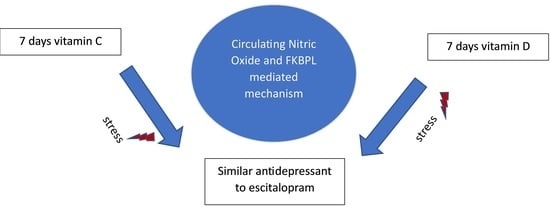
Vitamins C and D Exhibit Similar Antidepressant Effects to Escitalopram Mediated by NOx and FKBPL in a Stress-Induced Mice Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Study Design and Treatments
2.3. Acute Restraint Model
2.4. Behavioral Paradigms
2.4.1. Forced Swim Test
2.4.2. Tail Suspension Test
2.5. Elevated Plus Maze
2.6. Open Field Test
2.7. Western Blots
2.8. Nitric Oxide Assay
2.9. Statistical Analysis
3. Results
3.1. Forced Swim Test
3.2. Tail Suspension Test
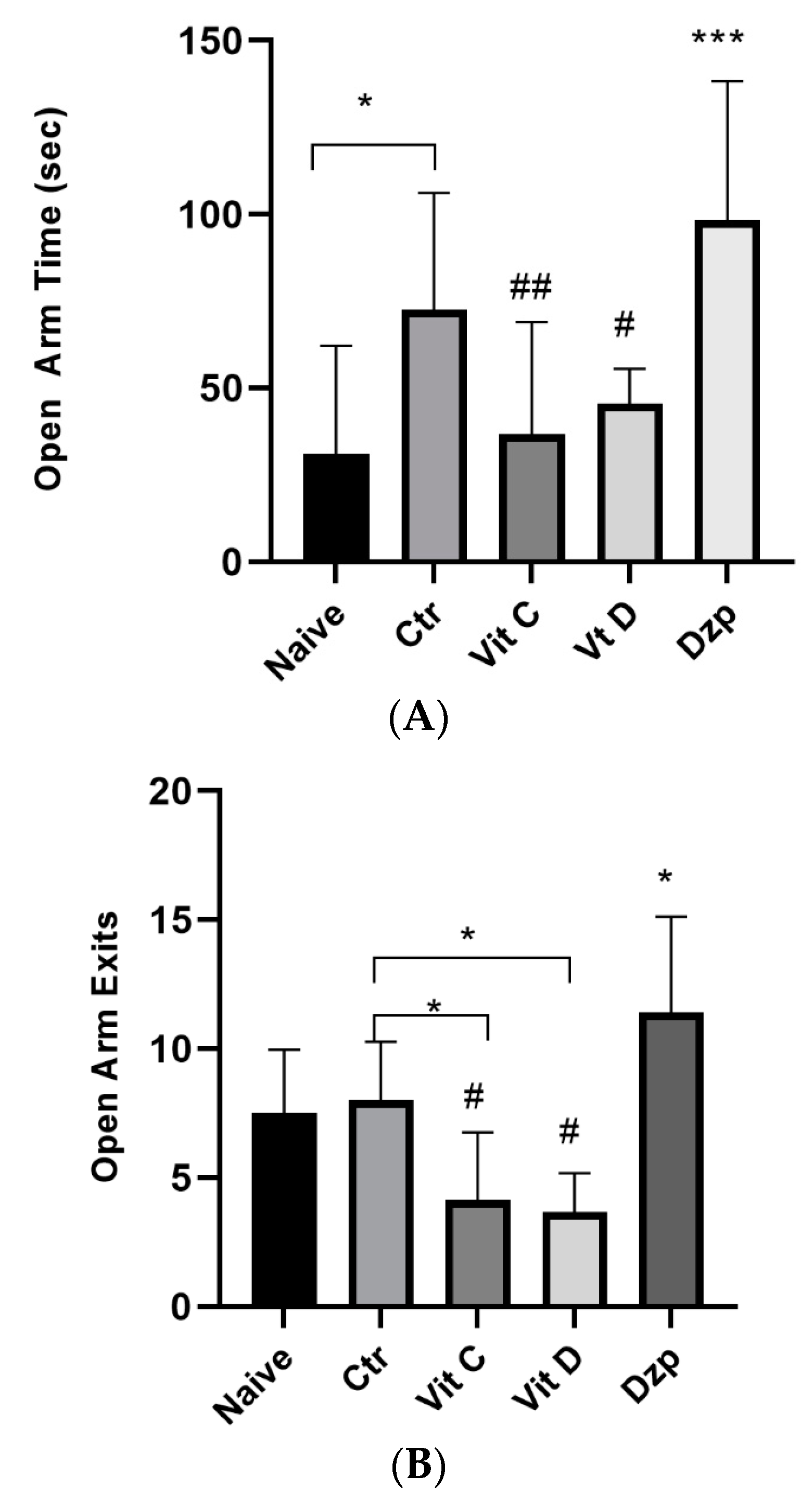
3.3. Elevated Plus Maze
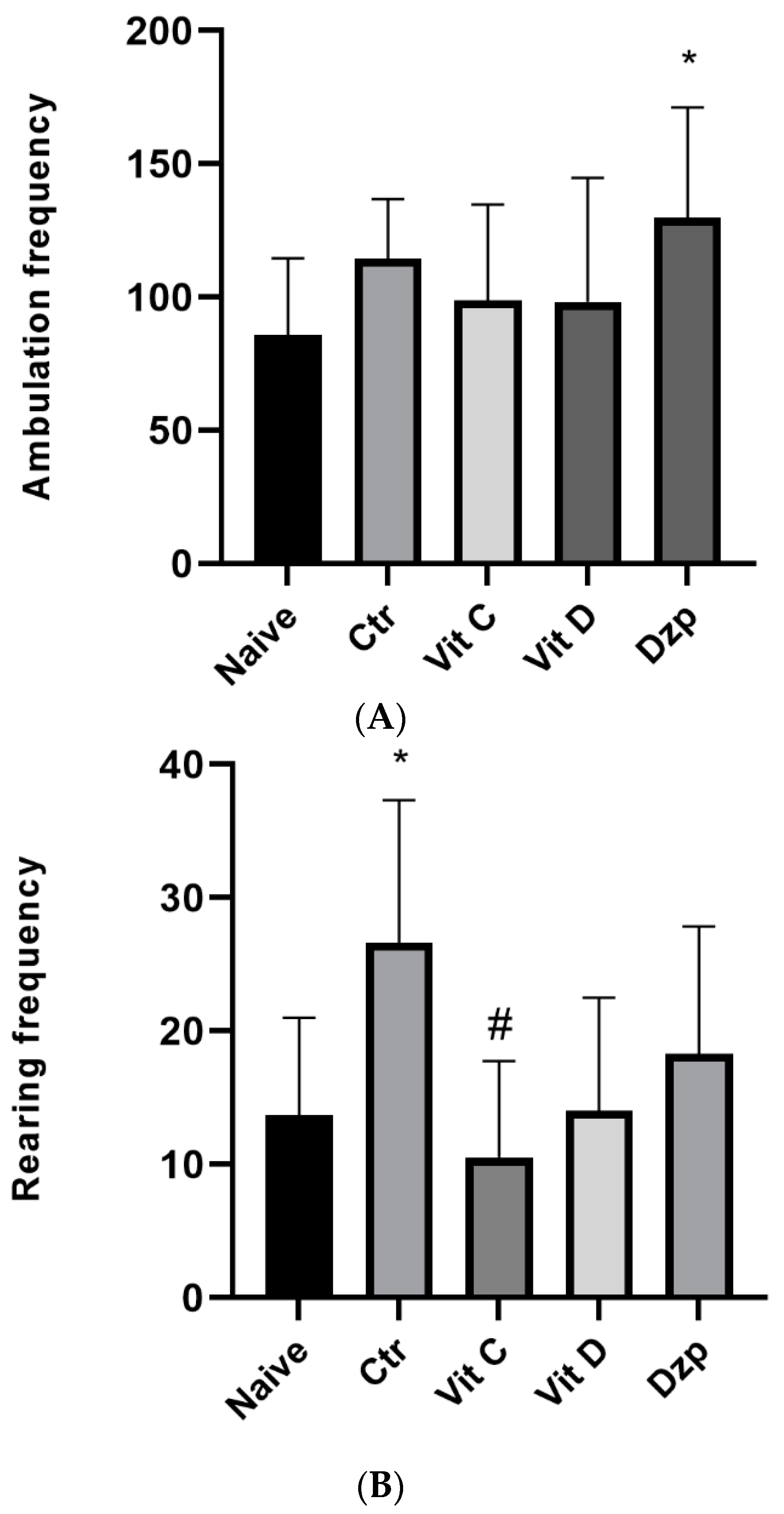
3.4. Open Field Test
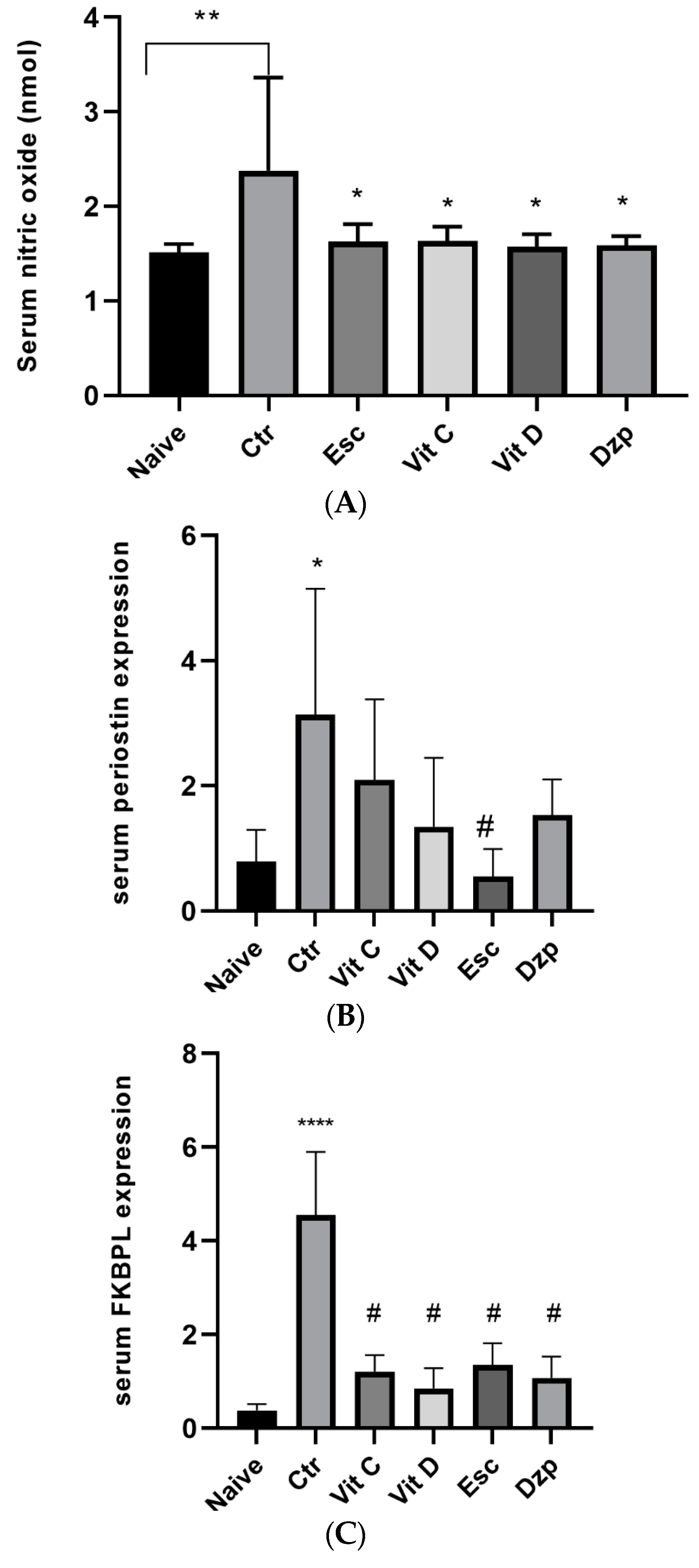
3.5. Nitric Oxide (NOx)
3.6. Periostin
3.7. FKBPL
4. Discussion
Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ettman, C.K.; Abdalla, S.M.; Cohen, G.H.; Sampson, L.; Vivier, P.M.; Galea, S. Prevalence of Depression Symptoms in US Adults before and during the COVID-19 Pandemic. JAMA Netw. Open 2020, 3, e2019686. [Google Scholar] [CrossRef] [PubMed]
- Kessler, R.C.; Bromet, E.J. The Epidemiology of Depression Across Cultures. Annu. Rev. Public Health 2013, 34, 119–138. [Google Scholar] [CrossRef] [Green Version]
- Mathers, C. The Global Burden of Disease: 2004 Update; World Health Organization: Lyon, France, 2008. [Google Scholar]
- Ressler, K.J.; Mayberg, H.S. Targeting Abnormal Neural Circuits in Mood and Anxiety Disorders: From the Laboratory to the Clinic. Nat. Neurosci. 2007, 10, 1116–1124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rakesh, G.; Pae, C.U.; Masand, P.S. Beyond Serotonin: Newer Antidepressants in the Future. Expert Rev. Neurother. 2017, 17, 777–790. [Google Scholar] [CrossRef]
- Amr, M.; El-Mogy, A.; Shams, T.; Vieira, K.; Lakhan, S.E. Efficacy of Vitamin C as an Adjunct to Fluoxetine Therapy in Pediatric Major Depressive Disorder: A Randomized, Double-Blind, Placebo-Controlled Pilot Study. Nutr. J. 2013, 12, 31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geng, C.; Shaikh, A.S.; Han, W.; Chen, D.; Guo, Y.; Jiang, P. Vitamin D and Depression: Mechanisms, Determination and Application. Asia Pac. J. Clin. Nutr. 2019, 28, 689–694. [Google Scholar] [CrossRef] [PubMed]
- Sahraian, A.; Ghanizadeh, A.; Kazemeini, F. Vitamin C as an Adjuvant for Treating Major Depressive Disorder and Suicidal Behavior, a Randomized Placebo-Controlled Clinical Trial. Trials 2015, 16, 4–11. [Google Scholar] [CrossRef] [Green Version]
- Guix, F.X.; Uribesalgo, I.; Coma, M.; Mun, F.J. The Physiology and Pathophysiology of Nitric Oxide in the Brain. Prog. Neurobiol. 2005, 76, 126–152. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Paik, J.; Lee, S.; Yoon, D.; Han, C.; Lee, B. Increased Plasma Nitric Oxide Level Associated with Suicide Attempt in Depressive Patients. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2006, 30, 1091–1096. [Google Scholar] [CrossRef]
- Lee, B.; Lee, S.; Yoon, D.; Lee, H. Increased Plasma Nitric Oxide Metabolites in Suicide Attempters. Neuropsychobiology 2006, 20, 127–132. [Google Scholar] [CrossRef]
- Chen, H.J.C.; Spiers, J.G.; Sernia, C.; Lavidis, N.A. Response of the Nitrergic System to Activation of the Neuroendocrine Stress Axis. Front. Neurosci. 2015, 9, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, D.; Zhou, W.; Wang, Z.; Wang, Y.; Liu, M.; Zhang, G.; Guo, X.; Kang, X. Periostin: An Emerging Molecule with a Potential Role in Spinal Degenerative Diseases. Front. Med. 2021, 8, 694800. [Google Scholar] [CrossRef] [PubMed]
- Bignold, R.E.; Johnson, J.R. Matricellular Protein Periostin Promotes Pericyte Migration in Fibrotic Airways. Front. Allergy 2021, 2, 95. [Google Scholar] [CrossRef] [PubMed]
- Alqudah, A.; AbuDalo, R.; Qnais, E.; Wedyan, M.; Oqal, M.; McClements, L. The Emerging Importance of Immunophilins in Fibrosis Development. Mol. Cell. Biochem. 2022, 478, 1281–1291. [Google Scholar] [CrossRef]
- Annett, S.; Spence, S.; Garciarena, C.; Campbell, C.; Dennehy, M.; Drakeford, C.; Lai, J.; Dowling, J.; Moore, G.; Yakkundi, A.; et al. Title: The Immunophilin Protein FKBPL and Its Peptide Derivatives Are Novel Regulators of Vascular Integrity and Inflammation via NF-ΚB Signaling. bioRxiv 2021. [Google Scholar] [CrossRef]
- Annett, S.; Moore, G.; Short, A.; Marshall, A.; McCrudden, C.; Yakkundi, A.; Das, S.; McCluggage, W.G.; Nelson, L.; Harley, I.; et al. FKBPL-Based Peptide, ALM201, Targets Angiogenesis and Cancer Stem Cells in Ovarian Cancer. Br. J. Cancer 2019, 122, 361–371. [Google Scholar] [CrossRef]
- Valentine, A.; O’Rourke, M.; Yakkundi, A.; Worthington, J.; Hookham, M.; Bicknell, R.; McCarthy, H.O.; McClelland, K.; McCallum, L.; Dyer, H.; et al. FKBPL and Peptide Derivatives: Novel Biological Agents That Inhibit Angiogenesis by a CD44-Dependent Mechanism. Clin. Cancer Res. 2011, 17, 1044–1056. [Google Scholar] [CrossRef] [Green Version]
- Robson, T.; Joiner, M.C.; Wilson, G.D.; McCullough, W.; Price, M.E.; Logan, I.; Jones, H.; McKeown, S.R.; Hirst, D.G. A Novel Human Stress Response-Related Gene with a Potential Role in Induced Radioresistance. Radiat. Res. 1999, 152, 451. [Google Scholar] [CrossRef]
- McKeen, H.D.; McAlpine, K.; Valentine, A.; Quinn, D.J.; McClelland, K.; Byrne, C.; O’Rourke, M.; Young, S.; Scott, C.J.; McCarthy, H.O.; et al. A Novel FK506-like Binding Protein Interacts with the Glucocorticoid Receptor and Regulates Steroid Receptor Signaling. Endocrinology 2008, 149, 5724–5734. [Google Scholar] [CrossRef] [Green Version]
- Sunnotel, O.; Hiripi, L.; Lagan, K.; McDaid, J.R.; De León, J.M.; Miyagawa, Y.; Crowe, H.; Kaluskar, S.; Ward, M.; Scullion, C.; et al. Alterations in the Steroid Hormone Receptor Co-Chaperone FKBPL Are Associated with Male Infertility: A Case-Control Study. Reprod. Biol. Endocrinol. 2010, 8, 22. [Google Scholar] [CrossRef] [Green Version]
- Huzard, D.; Rappeneau, V.; Meijer, O.C.; Touma, C.; Arango-Lievano, M.; Garabedian, M.J.; Jeanneteau, F. Experience and Activity-Dependent Control of Glucocorticoid Receptors during the Stress Response in Large-Scale Brain Networks. Stress 2020, 24, 130–153. [Google Scholar] [CrossRef] [PubMed]
- De Kloet, E.R.; Joëls, M. Mineralocorticoid Receptors and Glucocorticoid Receptors in HPA Stress Responses During Coping and Adaptation. Oxf. Res. Encycl. Neurosci. 2020, 560, 738458. [Google Scholar] [CrossRef]
- Gammoh, O.; Mayyas, F.; Darwish Elhajji, F. Chlorpheniramine and Escitalopram: Similar Antidepressant and Nitric Oxide Lowering Roles in a Mouse Model of Anxiety. Biomed. Rep. 2017, 6, 675–680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Machawal, L.; Kumar, A. Possible Involvement of Nitric Oxide Mechanism in the Neuroprotective Effect of Rutin against Immobilization Stress Induced Anxiety like Behaviour, Oxidative Damage in Mice. Pharmacol. Rep. 2014, 66, 15–21. [Google Scholar] [CrossRef]
- Porsolt, R.D.; Bertin, A.; Blavet, N.; Deniel, M.; Jalfre, M. Immobility Induced by Forced Swimming in Rats: Effects of Agents Which Modify Central Catecholamine and Serotonin Activity. Eur. J. Pharmacol. 1979, 57, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Binfaré, R.W.; Rosa, A.O.; Lobato, K.R.; Santos, A.R.S.; Rodrigues, A.L.S. Ascorbic Acid Administration Produces an Antidepressant-like Effect: Evidence for the Involvement of Monoaminergic Neurotransmission. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2009, 33, 530–540. [Google Scholar] [CrossRef]
- Dishman, R.K.; Armstrong, R.B.; Delp, M.D.; Graham, R.E.; Dunn, A.L. Open-Field Behavior Is Not Related to Treadmill Performance in Exercising Rats. Physiol. Behav. 1988, 43, 541–546. [Google Scholar] [CrossRef]
- Mirzaii-Dizgah, M.-H.; Mirzaii-Dizgah, M.-R.; Mirzaii-Dizgah, I. Serum and Saliva Total Tau Protein as a Marker for Relapsing-Remitting Multiple Sclerosis. Med. Hypotheses 2020, 135, 109476. [Google Scholar] [CrossRef]
- Green, L.C.; Wagner, D.A.; Glogowski, J.; Skipper, P.L.; Wishnok, J.S.; Tannenbaum, S.R. Analysis of Nitrate, Nitrite, and [15N]Nitrate in Biological Fluids. Anal. Biochem. 1982, 126, 131–138. [Google Scholar] [CrossRef]
- Majewska, M.D.; Bell, J.A. Ascorbic Acid Protects Neurons from Injury Induced by Glutamate and NMDA. Neuroreport 1990, 1, 194–196. [Google Scholar] [CrossRef]
- Levine, M.; Morita, K.; Heldman, E.; Pollard, H.B. Ascorbic Acid Regulation of Norepinephrine Biosynthesis in Isolated Chromaffin Granules from Bovine Adrenal Medulla. J. Biol. Chem. 1985, 260, 15598–15603. [Google Scholar] [CrossRef]
- Kuo, C.-H.; Hata, F.; Yoshida, H.; Yamatodani, A.; Wada, H. Effect of Ascorbic Acid on Release of Acetylcholine from Synaptic Vesicles Prepared from Different Species of Animals and Release of Noradrenaline from Synaptic Vesicles of Rat Brain. Life Sci. 1979, 24, 911–915. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.; Shen, T.; Wang, F.; Wu, P.; Chen, J. Preventive and Therapeutic Potential of Vitamin C in Mental Disorders. Curr. Med. Sci. 2018, 38, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kawaura, A.; Kitamura, Y.; Tanida, N.; Akiyama, J.; Mizutani, M.; Harada, K.; Morishita, M.; Inoue, S.; Kano, Y.; Okano, T.; et al. Antidepressant-like Effect of 1α-Hydroxyvitamin D3 on Mice in the Forced Swimming Test. J. Nutr. Sci. Vitaminol. 2017, 63, 81–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fedotova, J.; Dudnichenko, T.; Kruzliak, P.; Puchavskaya, Z. Different Effects of Vitamin D Hormone Treatment on Depression-like Behavior in the Adult Ovariectomized Female Rats. Biomed. Pharmacother. 2016, 84, 1865–1872. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Chen, Y.-H.; Chen, X.; Xu, S.; Yu, Z.; Xu, D.-X. Vitamin D Deficiency Impairs Neurobehavioral Development in Male Mice. Physiol. Behav. 2017, 179, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Berridge, M.J. Vitamin D, Reactive Oxygen Species and Calcium Signalling in Ageing and Disease. Philos. Trans. R. Soc. B Biol. Sci. 2016, 371, 20150434. [Google Scholar] [CrossRef] [Green Version]
- Moore, M.E.; Piazza, A.; McCartney, Y.; Lynch, M.A. Evidence That Vitamin D3 Reverses Age-Related Inflammatory Changes in the Rat Hippocampus. Biochem. Soc. Trans. 2005, 33, 573–577. [Google Scholar] [CrossRef] [Green Version]
- Matsunaga, E.; Nambu, S.; Oka, M.; Tanaka, M.; Taoka, M.; Iriki, A. Periostin, a Neurite Outgrowth-Promoting Factor, Is Expressed at High Levels in the Primate Cerebral Cortex. Dev. Growth Differ. 2015, 57, 200–208. [Google Scholar] [CrossRef]
- Ratajczak, T.; Ward, B.; Minchin, R. Immunophilin Chaperones in Steroid Receptor Signalling. Curr. Top. Med. Chem. 2003, 3, 1348–1357. [Google Scholar] [CrossRef]
- Boyle, M.P.; Brewer, J.A.; Funatsu, M.; Wozniak, D.F.; Tsien, J.Z.; Izumi, Y.; Muglia, L.J. Acquired Deficit of Forebrain Glucocorticoid Receptor Produces Depression-like Changes in Adrenal Axis Regulation and Behavior. Proc. Natl. Acad. Sci. USA 2005, 102, 473–478. [Google Scholar] [CrossRef] [Green Version]
- Pariante, C.M. Glucocorticoid Receptor Function In Vitro in Patients with Major Depression. Stress 2009, 7, 209–219. [Google Scholar] [CrossRef]
- Bellavance, M.A.; Rivest, S. The HPA-Immune Axis and the Immunomodulatory Actions of Glucocorticoids in the Brain. Front. Immunol. 2014, 5, 136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moraitis, A.G.; Block, T.; Nguyen, D.; Belanoff, J.K. The Role of Glucocorticoid Receptors in Metabolic Syndrome and Psychiatric Illness. J. Steroid Biochem. Mol. Biol. 2017, 165, 114–120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mazaira, G.I.; Echeverria, P.C.; Galigniana, M.D. Nucleocytoplasmic Shuttling of the Glucocorticoid Receptor Is Influenced by Tetratricopeptide Repeat-Containing Proteins. J. Cell Sci. 2020, 133, jcs238873. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gammoh, O.; Ibrahim, A.; Qnais, E.; Alqudah, A.; Altaber, S.; Aljabali, A.A.A.; Tambuwala, M.M. Vitamins C and D Exhibit Similar Antidepressant Effects to Escitalopram Mediated by NOx and FKBPL in a Stress-Induced Mice Model. Nutrients 2023, 15, 2692. https://doi.org/10.3390/nu15122692
Gammoh O, Ibrahim A, Qnais E, Alqudah A, Altaber S, Aljabali AAA, Tambuwala MM. Vitamins C and D Exhibit Similar Antidepressant Effects to Escitalopram Mediated by NOx and FKBPL in a Stress-Induced Mice Model. Nutrients. 2023; 15(12):2692. https://doi.org/10.3390/nu15122692
Chicago/Turabian StyleGammoh, Omar, Aseel Ibrahim, Esam Qnais, Abdelrahim Alqudah, Sara Altaber, Alaa A. A. Aljabali, and Murtaza M. Tambuwala. 2023. "Vitamins C and D Exhibit Similar Antidepressant Effects to Escitalopram Mediated by NOx and FKBPL in a Stress-Induced Mice Model" Nutrients 15, no. 12: 2692. https://doi.org/10.3390/nu15122692
APA StyleGammoh, O., Ibrahim, A., Qnais, E., Alqudah, A., Altaber, S., Aljabali, A. A. A., & Tambuwala, M. M. (2023). Vitamins C and D Exhibit Similar Antidepressant Effects to Escitalopram Mediated by NOx and FKBPL in a Stress-Induced Mice Model. Nutrients, 15(12), 2692. https://doi.org/10.3390/nu15122692