Muscle Traits, Sarcopenia, and Sarcopenic Obesity: A Vitamin D Mendelian Randomization Study
Abstract
1. Introduction
2. Methods
2.1. Grip Strength, Muscle Mass, and Probable Sarcopenia
2.2. Measured and Genetically Instrumented 25(OH)D
2.3. Statistical Methods
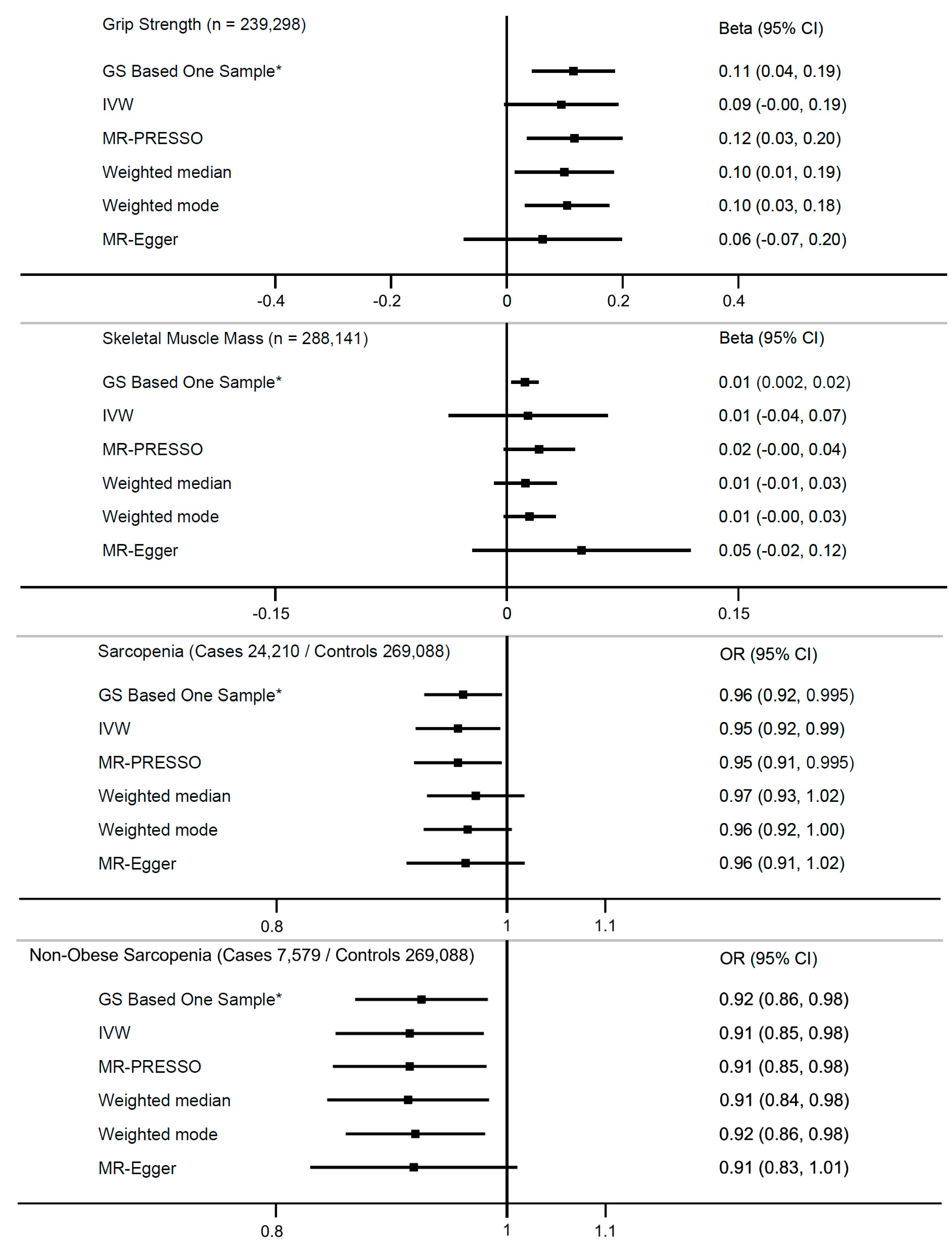
3. Results
3.1. Grip Strength
3.2. Probable Sarcopenia
3.3. Arm Skeletal Muscle Mass
3.4. Sensitivity Analyses
4. Discussion
Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ceglia, L. Vitamin D and its role in skeletal muscle. Curr. Opin. Clin. Nutr. Metab. Care 2009, 12, 628–633. [Google Scholar] [CrossRef] [PubMed]
- Uday, S.; Högler, W. Nutritional Rickets and Osteomalacia in the Twenty-first Century: Revised Concepts, Public Health, and Prevention Strategies. Curr. Osteoporos. Rep. 2017, 15, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Pilz, S.; Zittermann, A.; Trummer, C.; Theiler-Schwetz, V.; Lerchbaum, E.; Keppel, M.H.; Grübler, M.R.; März, W.; Pandis, M. Vitamin D testing and treatment: A narrative review of current evidence. Endocr. Connect. 2019, 8, R27–R43. [Google Scholar] [CrossRef] [PubMed]
- Iannuzzi-Sucich, M.; Prestwood, K.M.; Kenny, A.M. Prevalence of Sarcopenia and Predictors of Skeletal Muscle Mass in Healthy, Older Men and Women. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2002, 57, M772–M777. [Google Scholar] [CrossRef]
- Torres, C.F.; Forbes, G.B.; Decancq, G.H. Muscle weakness in infants with rickets: Distribution, course, and recovery. Pediatr. Neurol. 1986, 2, 95–98. [Google Scholar] [CrossRef]
- Remelli, F.; Vitali, A.; Zurlo, A.; Volpato, S. Vitamin D Deficiency and Sarcopenia in Older Persons. Nutrients 2019, 11, 2861. [Google Scholar] [CrossRef]
- Di Filippo, L.; De Lorenzo, R.; Giustina, A.; Rovere-Querini, P.; Conte, C. Vitamin D in Osteosarcopenic Obesity. Nutrients 2022, 14, 1816. [Google Scholar] [CrossRef]
- Endo, I.; Inoue, D.; Mitsui, T.; Umaki, Y.; Akaike, M.; Yoshizawa, T.; Kato, S.; Matsumoto, T. Deletion of Vitamin D Receptor Gene in Mice Results in Abnormal Skeletal Muscle Development with Deregulated Expression of Myoregulatory Transcription Factors. Endocrinology 2003, 144, 5138–5144. [Google Scholar] [CrossRef]
- Girgis, C.M.; Cha, K.M.; So, B.; Tsang, M.; Chen, J.; Houweling, P.J.; Schindeler, A.; Stokes, R.; Swarbrick, M.M.; Evesson, F.J.; et al. Mice with myocyte deletion of vitamin D receptor have sarcopenia and impaired muscle function. J. Cachexia Sarcopenia Muscle 2019, 10, 1228–1240. [Google Scholar] [CrossRef]
- Chiang, C.-M.; Ismaeel, A.; Griffis, R.B.; Weems, S. Effects of Vitamin D Supplementation on Muscle Strength in Athletes: A Systematic Review. J. Strength Cond. Res. 2017, 31, 566–574. [Google Scholar] [CrossRef]
- Bolland, M.J.; Grey, A.; Avenell, A. Assessment of research waste part 2: Wrong study populations- an exemplar of baseline vitamin D status of participants in trials of vitamin D supplementation. BMC Med. Res. Methodol. 2018, 18, 101. [Google Scholar] [CrossRef]
- Davies, N.M.; Holmes, M.V.; Davey Smith, G. Reading Mendelian randomisation studies: A guide, glossary, and checklist for clinicians. BMJ 2018, 362, k601. [Google Scholar] [CrossRef]
- Schaefer, L.; Plotnikoff, R.C.; Majumdar, S.R.; Mollard, R.; Woo, M.; Sadman, R.; Rinaldi, R.L.; Boulé, N.; Torrance, B.; Ball, G.D.; et al. Outdoor Time Is Associated with Physical Activity, Sedentary Time, and Cardiorespiratory Fitness in Youth. J. Pediatr. 2014, 165, 516–521. [Google Scholar] [CrossRef] [PubMed]
- Mason, R.S.; Rybchyn, M.S.; Abboud, M.; Brennan-Speranza, T.C.; Fraser, D.R. The Role of Skeletal Muscle in Maintaining Vitamin D Status in Winter. Curr. Dev. Nutr. 2019, 3, nzz087. [Google Scholar] [CrossRef] [PubMed]
- Pinto Pereira, S.M.; Garfield, V.; Norris, T.; Burgess, S.; Williams, D.M.; Dodds, R.; Sayer, A.A.; Robinson, S.M.; Cooper, R. Linear and Nonlinear Associations Between Vitamin D and Grip Strength: A Mendelian Randomization Study in UK Biobank. J. Gerontol. Ser. A, 2022; in press. [Google Scholar] [CrossRef] [PubMed]
- Mazidi, M.; Davies, I.G.; Penson, P.; Rikkonen, T.; Isanejad, M. Lifetime serum concentration of 25-hydroxyvitamin D 25 (OH) is associated with hand grip strengths: Insight from a Mendelian randomisation. Age Ageing 2022, 51, afac079. [Google Scholar] [CrossRef] [PubMed]
- Wind, A.E.; Takken, T.; Helders, P.J.M.; Engelbert, R.H.H. Is grip strength a predictor for total muscle strength in healthy children, adolescents, and young adults? Eur. J. Pediatr. 2010, 169, 281–287. [Google Scholar] [CrossRef]
- Kirwan, R.; Isanejad, M.; Davies, I.G.; Mazidi, M. Genetically Determined Serum 25-Hydroxyvitamin D Is Associated with Total, Trunk, and Arm Fat-Free Mass: A Mendelian Randomization Study. J. Nutr. Health Aging 2022, 26, 46–51. [Google Scholar] [CrossRef]
- Sha, T.; Li, W.; He, H.; Wu, J.; Wang, Y.; Li, H. Causal relationship of genetically predicted serum micronutrients levels with sarcopenia: A Mendelian randomization study. Front. Nutr. 2022, 9, 913155. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef]
- Dodds, R.M.; Granic, A.; Robinson, S.M.; Sayer, A.A. Sarcopenia, long-term conditions, and multimorbidity: Findings from UK Biobank participants. J. Cachexia Sarcopenia Muscle 2020, 11, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Sudlow, C.; Gallacher, J.; Allen, N.; Beral, V.; Burton, P.; Danesh, J.; Downey, P.; Elliott, P.; Green, J.; Landray, M.; et al. UK biobank: An open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 2015, 12, e1001779. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, J.; Zhou, A.; Hypponen, E. Muscle traits, Sarcopenia, and Sarcopenic Obesity: A Vitamin D Mendelian Randomization Study—Supplementary Appendix. Available online: https://data.mendeley.com/ (accessed on 6 June 2023).
- Deurenberg, P.; Yap, M.; Van Staveren, W. Body mass index and percent body fat: A meta analysis among different ethnic groups. Int. J. Obes. 1998, 22, 1164–1171. [Google Scholar] [CrossRef]
- Townsend, P.; Phillimore, P.; Beattie, A. Health and Deprivation: Inequality and the North; Routledge: London, UK, 1988. [Google Scholar]
- Revez, J.A.; Lin, T.; Qiao, Z.; Xue, A.; Holtz, Y.; Zhu, Z.; Zeng, J.; Wang, H.; Sidorenko, J.; Kemper, K.E.; et al. Genome-wide association study identifies 143 loci associated with 25 hydroxyvitamin D concentration. Nat. Commun. 2020, 11, 1647. [Google Scholar] [CrossRef] [PubMed]
- Zhou, A.; Selvanayagam, J.B.; Hyppönen, E. Non-linear Mendelian randomization analyses support a role for vitamin D deficiency in cardiovascular disease risk. Eur. Heart J. 2022, 43, 1731–1739. [Google Scholar] [CrossRef]
- Jiang, X.; O’Reilly, P.F.; Aschard, H.; Hsu, Y.-H.; Richards, J.B.; Dupuis, J.; Ingelsson, E.; Karasik, D.; Pilz, S.; Berry, D.; et al. Genome-wide association study in 79,366 European-ancestry individuals informs the genetic architecture of 25-hydroxyvitamin D levels. Nat. Commun. 2018, 9, 260. [Google Scholar] [CrossRef]
- Royston, P.; Ambler, G.; Sauerbrei, W. The use of fractional polynomials to model continuous risk variables in epidemiology. Int. J. Epidemiol. 1999, 28, 964–974. [Google Scholar] [CrossRef]
- Staley, J.R.; Burgess, S. Semiparametric methods for estimation of a nonlinear exposure-outcome relationship using instrumental variables with application to Mendelian randomization. Genet. Epidemiol. 2017, 41, 341–352. [Google Scholar] [CrossRef]
- Burgess, S.; Mason, A.M.; Grant, A.J.; Slob, E.A.; Gkatzionis, A.; Zuber, V.; Patel, A.; Tian, H.; Liu, C.; Haynes, W.G.; et al. Using genetic association data to guide drug discovery and development: Review of methods and applications. Am. J. Hum. Genet. 2023, 110, 195–214. [Google Scholar] [CrossRef]
- Burgess, S.; Davey Smith, G.; Davies, N.M.; Dudbridge, F.; Gill, D.; Glymour, M.M.; Hartwig, F.P.; Holmes, M.V.; Minelli, C.; Relton, C.L.; et al. Guidelines for performing Mendelian randomization investigations. Wellcome Open Res. 2020, 4, 186. [Google Scholar] [CrossRef] [PubMed]
- Burgess, S.; Gill, D. Genetic evidence for vitamin D and cardiovascular disease: Choice of variants is critical. Eur. Heart J. 2022, 43, 1740–1742. [Google Scholar] [CrossRef]
- McCormick, R.; Vasilaki, A. Age-related changes in skeletal muscle: Changes to life-style as a therapy. Biogerontology 2018, 19, 519–536. [Google Scholar] [CrossRef]
- Yeung, S.S.Y.; Reijnierse, E.M.; Pham, V.K.; Trappenburg, M.C.; Lim, W.K.; Meskers, C.G.M.; Maier, A.B. Sarcopenia and its association with falls and fractures in older adults: A systematic review and meta-analysis. J. Cachexia Sarcopenia Muscle 2019, 10, 485–500. [Google Scholar] [CrossRef]
- Bruyère, O.; Beaudart, C.; Ethgen, O.; Reginster, J.-Y.; Locquet, M. The health economics burden of sarcopenia: A systematic review. Maturitas 2019, 119, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Pinedo-Villanueva, R.; Westbury, L.D.; Syddall, H.E.; Sanchez-Santos, M.T.; Dennison, E.M.; Robinson, S.M.; Cooper, C. Health Care Costs Associated with Muscle Weakness: A UK Population-Based Estimate. Calcif. Tissue Int. 2019, 104, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Steffl, M.; Sima, J.; Shiells, K.; Holmerova, I. The increase in health care costs associated with muscle weakness in older people without long-term illnesses in the Czech Republic: Results from the Survey of Health, Ageing and Retirement in Europe (SHARE). Clin. Interv. Aging 2017, 12, 2003–2007. [Google Scholar] [CrossRef] [PubMed]
- Trantham, L.; Sikirica, M.V.; Candrilli, S.D.; Benson, V.S.; Mohan, D.; Neil, D.; Joshi, A.V. Healthcare costs and utilization associated with muscle weakness diagnosis codes in patients with chronic obstructive pulmonary disease: A United States claims analysis. J. Med. Econ. 2019, 22, 319–327. [Google Scholar] [CrossRef]
- Beaudart, C.; Rizzoli, R.; Bruyère, O.; Reginster, J.-Y.; Biver, E. Sarcopenia: Burden and challenges for public health. Arch. Public Health 2014, 72, 45. [Google Scholar] [CrossRef]
- Sousa, A.S.; Guerra, R.S.; Fonseca, I.; Pichel, F.; Ferreira, S.; Amaral, T.F. Financial impact of sarcopenia on hospitalization costs. Eur. J. Clin. Nutr. 2016, 70, 1046–1051. [Google Scholar] [CrossRef]
- Schrager, M.A.; Metter, E.J.; Simonsick, E.; Ble, A.; Bandinelli, S.; Lauretani, F.; Ferrucci, L. Sarcopenic obesity and inflammation in the InCHIANTI study. J. Appl. Physiol. 2007, 102, 919–925. [Google Scholar] [CrossRef]
- Silberstein, M. COVID-19 and IL-6: Why vitamin D (probably) helps but tocilizumab might not. Eur. J. Pharmacol. 2021, 899, 174031. [Google Scholar] [CrossRef]
- Kominsky, D.J.; Campbell, E.L.; Colgan, S.P. Metabolic shifts in immunity and inflammation. J. Immunol. 2010, 184, 4062–4068. [Google Scholar] [CrossRef]
- Siddik, M.A.B.; Shin, A.C. Recent Progress on Branched-Chain Amino Acids in Obesity, Diabetes, and Beyond. Endocrinol. Metab. 2019, 34, 234. [Google Scholar] [CrossRef] [PubMed]
- Katsuhara, S.; Yokomoto-Umakoshi, M.; Umakoshi, H.; Matsuda, Y.; Iwahashi, N.; Kaneko, H.; Ogata, M.; Fukumoto, T.; Terada, E.; Sakamoto, R.; et al. Impact of Cortisol on Reduction in Muscle Strength and Mass: A Mendelian Randomization Study. J. Clin. Endocrinol. Metab. 2022, 107, e1477–e1487. [Google Scholar] [CrossRef]
- Ko, D.H.; Kim, Y.K. The Prevalence of Metabolic Syndrome According to Grip Strength in Teenagers. Children 2021, 8, 108. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Guo, G.; Xia, L.; Yang, X.; Zhang, B.; Liu, F.; Ma, J.; Hu, Z.; Li, Y.; Li, W.; et al. Relative handgrip strength is inversely associated with metabolic profile and metabolic disease in the general population in China. Front. Physiol. 2018, 9, 59. [Google Scholar] [CrossRef] [PubMed]
- Sutandyo, N.; Rinaldi, I.; Sari, N.K.; Winston, K. Prevalence of Anemia and Factors Associated with Handgrip Strength in Indonesian Elderly Population. Cureus 2022, 14, e25290. [Google Scholar] [CrossRef]
- Gi, Y.-M.; Jung, B.; Kim, K.-W.; Cho, J.-H.; Ha, I.-H. Low handgrip strength is closely associated with anemia among adults: A cross-sectional study using Korea National Health and Nutrition Examination Survey (KNHANES). PLoS ONE 2020, 15, e0218058. [Google Scholar] [CrossRef]
- Malczewska-Lenczowska, J.; Sitkowski, D.; Surała, O.; Orysiak, J.; Szczepańska, B.; Witek, K. The Association between Iron and Vitamin D Status in Female Elite Athletes. Nutrients 2018, 10, 167. [Google Scholar] [CrossRef]
- Sakuma, K.; Yamaguchi, A. Sarcopenia and Age-Related Endocrine Function. Int. J. Endocrinol. 2012, 2012, 127362. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, C.; Dou, Q.; Zhang, W.; Yang, Y.; Xie, X. Sarcopenia as a predictor of all-cause mortality among older nursing home residents: A systematic review and meta-analysis. BMJ Open 2018, 8, e021252. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, J.P.; Zhou, A.; Hyppönen, E. Vitamin D Deficiency Increases Mortality Risk in the UK Biobank: A Nonlinear Mendelian Randomization Study. Ann. Intern. Med. 2022, 175, 1552–1559. [Google Scholar] [CrossRef] [PubMed]
- Sofianopoulou, E.; Kaptoge, S.K.; Afzal, S.; Jiang, T.; Gill, D.; Gundersen, T.E.; Bolton, T.R.; Allara, E.; Arnold, M.G.; Mason, A.M.; et al. Estimating dose-response relationships for vitamin D with coronary heart disease, stroke, and all-cause mortality: Observational and Mendelian randomisation analyses. Lancet Diabetes Endocrinol. 2021, 9, 837–846. [Google Scholar] [CrossRef] [PubMed]
- Dzik, K.P.; Kaczor, J.J. Mechanisms of vitamin D on skeletal muscle function: Oxidative stress, energy metabolism and anabolic state. Eur. J. Appl. Physiol. 2019, 119, 825–839. [Google Scholar] [CrossRef]
- Abrams, G.D.; Feldman, D.; Safran, M.R. Effects of vitamin D on skeletal muscle and athletic performance. JAAOS J. Am. Acad. Orthop. Surg. 2018, 26, 278–285. [Google Scholar] [CrossRef]
- Bohannon, R.W. Grip Strength: An Indispensable Biomarker for Older Adults. Clin. Interv. Aging. 2019, 14, 1681–1691. [Google Scholar] [CrossRef]
- Batty, G.D.; Gale, C.R.; Kivimäki, M.; Deary, I.J.; Bell, S. Comparison of risk factor associations in UK Biobank against representative, general population based studies with conventional response rates: Prospective cohort study and individual participant meta-analysis. BMJ 2020, 368, m131. [Google Scholar] [CrossRef]
- Gkatzionis, A.; Burgess, S. Contextualizing selection bias in Mendelian randomization: How bad is it likely to be? Int. J. Epidemiol. 2019, 48, 691–701. [Google Scholar] [CrossRef]
- Hyppönen, E.; Vimaleswaran, K.S.; Zhou, A. Genetic Determinants of 25-Hydroxyvitamin D Concentrations and Their Relevance to Public Health. Nutrients 2022, 14, 4408. [Google Scholar] [CrossRef]
- DiaSorin. LIAISON—25 OH Vitamin D TOTAL Assay 2019. Available online: https://www.diasorin.com/sites/default/files/allegati_prodotti/25_oh_vit._d_total_m0870004213_e.pdf (accessed on 8 June 2020).
- Vickers, D.; Rees, P. Creating the UK National Statistics 2001 output area classification. J. R. Stat. Soc. 2007, 170, 379–403. [Google Scholar] [CrossRef]
- Nuttall, F.Q. Body Mass Index. Nutr. Today 2015, 50, 117–128. [Google Scholar] [CrossRef]
- Burgess, S.; Thompson, S.G. Use of allele scores as instrumental variables for Mendelian randomization. Int. J. Epidemiol. 2013, 42, 1134–1144. [Google Scholar] [CrossRef] [PubMed]
- Palmer, T.M.; Sterne, J.A.; Harbord, R.M.; Lawlor, D.A.; Sheehan, N.A.; Meng, S.; Granell, R.; Smith, G.D.; Didelez, V. Instrumental Variable Estimation of Causal Risk Ratios and Causal Odds Ratios in Mendelian Randomization Analyses. Am. J. Epidemiol. 2011, 173, 1392–1403. [Google Scholar] [CrossRef]
- Burgess, S.; Davies, N.M. Thompson SG. Bias due to participant overlap in two-sample Mendelian randomization. Genet. Epidemiol. 2016, 40, 597–608. [Google Scholar] [CrossRef]
- Burgess, S.; Butterworth, A.; Thompson, S.G. Mendelian Randomization Analysis with Multiple Genetic Variants Using Summarized Data. Genet. Epidemiol. 2013, 37, 658–665. [Google Scholar] [CrossRef]
- Bowden, J.; Davey Smith, G.; Burgess, S. Mendelian Randomization with Invalid Instruments: Effect Estimation and Bias Detection through Egger Regression. Int. J. Epidemiol. 2015, 44, 512–525. [Google Scholar] [CrossRef] [PubMed]
- Verbanck, M.; Chen, C.Y.; Neale, B.; Do, R. Detection of Widespread Horizontal Pleiotropy in Causal Relationships Inferred from Mendelian Randomization between Complex Traits and Diseases. Nat. Genet. 2018, 50, 693–698. [Google Scholar] [CrossRef] [PubMed]
- Hartwig, F.P.; Davey Smith, G.; Bowden, J. Robust Inference in Summary Data Mendelian Randomization via the Zero Modal Pleiotropy Assumption. Int. J. Epidemiol. 2017, 46, 1985–1998. [Google Scholar] [CrossRef] [PubMed]
- Bowden, J.; Davey Smith, G.; Haycock, P.C.; Burgess, S. Consistent Estimation in Mendelian Randomization with Some Invalid Instruments Using a Weighted Median Estimator. Genet. Epidemiol. 2016, 40, 304–314. [Google Scholar] [CrossRef] [PubMed]
- Kamat, M.A.; Blackshaw, J.A.; Young, R.; Surendran, P.; Burgess, S.; Danesh, J.; Butterworth, A.S.; Staley, J.R. PhenoScanner V2: An expanded tool for searching human genotype-phenotype associations. Bioinformatics 2019, 35, 4851–4853. [Google Scholar] [CrossRef]

| 25(OH)D | 25(OH)D <25 nmol/L | Grip Strength a | Probable Sarcopenia | Sarcopenic Obesity | Arm Skeletal Muscle Mass b | ||
|---|---|---|---|---|---|---|---|
| N = 307,281 | N = 36,009 | N = 306,967 c | N = 25,414 | N = 16,520 | N = 302,112 d | ||
| N (%) | Mean (SD) | % | Mean (SD) | % | % | Mean (SD) | |
| All | 307,281 | 49.82 (20.96) | 11.70 | 31.04 (11.03) | 8.28 | 5.54 | 5.51 (1.58) |
| Sex | |||||||
| Men | 144,538 (47.4) | 49.86 (21.03) | 11.60 | 39.61 (8.74) | 6.39 | 3.98 | 6.92 (1.09) |
| Women | 162,743 (53.0) | 49.78 (20.89) | 11.80 | 23.43 (6.22) | 9.96 | 6.94 | 4.26 (0.60) |
| Age | |||||||
| <60 | 169,594 (55.2) | 48.38 (21.12) | 13.46 | 32.48 (11.18) | 5.46 | 3.40 | 5.56 (1.66) |
| ≥60 | 137,687 (44.8) | 51.57 (20.62) | 9.54 | 29.28 (10.58) | 11.75 | 8.23 | 5.44 (1.48) |
| BMI | |||||||
| Low 25%, 12.1–24.0 | 76,633 (24.9) | 53.04 (22.09) | 10.19 | 28.62 (9.62) | 7.61 | 1.21 | 4.53 (1.13) |
| Mid 50%, 24.1–29.8 | 153,312 (50.0) | 50.95 (20.68) | 10.05 | 32.19 (11.17) | 7.55 | 5.46 | 5.57 (1.44) |
| High 25% 29.8–74.7 | 76,667 (25.0) | 44.42 (19.23) | 16.40 | 31.23 (11.63) | 10.18 | 9.77 | 6.36 (1.71) |
| Missing | 669 (0.2) | 40.69 (21.11) | 26.01 | 25.51 (12.86) | 33.73 | 4.33 | 5.34 (1.48) |
| Location e | |||||||
| South, ≤51° Lat | 102,226 (33.3) | 51.43 (20.49) | 9.29 | 30.77 (10.84) | 8.35 | 5.20 | 5.53 (1.58) |
| Mid, 52–53° Lat | 144,470 (47.0) | 49.93 (20.88) | 11.35 | 31.10 (11.11) | 8.42 | 5.80 | 5.51 (1.58) |
| North, 54–≥55° Lat | 60,585 (19.7) | 46.82 (21.59) | 16.62 | 31.36 (11.16) | 7.83 | 2.49 | 5.45 (1.58) |
| Smoking | |||||||
| Non-smokers | 167,537 (54.5) | 50.04 (20.63) | 10.92 | 30.48 (11.00) | 8.07 | 5.22 | 5.36 (1.55) |
| Ex-smokers | 108,015 (35.2) | 50.77 (21.04) | 10.66 | 31.61 (10.99) | 8.37 | 6.05 | 5.69 (1.60) |
| Current smokers | 30,673 (10.0) | 45.22 (21.80) | 19.63 | 32.23 (11.17) | 8.91 | 5.31 | 5.66 (1.60) |
| Missing | 1056 (0.3) | 50.17 (21.77) | 12.41 | 28.95 (11.14) | 14.58 | 11.22 | 5.57 (1.60) |
| Alcohol | |||||||
| Daily | 65,476 (21.3) | 51.22 (21.51) | 11.03 | 33.09 (10.82) | 6.51 | 3.93 | 5.71 (1.56) |
| 1 to 4 times wk | 155,474 (50.6) | 50.87 (20.80) | 10.19 | 31.85 (11.01) | 7.11 | 4.67 | 5.58 (1.60) |
| 1 to 3 times mo | 34,061 (11.1) | 48.08 (20.26) | 12.91 | 29.56 (10.73) | 8.46 | 5.85 | 5.35 (1.58) |
| Special occasion | 32,125 (10.5) | 46.18 (20.40) | 15.74 | 27.05 (10.43) | 12.85 | 9.37 | 5.13 (1.48) |
| Never | 19,934 (6.5) | 45.91 (20.95) | 17.06 | 27.07 (10.66) | 15.47 | 11.11 | 5.19 (1.45) |
| Missing | 211 (0.07) | 44.97 (21.05) | 16.59 | 28.54 (11.32) | 17.54 | 13.86 | 5.39 (1.57) |
| Physical activity | |||||||
| Low | 91,911 (29.9) | 46.30 (20.20) | 15.18 | 29.78 (10.96) | 10.39 | 7.49 | 5.47 (1.59) |
| Moderate | 149,064 (48.5) | 50.60 (20.80) | 10.50 | 31.31 (10.88) | 7.17 | 4.65 | 5.47 (1.57) |
| High | 59,518 (19.4) | 54.01 (21.41) | 8.11 | 32.65 (11.09) | 6.37 | 3.78 | 5.62 (1.60) |
| Missing | 6788 (2.2) | 43.33 (21.32) | 22.48 | 28.32 (12.19) | 20.78 | 14.80 | 5.77 (1.70) |
| Education | |||||||
| None | 52,119 (17.0) | 50.40 (21.43) | 11.89 | 28.56 (10.98) | 14.47 | 10.74 | 5.43 (1.55) |
| NVQ/CSE/A-Lev. | 109,007 (35.5) | 50.55 (21.19) | 11.22 | 31.02 (11.18) | 7.90 | 5.33 | 5.51 (1.61) |
| Deg./professional | 143,586 (46.7) | 49.04 (20.57) | 12.00 | 31.99 (10.79) | 6.23 | 3.79 | 5.54 (1.57) |
| Missing | 2569 (0.84) | 50.33 (20.90) | 11.60 | 29.48 (11.22) | 13.36 | 9.04 | 5.52 (1.59) |
| Townsend index | |||||||
| Q1 Deprivation low | 76,746 (25.0) | 51.91 (20.71) | 9.16 | 31.71 (11.06) | 6.60 | 4.21 | 5.50 (1.57) |
| Q2 | 76,745 (25.0) | 51.50 (20.70) | 9.35 | 31.31 (11.10) | 7.37 | 4.82 | 5.49 (1.58) |
| Q3 | 76,719 (25.0) | 49.91 (20.79) | 11.20 | 30.92 (10.98) | 8.25 | 5.55 | 5.50 (1.59) |
| Q4 Deprivation high | 76,711 (25.0) | 45.94 (21.08) | 17.11 | 30.24 (10.93) | 10.90 | 7.62 | 5.54 (1.59) |
| Missing | 360 (0.1) | 50.02 (20.53) | 11.39 | 31.37 (11.16) | 7.22 | 4.84 | 5.61 (1.66) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sutherland, J.P.; Zhou, A.; Hyppönen, E. Muscle Traits, Sarcopenia, and Sarcopenic Obesity: A Vitamin D Mendelian Randomization Study. Nutrients 2023, 15, 2703. https://doi.org/10.3390/nu15122703
Sutherland JP, Zhou A, Hyppönen E. Muscle Traits, Sarcopenia, and Sarcopenic Obesity: A Vitamin D Mendelian Randomization Study. Nutrients. 2023; 15(12):2703. https://doi.org/10.3390/nu15122703
Chicago/Turabian StyleSutherland, Joshua P., Ang Zhou, and Elina Hyppönen. 2023. "Muscle Traits, Sarcopenia, and Sarcopenic Obesity: A Vitamin D Mendelian Randomization Study" Nutrients 15, no. 12: 2703. https://doi.org/10.3390/nu15122703
APA StyleSutherland, J. P., Zhou, A., & Hyppönen, E. (2023). Muscle Traits, Sarcopenia, and Sarcopenic Obesity: A Vitamin D Mendelian Randomization Study. Nutrients, 15(12), 2703. https://doi.org/10.3390/nu15122703






