Analysis of Network Pharmacological Efficacy and Therapeutic Effectiveness in Animal Models for Functional Dyspepsia of Foeniculi fructus
Abstract
:1. Introduction
2. Materials and Methods
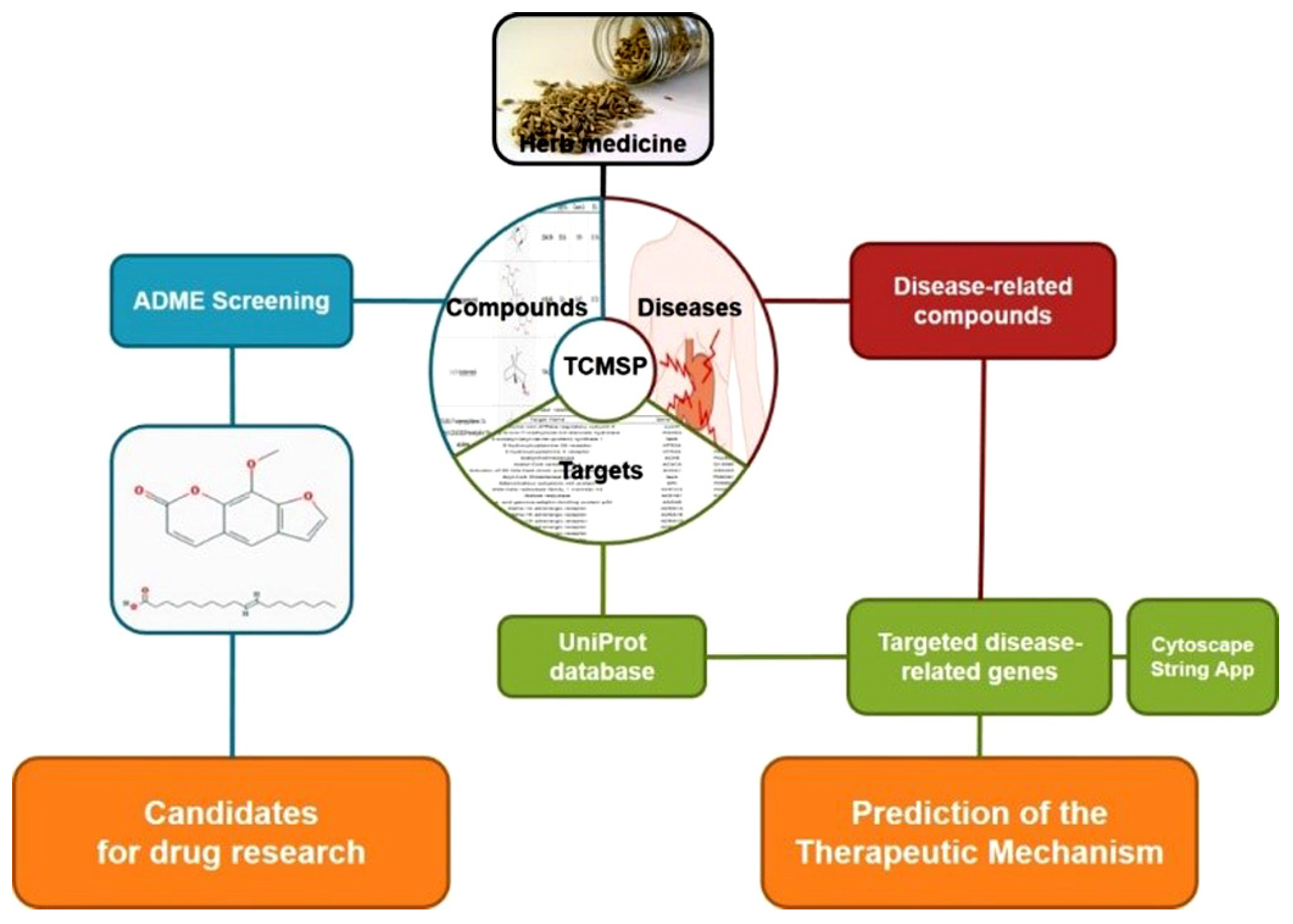
2.1. Analysis of Network Pharmacology
2.1.1. Identifying Compounds of F. fructus
2.1.2. Target Network
2.1.3. Analysis of Network
2.1.4. Screening of Active Compound
2.2. Analysis of F. fructus
2.2.1. Instrument and Reagent
2.2.2. Preparation of the Standard Solution
2.2.3. Preparation of the Test Liquid for Quantitative Analysis
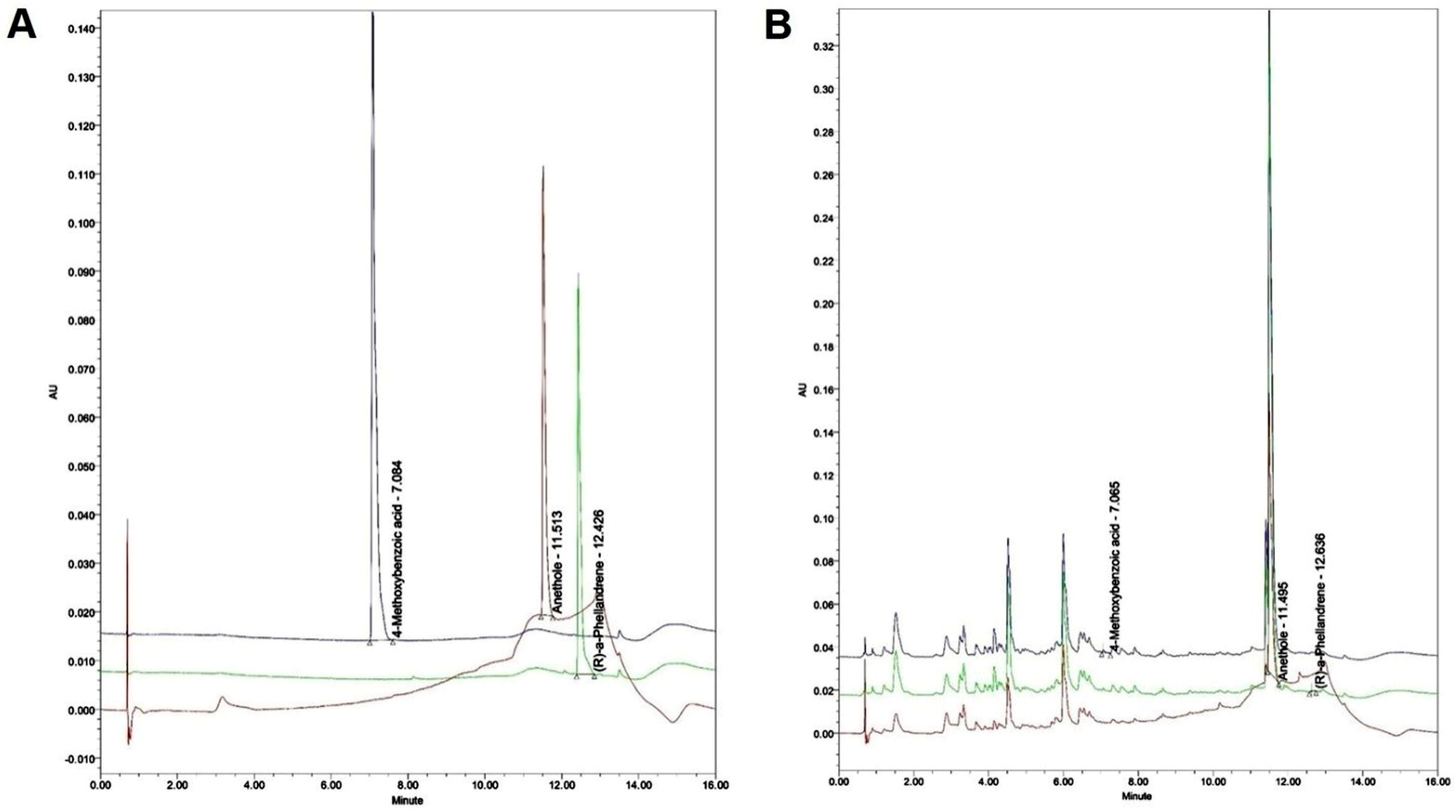
2.2.4. Quantitation of the F. fructus Extract
2.3. Animal Testing
2.3.1. Design of Animal Experiment
2.3.2. Assessment of Gastric Weight and Gastric Emptying
2.3.3. Assessment of Intestinal Transit Rate by Evans Blue
2.3.4. Western Blot Analysis for Check of Protein Level
2.3.5. Quantitative Real-Time PCR to Evaluate Gene Expression
3. Results
3.1. Target Information Derived by Examining Correlations between Compounds and Targets
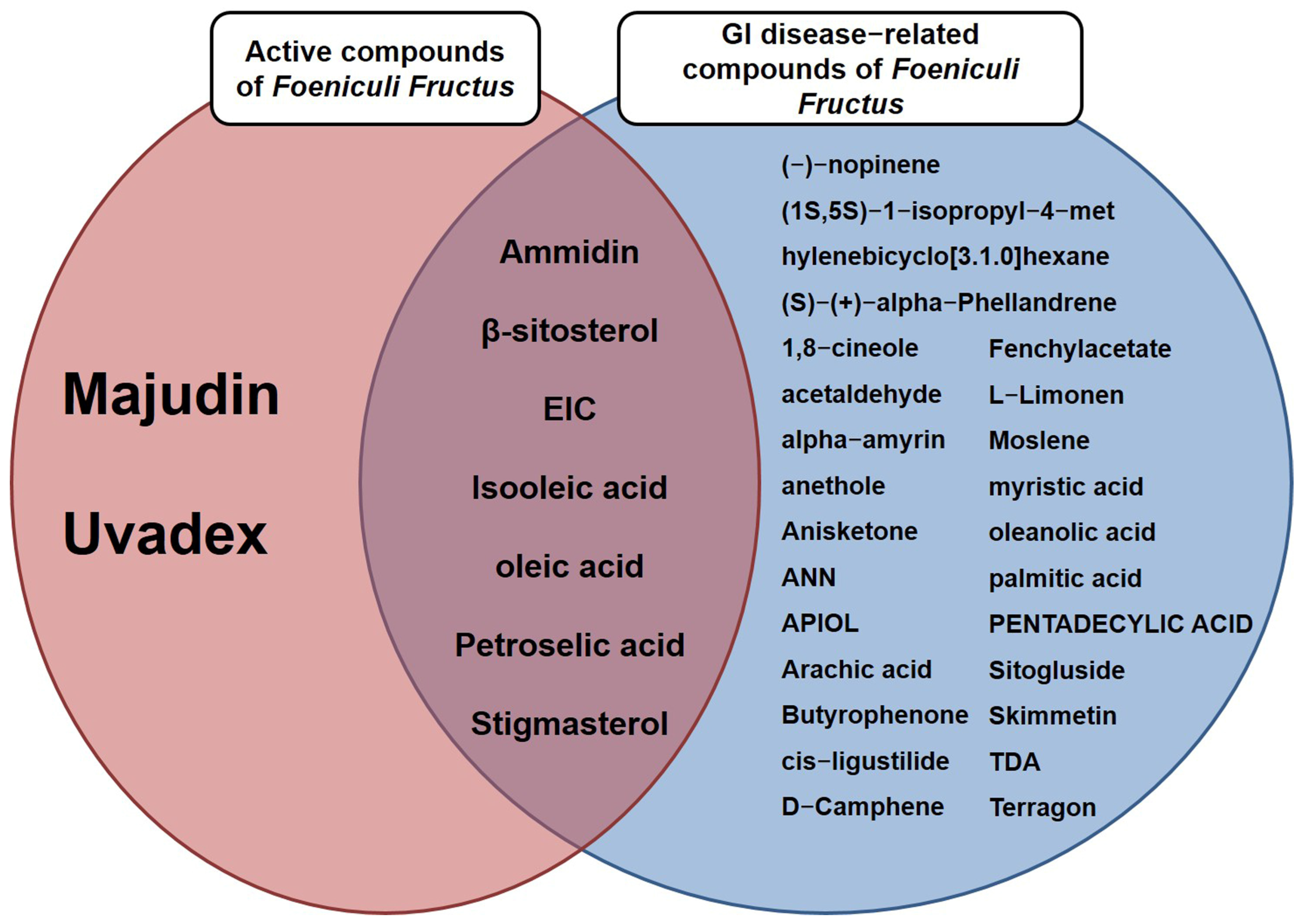
3.2. A Total of Nine Compounds Met ADME Requirements for Active Compounds
3.3. Thirty-Two Compounds Associated with Gastrointestinal (GI) Diseases Were Identified in F. fructus
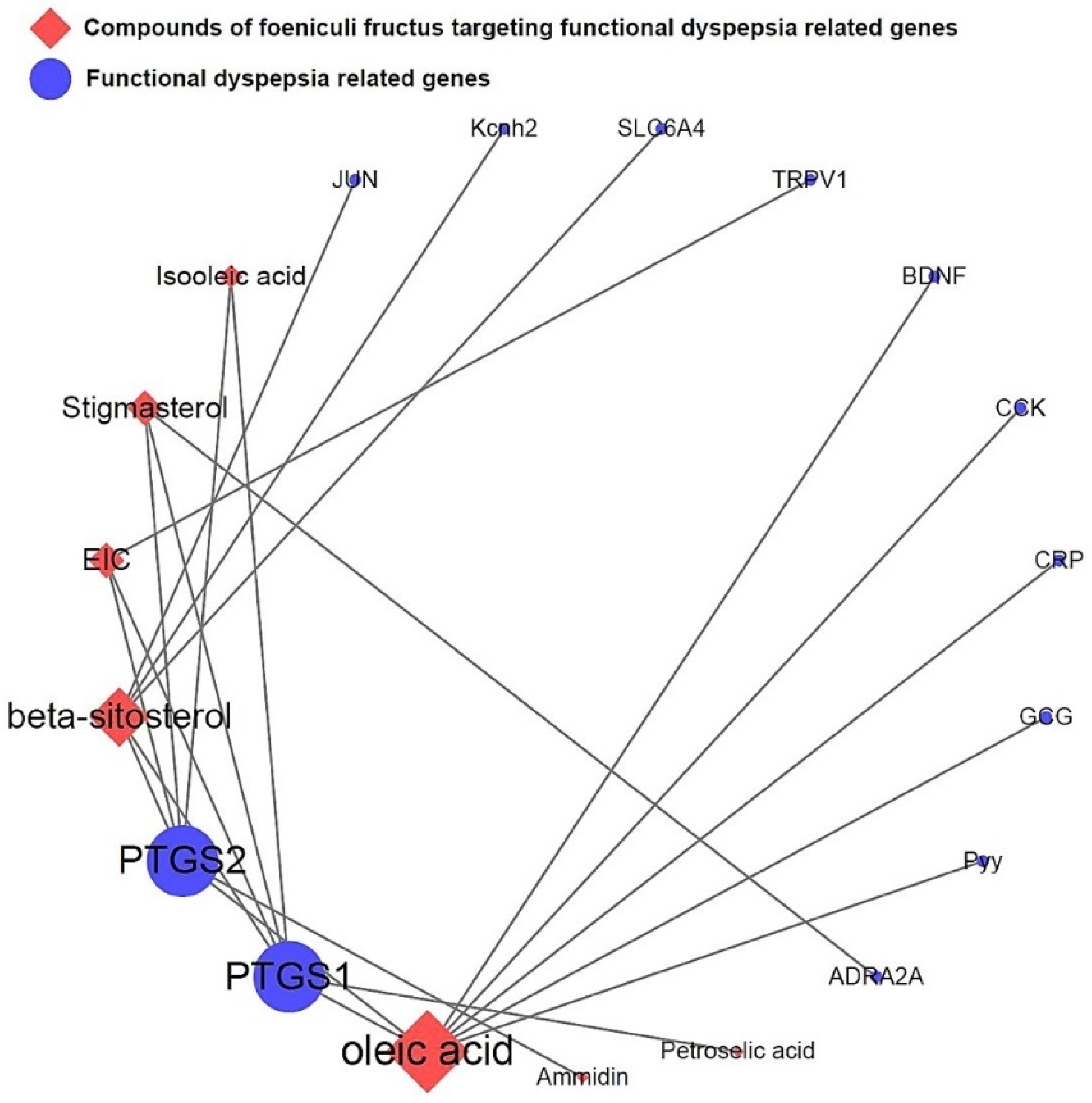
3.4. All 31 GI Disease-Related Compounds in F. fructus except Oleanolic Acid Were Associated with Functional Dyspepsia
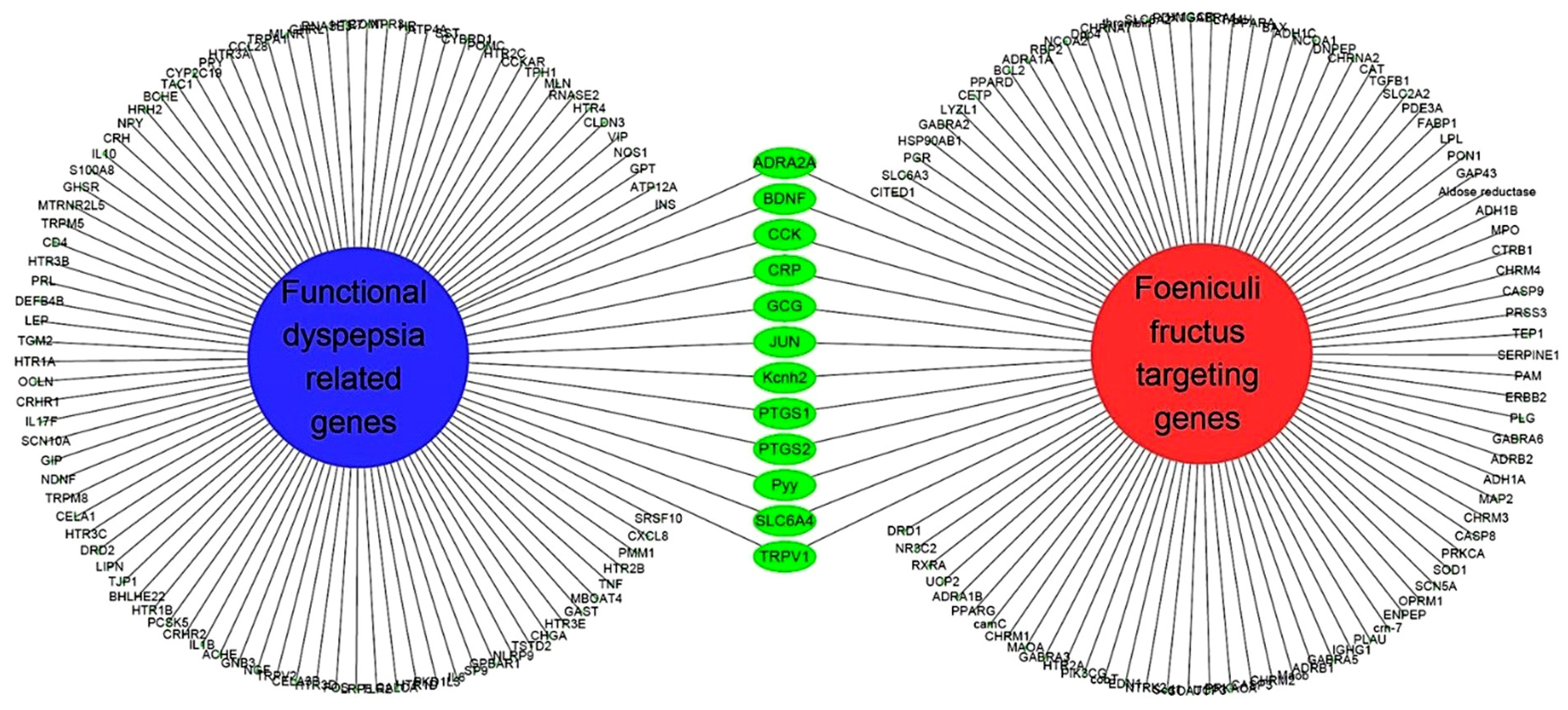
3.5. Network of Functional Dyspepsia-Associated Genes and Compounds
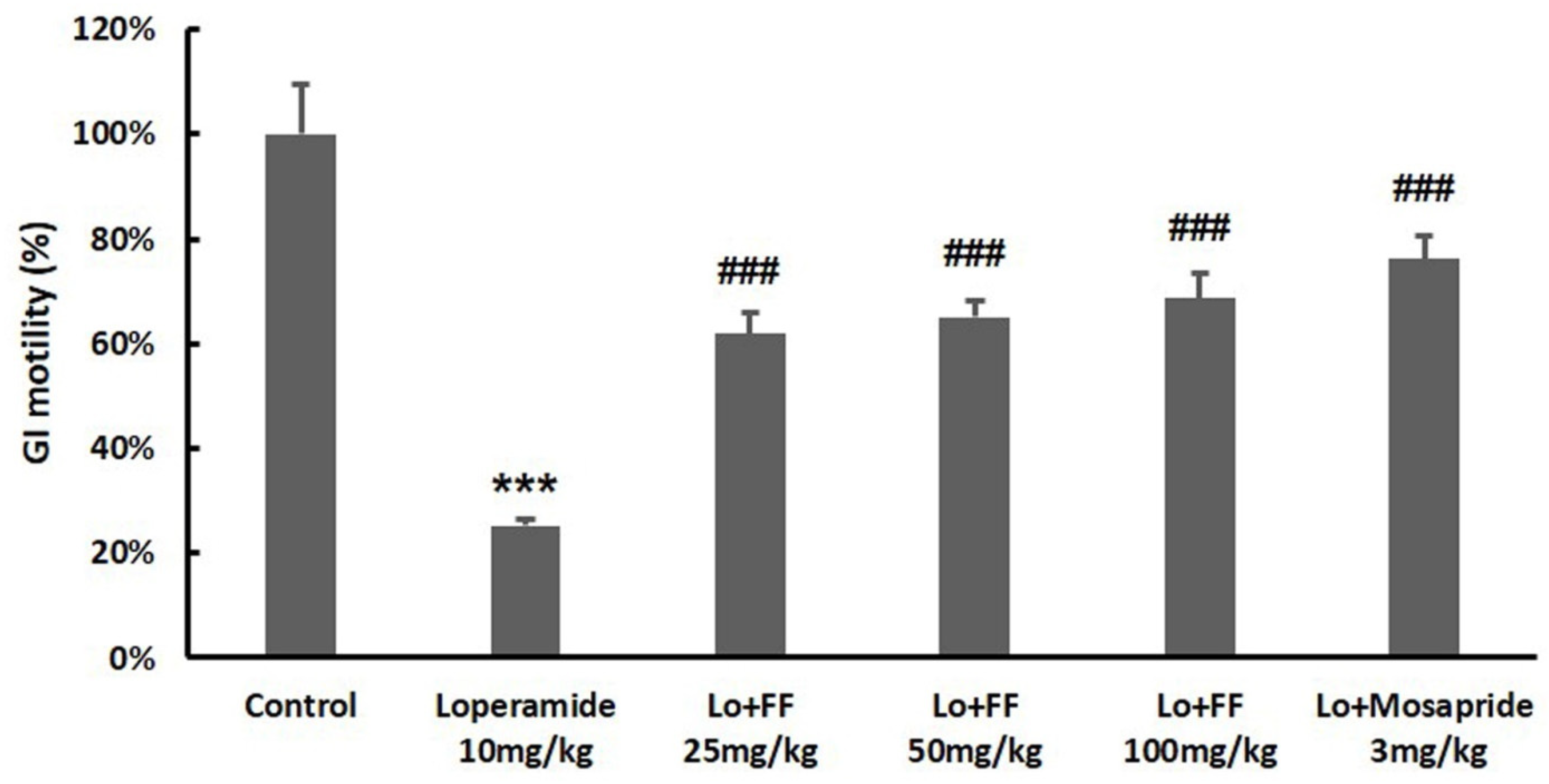
3.6. Mouse Experiment on Delayed Gastric Emptying
3.7. Mouse Experiment on Molecules Involved in Gastrointestinal Motility
3.8. Mouse Experiment on Intestinal Motility
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lacy, B.E.; Chase, R.C.; Cangemi, D.J. The treatment of functional dyspepsia: Present and future. Expert Rev. Gastroenterol. Hepatol. 2023, 17, 9–20. [Google Scholar] [CrossRef]
- Li, J.; Wang, F.; Lv, L.; Xu, L.; Zeng, E.; Tang, X. Histamine H2 antagonists for functional dyspepsia: A protocol for a systematic review and meta-analysis. Medicine 2019, 98, e18128. [Google Scholar] [CrossRef]
- Potter, M.D.E.; Wood, N.K.; Walker, M.M.; Jones, M.P.; Talley, N.J. Proton pump inhibitors and suppression of duodenal eosinophilia in functional dyspepsia. Gut 2019, 68, 1339–1340. [Google Scholar] [CrossRef]
- Ebik, B.; Aslan, N.; Ekin, N.; Bacaksiz, F.; Arpa, M.; Neselioglu, S.; Erel, O.; Ucmak, F. Oxidative stress and the importance of H. pylori eradication in patients with functional dyspepsia. Saudi J. Gastroenterol. 2022, 28, 434–440. [Google Scholar] [CrossRef] [PubMed]
- Das, B.; Rabalais, J.; Kozan, P.; Lu, T.; Durali, N.; Okamoto, K.; McGeough, M.D.; Lee, B.J.; Barrett, K.E.; Marchelletta, R.; et al. The effect of a fennel seed extract on the STAT signaling and intestinal barrier function. PLoS ONE 2022, 17, e0271045. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.M.; Hwang, J.K. Antiinflammatory, analgesic and antioxidant activities of the fruit of Foeniculum vulgare. Fitoterapia 2004, 75, 557–565. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, R.; Barros, L.; Carvalho, A.M.; Ferreira, I.C.F.R. Infusions and Decoctions of Mixed Herbs used in Folk Medicine: Synergism in Antioxidant Potential: Synergism in antioxidant potential of mixed herbs from folk medicine. Phytother. Res. 2011, 25, 1209–1214. [Google Scholar] [CrossRef]
- Ghaffari, P.; Hosseininik, M.; Afrasiabifar, A.; Sadeghi, H.; Hosseininik, A.; Tabatabaei, S.M.; Hosseini, N. The effect of Fennel seed powder on estradiol levels, menopausal symptoms, and sexual desire in postmenopausal women. Menopause 2020, 27, 1281–1286. [Google Scholar] [CrossRef]
- Mokaberinejad, R.; Rampisheh, Z.; Aliasl, J.; Akhtari, E. The comparison of fennel infusion plus dry cupping versus metformin in management of oligomenorrhoea in patients with polycystic ovary syndrome: A randomised clinical trial. J. Obstet. Gynaecol. 2019, 39, 652–658. [Google Scholar] [CrossRef]
- Savino, F.; Cresi, F.; Castagno, E.; Silvestro, L.; Oggero, R. A randomized double-blind placebo-controlled trial of a standardized extract of Matricariaerecutita, Foeniculum vulgare and Melissa officinalis (ColiMil®) in the treatment of breastfed colicky infants. Phytother. Res. 2005, 19, 335–340. [Google Scholar] [CrossRef]
- Özbek, H.; Uğraş, S.; Dülger, H.; Bayram, İ.; Tuncer, İ.; Öztürk, G.; Öztürk, A. Hepatoprotective effect of Foeniculum vulgare essential oil. Fitoterapia 2003, 74, 317–319. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Lee, D.U.; Kim, Y.S.; Kim, H.P. 5-Lipoxygenase Inhibition of the Fructus of Foeniculum vulgare and Its Constituents. Biomol. Ther. 2012, 20, 113–117. [Google Scholar] [CrossRef] [Green Version]
- Badgujar, S.B.; Patel, V.V.; Bandivdekar, A.H. Foeniculum vulgare Mill: A Review of Its Botany, Phytochemistry, Pharmacology, Contemporary Application, and Toxicology. BioMed Res. Int. 2014, 2014, 842674. [Google Scholar] [CrossRef] [Green Version]
- Ma, H.; Zhao, J.; Zhao, X. The Effect of Fennel Tea Drinking on Postoperative Gut Recovery after Gynecological Malignancies Operation. Sichuan Da Xue Xue Bao Yi Xue Ban 2015, 46, 940–943. [Google Scholar] [PubMed]
- Poornima, P.; Kumar, J.D.; Zhao, Q.; Blunder, M.; Efferth, T. Network pharmacology of cancer: From understanding of complex interactomes to the design of multi-target specific therapeutics from nature. Pharmacol. Res. 2016, 111, 290–302. [Google Scholar] [CrossRef]
- Lee, W.Y.; Lee, C.Y.; Kim, Y.S.; Kim, C.E. The Methodological Trends of Traditional Herbal Medicine Employing Network Pharmacology. Biomolecules 2019, 9, 362. [Google Scholar] [CrossRef] [Green Version]
- He, R.; Ou, S.; Chen, S.; Ding, S. Network Pharmacology-Based Study on the Molecular Biological Mechanism of Action for Compound Kushen Injection in Anti-Cancer Effect. Med. Sci. Monit. 2020, 26, e918520. [Google Scholar] [CrossRef] [PubMed]
- Mi, J.L.; Liu, C.; Xu, M.; Wang, R.S. Network Pharmacology to Uncover the Molecular Mechanisms of Action of LeiGongTeng for the Treatment of Nasopharyngeal Carcinoma. Med. Sci. Monit. 2020, 26, e923431. [Google Scholar] [CrossRef]
- Wang, Y.; Dong, B.; Xue, W.; Feng, Y.; Yang, C.; Liu, P.; Cao, J.; Zhu, C. Anticancer Effect of Radix Astragali on Cholangiocarcinoma In Vitro and Its Mechanism via Network Pharmacology. Med. Sci. Monit. 2020, 26, e922837. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Wang, Q.; Liu, M. A Network Pharmacology Approach to Explore the Potential Mechanisms of Huangqin-Baishao Herb Pair in Treatment of Cancer. Med. Sci. Monit. 2020, 26, e923199. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.Q.; Xu, H.B.; Zhang, S.J.; Li, X.Y. Identification of the Active Compounds and Significant Pathways of Artemisia Annua in the Treatment of Non-Small Cell Lung Carcinoma based on Network Pharmacology. Med. Sci. Monit. 2020, 26, e924. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Lee, I.H.; Park, S.I.; Lee, D.Y. Network Pharmacology-Based Investigation of the System-Level Molecular Mechanisms of the Hematopoietic Activity of Samul-Tang, a Traditional Korean Herbal Formula. Evid. Based Complement. Altern. Med. 2020, 2020, 9048089. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Yang, M.; Yang, L.; Xie, C.; Gao, H.; Fu, X.; Xie, H.; Liu, Y. Network Pharmacology-Based Identification of the Mechanisms of Shen-Qi Compound Formula in Treating Diabetes Mellitus. Evid. Based Complement. Altern. Med. 2020, 2020, 5798764. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhong, M.; Long, F.; Yang, R. Deciphering the Active Ingredients and Molecular Mechanisms of Tripterygium hypoglaucum (Levl.) Hutch against Rheumatoid Arthritis Based on Network Pharmacology. Evid. Based Complement. Altern. Med. 2020, 2020, 2361865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, D.H.; Su, Y.F.; Sun, C.X.; Fan, H.F.; Gao, W.J. A Network Pharmacology-Based Identification Study on the Mechanism of Xiao-Xu-Ming Decoction for Cerebral Ischemic Stroke. Evid. Based Complement. Altern. Med. 2020, 2020, 2507074. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Fan, Y.; Tian, C.; Jin, Y.; Du, S.; Zeng, P.; Wang, A. Deciphering the Molecular Targets and Mechanisms of HGWD in the Treatment of Rheumatoid Arthritis via Network Pharmacology and Molecular Docking. Evid. Based Complement. Altern. Med. 2020, 2020, 7151634. [Google Scholar] [CrossRef]
- Qian, H.; Jin, Q.; Liu, Y.; Wang, N.; Chu, Y.; Liu, B.; Liu, Y.; Jiang, W.; Song, Y. Study on the Multitarget Mechanism of Sanmiao Pill on Gouty Arthritis Based on Network Pharmacology. Evid. Based Complement. Altern. Med. 2020, 2020, 9873739. [Google Scholar] [CrossRef]
- Ren, B.; Tan, L.; Xiong, Y.; Ji, W.; Mu, J.; Pei, Y.; Cheng, F.; Wang, X.; Wang, Q. Integrated Analysis of the Mechanisms of Da-Chai-Hu Decoction in Type 2 Diabetes Mellitus by a Network Pharmacology Approach. Evid. Based Complement. Altern. Med. 2020, 2020, 9768414. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, Y.; Luo, J.; Wang, R.; Tang, C.; Zhang, Y.; Borgatti, M. Virtual Screening Technique Used to Estimate the Mechanism of AdhatodavasicaNees for the Treatment of Rheumatoid Arthritis Based on Network Pharmacology and Molecular Docking. Evid. Based Complement. Altern. Med. 2020, 2020, 5872980. [Google Scholar]
- Xiao, K.; Li, K.; Long, S.; Kong, C.; Zhu, S. Potential Molecular Mechanisms of Chaihu-Shugan-San in Treatment of Breast Cancer Based on Network Pharmacology. Evid. Based Complement. Altern. Med. 2020, 2020, 3670309. [Google Scholar] [CrossRef]
- Yang, K.; Zeng, L.; Ge, J. Exploring the Pharmacological Mechanism of DanzhiXiaoyao Powder on ER-Positive Breast Cancer by a Network Pharmacology Approach. Evid. Based Complement. Altern. Med. 2018, 2018, 5059743. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Liao, Y.; Liu, L.; Sun, Y.; Lin, S.; Lan, J.; Mao, H.; Chen, H.; Zhao, Y. A Network Pharmacology Approach to Investigate the Active Compounds and Mechanisms of Musk for Ischemic Stroke. Evid. Based Complement. Altern. Med. 2020, 2020, 4063180. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Wang, Q.; Xiang, Z.; Tong, Q.; Pan, J.; Wan, L.; Chen, J. Network Pharmacology Analysis of Traditional Chinese Medicine Formula Xiao Ke Yin Shui Treating Type 2 Diabetes Mellitus. Evid. Based Complement. Altern. Med. 2019, 2019, 4202563. [Google Scholar] [CrossRef] [Green Version]
- Ru, J.; Li, P.; Wang, J.; Zhou, W.; Li, B.; Huang, C.; Li, P.; Guo, Z.; Tao, W.; Yang, Y.; et al. TCMSP: A database of systems pharmacology for drug discovery from herbal medicines. J. Cheminform. 2014, 6, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- The UniProt Consortium. UniProt: A worldwide hub of protein knowledge. Nucleic Acids Res. 2019, 47, D506–D515. [Google Scholar] [CrossRef] [Green Version]
- Choi, N.R.; Lee, K.; Seo, M.; Ko, S.J.; Choi, W.G.; Kim, S.C.; Kim, J.; Park, J.W.; Kim, B.J. Network Pharmacological Analysis and Experimental Validation of the Effect of Smilacis Glabrae Rhixoma on Gastrointestinal Motility Disorder. Plants 2023, 12, 1509. [Google Scholar] [CrossRef]
- Doncheva, N.T.; Morris, J.H.; Gorodkin, J.; Jensen, L.J. CytoscapeStringApp: Network Analysis and Visualization of Proteomics Data. J. Proteome Res. 2019, 18, 623–632. [Google Scholar] [CrossRef]
- Reagan-Shaw, S.; Nihal, M.; Ahmad, N. Dose translation from animal to human studies revisited. FASEB J. 2008, 22, 659–661. [Google Scholar] [CrossRef] [Green Version]
- Ekor, M. The growing use of herbal medicines: Issues relating to adverse reactions and challenges in monitoring safety. Front. Pharmacol. 2014, 4, 177. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Y.; Zheng, M.; Shao, J.; Jiang, C.; Shen, J.; Tao, R.; Deng, Y.; Xu, Y.; Lu, Y. Upregulation of claudin-4 by Chinese traditional medicine Shenfu attenuates lung tissue damage by acute lung injury aggravated by acute gastrointestinal injury. Pharm. Biol. 2022, 60, 1981–1993. [Google Scholar] [CrossRef]
- Asano, T.; Aida, S.; Suemasu, S.; Mizushima, T. Anethole restores delayed gastric emptying and impaired gastric accommodation in rodents. Biochem. Biophys. Res. Commun. 2016, 472, 125–130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sprouse, J.; Sampath, C.; Gangula, P. 17β-Estradiol Suppresses Gastric Inflammatory and Apoptotic Stress Responses and Restores nNOS-Mediated Gastric Emptying in Streptozotocin (STZ)-Induced Diabetic Female Mice. Antioxidants 2023, 12, 758. [Google Scholar] [CrossRef] [PubMed]
- Nunes Marona, H.R.; Bastos Lucchesi, M.B. Protocol to refine intestinal motility test in mice. Lab. Anim. 2004, 38, 257–260. [Google Scholar] [CrossRef]
- Mittelstadt, S.W.; Hemenway, C.L.; Spruell, R.D. Effects of fasting on evaluation of gastrointestinal transit with charcoal meal. J. Pharmacol. Toxicol. Methods 2005, 52, 154–158. [Google Scholar] [CrossRef] [PubMed]
- El-Soud, N.A.; El-Laithy, N.; El-Saeed, G.; Wahby, M.S.; Khalil, M.; Morsy, F.; Shaffie, N. Antidiabetic Activities of Foeniculum vulgare Mill. Essential Oil in Streptozotocin-Induced Diabetic Rats 8. Maced. J. Med. Sci. 2011, 4, 139–146. [Google Scholar]
- Pourjafari, F.; Haghpanah, T.; Nematollahi-Mahani, S.N.; Pourjafari, F.; Ezzatabadipour, M. Hydroalcoholic extract and seed of Foeniculum vulgare improve folliculogenesis and total antioxidant capacity level in F1 female mice offspring. BMC Complement. Med. Ther. 2020, 20, 294. [Google Scholar] [CrossRef]
- Oktay, M.; Gülçin, İ.; Küfrevioğlu, Ö.İ. Determination of in vitro antioxidant activity of fennel (Foeniculum vulgare) seed extracts. LWT Food Sci. Technol. 2003, 36, 263–271. [Google Scholar] [CrossRef]
- Pradhan, M.; Sribhuwaneswari, S.; Karthikeyan, D.; Minz, S.; Sure, P.; Chandu, A.N.; Mishra, U.; Kamalakannan, K.; Saravanankumar, A.; Sivakumar, T. In-vitro Cytoprotection Activity of Foeniculum vulgare and Helicteresisora in Cultured Human Blood Lymphocytes and Antitumour Activity against B16F10 Melanoma Cell Line. Res. J. Pharm. Tech. 2008, 1, 450–452. [Google Scholar]
- Orhan, İ.E.; ÖzçelİK, B.; Kartal, M.; Kan, Y. Antimicrobial and antiviral effects of essential oils from selected Umbelliferae and Labiatae plants and individual essential oil components. Turk. J. Biol. 2012, 36, 239–246. [Google Scholar]
- Thaler, K.; Kaminski, A.; Chapman, A.; Langley, T.; Gartlehner, G. Bach Flower Remedies for psychological problems and pain: A systematic review. BMC Complement. Altern. Med. 2009, 9, 16. [Google Scholar] [CrossRef] [Green Version]
- Morales, P.; Carvalho, A.M.; Sánchez-Mata, M.C.; Cámara, M.; Molina, M.; Ferreira, I.C.F.R. Tocopherol composition and antioxidant activity of Spanish wild vegetables. Genet. Resour. Crop. Evol. 2012, 59, 851–863. [Google Scholar] [CrossRef]
- Birdane, F.M.; Cemek, M.; Birdane, Y.O.; Gülçin, I.; Büyükokuroğlu, M.E. Beneficial effects of Foeniculum vulgare on ethanol-induced acute gastric mucosal injury in rats. World J. Gastroenterol. 2007, 13, 607–611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noreen, S.; Tufail, T.; Badar Ul Ain, H.; Awuchi, C.G. Pharmacological, nutraceutical, functional and therapeutic properties of fennel (foeniculum vulgare). Int. J. Food Prop. 2023, 26, 915–927. [Google Scholar] [CrossRef]
- Chan, F.K.; To, K.F.; Ng, Y.P.; Lee, T.L.; Cheng, A.S.; Leung, W.K.; Sung, J.J. Expression and cellular localization of COX-1 and -2 in Helicobacter pylori gastritis. Aliment. Pharmacol. Ther. 2001, 15, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Vane, J.R.; Bakhle, Y.S.; Botting, R.M. Cyclooxygenases 1 and 2. Annu. Rev. Pharmacol. Toxicol. 1998, 38, 97–120. [Google Scholar] [CrossRef]
- Wallace, J.L.; Devchand, P.R. Emerging roles for cyclooxygenase-2 in gastrointestinal mucosal defense. Br. J. Pharmacol. 2005, 145, 275–282. [Google Scholar] [CrossRef] [Green Version]
- Lin, H.C.; van Citters, G.W.; Heimer, F.; Bonorris, G. Slowing of gastrointestinal transit by oleic acid: A preliminary report of a novel, nutrient-based treatment in humans. Dig. Dis. Sci. 2001, 46, 223–229. [Google Scholar] [CrossRef]
- Ding, K.; Tan, Y.; Ding, Y.; Fang, Y.; Yang, X.; Fang, J.; Xu, D.; Zhang, H.; Lu, W.; Li, M.; et al. β-Sitosterol improves experimental colitis in mice with a target against pathogenic bacteria. J. Cell. Biochem. 2019, 120, 5687–5694. [Google Scholar] [CrossRef]
- Eckburg, P.B.; Bik, E.M.; Bernstein, C.N.; Purdom, E.; Dethlefsen, L.; Sargent, M.; Gill, S.R.; Nelson, K.E.; Relman, D.A. Diversity of the human intestinal microbial flora. Science 2005, 308, 1635–1638. [Google Scholar] [CrossRef] [Green Version]
- Backhed, F.; Ley, R.E.; Sonnenburg, J.L.; Peterson, D.A.; Gordon, J.I. Host-bacterial mutualism in the human intestine. Science 2005, 307, 1915–1920. [Google Scholar] [CrossRef] [Green Version]
- Lynch, S.V.; Pedersen, O. The Human Intestinal Microbiome in Health and Disease. N. Engl. J. Med. 2016, 375, 2369–2379. [Google Scholar] [CrossRef] [Green Version]
- Barbara, G.; Feinle-Bisset, C.; Ghoshal, U.C.; Quigley, E.M.; Santos, J.; Vanner, S.; Vergnolle, N.; Zoetendal, E.G. The Intestinal Microenvironment and Functional Gastrointestinal Disorders. Gastroenterology 2016, 150, 1305–1318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gkolfakis, P.; Dimitriadis, G.; Triantafyllou, K. Gut microbiota and non-alcoholic fatty liver disease. Hepatobiliary Pancreat. Dis. Int. 2015, 14, 572–581. [Google Scholar] [CrossRef] [PubMed]
- Talley, N.J. What Causes Functional Gastrointestinal Disorders? A Proposed Disease Model. Am. J. Gastroenterol. 2020, 115, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Tziatzios, G.; Giamarellos-Bourboulis, E.J.; Papanikolaou, I.S.; Pimentel, M.; Dimitriadis, G.D.; Triantafyllou, K. Is small intestinal bacterial overgrowth involved in the pathogenesis of functional dyspepsia? Med. Hypotheses 2017, 106, 26–32. [Google Scholar] [CrossRef]
- Lee, M.C.; Ha, W.; Park, J.; Kim, J.; Jung, Y.; Kim, B.J. Effects of Lizhong Tang on gastrointestinal motility in mice. World J. Gastroenterol. 2016, 22, 7778. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hafeez, M.; Hussain, F.; Salamat, A.; Khan, M.B. Gastric emptying scintigraphy in postprandial distress syndrome. Pak. J. Med. Sci. 2018, 34, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Shanahan, E.R.; Kang, S.; Staudacher, H.; Shah, A.; Do, A.; Burns, G.; Chachay, V.S.; Koloski, N.A.; Keely, S.; Walker, M.M.; et al. Alterations to the duodenal microbiota are linked to gastric emptying and symptoms in functional dyspepsia. Gut 2023, 72, 929–938. [Google Scholar] [CrossRef]
- Futagami, S.; Yamawaki, H.; Shimpuku, M.; Izumi, N.; Wakabayashi, T.; Kodaka, Y.; Nagoya, H.; Shindo, T.; Kawagoe, T.; Sakamoto, C. Impact of Coexisting Irritable Bowel Syndrome and Non-erosive Reflux Disease on Postprandial Abdominal Fullness and Sleep Disorders in Functional Dyspepsia. J. Nippon. Med. Sch. 2013, 80, 362–370. [Google Scholar] [CrossRef] [Green Version]
- Kaji, M.; Fujiwara, Y.; Shiba, M.; Kohata, Y.; Yamagami, H.; Tanigawa, T.; Watanabe, K.; Watanabe, T.; Tominaga, K.; Arakawa, T. Prevalence of overlaps between GERD, FD and IBS and impact on health-related quality of life: Overlap of FGIDs and HR-QOL. J. Gastroenterol. Hepatol. 2010, 25, 1151–1156. [Google Scholar] [CrossRef]
- Choi, Y.J.; Kim, N.; Yoon, H.; Shin, C.M.; Park, Y.S.; Kim, J.W.; Kim, Y.S.; Lee, D.H.; Jung, H.C. Overlap between irritable bowel syndrome and functional dyspepsia including subtype analyses: Overlap of IBS and dyspepsia. J. Gastroenterol. Hepatol. 2017, 32, 1553–1561. [Google Scholar] [CrossRef]
- Terauchi, A.; Kobayashi, D.; Mashimo, H. Distinct roles of nitric oxide synthases and interstitial cells of Cajal in rectoanal relaxation. Am. J. Physiol. Gastrointest. Liver Physiol. 2005, 289, G291–G299. [Google Scholar] [CrossRef] [Green Version]
- Park, J.M.; Baeg, M.K.; Lim, C.H.; Cho, Y.K.; Choi, M.G. Nitric Oxide Synthase Gene Polymorphisms in Functional Dyspepsia. Dig. Dis. Sci. 2014, 59, 72–77. [Google Scholar] [CrossRef]
- Gomez-Pinilla, P.J.; Gibbons, S.J.; Bardsley, M.R.; Lorincz, A.; Pozo, M.J.; Pasricha, P.J.; de Rijn, M.V.; West, R.B.; Sarr, M.G.; Kendrick, M.L.; et al. Ano1 is a selective marker of interstitial cells of Cajal in the human and mouse gastrointestinal tract. Am. J. Physiol. Gastrointest. Liver Physiol. 2009, 296, G1370–G1381. [Google Scholar] [CrossRef] [Green Version]
- Sanders, K.M.; Hwang, S.J.; Ward, S.M. Neuroeffector apparatus in gastrointestinal smooth muscle organs: Neural regulation of GI smooth muscle. J. Physiol. 2010, 588, 4621–4639. [Google Scholar] [CrossRef] [PubMed]
- Saeki, T.; Kimura, T.; Hashidume, K.; Murayama, T.; Yamamura, H.; Ohya, S.; Suzuki, Y.; Nakayama, S.; Imaizumi, Y. Conversion of Ca2+ oscillation into propagative electrical signals by Ca2+-activated ion channels and connexin as a reconstituted Ca2+ clock model for the pacemaker activity. Biochem. Biophys. Res. Commun. 2019, 510, 242–247. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.N.; Ohya, S.; Wang, J.; Imaizumi, Y.; Nakayama, S. Involvement of ryanodine receptors in pacemaker Ca2+ oscillation in murine gastric ICC. Biochem. Biophys. Res. Commun. 2005, 328, 640–646. [Google Scholar] [CrossRef]
- He, W.; Peng, Y.; Zhang, W.; Lv, N.; Tang, J.; Chen, C.; Zhang, C.; Gao, S.; Chen, H.; Zhi, G.; et al. Myosin Light Chain Kinase Is Central to Smooth Muscle Contraction and Required for Gastrointestinal Motility in Mice. Gastroenterology 2008, 135, 610–620.e2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, W.; Feng, P. Myosin Light Chain Kinase Is Involved in the Mechanism of Gastrointestinal Dysfunction in Diabetic Rats. Dig. Dis. Sci. 2012, 57, 1197–1202. [Google Scholar] [CrossRef]
- Mazzone, A.; Bernard, C.E.; Strege, P.R.; Beyder, A.; Galietta, L.J.V.; Pasricha, P.J.; Rae, J.L.; Parkman, H.P.; Linden, D.R.; Szurszewski, J.H.; et al. Altered Expression of Ano1 Variants in Human Diabetic Gastroparesis. J. Biol. Chem. 2011, 286, 13393–13403. [Google Scholar] [CrossRef] [Green Version]



| Time (min) | 0.1% FA/Water (%) | 0.1% FA/Acetonitrile (%) | Flow Rate (mL/min) |
|---|---|---|---|
| 0 | 98 | 2 | 0.40 |
| 1.0 | 98 | 2 | 0.40 |
| 3.0 | 85 | 15 | 0.40 |
| 5.0 | 75 | 25 | 0.40 |
| 6.0 | 55 | 45 | 0.40 |
| 8.0 | 50 | 50 | 0.40 |
| 9.0 | 30 | 70 | 0.40 |
| 10.0 | 10 | 90 | 0.40 |
| 12.0 | 2 | 98 | 0.40 |
| 14.0 | 98 | 2 | 0.40 |
| 16.0 | 98 | 2 | 0.40 |
| F. fructus (Unit: mg/kg) | |
|---|---|
| 4-Methoxybenzoic acid | 0.219 ± 0.042 |
| Anethole | 63.029 ± 2.076 |
| R-(a)-Phellandrene | 0.792 ± 0.059 |
| Gene | Primer | Sequence (5′ to 3′) | Product Length (bp) |
|---|---|---|---|
| 5HT4R | Forward | AGTTCCAACGAGGGTTTCAGG | 92 |
| Reverse | CAGCAGGTTGCCCAAGATG | ||
| ANO1 | Forward | GGCATTTGTCATTGTCTTCCAG | 140 |
| Reverse | TCCTCACGCATAAACAGCTC | ||
| RYR3 | Forward | GGCCAAGAACATCAGAGTGACTAA | 79 |
| Reverse | TCACTTCTGCCCTGTCAGTTTC | ||
| smMLCK | Forward | AGAAGTCAAGGAGGTAAAGAATGATGT | 76 |
| Reverse | CGGGTCGCTTTTCATTGC | ||
| GAPDH | Forward | CATGGCCTTCCGTGTTCCT | 103 |
| Reverse | CCTGCTTCACCACCTTCTTGA |
| Molecule Name | Structure | MW | OB(%) | Caco-2 | DL |
|---|---|---|---|---|---|
| Ammidin | 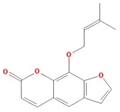 | 270.3 | 34.55 | 1.13 | 0.22 |
| beta-sitosterol |  | 414.79 | 36.91 | 1.32 | 0.75 |
| EIC | 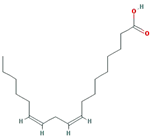 | 280.5 | 41.9 | 1.16 | 0.14 |
| Isooleic acid | 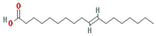 | 282.52 | 33.13 | 1.15 | 0.14 |
| Majudin |  | 216.2 | 42.21 | 0.94 | 0.13 |
| oleic acid |  | 282.52 | 33.13 | 1.17 | 0.14 |
| Petroselic acid | 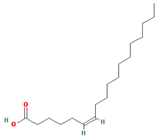 | 282.52 | 33.13 | 1.17 | 0.14 |
| Stigmasterol | 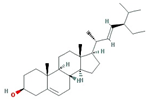 | 412.77 | 43.83 | 1.44 | 0.76 |
| Uvadex | 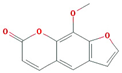 | 216.2 | 35.3 | 1.05 | 0.13 |
| Molecule Name | Gene Name | Disease Name |
|---|---|---|
| (−)-nopinene | PTGS1 | * Functional dyspepsia |
| PTGS2 | * Functional dyspepsia Colorectal cancer Peutz–Jeghers syndrome Oropharyngeal squamous cell carcinoma | |
| (1S,5S)-1-isopropyl-4-methylenebicyclo [3.1.0]hexane | PTGS2 | * Functional dyspepsia Colorectal cancer Peutz–Jeghers syndrome Oropharyngeal squamous cell carcinoma |
| (S)-(+)-alpha-Phellandrene | ACHE | * Functional dyspepsia |
| 1,8-cineole | NOS3 | Colorectal cancer |
| PTGS2 | * Functional dyspepsia Colorectal cancer Peutz–Jeghers syndrome Oropharyngeal squamous cell carcinoma | |
| acetaldehyde | BCHE | * Functional dyspepsia |
| CTNNB1 | Colorectal cancer | |
| FOS | * Functional dyspepsia | |
| IL1B | * Functional dyspepsia | |
| IL6 | * Functional dyspepsia | |
| JUN | * Functional dyspepsia | |
| LTA4H | Esophageal cancer | |
| MAPK8 | Crohn’s Disease, unspecified | |
| MAPK9 | Crohn’s Disease, unspecified | |
| MMP1 | Kaposi’s Sarcoma Pancreatic Cancer | |
| Mmp12 | Crohn’s Disease, unspecified Gastro-intestinal ulcers Ulcerative colitis | |
| MMP2 | Kaposi’s Sarcoma Pancreatic Cancer | |
| MMP3 | Pancreatic Cancer | |
| NOS1 | * Functional dyspepsia | |
| OPRK1 | Diarrhea | |
| POMC | * Functional dyspepsia | |
| PTGS1 | * Functional dyspepsia | |
| PTGS2 | * Functional dyspepsia Colorectal cancer Peutz–Jeghers syndrome Oropharyngeal squamous cell carcinoma | |
| RARB | Pancreatic Cancer | |
| RRM1 | Pancreatic Neoplasms | |
| TNF | * Functional dyspepsia Crohn’s Disease, unspecified | |
| alpha-amyrin | PTGS2 | * Functional dyspepsia Colorectal cancer Peutz–Jeghers syndrome Oropharyngeal squamous cell carcinoma |
| Ammidin | PTGS2 | * Functional dyspepsia Colorectal cancer Peutz–Jeghers syndrome Oropharyngeal squamous cell carcinoma |
| anethole | JUN | * Functional dyspepsia |
| Anisketone | ACHE | * Functional dyspepsia |
| ADRA2A | * Functional dyspepsia | |
| CA2 | Pancreatic Cancer | |
| NOS3 | Colon cancer | |
| PTGS1 | * Functional dyspepsia | |
| PTGS2 | * Functional dyspepsia Colorectal cancer Peutz–Jeghers syndrome Oropharyngeal squamous cell carcinoma | |
| ANN | PTGS1 | * Functional dyspepsia |
| PTGS2 | * Functional dyspepsia Colorectal cancer Peutz–Jeghers syndrome Oropharyngeal squamous cell carcinoma | |
| APIOL | ADRA2A | * Functional dyspepsia |
| LTA4H | Esophageal cancer | |
| NOS3 | Colon cancer | |
| PTGS1 | * Functional dyspepsia | |
| PTGS2 | * Functional dyspepsia Colorectal cancer Peutz–Jeghers syndrome Oropharyngeal squamous cell carcinoma | |
| SLC6A4 | * Functional dyspepsia | |
| Arachic acid | HSP90AB1 | Gastrointestinal Stromal Tumors (GIST) |
| PTGS1 | * Functional dyspepsia | |
| PTGS2 | * Functional dyspepsia Colorectal cancer Peutz–Jeghers syndrome Oropharyngeal squamous cell carcinoma | |
| beta-sitosterol | HSP90AB1 | Gastrointestinal Stromal Tumors (GIST) |
| JUN | * Functional dyspepsia | |
| Kcnh2 | * Functional dyspepsia | |
| OPRM1 | Diarrhea Opioid-induced bowel dysfunction | |
| PTGS1 | * Functional dyspepsia | |
| PTGS2 | * Functional dyspepsia Colorectal cancer Peutz–Jeghers syndrome Oropharyngeal squamous cell carcinoma | |
| SLC6A4 | * Functional dyspepsia | |
| Butyrophenone | CA2 | Pancreatic Cancer |
| PTGS1 | * Functional dyspepsia | |
| PTGS2 | * Functional dyspepsia Colorectal cancer Peutz–Jeghers syndrome Oropharyngeal squamous cell carcinoma | |
| cis-ligustilide | PTGS2 | * Functional dyspepsia Colorectal cancer Peutz–Jeghers syndrome Oropharyngeal squamous cell carcinoma |
| D-Camphene | Kcnh2 | * Functional dyspepsia |
| PTGS2 | * Functional dyspepsia Colorectal cancer Peutz–Jeghers syndrome Oropharyngeal squamous cell carcinoma | |
| EIC | PTGS1 | * Functional dyspepsia |
| PTGS2 | * Functional dyspepsia Colorectal cancer Peutz–Jeghers syndrome Oropharyngeal squamous cell carcinoma | |
| TRPV1 | * Functional dyspepsia | |
| Fenchylacetate | PTGS2 | * Functional dyspepsia Colorectal cancer Peutz–Jeghers syndrome Oropharyngeal squamous cell carcinoma |
| Isooleic acid | PTGS1 | * Functional dyspepsia |
| PTGS2 | * Functional dyspepsia Colorectal cancer Peutz–Jeghers syndrome Oropharyngeal squamous cell carcinoma | |
| L-Limonen | PTGS2 | * Functional dyspepsia Colorectal cancer Peutz–Jeghers syndrome Oropharyngeal squamous cell carcinoma |
| Moslene | ACHE | * Functional dyspepsia |
| PTGS2 | * Functional dyspepsia Colorectal cancer Peutz–Jeghers syndrome Oropharyngeal squamous cell carcinoma | |
| myristic acid | BCHE | * Functional dyspepsia |
| PTGS1 | * Functional dyspepsia | |
| PTGS2 | * Functional dyspepsia Colorectal cancer Peutz–Jeghers syndrome Oropharyngeal squamous cell carcinoma | |
| oleanolic acid | AMY2A | Pancreatic disease |
| oleic acid | BDNF | * Functional dyspepsia |
| CCk | * Functional dyspepsia | |
| CRP | * Functional dyspepsia | |
| GCG | * Functional dyspepsia | |
| PTGS1 | * Functional dyspepsia | |
| PTGS2 | * Functional dyspepsia Colorectal cancer Peutz–Jeghers syndrome Oropharyngeal squamous cell carcinoma | |
| Pyy | * Functional dyspepsia | |
| palmitic acid | IL10 | * Functional dyspepsia |
| PTGS1 | * Functional dyspepsia | |
| PTGS2 | * Functional dyspepsia Colorectal cancer Peutz–Jeghers syndrome Oropharyngeal squamous cell carcinoma | |
| TNF | * Functional dyspepsia Crohns’s Disease, unspecified | |
| PENTADECYLIC ACID | PTGS1 | * Functional dyspepsia |
| PTGS2 | * Functional dyspepsia Colorectal cancer Peutz–Jeghers syndrome Oropharyngeal squamous cell carcinoma | |
| Petroselic acid | PTGS1 | * Functional dyspepsia |
| Sitogluside | HSP90AB1 | Gastrointestinal Stromal Tumors (GIST) |
| HTR3A | * Functional dyspepsia Diarrhea Postoperative nausea and vomiting Irritable bowel syndrome Chemotherapy-induced nausea and vomiting | |
| Kcnh2 | * Functional dyspepsia | |
| PTGS1 | * Functional dyspepsia | |
| PTGS2 | * Functional dyspepsia Colorectal cancer Peutz–Jeghers syndrome Oropharyngeal squamous cell carcinoma | |
| Skimmetin | ADRA2A | * Functional dyspepsia |
| LTA4H | Esophageal cancer | |
| PTGS1 | * Functional dyspepsia | |
| PTGS2 | * Functional dyspepsia Colorectal cancer Peutz–Jeghers syndrome Oropharyngeal squamous cell carcinoma | |
| Stigmasterol | ADRA2A | * Functional dyspepsia |
| LTA4H | Esophageal cancer | |
| PTGS1 | * Functional dyspepsia | |
| PTGS2 | * Functional dyspepsia Colorectal cancer Peutz–Jeghers syndrome Oropharyngeal squamous cell carcinoma | |
| TDA | PTGS1 | * Functional dyspepsia |
| Terragon | ADRA2A | * Functional dyspepsia |
| PTGS1 | * Functional dyspepsia | |
| PTGS2 | * Functional dyspepsia Colorectal cancer Peutz–Jeghers syndrome Oropharyngeal squamous cell carcinoma |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, N.-R.; Jung, D.; Kim, S.-C.; Park, J.-W.; Choi, W.-G.; Kim, B.-J. Analysis of Network Pharmacological Efficacy and Therapeutic Effectiveness in Animal Models for Functional Dyspepsia of Foeniculi fructus. Nutrients 2023, 15, 2644. https://doi.org/10.3390/nu15122644
Choi N-R, Jung D, Kim S-C, Park J-W, Choi W-G, Kim B-J. Analysis of Network Pharmacological Efficacy and Therapeutic Effectiveness in Animal Models for Functional Dyspepsia of Foeniculi fructus. Nutrients. 2023; 15(12):2644. https://doi.org/10.3390/nu15122644
Chicago/Turabian StyleChoi, Na-Ri, Daehwa Jung, Sang-Chan Kim, Jae-Woo Park, Woo-Gyun Choi, and Byung-Joo Kim. 2023. "Analysis of Network Pharmacological Efficacy and Therapeutic Effectiveness in Animal Models for Functional Dyspepsia of Foeniculi fructus" Nutrients 15, no. 12: 2644. https://doi.org/10.3390/nu15122644
APA StyleChoi, N.-R., Jung, D., Kim, S.-C., Park, J.-W., Choi, W.-G., & Kim, B.-J. (2023). Analysis of Network Pharmacological Efficacy and Therapeutic Effectiveness in Animal Models for Functional Dyspepsia of Foeniculi fructus. Nutrients, 15(12), 2644. https://doi.org/10.3390/nu15122644







