMN.pdf (accessed on 31 May 2023).
- Puett, C.; Guerrero, S. Barriers to access for severe acute malnutrition treatment services in Pakistan and Ethiopia: A comparative qualitative analysis. Public Health Nutr. 2015, 18, 1873–1882. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- UNICEF. Treatment of Wasting Using Simplified Approaches—A Rapid Evidence Review. 2021. Available online: https://www.unicef.org/documents/rapid-review-treatment-wasting-using-simplified-approaches (accessed on 31 May 2023).
- Bailey, J.; Opondo, C.; Lelijveld, N.; Marron, B.; Onyo, P.; Musyoki, E.N.; Adongo, S.W.; Manary, M.; Briend, A.; Kerac, M. A simplified, combined protocol versus standard treatment for acute malnutrition in children 6–59 months (ComPAS trial): A cluster-randomized controlled non-inferiority trial in Kenya and South Sudan. PLoS Med. 2020, 17, e1003192. [Google Scholar] [CrossRef] [PubMed]
- Bailey, J.; Lelijveld, N.; Marron, B.; Onyoo, P.; Ho, L.S.; Manary, M.; Briend, A.; Opondo, C.; Kerac, M. Combined Protocol for Acute Malnutrition Study (ComPAS) in rural South Sudan and urban Kenya: Study protocol for a randomized controlled trial. Trials 2018, 19, 251. [Google Scholar] [CrossRef] [Green Version]
- Van Boetzelaer, E.; Zhou, A.; Tesfai, C.; Kozuki, N. Performance of low-literate community health workers treating severe acute malnutrition in South Sudan. Matern. Child Nutr. 2019, 15, e12716. [Google Scholar] [CrossRef] [Green Version]
- Alvarez Morán, J.L.; Alé, F.G.B.; Rogers, E.; Guerrero, S. Quality of care for treatment of uncomplicated severe acute malnutrition delivered by community health workers in a rural area of Mali. Matern. Child Nutr. 2018, 14, e12449. [Google Scholar] [CrossRef] [Green Version]
- Lopez-Ejeda, N. Severe Acute Malnutrition Treatment Delivered by Community Health Workers in Emergency Settings of Mali (iCCM + Project). 2020. Available online: http://www.isrctn.com/ISRCTN60973756 (accessed on 24 November 2021).
- López-Ejeda, N.; Charle Cuellar, P.; Vargas, A.; Guerrero, S. Can community health workers manage uncomplicated severe acute malnutrition? A review of operational experiences in delivering severe acute malnutrition treatment through community health platforms. Matern. Child Nutr. 2019, 15, e12719. [Google Scholar] [CrossRef]
- Alé, F.G.B.; Phelan, K.P.Q.; Issa, H.; Defourny, I.; Le Duc, G.; Harczi, G.; Issaley, K.; Sayadi, S.; Ousmane, N.; Yahaya, I.; et al. Mothers screening for malnutrition by mid-upper arm circumference is non-inferior to community health workers: Results from a large-scale pragmatic trial in rural Niger. Arch. Public Health. 2016, 74, 38. [Google Scholar] [CrossRef] [Green Version]
- Blackwell, N.; Myatt, M.; Allafort-Duverger, T.; Balogoun, A.; Ibrahim, A.; Briend, A. Mothers Understand And Can do it (MUAC): A comparison of mothers and community health workers determining mid-upper arm circumference in 103 children aged from 6 months to 5 years. Arch. Public Health. 2015, 73, 26. [Google Scholar] [CrossRef] [Green Version]
- Bliss, J.; Lelijveld, N.; Briend, A.; Kerac, M.; Manary, M.; McGrath, M.; Weise Prinzo, Z.; Shepherd, S.; Marie Zagre, N.; Woodhead, S.; et al. Use of Mid-Upper Arm Circumference by Novel Community Platforms to Detect, Diagnose, and Treat Severe Acute Malnutrition in Children: A Systematic Review. Glob. Health Sci. Pract. 2018, 6, 552–564. [Google Scholar] [CrossRef] [Green Version]
- Isanaka, S.; Berthé, F.; Nackers, F.; Tang, K.; Hanson, K.E.; Grais, R.F. Feasibility of engaging caregivers in at-home surveillance of children with uncomplicated severe acute malnutrition. Matern. Child Nutr. 2020, 16, e12876. [Google Scholar] [CrossRef]
- Kangas, S.T.; Marron, B.; Tausanovitch, Z.; Radin, E.; Andrianarisoa, J.; Dembele, S.; Ouédraogo, C.T.; Coulibaly, I.N.; Biotteau, M.; Ouologuem, B.; et al. Effectiveness of Acute Malnutrition Treatment at Health Center and Community Levels with a Simplified, Combined Protocol in Mali: An Observational Cohort Study. Nutrients 2022, 14, 4923. [Google Scholar] [CrossRef]
- Lelijveld, N.; Musyoki, E.; Adongo, S.W.; Mayberry, A.; Wells, J.C.; Opondo, C.; Kerac, M.; Bailey, J. Relapse and post-discharge body composition of children treated for acute malnutrition using a simplified, combined protocol: A nested cohort from the ComPAS RCT. PLoS ONE 2021, 16, e0245477. [Google Scholar] [CrossRef]
- CORTASAM. A Research Agenda for Malnutrition: A Statement from the Council of Research & Technical Advice on Acute Malnutrition (CORTASAM). 2018. Available online: https://acutemalnutrition.org/en/resource-library/5emJ22EhFYwQy60OsECqGe (accessed on 16 May 2023).
- USAID, USGS. Land use and land cover Trends in West Africa. 2023. Available online: https://www.usgs.gov/centers/eros/science/land-use-and-land-cover-trends-west-africa (accessed on 31 May 2023).
- USAID FEWS NET. Mali Livelihood Zones. 2014. Available online: https://fews.net/sites/default/files/documents/reports/ML_Livelihoods_2014.pdf (accessed on 31 May 2023).
- FEWS NET. Mali Food Security Outlook. June 2020 to January 2021. 2020. Available online: https://fews.net/sites/default/files/documents/reports/MALI%20Food%20Security%20Outlook%20June%202020_January%202021_EN.pdf (accessed on 31 May 2023).
- Ministère de la Santé et de l’Hygiène Publique. Annuaire Statistique 2018 Du Systeme Local D’information Sanitaire Du Mali. 2018. Available online: http://www.sante.gov.ml/docs/AnnuaireSLIS2018VFdu27avril.pdf (accessed on 31 May 2023).
- Institut National de la Statistique (INSTAT); Cellule de Planification et de Statistique Secteur Santé-Développement Social et Promotion de la Famille (CSP/SS-DS-PF); ICF. Enquête Démographique et de Santé au Mali 2018; INSTAT: Bamako, Mali, 2019. [Google Scholar]
- Institut National de la Statistique (INSTAT); Direction NAtional de la Santé (DNS). Enquête Nutritionnelle et de Mortalité Rétrospective Suivant la Méthodologie SMART au Mali 2019; INSTAT: Bamako, Mali, 2019. [Google Scholar]
- Ballard, T.; Coates, J.; Swindale, A.; Deitchler, M. Household Hunger Scale: Indicator Definition and Measurement Guide. [Internet]. Washington, DC: Food and Nutrition Technical Assistance II Project, FHI 360. 2011. Available online: https://www.fantaproject.org/sites/default/files/resources/HHS-Indicator-Guide-Aug2011.pdf (accessed on 31 May 2023).
- World Health Organization; United Nations Children’s Fund (UNICEF). Indicators for Assessing Infant and Young Child Feeding Practices. Definitions and Measurement Methods. 2021. Available online: https://data.unicef.org/resources/indicators-for-assessing-infant-and-young-child-feeding-practices/ (accessed on 31 May 2023).
- Daures, M.; Phelan, K.; Issoufou, M.; Sawadogo, O.; Akpakpo, B.; Kinda, M.; Shepherd, S.; Becquet, R. Incidence of relapse following a new approach to simplifying and optimising acute malnutrition treatment in children aged 6–59 months: A prospective cohort in rural Northern Burkina Faso. J. Nutr. Sci. 2021, 10, e27. [Google Scholar] [CrossRef]
- Kangas, S.T.; Salpéteur, C.; Nikièma, V.; Talley, L.; Ritz, C.; Friis, H.; Briend, A.; Kaestel, P. Impact of reduced dose of ready-to-use therapeutic foods in children with uncomplicated severe acute malnutrition: A randomised non-inferiority trial in Burkina Faso. PLoS Med. 2019, 16, e1002887. [Google Scholar] [CrossRef] [Green Version]
- Chang, C.Y.; Trehan, I.; Wang, R.J.; Thakwalakwa, C.; Maleta, K.; Deitchler, M.; Manary, M.J. Children successfully treated for moderate acute malnutrition remain at risk for malnutrition and death in the subsequent year after recovery. J. Nutr. 2013, 143, 215–220. [Google Scholar] [CrossRef] [Green Version]
- Adegoke, O.; Arif, S.; Bahwere, P.; Harb, J.; Hug, J.; Jasper, P.; Mudzongo, P.; Nanama, S.; Olisenekwu, G.; Visram, A. Incidence of severe acute malnutrition after treatment: A prospective matched cohort study in Sokoto, Nigeria. Matern. Child Nutr. 2021, 17, e13070. [Google Scholar] [CrossRef]
- Binns, P.J.; Dale, N.M.; Banda, T.; Banda, C.; Shaba, B.; Myatt, M. Safety and practicability of using mid-upper arm circumference as a discharge criterion in community based management of severe acute malnutrition in children aged 6 to 59 months programmes. Arch. Public Health. 2016, 74, 24. [Google Scholar] [CrossRef] [Green Version]
- Guesdon, B.; Katwal, M.; Poudyal, A.K.; Bhandari, T.R.; Counil, E.; Nepali, S. Anthropometry at discharge and risk of relapse in children treated for severe acute malnutrition: A prospective cohort study in rural Nepal. Nutr. J. 2021, 20, 32. [Google Scholar] [CrossRef]
- Myatt, M.; Khara, T.; Dolan, C.; Garenne, M.; Briend, A. Improving screening for malnourished children at high risk of death: A study of children aged 6–59 months in rural Senegal. Public Health Nutr. 2019, 22, 862–871. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khara, T.; Myatt, M.; Sadler, K.; Bahwere, P.; Berkley, J.A.; Black, R.E.; Boyd, E.; Garenne, M.; Isanaka, S.; Lelijveld, N.; et al. Anthropometric criteria for best-identifying children at high risk of mortality: A pooled analysis of twelve cohorts. Public Health Nutr. 2023, 26, 803–819. [Google Scholar] [CrossRef] [PubMed]
- Myatt, M.; Khara, T.; Schoenbuchner, S.; Pietzsch, S.; Dolan, C.; Lelijveld, N.; Briend, A. Children who are both wasted and stunted are also underweight and have a high risk of death: A descriptive epidemiology of multiple anthropometric deficits using data from 51 countries. Arch. Public Health. 2018, 76, 28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- UNICEF. Management of Child Wasting in the Context of COVID-19; UNICEF: New York, NY, USA, 2020. [Google Scholar]
- Kangas, S.T.; Kaestel, P.; Salpéteur, C.; Nikièma, V.; Talley, L.; Briend, A.; Ritz, C.; Friis, H.; Wells, J.C. Body composition during outpatient treatment of severe acute malnutrition: Results from a randomised trial testing different doses of ready-to-use therapeutic foods. Clin. Nutr. 2020, 39, 3426–3433. [Google Scholar] [CrossRef] [Green Version]
- Kangas, S.T.; Salpéteur, C.; Nikièma, V.; Talley, L.; Briend, A.; Ritz, C.; Friis, H.; Kaestel, P. Vitamin A and iron status of children before and after treatment of uncomplicated severe acute malnutrition. Clin. Nutr. 2020, 39, 3512–3519. [Google Scholar] [CrossRef]
- Sigh, S.; Roos, N.; Chhoun, C.; Laillou, A.; Wieringa, F.T. Ready-to-Use Therapeutic Foods Fail to Improve Vitamin A and Iron Status Meaningfully during Treatment for Severe Acute Malnutrition in 6–59-month-old Cambodian Children. Nutrients 2023, 15, 905. [Google Scholar] [CrossRef]
- Akomo, P.; Bahwere, P.; Murakami, H.; Banda, C.; Maganga, E.; Kathumba, S.; Sadler, S.; Collins, S. Soya, maize and sorghum ready-to-use therapeutic foods are more effective in correcting anaemia and iron deficiency than the standard ready-to-use therapeutic food: Randomized controlled trial. BMC Public Health 2019, 19, 806. [Google Scholar] [CrossRef]
- Schaible, U.E.; Kaufmann, S.H.E. Malnutrition and Infection: Complex Mechanisms and Global Impacts. PLoS Med. 2007, 4, e115. [Google Scholar] [CrossRef] [Green Version]
- Patlán-Hernández, A.R.; Stobaugh, H.C.; Cumming, O.; Angioletti, A.; Pantchova, D.; Lapègue, J.; Stern, S.; N’Diaye, D.S. Water, sanitation and hygiene interventions and the prevention and treatment of childhood acute malnutrition: A systematic review. Matern. Child Nutr. 2022, 18, e13257. [Google Scholar] [CrossRef]
- Makamto Sobgui, C.; Kamedjie Fezeu, L.; Diawara, F.; Diarra, H.; Afari-Sefa, V.; Tenkouano, A. Predictors of poor nutritional status among children aged 6–24 months in agricultural regions of Mali: A cross-sectional study. BMC Nutr. 2018, 4, 18. [Google Scholar] [CrossRef] [Green Version]
- Famine Early Warning System. Mali. Seasonal Calendar. Typical Year. 2013. Available online: https://fews.net/west-africa/mali/seasonal-calendar/december-2013 (accessed on 31 May 2023).
- Somassè, Y.E.; Dramaix, M.; Bahwere, P.; Donnen, P. Relapses from acute malnutrition and related factors in a community-based management programme in Burkina Faso: Relapses in a community-based management programme of acute malnutrition. Matern. Child Nutr. 2016, 12, 908–917. [Google Scholar] [CrossRef] [Green Version]
- Seror, V.; Cortaredona, S.; Ly, E.Y.; Ndiaye, S.; Gaye, I.; Fall, M.; Peretti-Watel, P. Vaccination card availability and childhood immunization in Senegal. BMC Public Health 2020, 20, 658. [Google Scholar] [CrossRef]
- UNICEF. Strategy for Improved Nutrition of Children and Women in Developing Countries; UNICEF: New York, NY, USA, 1990. [Google Scholar]
- Grellety, E.; Krause, L.K.; Shams Eldin, M.; Porten, K.; Isanaka, S. Comparison of weight-for-height and mid-upper arm circumference (MUAC) in a therapeutic feeding programme in South Sudan: Is MUAC alone a sufficient criterion for admission of children at high risk of mortality? Public Health Nutr. 2015, 18, 2575–2581. [Google Scholar] [CrossRef] [Green Version]
- Takele, K.; Zewotir, T.; Ndanguza, D. Risk factors of morbidity among children under age five in Ethiopia. BMC Public Health 2019, 19, 942. [Google Scholar] [CrossRef] [Green Version]
- Stewart, C.P.; Wessells, K.R.; Arnold, C.D.; Huybregts, L.; Ashorn, P.; Becquey, E.; Humphrey, J.H.; Dewey, K.G. Lipid-based nutrient supplements and all-cause mortality in children 6–24 months of age: A meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2020, 111, 207–218. [Google Scholar] [CrossRef] [Green Version]
- Dewey, K.G.; Wessells, K.R.; Arnold, C.D.; Prado, E.L.; Abbeddou, S.; Adu-Afarwuah, S.; Ali, H.; Arnold, B.F.; Ashorn, P.; Ashorn, U.; et al. Characteristics that modify the effect of small-quantity lipid-based nutrient supplementation on child growth: An individual participant data meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2021, 114, 15S–42S. [Google Scholar] [CrossRef]
- Wessells, K.R.; Arnold, C.D.; Stewart, C.P.; Prado, E.L.; Abbeddou, S.; Adu-Afarwuah, S.; Arnold, B.F.; Ashorn, P.; Ashorn, U.; Becquey, E.; et al. Characteristics that modify the effect of small-quantity lipid-based nutrient supplementation on child anemia and micronutrient status: An individual participant data meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2021, 114, 68S–94S. [Google Scholar] [CrossRef]
- Prado, E.L.; Arnold, C.D.; Wessells, K.R.; Stewart, C.P.; Abbeddou, S.; Adu-Afarwuah, S.; Arnold, B.F.; Ashorn, U.; Ashorn, P.; Becquey, E.; et al. Small-quantity lipid-based nutrient supplements for children age 6–24 months: A systematic review and individual participant data meta-analysis of effects on developmental outcomes and effect modifiers. Am. J. Clin. Nutr. 2021, 114, 43S–67S. [Google Scholar] [CrossRef]
| N Not Missing | Value | |
|---|---|---|
| 1. Demographic and admission characteristics to malnutrition treatment | ||
| Male, % (n) | 420 | 48.1 (202) |
| Age (months), median [IQR] | 420 | 12.9 [10.0; 22.4] |
| WHZ, mean ± SD | 270 * | −2.6 ± 1.2 |
| WHZ < −3, % (n) | 270 * | 31.1 (84) |
| WAZ, mean ± SD | 420 | −2.9 ± 1.0 |
| WAZ < −3, % (n) | 420 | 45 (189) |
| HAZ, mean ± SD | 270 * | −2.0 ± 1.5 |
| HAZ < −3, % (n) | 270 * | 26.3 (71) |
| Admission category, % (n) | 420 | |
| MUAC < 115 mm and/or edema | 26.7 (112) | |
| MUAC ≥ 115 mm < 125 mm | 73.3 (308) | |
| Admission site, % (n) | 420 | |
| Treated at health center level | 64.8 (272) | |
| Treated at CHW site level | 35.2 (148) | |
| 2. Characteristics at discharge recovered from treatment | ||
| WHZ, mean ± SD | 326 * | −1.2 ± 0.9 |
| WHZ < −2, % (n) | 326 * | 16.0 (52) |
| WAZ, mean ± SD | 413 | −2.0 ± 0.9 |
| WAZ < −3, % (n) | 413 | 11.9 (49) |
| HAZ, mean ± SD | 327 * | −2.1 ± 1.3 |
| HAZ < −3, % (n) | 327 * | 26.3 (86) |
| MUAC (mm), mean ± SD | 420 | 128.3 ± 2.6 |
| MUAC < 130 mm, % (n) | 420 | 68.6 (288) |
| Child’s vaccination card is available at enrolment, % (n) | 420 | 38.8 (163) |
| Child’s vaccine status is up to date at enrolment, % (n) | 163 | 53.4 (87) |
| Length of stay in treatment, median [IQR] | 420 | 34 [23; 49] |
| 3. Socio-economic characteristics collected at first home visit | ||
| Household size, mean ± SD | 413 | 20.9 ± 14.1 |
| Number of children under 5 years of age in the household, mean ± SD | 413 | 5.2 ± 3.7 |
| Main source of income is from agriculture, % (n) | 413 | 81.4 (336) |
| Caregiver has no other occupation than housewife duties, % (n) | 413 | 73.6 (304) |
| Household uses an improved water source, % (n) | 413 | 68.0 (281) |
| Household uses a product to treat drinking water, % (n) | 413 | 17.4 (72) |
| Household uses a latrine, % (n) | 413 | 54.2 (224) |
| Child is unclean, % (n) | 415 | 27.5 (114) |
| Little or no hunger in household, % (n) | 396 | 96.7 (383) |
| 4. Dietary habits collected at first home visit | ||
| Breastfeeding, % (n) | 415 | 58.8 (244) |
| Number of food groups eaten, mean ± SD | 415 | 1.7 ± 0.8 |
| Frequency of feeding solids or semi-solids, mean ± SD | 415 | 3.2 ± 1.2 |
| 5. Morbidity during post-discharge follow-up | ||
| Total number of days sick during follow-up, mean ± SD | 415 | 15.0 ± 10.2 |
| Total number of days sick per month of follow-up, mean ± SD | 415 | 3.5 ± 3.1 |
| Child was sick at least once during follow-up, % (n) | 415 | 95.9 (398) |
| Caregiver has sought formal care for an illness during follow-up in case child has been sick, % (n) | 398 | 15.1 (60) |
| 6. Changes in the household level during follow-up | ||
| Income has decreased during follow-up, % (n) | 402 | 17.7 (71) |
| Caregivers activities have increased during follow-up, % (n) | 402 | 26.9 (108) |
| Hunger has increased during the follow-up, % (n) | 402 | 3 (12) |
| MUAC and Edema Status at Admission to Treatment | ||||||
|---|---|---|---|---|---|---|
| MUAC < 125 mm and/or Edema | MUAC < 115 mm and/or Edema | MUAC 115 mm to MUAC < 125 mm | ||||
| n/N | % [95%CI] | n/N | % [95%CI] | n/N | % [95%CI] | |
| Cumulative incidence over the 6-month follow-up | ||||||
| All children enrolled | 99/420 | 23.6 [19.6; 27.9] | 26/113 | 23.0 [15.6; 31.9] | 73/307 | 23.8 [19.1; 28.9] |
| Non-drop outs | 99/380 | 26.1 [21.7; 30.8] | 26/102 | 25.5 [17.4; 35.1] | 73/278 | 26.3 [21.2; 31.8] |
| Incidence rates (per 100 child-months) | ||||||
| First 3 months | 59/1136 | 5.2 [4.0; 6.7] | 19/305 | 6.2 [4.0; 9.8] | 40/830 | 4.8 [3.5; 6.6] |
| Latter 3 months | 40/909 | 4.4 [3.2; 6.0] | 7/235 | 3.0 [1.4; 6.3] | 33/674 | 4.9 [3.5; 6.9] |
| Full 6 months | 99/2045 | 4.8 [4.0; 5.9] | 26/540 | 4.8 [3.3; 7.1] | 73/1505 | 4.9 [3.9; 6.1] |
| Unadjusted | Sex and Age Adjusted | |||
|---|---|---|---|---|
| HR [95%CI] | p-Value | HR [95%CI] | p-Value | |
| 1. Demographic and admission characteristics to malnutrition treatment | ||||
| Age (months) | 0.94 [0.91; 0.97] | <0.001 | 0.65 [0.51; 0.84] * | 0.001 |
| WHZ | 0.89 [0.75; 1.06] | 0.203 | 0.82 [0.69; 0.99] | 0.040 |
| WAZ | 0.91 [0.75; 1.12] | 0.37 | 0.75 [0.60; 0.93] | 0.009 |
| HAZ | 1.05 [0.91; 1.20] | 0.51 | 0.94 [0.80; 1.09] | 0.39 |
| MUAC (mm) | 0.97 [0.94; 1.00] | 0.081 | 0.97 [0.93; 1.00] | 0.072 |
| 2. Characteristics at discharge recovered from treatment | ||||
| WHZ | 0.71 [0.56; 0.90] | 0.005 | 0.63 [0.49; 0.80] | <0.001 |
| WAZ | 0.75 [0.59; 0.94] | 0.014 | 0.52 [0.40; 0.68] | <0.001 |
| HAZ | 0.99 [0.85; 1.15] | 0.87 | 0.86 [0.73; 1.02] | 0.083 |
| MUAC (mm) | 0.75 [0.67; 0.84] | <0.001 | 0.77 [0.69; 0.86] | <0.001 |
| Length of stay (d) | 1.01 [1.01; 1.02] | <0.001 | 1.01 [1.01; 1.02] | 0.001 |
| 3. Socio-economic characteristics | ||||
| Household size | 0.99 [0.97; 1.00] | 0.14 | 0.99 [0.97; 1.00] | 0.13 |
| Number of children U5 in the household | 0.96 [0.90; 1.02] | 0.22 | 0.96 [0.90; 1.02] | 0.19 |
| 4. Dietary habits collected at first home visit | ||||
| Number of food groups eaten | 0.77 [0.57; 1.05] | 0.103 | 0.84 [0.62; 1.15] | 0.28 |
| Frequency of feeding solids or semi-solids | 0.81 [0.68; 0.96] | 0.013 | 0.90 [0.75; 1.08] | 0.26 |
| 5. Morbidity during post-discharge FU | ||||
| Total number of days sick during FU | 0.99 [0.97; 1.01] | 0.274 | 0.98 [0.96; 1.00] | 0.09 |
| Total number of days sick per month of FU | 1.31 [1.25; 1.36] | <0.001 | 1.30 [1.24; 1.35] | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kangas, S.T.; Coulibaly, I.N.; Tausanovitch, Z.; Ouologuem, B.; Marron, B.; Radin, E.; Ritz, C.; Dembele, S.; Ouédraogo, C.T.; Bailey, J. Post-Recovery Relapse of Children Treated with a Simplified, Combined Nutrition Treatment Protocol in Mali: A Prospective Cohort Study. Nutrients 2023, 15, 2636. https://doi.org/10.3390/nu15112636
Kangas ST, Coulibaly IN, Tausanovitch Z, Ouologuem B, Marron B, Radin E, Ritz C, Dembele S, Ouédraogo CT, Bailey J. Post-Recovery Relapse of Children Treated with a Simplified, Combined Nutrition Treatment Protocol in Mali: A Prospective Cohort Study. Nutrients. 2023; 15(11):2636. https://doi.org/10.3390/nu15112636
Chicago/Turabian StyleKangas, Suvi T., Issa Niamanto Coulibaly, Zachary Tausanovitch, Bareye Ouologuem, Bethany Marron, Elizabeth Radin, Christian Ritz, Salimou Dembele, Césaire T. Ouédraogo, and Jeanette Bailey. 2023. "Post-Recovery Relapse of Children Treated with a Simplified, Combined Nutrition Treatment Protocol in Mali: A Prospective Cohort Study" Nutrients 15, no. 11: 2636. https://doi.org/10.3390/nu15112636
APA StyleKangas, S. T., Coulibaly, I. N., Tausanovitch, Z., Ouologuem, B., Marron, B., Radin, E., Ritz, C., Dembele, S., Ouédraogo, C. T., & Bailey, J. (2023). Post-Recovery Relapse of Children Treated with a Simplified, Combined Nutrition Treatment Protocol in Mali: A Prospective Cohort Study. Nutrients, 15(11), 2636. https://doi.org/10.3390/nu15112636







