New Pieces for an Old Puzzle: Approaching Parkinson’s Disease from Translatable Animal Models, Gut Microbiota Modulation, and Lipidomics
Abstract
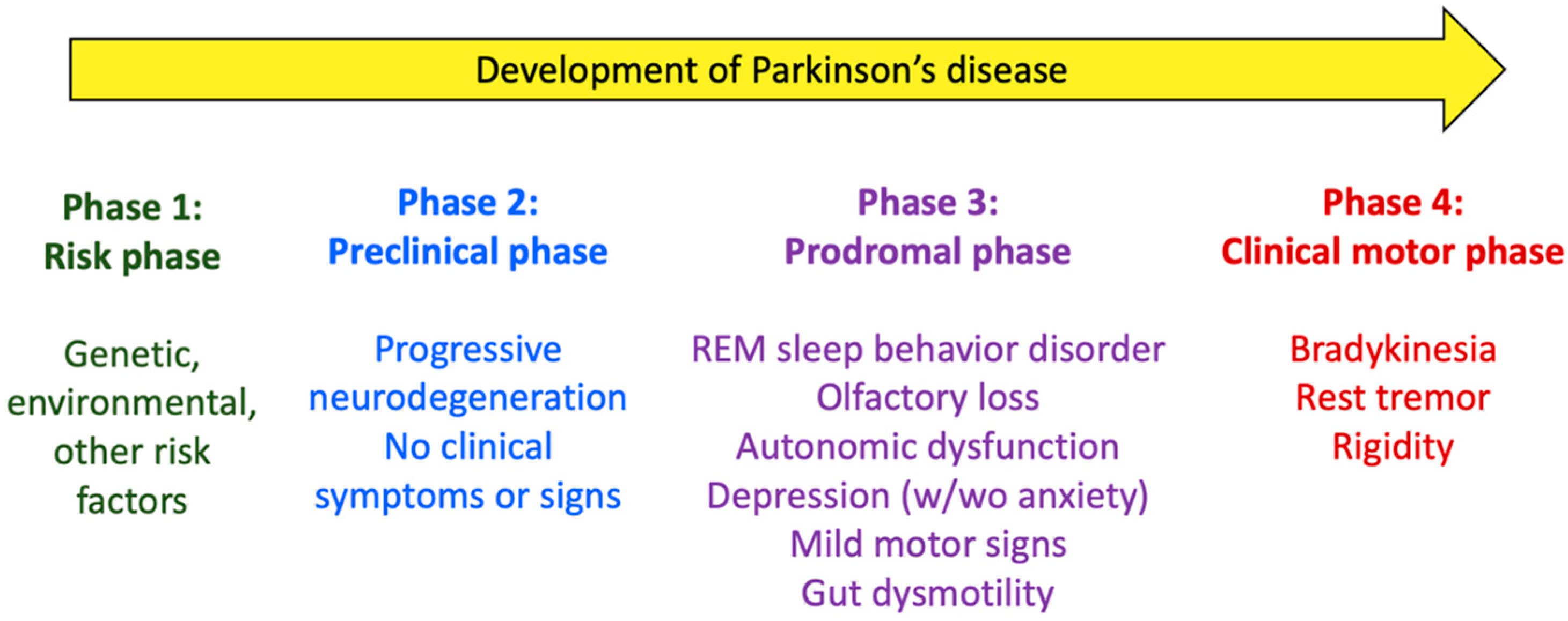
:1. Introduction
2. Parkinson’s Disease: Pathogeny, Risk/Protection Factors, and Gut Involvement
3. Gastrointestinal Motor Function in Animal Models of Parkinson’s Disease
3.1. Rodent-Based Animal Models of PD
3.1.1. Genetic Models
3.1.2. Neurotoxin-Based Models
6-Hydroxydopamine
MPTP
Pesticides
- (a)
- Rotenone
- (b)
- Paraquat
Other Neurotoxins
3.2. Evaluation of GI Motor Function in PD Models
4. Intestinal Microbiota and PD
4.1. Underlying Mechanisms Associating Intestinal Dysbiosis and PD
4.2. Microbiota-Based Therapies for PD
5. Lipidomics as a New Tool to Identify Biomarkers in Parkinson’s Disease
5.1. Lipidomics Approaches
5.2. Lipids and Parkinson’s Disease
5.2.1. Fatty Acyls
5.2.2. Glycerolipids, Glycerophospholipids, and Sphingolipids
5.2.3. Sterols
5.3. Lipidomics Analysis in Parkinson’s Disease
5.4. Microbiota, Probiotics, and Lipidomics in Parkinson’s Disease
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Poewe, W.; Seppi, K.; Tanner, C.M.; Halliday, G.M.; Brundin, P.; Volkmann, J.; Schrag, A.-E.; Lang, A.E. Parkinson Disease. Nat. Rev. Dis. Primers 2017, 3, 17013. [Google Scholar] [CrossRef]
- Van Den Eeden, S.K.; Tanner, C.M.; Bernstein, A.L.; Fross, R.D.; Leimpeter, A.; Bloch, D.A.; Nelson, L.M. Incidence of Parkinson’s Disease: Variation by Age, Gender, and Race/Ethnicity. Am. J. Epidemiol. 2003, 157, 1015–1022. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tysnes, O.-B.; Storstein, A. Epidemiology of Parkinson’s Disease. J. Neural. Transm. 2017, 124, 901–905. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Wang, P.; Jankovic, J. The Genetics of Parkinson Disease. Ageing Res. Rev. 2018, 42, 72–85. [Google Scholar] [CrossRef]
- Ou, Z.; Pan, J.; Tang, S.; Duan, D.; Yu, D.; Nong, H.; Wang, Z. Global Trends in the Incidence, Prevalence, and Years Lived With Disability of Parkinson’s Disease in 204 Countries/Territories From 1990 to 2019. Front. Public Health 2021, 9, 776847. [Google Scholar] [CrossRef]
- GBD 2016 Parkinson’s Disease Collaborators Global, Regional, and National Burden of Parkinson’s Disease, 1990–2016: A Systematic Analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2018, 17, 939–953. [CrossRef] [Green Version]
- Aarsland, D.; Batzu, L.; Halliday, G.M.; Geurtsen, G.J.; Ballard, C.; Ray Chaudhuri, K.; Weintraub, D. Parkinson Disease-Associated Cognitive Impairment. Nat. Rev. Dis. Primers 2021, 7, 47. [Google Scholar] [CrossRef]
- Yang, W.; Hamilton, J.L.; Kopil, C.; Beck, J.C.; Tanner, C.M.; Albin, R.L.; Ray Dorsey, E.; Dahodwala, N.; Cintina, I.; Hogan, P.; et al. Current and Projected Future Economic Burden of Parkinson’s Disease in the U.S. NPJ Park. Dis. 2020, 6, 15. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, E.; Graybiel, A.M.; Agid, Y.A. Melanized Dopaminergic Neurons Are Differentially Susceptible to Degeneration in Parkinson’s Disease. Nature 1988, 334, 345–348. [Google Scholar] [CrossRef] [PubMed]
- Masato, A.; Plotegher, N.; Boassa, D.; Bubacco, L. Impaired Dopamine Metabolism in Parkinson’s Disease Pathogenesis. Mol. Neurodegener. 2019, 14, 35. [Google Scholar] [CrossRef] [Green Version]
- Berg, D.; Postuma, R.B.; Bloem, B.; Chan, P.; Dubois, B.; Gasser, T.; Goetz, C.G.; Halliday, G.M.; Hardy, J.; Lang, A.E.; et al. Time to Redefine PD? Introductory Statement of the MDS Task Force on the Definition of Parkinson’s Disease. Mov. Disord. 2014, 29, 454–462. [Google Scholar] [CrossRef] [PubMed]
- Zacharias, H.U.; Kaleta, C.; Cossais, F.; Schaeffer, E.; Berndt, H.; Best, L.; Dost, T.; Glüsing, S.; Groussin, M.; Poyet, M.; et al. Microbiome and Metabolome Insights into the Role of the Gastrointestinal-Brain Axis in Parkinson’s and Alzheimer’s Disease: Unveiling Potential Therapeutic Targets. Metabolites 2022, 12, 1222. [Google Scholar] [CrossRef] [PubMed]
- Berg, D.; Postuma, R.B.; Adler, C.H.; Bloem, B.R.; Chan, P.; Dubois, B.; Gasser, T.; Goetz, C.G.; Halliday, G.; Joseph, L.; et al. MDS Research Criteria for Prodromal Parkinson’s Disease. Mov. Disord. 2015, 30, 1600–1611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Youdim, M.B.H. Why Do We Need Multifunctional Neuroprotective and Neurorestorative Drugs for Parkinson’s and Alzheimer’s Diseases as Disease Modifying Agents. Exp. Neurobiol. 2010, 19, 1–14. [Google Scholar] [CrossRef]
- Fasano, A.; Daniele, A.; Albanese, A. Treatment of Motor and Non-Motor Features of Parkinson’s Disease with Deep Brain Stimulation. Lancet Neurol. 2012, 11, 429–442. [Google Scholar] [CrossRef]
- Alarcón, T.A.; Presti-Silva, S.M.; Simões, A.P.T.; Ribeiro, F.M.; Pires, R.G.W. Molecular Mechanisms Underlying the Neuroprotection of Environmental Enrichment in Parkinson’s Disease. Neural Regen. Res. 2023, 18, 1450–1456. [Google Scholar] [CrossRef]
- Zhang, T.D.; Kolbe, S.C.; Beauchamp, L.C.; Woodbridge, E.K.; Finkelstein, D.I.; Burrows, E.L. How Well Do Rodent Models of Parkinson’s Disease Recapitulate Early Non-Motor Phenotypes? A Systematic Review. Biomedicines 2022, 10, 3026. [Google Scholar] [CrossRef]
- Giuliano, C.; Cerri, S.; Cesaroni, V.; Blandini, F. Relevance of Biochemical Deep Phenotyping for a Personalised Approach to Parkinson’s Disease. Neuroscience 2022, 511, 100–109. [Google Scholar] [CrossRef]
- Spillantini, M.G.; Schmidt, M.L.; Lee, V.M.; Trojanowski, J.Q.; Jakes, R.; Goedert, M. Alpha-Synuclein in Lewy Bodies. Nature 1997, 388, 839–840. [Google Scholar] [CrossRef]
- Pizarro-Galleguillos, B.M.; Kunert, L.; Brüggemann, N.; Prasuhn, J. Iron- and Neuromelanin-Weighted Neuroimaging to Study Mitochondrial Dysfunction in Patients with Parkinson’s Disease. Int. J. Mol. Sci. 2022, 23, 13678. [Google Scholar] [CrossRef]
- Kulkarni, A.; Preeti, K.; Tryphena, K.P.; Srivastava, S.; Singh, S.B.; Khatri, D.K. Proteostasis in Parkinson’s Disease: Recent Development and Possible Implication in Diagnosis and Therapeutics. Ageing Res. Rev. 2023, 84, 101816. [Google Scholar] [CrossRef] [PubMed]
- Mächtel, R.; Annamaria Boros, F.; Philipp Dobert, J.; Arnold, P.; Zunke, F. From Lysosomal Storage Disorders to Parkinson’s Disease—Challenges and Opportunities. J. Mol. Biol. 2022, 435, 167932. [Google Scholar] [CrossRef]
- Zheng, Z.; Zhang, S.; Zhang, H.; Gao, Z.; Wang, X.; Liu, X.; Xue, C.; Yao, L.; Lu, G. Mechanisms of Autoimmune Cell in DA Neuron Apoptosis of Parkinson’s Disease: Recent Advancement. Oxidative Med. Cell. Longev. 2022, 2022, 7965433. [Google Scholar] [CrossRef] [PubMed]
- Fox, S.H.; Katzenschlager, R.; Lim, S.-Y.; Barton, B.; de Bie, R.M.A.; Seppi, K.; Coelho, M.; Sampaio, C. Movement Disorder Society Evidence-Based Medicine Committee International Parkinson and Movement Disorder Society Evidence-Based Medicine Review: Update on Treatments for the Motor Symptoms of Parkinson’s Disease. Mov. Disord. 2018, 33, 1248–1266. [Google Scholar] [CrossRef] [PubMed]
- Jankovic, J.; Tan, E.K. Parkinson’s Disease: Etiopathogenesis and Treatment. J. Neurol. Neurosurg. Psychiatry 2020, 91, 795–808. [Google Scholar] [CrossRef]
- Miguelez, C.; De Deurwaerdère, P.; Sgambato, V. Editorial: Non-Dopaminergic Systems in Parkinson’s Disease. Front. Pharmacol. 2020, 11, 593822. [Google Scholar] [CrossRef]
- Giguère, N.; Burke Nanni, S.; Trudeau, L.-E. On Cell Loss and Selective Vulnerability of Neuronal Populations in Parkinson’s Disease. Front. Neurol. 2018, 9, 455. [Google Scholar] [CrossRef]
- Blesa, J.; Foffani, G.; Dehay, B.; Bezard, E.; Obeso, J.A. Motor and Non-Motor Circuit Disturbances in Early Parkinson Disease: Which Happens First? Nat. Rev. Neurosci. 2022, 23, 115–128. [Google Scholar] [CrossRef]
- Braak, H.; Del Tredici, K.; Rüb, U.; de Vos, R.A.I.; Jansen Steur, E.N.H.; Braak, E. Staging of Brain Pathology Related to Sporadic Parkinson’s Disease. Neurobiol. Aging 2003, 24, 197–211. [Google Scholar] [CrossRef]
- Franceschi, C.; Campisi, J. Chronic Inflammation (Inflammaging) and Its Potential Contribution to Age-Associated Diseases. J. Gerontol. A Biol. Sci. Med. Sci. 2014, 69 (Suppl. 1), S4–S9. [Google Scholar] [CrossRef] [PubMed]
- Reeve, A.; Simcox, E.; Turnbull, D. Ageing and Parkinson’s Disease: Why Is Advancing Age the Biggest Risk Factor? Ageing Res. Rev. 2014, 14, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Bellou, V.; Belbasis, L.; Tzoulaki, I.; Evangelou, E.; Ioannidis, J.P.A. Environmental Risk Factors and Parkinson’s Disease: An Umbrella Review of Meta-Analyses. Park. Relat. Disord. 2016, 23, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, A.M.; Ou, Z.-Y.A.; Gordon, R.; Saminathan, H. Environmental Neurotoxicants and Inflammasome Activation in Parkinson’s Disease—A Focus on the Gut-Brain Axis. Int. J. Biochem. Cell Biol. 2022, 142, 106113. [Google Scholar] [CrossRef]
- Pezzoli, G.; Cereda, E. Exposure to Pesticides or Solvents and Risk of Parkinson Disease. Neurology 2013, 80, 2035–2041. [Google Scholar] [CrossRef]
- Tanner, C.M.; Kamel, F.; Ross, G.W.; Hoppin, J.A.; Goldman, S.M.; Korell, M.; Marras, C.; Bhudhikanok, G.S.; Kasten, M.; Chade, A.R.; et al. Rotenone, Paraquat, and Parkinson’s Disease. Environ. Health Perspect. 2011, 119, 866–872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goldman, S.M. Environmental Toxins and Parkinson’s Disease. Annu. Rev. Pharmacol. Toxicol. 2014, 54, 141–164. [Google Scholar] [CrossRef] [PubMed]
- Lorscheider, F.L.; Vimy, M.J.; Summers, A.O. Mercury Exposure from “Silver” Tooth Fillings: Emerging Evidence Questions a Traditional Dental Paradigm. FASEB J. 1995, 9, 504–508. [Google Scholar] [CrossRef]
- Pyatha, S.; Kim, H.; Lee, D.; Kim, K. Association between Heavy Metal Exposure and Parkinson’s Disease: A Review of the Mechanisms Related to Oxidative Stress. Antioxidants 2022, 11, 2467. [Google Scholar] [CrossRef]
- Pan, B.-B.; Yang, Y.; Liu, H.-Z.; Li, Y.-H.; Su, X.-C. Coordination of Platinum to α-Synuclein Inhibits Filamentous Aggregation in Solution. Chembiochem 2019, 20, 1953–1958. [Google Scholar] [CrossRef]
- Reichmann, H.; Csoti, I.; Koschel, J.; Lorenzl, S.; Schrader, C.; Winkler, J.; Wüllner, U. Life Style and Parkinson’s Disease. J. Neural. Transm. 2022, 129, 1235–1245. [Google Scholar] [CrossRef]
- van Wamelen, D.J.; Wan, Y.-M.; Ray Chaudhuri, K.; Jenner, P. Stress and Cortisol in Parkinson’s Disease. Int. Rev. Neurobiol. 2020, 152, 131–156. [Google Scholar] [CrossRef] [PubMed]
- Luthra, N.S.; Clow, A.; Corcos, D.M. The Interrelated Multifactorial Actions of Cortisol and Klotho: Potential Implications in the Pathogenesis of Parkinson’s Disease. Brain Sci. 2022, 12, 1695. [Google Scholar] [CrossRef] [PubMed]
- Scott-Massey, A.; Boag, M.K.; Magnier, A.; Bispo, D.P.C.F.; Khoo, T.K.; Pountney, D.L. Glymphatic System Dysfunction and Sleep Disturbance May Contribute to the Pathogenesis and Progression of Parkinson’s Disease. Int. J. Mol. Sci. 2022, 23, 12928. [Google Scholar] [CrossRef] [PubMed]
- Ono, K.; Mochizuki, H.; Ikeda, T.; Nihira, T.; Takasaki, J.; Teplow, D.B.; Yamada, M. Effect of Melatonin on α-Synuclein Self-Assembly and Cytotoxicity. Neurobiol. Aging 2012, 33, 2172–2185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clark, I.; Landolt, H.P. Coffee, Caffeine, and Sleep: A Systematic Review of Epidemiological Studies and Randomized Controlled Trials. Sleep Med. Rev. 2017, 31, 70–78. [Google Scholar] [CrossRef] [Green Version]
- Reichert, C.F.; Deboer, T.; Landolt, H.-P. Adenosine, Caffeine, and Sleep-Wake Regulation: State of the Science and Perspectives. J. Sleep Res. 2022, 31, e13597. [Google Scholar] [CrossRef]
- Obeso, J.A.; Stamelou, M.; Goetz, C.G.; Poewe, W.; Lang, A.E.; Weintraub, D.; Burn, D.; Halliday, G.M.; Bezard, E.; Przedborski, S.; et al. Past, Present, and Future of Parkinson’s Disease: A Special Essay on the 200th Anniversary of the Shaking Palsy. Mov. Disord. 2017, 32, 1264–1310. [Google Scholar] [CrossRef]
- Leite Silva, A.B.R.; Gonçalves de Oliveira, R.W.; Diógenes, G.P.; de Castro Aguiar, M.F.; Sallem, C.C.; Lima, M.P.P.; de Albuquerque Filho, L.B.; Peixoto de Medeiros, S.D.; Penido de Mendonça, L.L.; de Santiago Filho, P.C.; et al. Premotor, Nonmotor and Motor Symptoms of Parkinson’s Disease: A New Clinical State of the Art. Ageing Res. Rev. 2023, 84, 101834. [Google Scholar] [CrossRef]
- Warnecke, T.; Schäfer, K.-H.; Claus, I.; Del Tredici, K.; Jost, W.H. Gastrointestinal Involvement in Parkinson’s Disease: Pathophysiology, Diagnosis, and Management. NPJ Park. Dis. 2022, 8, 31. [Google Scholar] [CrossRef]
- Xu, J.; Wang, L.; Chen, X.; Le, W. New Understanding on the Pathophysiology and Treatment of Constipation in Parkinson’s Disease. Front. Aging Neurosci. 2022, 14, 917499. [Google Scholar] [CrossRef]
- Meng, L.; Yuan, X.; Cao, X.; Zhang, Z. The Gut-Brain Axis in the Pathogenesis of Parkinson’s Disease. Brain Sci. Adv. 2019, 5, 73–81. [Google Scholar] [CrossRef] [Green Version]
- Menozzi, E.; Macnaughtan, J.; Schapira, A.H.V. The Gut-Brain Axis and Parkinson Disease: Clinical and Pathogenetic Relevance. Ann. Med. 2021, 53, 611–625. [Google Scholar] [CrossRef] [PubMed]
- Hawkes, C.H.; Del Tredici, K.; Braak, H. A Timeline for Parkinson’s Disease. Park. Relat. Disord. 2010, 16, 79–84. [Google Scholar] [CrossRef]
- Shannon, K.M.; Keshavarzian, A.; Dodiya, H.B.; Jakate, S.; Kordower, J.H. Is Alpha-Synuclein in the Colon a Biomarker for Premotor Parkinson’s Disease? Evidence from 3 Cases. Mov. Disord. 2012, 27, 716–719. [Google Scholar] [CrossRef]
- O’Day, C.; Finkelstein, D.I.; Diwakarla, S.; McQuade, R.M. A Critical Analysis of Intestinal Enteric Neuron Loss and Constipation in Parkinson’s Disease. J. Park. Dis. 2022, 12, 1841–1861. [Google Scholar] [CrossRef] [PubMed]
- Shannon, K.; Vanden Berghe, P. The Enteric Nervous System in PD: Gateway, Bystander Victim, or Source of Solutions. Cell Tissue Res. 2018, 373, 313–326. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.-J.; Lin, C.-H. Gut Microenvironmental Changes as a Potential Trigger in Parkinson’s Disease through the Gut-Brain Axis. J. Biomed. Sci. 2022, 29, 54. [Google Scholar] [CrossRef]
- Dutta, S.K.; Verma, S.; Jain, V.; Surapaneni, B.K.; Vinayek, R.; Phillips, L.; Nair, P.P. Parkinson’s Disease: The Emerging Role of Gut Dysbiosis, Antibiotics, Probiotics, and Fecal Microbiota Transplantation. J. Neurogastroenterol. Motil. 2019, 25, 363–376. [Google Scholar] [CrossRef] [Green Version]
- Pavan, S.; Prabhu, A.N.; Prasad Gorthi, S.; Das, B.; Mutreja, A.; Shetty, V.; Ramamurthy, T.; Ballal, M. Exploring the Multifactorial Aspects of Gut Microbiome in Parkinson’s Disease. Folia Microbiol. 2022, 67, 693–706. [Google Scholar] [CrossRef]
- Zhu, M.; Liu, X.; Ye, Y.; Yan, X.; Cheng, Y.; Zhao, L.; Chen, F.; Ling, Z. Gut Microbiota: A Novel Therapeutic Target for Parkinson’s Disease. Front. Immunol. 2022, 13, 937555. [Google Scholar] [CrossRef]
- Cenci, M.A.; Björklund, A. Animal models for preclinical Parkinson’s research: An update and critical appraisal. In Progress in Brain Research; Elsevier: Amsterdam, The Netherlands, 2020; Volume 252, pp. 27–59. ISBN 978-0-444-64260-8. [Google Scholar]
- Blesa, J.; Przedborski, S. Parkinson’s Disease: Animal Models and Dopaminergic Cell Vulnerability. Front. Neuroanat. 2014, 8, 155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reed, X.; Bandrés-Ciga, S.; Blauwendraat, C.; Cookson, M.R. The Role of Monogenic Genes in Idiopathic Parkinson’s Disease. Neurobiol. Dis. 2019, 124, 230–239. [Google Scholar] [CrossRef] [PubMed]
- Polymeropoulos, M.H.; Higgins, J.J.; Golbe, L.I.; Johnson, W.G.; Ide, S.E.; Iorio, G.; Sanges, G.; Stenroos, E.S.; Pho, L.T.; Schaffer, A.A. Mapping of a Gene for Parkinson’s Disease to Chromosome 4q21-Q23. Science 1996, 274, 1197–1199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gubellini, P.; Kachidian, P. Animal Models of Parkinson’s Disease: An Updated Overview. Rev. Neurol. 2015, 171, 750–761. [Google Scholar] [CrossRef]
- Pingale, T.; Gupta, G.L. Classic and Evolving Animal Models in Parkinson’s Disease. Pharmacol. Biochem. Behav. 2020, 199, 173060. [Google Scholar] [CrossRef] [PubMed]
- Diwakarla, S.; McQuade, R.M.; Constable, R.; Artaiz, O.; Lei, E.; Barnham, K.J.; Adlard, P.A.; Cherny, R.A.; Di Natale, M.R.; Wu, H.; et al. ATH434 Reverses Colorectal Dysfunction in the A53T Mouse Model of Parkinson’s Disease. J. Park. Dis. 2021, 11, 1821–1832. [Google Scholar] [CrossRef]
- Wang, W.; Song, N.; Jia, F.; Tang, T.; Bao, W.; Zuo, C.; Xie, J.; Jiang, H. Genomic DNA Levels of Mutant Alpha-Synuclein Correlate with Non-Motor Symptoms in an A53T Parkinson’s Disease Mouse Model. Neurochem. Int. 2018, 114, 71–79. [Google Scholar] [CrossRef]
- Kuo, Y.-M.; Li, Z.; Jiao, Y.; Gaborit, N.; Pani, A.K.; Orrison, B.M.; Bruneau, B.G.; Giasson, B.I.; Smeyne, R.J.; Gershon, M.D.; et al. Extensive Enteric Nervous System Abnormalities in Mice Transgenic for Artificial Chromosomes Containing Parkinson Disease-Associated Alpha-Synuclein Gene Mutations Precede Central Nervous System Changes. Hum. Mol. Genet. 2010, 19, 1633–1650. [Google Scholar] [CrossRef]
- Kuo, Y.-M.; Nwankwo, E.I.; Nussbaum, R.L.; Rogers, J.; Maccecchini, M.L. Translational Inhibition of α-Synuclein by Posiphen Normalizes Distal Colon Motility in Transgenic Parkinson Mice. Am. J. Neurodegener. Dis. 2019, 8, 1–15. [Google Scholar]
- Gries, M.; Christmann, A.; Schulte, S.; Weyland, M.; Rommel, S.; Martin, M.; Baller, M.; Röth, R.; Schmitteckert, S.; Unger, M.; et al. Parkinson Mice Show Functional and Molecular Changes in the Gut Long before Motoric Disease Onset. Mol. Neurodegener. 2021, 16, 34. [Google Scholar] [CrossRef]
- Ghaisas, S.; Langley, M.R.; Palanisamy, B.N.; Dutta, S.; Narayanaswamy, K.; Plummer, P.J.; Sarkar, S.; Ay, M.; Jin, H.; Anantharam, V.; et al. MitoPark Transgenic Mouse Model Recapitulates the Gastrointestinal Dysfunction and Gut-Microbiome Changes of Parkinson’s Disease. Neurotoxicology 2019, 75, 186–199. [Google Scholar] [CrossRef] [PubMed]
- Singh, Y.; Trautwein, C.; Dhariwal, A.; Salker, M.S.; Alauddin, M.; Zizmare, L.; Pelzl, L.; Feger, M.; Admard, J.; Casadei, N.; et al. DJ-1 (Park7) Affects the Gut Microbiome, Metabolites and the Development of Innate Lymphoid Cells (ILCs). Sci. Rep. 2020, 10, 16131. [Google Scholar] [CrossRef] [PubMed]
- Liang, F.; Chen, C.-Y.; Li, Y.-P.; Ke, Y.-C.; Ho, E.-P.; Jeng, C.-F.; Lin, C.-H.; Chen, S.-K. Early Dysbiosis and Dampened Gut Microbe Oscillation Precede Motor Dysfunction and Neuropathology in Animal Models of Parkinson’s Disease. J. Park. Dis. 2022, 12, 2423–2440. [Google Scholar] [CrossRef] [PubMed]
- Ungerstedt, U. 6-hydroxy-dopamine induced degeneration of central monoamine neurons. Eur. J. Pharmacol. 1968, 5, 107–110. [Google Scholar] [CrossRef] [PubMed]
- Kin, K.; Yasuhara, T.; Kameda, M.; Date, I. Animal Models for Parkinson’s Disease Research: Trends in the 2000s. Int. J. Mol. Sci. 2019, 20, 5402. [Google Scholar] [CrossRef] [Green Version]
- Vegezzi, G.; Al Harraq, Z.; Levandis, G.; Cerri, S.; Blandini, F.; Gnudi, G.; Miduri, F.; Blandizzi, C.; Domenichini, G.; Bertoni, S.; et al. Radiological Analysis of Gastrointestinal Dysmotility in a Model of Central Nervous Dopaminergic Degeneration: Comparative Study with Conventional in Vivo Techniques in the Rat. J. Pharmacol. Toxicol. Methods 2014, 70, 163–169. [Google Scholar] [CrossRef]
- William Langston, J.; Ballard, P.; Tetrud, J.W.; Irwin, I. Chronic parkinsonism in humans due to a product of meperidine-analog synthesis. Science 1983, 219, 979–980. [Google Scholar] [CrossRef] [Green Version]
- Sundström, E.; Samuelsson, E.B. Comparison of Key Steps in 1-Methyl-4-Phenyl-1,2,3,6-Tetrahydropyridine (MPTP) Neurotoxicity in Rodents. Pharmacol. Toxicol. 1997, 81, 226–231. [Google Scholar] [CrossRef] [Green Version]
- Meredith, G.E.; Kang, U.J. Behavioral models of Parkinsons disease in rodents: A new look at an old problem. Mov. Disord. 2006, 21, 1595–1606. [Google Scholar] [CrossRef]
- Anderson, G.; Noorian, A.R.; Taylor, G.; Anitha, M.; Bernhard, D.; Srinivasan, S.; Greene, J.G. Loss of Enteric Dopaminergic Neurons and Associated Changes in Colon Motility in an MPTP Mouse Model of Parkinson’s Disease. Exp. Neurol. 2007, 207, 4–12. [Google Scholar] [CrossRef] [Green Version]
- Delamarre, A.; MacSweeney, C.; Suzuki, R.; Brown, A.J.; Li, Q.; Pioli, E.Y.; Bezard, E. Gastrointestinal and Metabolic Function in the MPTP-Treated Macaque Model of Parkinson’s Disease. Heliyon 2020, 6, e05771. [Google Scholar] [CrossRef]
- Ascherio, A.; Schwarzschild, M.A. The Epidemiology of Parkinson’s Disease: Risk Factors and Prevention. Lancet Neurol. 2016, 15, 1257–1272. [Google Scholar] [CrossRef]
- Ferrante, R.J.; Schulz, J.B.; Kowall, N.W.; Beal, M.F. Systemic Administration of Rotenone Produces Selective Damage in the Striatum and Globus Pallidus, but Not in the Substantia Nigra. Brain Res. 1997, 753, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Pienaar, I.S.; Lu, B.; Schallert, T. Closing the Gap between Clinic and Cage: Sensori-Motor and Cognitive Behavioural Testing Regimens in Neurotoxin-Induced Animal Models of Parkinson’s Disease. Neurosci. Biobehav. Rev. 2012, 36, 2305–2324. [Google Scholar] [CrossRef] [PubMed]
- Schaffernicht, G.; Shang, Q.; Stievenard, A.; Bötzel, K.; Dening, Y.; Kempe, R.; Toussaint, M.; Gündel, D.; Kranz, M.; Reichmann, H.; et al. Pathophysiological Changes in the Enteric Nervous System of Rotenone-Exposed Mice as Early Radiological Markers for Parkinson’s Disease. Front. Neurol. 2021, 12, 642604. [Google Scholar] [CrossRef] [PubMed]
- Ahn, E.H.; Kang, S.S.; Liu, X.; Chen, G.; Zhang, Z.; Chandrasekharan, B.; Alam, A.M.; Neish, A.S.; Cao, X.; Ye, K. Initiation of Parkinson’s Disease from Gut to Brain by δ-Secretase. Cell Res. 2020, 30, 70–87. [Google Scholar] [CrossRef] [PubMed]
- Brooks, A.I.; Chadwick, C.A.; Gelbard, H.A.; Cory-Slechta, D.A.; Federoff, H.J. Paraquat Elicited Neurobehavioral Syndrome Caused by Dopaminergic Neuron Loss. Brain Res. 1999, 823, 1–10. [Google Scholar] [CrossRef]
- McCormack, A.L.; Thiruchelvam, M.; Manning-Bog, A.B.; Thiffault, C.; Langston, J.W.; Cory-Slechta, D.A.; Monte, D.A. Environmental Risk Factors and Parkinson’s Disease: Selective Degeneration of Nigral Dopaminergic Neurons Caused by the Herbicide Paraquat. Neurobiol. Dis. 2002, 10, 119–127. [Google Scholar] [CrossRef] [Green Version]
- Hou, L.; Huang, R.; Sun, F.; Zhang, L.; Wang, Q. NADPH Oxidase Regulates Paraquat and Maneb-Induced Dopaminergic Neurodegeneration through Ferroptosis. Toxicology 2019, 417, 64–73. [Google Scholar] [CrossRef]
- Bastías-Candia, S.; Zolezzi, J.M.; Inestrosa, N.C. Revisiting the Paraquat-Induced Sporadic Parkinson’s Disease-Like Model. Mol. Neurobiol. 2019, 56, 1044–1055. [Google Scholar] [CrossRef] [PubMed]
- Manning-Bog, A.B.; McCormack, A.L.; Li, J.; Uversky, V.N.; Fink, A.L.; Monte, D.A. The Herbicide Paraquat Causes Up-Regulation and Aggregation of α-Synuclein in Mice: Paraquat and α-Synuclein. J. Biol. Chem. 2002, 277, 1641–1644. [Google Scholar] [CrossRef] [Green Version]
- Naudet, N.; Antier, E.; Gaillard, D.; Morignat, E.; Lakhdar, L.; Baron, T.; Bencsik, A. Oral Exposure to Paraquat Triggers Earlier Expression of Phosphorylated α-Synuclein in the Enteric Nervous System of A53T Mutant Human α-Synuclein Transgenic Mice. J. Neuropathol. Exp. Neurol. 2017, 76, 1046–1057. [Google Scholar] [CrossRef] [Green Version]
- Cristóvão, A.C.; Campos, F.L.; Je, G.; Esteves, M.; Guhathakurta, S.; Yang, L.; Beal, M.F.; Fonseca, B.M.; Salgado, A.J.; Queiroz, J. Characterization of a Parkinson’s disease rat model using an upgraded paraquat exposure paradigm. Eur. J. Neurosci. 2020, 52, 3242–3255. [Google Scholar] [CrossRef]
- Anselmi, L.; Bove, C.; Coleman, F.H.; Le, K.; Subramanian, M.P.; Venkiteswaran, K.; Subramanian, T.; Travagli, R.A. Ingestion of Subthreshold Doses of Environmental Toxins Induces Ascending Parkinsonism in the Rat. NPJ Park. Dis. 2018, 4, 30. [Google Scholar] [CrossRef] [Green Version]
- García-Revilla, J.; Herrera, A.J.; de Pablos, R.M.; Venero, J.L. Inflammatory Animal Models of Parkinson’s Disease. J. Park. Dis. 2002, 12, S165–S182. [Google Scholar] [CrossRef]
- Herrera, A.J.; Castaño, A.; Venero, J.L.; Cano, J.; Machado, A. The Single Intranigral Injection of LPS as a New Model for Studying the Selective Effects of Inflammatory Reactions on Dopaminergic System. Neurobiol. Dis. 2000, 7, 429–447. [Google Scholar] [CrossRef] [Green Version]
- Hunter, R.L.; Choi, D.-Y.; Kincer, J.F.; Cass, W.A.; Bing, G.; Gash, D.M. Fenbendazole Treatment May Influence Lipopolysaccharide Effects in Rat Brain. Comp. Med. 2007, 57, 487–492. [Google Scholar] [PubMed]
- Hunter, R.L.; Cheng, B.; Choi, D.-Y.; Liu, M.; Liu, S.; Cass, W.A.; Bing, G. Intrastriatal Lipopolysaccharide Injection Induces Parkinsonism in C57/B6 Mice. J. Neurosci. Res. 2009, 87, 1913–1921. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, B.; Xu, P.; Wu, S.; Jiang, Z.; Huang, Z.; Li, Q.; Chen, D. Diosgenin Attenuates Lipopolysaccharide-Induced Parkinson’s Disease by Inhibiting the TLR/NF-ΚB Pathway. J. Alzheimers Dis. 2018, 64, 943–955. [Google Scholar] [CrossRef]
- Deng, I.; Corrigan, F.; Garg, S.; Zhou, X.-F.; Bobrovskaya, L. Further Characterization of Intrastriatal Lipopolysaccharide Model of Parkinson’s Disease in C57BL/6 Mice. Int. J. Mol. Sci. 2021, 22, 7380. [Google Scholar] [CrossRef] [PubMed]
- Quintana, E.; Hernández, C.; Alvarez-Barrientos, A.; Esplugues, J.V.; Barrachina, M.D. Synthesis of Nitric Oxide in Postganglionic Myenteric Neurons during Endotoxemia: Implications for Gastric Motor Function in Rats. FASEB J. 2004, 18, 531–533. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-Y.; Li, Y.-N.; Ni, J.-B.; Chen, C.-J.; Lv, S.; Chai, S.-Y.; Wu, R.-H.; Yüce, B.; Storr, M. Involvement of Cannabinoid-1 and Cannabinoid-2 Receptors in Septic Ileus. Neurogastroenterol. Motil. 2010, 22, 350-e88. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, W.; Song, N.; Jiao, L.; Jia, F.; Du, X.; Chen, X.; Yan, C.; Jiao, J.; Jiao, Q.; et al. Ghrelin Bridges DMV Neuropathology and GI Dysfunction in the Early Stages of Parkinson’s Disease. Adv. Sci. 2022, 9, e2203020. [Google Scholar] [CrossRef]
- Cabezos, P.A.; Vera, G.; Castillo, M.; Fernández-Pujol, R.; Martín, M.I.; Abalo, R. Radiological Study of Gastrointestinal Motor Activity after Acute Cisplatin in the Rat. Temporal Relationship with Pica. Auton. Neurosci. 2008, 141, 54–65. [Google Scholar] [CrossRef]
- Toti, L.; Travagli, R.A. Gastric Dysregulation Induced by Microinjection of 6-OHDA in the Substantia Nigra Pars Compacta of Rats Is Determined by Alterations in the Brain-Gut Axis. Am. J. Physiol. -Gastrointest. Liver Physiol. 2014, 307, G1013–G1023. [Google Scholar] [CrossRef]
- Stott, S.; Broza, Y.Y.; Gharra, A.; Wang, Z.; Barker, R.A.; Haick, H. The Utility of Breath Analysis in the Diagnosis and Staging of Parkinson’s Disease. J. Park. Dis. 2022, 12, 993–1002. [Google Scholar] [CrossRef]
- Anselmi, L.; Toti, L.; Bove, C.; Hampton, J.; Travagli, R.A. A Nigro−Vagal Pathway Controls Gastric Motility and Is Affected in a Rat Model of Parkinsonism. Gastroenterology 2017, 153, 1581–1593. [Google Scholar] [CrossRef]
- Chai, X.-Y.; Diwakarla, S.; Pustovit, R.V.; McQuade, R.M.; Di Natale, M.; Ermine, C.M.; Parish, C.L.; Finkelstein, D.I.; Furness, J.B. Investigation of Nerve Pathways Mediating Colorectal Dysfunction in Parkinson’s Disease Model Produced by Lesion of Nigrostriatal Dopaminergic Neurons. Neurogastroenterol. Motil. 2020, 32, e13893. [Google Scholar] [CrossRef]
- Levandis, G.; Balestra, B.; Siani, F.; Rizzo, V.; Ghezzi, C.; Ambrosi, G.; Cerri, S.; Bonizzi, A.; Vicini, R.; Vairetti, M.; et al. Response of Colonic Motility to Dopaminergic Stimulation Is Subverted in Rats with Nigrostriatal Lesion: Relevance to Gastrointestinal Dysfunctions in Parkinson’s Disease. Neurogastroenterol. Motil. 2015, 27, 1783–1795. [Google Scholar] [CrossRef] [PubMed]
- Nakamori, H.; Noda, K.; Mitsui, R.; Hashitani, H. Role of Enteric Dopaminergic Neurons in Regulating Peristalsis of Rat Proximal Colon. Neurogastroenterol. Motil. 2021, 33, e14127. [Google Scholar] [CrossRef] [PubMed]
- Rota, L.; Pellegrini, C.; Benvenuti, L.; Antonioli, L.; Fornai, M.; Blandizzi, C.; Cattaneo, A.; Colla, E. Constipation, Deficit in Colon Contractions and Alpha-Synuclein Inclusions within the Colon Precede Motor Abnormalities and Neurodegeneration in the Central Nervous System in a Mouse Model of Alpha-Synucleinopathy. Transl. Neurodegener. 2019, 8, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McQuade, R.M.; Singleton, L.M.; Wu, H.; Lee, S.; Constable, R.; Di Natale, M.; Ringuet, M.T.; Berger, J.P.; Kauhausen, J.; Parish, C.L.; et al. The Association of Enteric Neuropathy with Gut Phenotypes in Acute and Progressive Models of Parkinson’s Disease. Sci. Rep. 2021, 11, 7934. [Google Scholar] [CrossRef]
- Nie, P.; Li, Z.; Wang, Y. Gut microbiome interventions in human health and diseases. Med. Res. Rev. 2019, 39, 2286–2313. [Google Scholar] [CrossRef]
- Sommer, F.; Bäckhed, F. The Gut Microbiota–Masters of Host Development and Physiology. Nat. Rev. Microbiol. 2013, 11, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Drago, L.; Valentina, C.; Fabio, P. Gut Microbiota, Dysbiosis and Colon Lavage. Dig. Liver Dis. 2019, 51, 1209–1213. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.-Y.; Li, H.-X.; Xu, R.-C. Potential Roles of Gut Microbiota and Microbial Metabolites in Parkinson’s Disease. Ageing Res. Rev. 2021, 69, 101347. [Google Scholar] [CrossRef]
- Toh, T.S.; Chong, C.W.; Lim, S.Y. Gut Microbiome in Parkinson’s Disease: New Insights from Meta-Analysis. Park. Relat. Disord. 2022, 94, 1–9. [Google Scholar] [CrossRef]
- O’Donovan, S.M.; Crowley, E.K.; Brown, J.R.M.; O’Sullivan, O.; O’Leary, O.F.; Timmons, S.; Nolan, Y.M.; Clarke, D.J.; Hyland, N.P.; Joyce, S.A.; et al. Nigral Overexpression of α-Synuclein in a Rat Parkinson’s Disease Model Indicates Alterations in the Enteric Nervous System and the Gut Microbiome. Neurogastroenterol. Motil. 2020, 32, e13726. [Google Scholar] [CrossRef]
- Wang, W.; Zhu, G.; Wang, Y.; Li, W.; Yi, S.; Wang, K.; Fan, L.; Tang, J.; Chen, R. Multi-Omics Integration in Mice with Parkinson’s Disease and the Intervention Effect of Cyanidin-3-O-Glucoside. Front. Aging Neurosci. 2022, 14, 877078. [Google Scholar] [CrossRef]
- Radisavljevic, N.; Cirstea, M.; Bauer, K.; Lo, C.; Metcalfe-Roach, A.; Bozorgmehr, T.; Bar-Yoseph, H.; Brett Finlay, B. Effects of Gut Microbiota Alterations on Motor, Gastrointestinal, and Behavioral Phenotype in a Mouse Model of Parkinson’s Disease. J. Park. Dis. 2022, 12, 1479–1495. [Google Scholar] [CrossRef]
- Yang, X.; Qian, Y.; Xu, S.; Song, Y.; Xiao, Q. Longitudinal analysis of fecal microbiome and pathologic processes in a rotenone induced mice model of Parkinson’s disease. Front. Aging Neurosci. 2018, 9, 441. [Google Scholar] [CrossRef] [Green Version]
- Dănău, A.; Dumitrescu, L.; Lefter, A.; Tulbă, D.; Popescu, B.O. Small Intestinal Bacterial Overgrowth as Potential Therapeutic Target in Parkinson’s Disease. Int. J. Mol. Sci. 2021, 22, 11663. [Google Scholar] [CrossRef]
- Fasano, A.; Bove, F.; Gabrielli, M.; Petracca, M.; Zocco, M.A.; Ragazzoni, E.; Barbaro, F.; Piano, C.; Fortuna, S.; Tortora, A.; et al. The Role of Small Intestinal Bacterial Overgrowth in Parkinson’s Disease. Mov. Disord. 2013, 28, 1241–1249. [Google Scholar] [CrossRef]
- Tan, A.H.; Mahadeva, S.; Thalha, A.M. Small Intestinal Bacterial Overgrowth in Parkinson’s Disease. Park. Relat. Disord. 2014, 20, 535–540. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Feng, X.; Jiang, Z.; Jiang, Z. Association of Small Intestinal Bacterial Overgrowth with Parkinson’s Disease: A Systematic Review and Meta-Analysis. Gut Pathog. 2021, 13, 25. [Google Scholar] [CrossRef] [PubMed]
- Stolzenberg, E.; Berry, D.; Yang, D. A Role for Neuronal Alpha-Synuclein in Gastrointestinal Immunity. J. Innate Immun. 2017, 9, 456–463. [Google Scholar] [CrossRef]
- Nerius, M.; Doblhammer, G.; Tamgüney, G. GI Infections Are Associated with an Increased Risk of Parkinson’s Disease. Gut 2020, 69, 1154–1156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sampson, T.R.; Debelius, J.W.; Thron, T. Gut Microbiota Regulate Motor Deficits and Neuroinflammation in a Model of Par-Kinson’s Disease. Cell 2016, 167, 1469–1480. [Google Scholar] [CrossRef] [Green Version]
- Gonçalves, F.B.; Morais, V.A. PINK1: A Bridge between Mitochondria and Parkinson’s Disease. Life 2021, 11, 371. [Google Scholar] [CrossRef] [PubMed]
- Matheoud, D.; Cannon, T.; Voisin, A. Intestinal Infection Triggers Parkinson’s Disease-like Symptoms in Pink1−/− Mice. Nature 2019, 571, 565–569. [Google Scholar] [CrossRef]
- Unger, M.M.; Spiegel, J.; Dillmann, K.U. Short Chain Fatty Acids and Gut Microbiota Differ between Patients with Parkinson’s Disease and Age-Matched Controls. Park. Relat. Disord. 2016, 32, 66–72. [Google Scholar] [CrossRef]
- Nuzum, N.D.; Loughman, A.; Szymlek-Gay, E.A.; Hendy, A.; Teo, W.P.; Macpherson, H. Gut Microbiota Differences between Healthy Older Adults and Individuals with Parkinson’s Disease: A Systematic Review. Neurosci. Biobehav. Rev. 2020, 112, 227–241. [Google Scholar] [CrossRef]
- Hopfner, F.; Künstner, A.; Müller, S.H. Gut Microbiota in Parkinson Disease in a Northern German Cohort. Brain Res. 2017, 1667, 41–45. [Google Scholar] [CrossRef]
- Gerhardt, S.; Mohajeri, M.H. Changes of Colonic Bacterial Composition in Parkinson’s Disease and Other Neurodegenerative Diseases. Nutrients 2018, 10, 708. [Google Scholar] [CrossRef] [Green Version]
- Jin, M.; Li, J.; Liu, F.; Lyu, N. Analysis of the Gut Microflora in Patients with Parkinson’s Disease. Front. Neurosci. 2019, 13, 1184. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Cui, L.; Yang, Y. Gut Microbiota Differs between Parkinson’s Disease Patients and Healthy Controls in Northeast China. Front. Mol. Neurosci. 2019, 12, 171. [Google Scholar] [CrossRef] [Green Version]
- Cirstea, M.S.; Sundvick, K.; Golz, E.; Yu, A.C.; Boutin, R.C.T.; Kliger, D.; Finlay, B.B.; Appel-Cresswell, S. The Gut Mycobiome in Parkinson’s Disease. J. Park. Dis. 2021, 11, 153–158. [Google Scholar] [CrossRef]
- Guo, X.; Tang, P.; Hou, C.; Chong, L.; Zhang, X.; Liu, P.; Chen, L.; Liu, Y.; Zhang, L.; Li, R. Integrated Microbiome and Host Transcriptome Profiles Link Parkinson’s Disease to Blautia Genus: Evidence From Feces, Blood, and Brain. Front. Microbiol. 2022, 13, 875101. [Google Scholar] [CrossRef] [PubMed]
- Lubomski, M.; Xu, X.; Holmes, A.J.; Muller, S.; Yang, J.Y.H.; Davis, R.L.; Sue, C.M. The Gut Microbiome in Parkinson’s Disease: A Longitudi-Nal Study of the Impacts on Disease Progression and the Use of Device-Assisted Therapies. Front. Aging Neurosci. 2022, 14, 414. [Google Scholar] [CrossRef] [PubMed]
- Pietrucci, D.; Cerroni, R.; Unida, V. Dysbiosis of gut microbiota in a selected population of Parkinson’s patients. Park. Relat. Disord. 2019, 65, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Shen, L. Gut, Oral and Nasal Microbiota and Parkinson’s Disease. Microb. Cell Fact. 2020, 19, 50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wallen, Z.D.; Appah, M.; Dean, M.N. Characterizing dysbiosis of gut microbiome in PD: Evidence for overabundance of op-portunistic pathogens. NPJ Park. Dis. 2020, 6, 11. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Lu, G.; Li, Z. Altered Actinobacteria and Firmicutes Phylum Associated Epitopes in Patients With Parkinson’s Disease. Front. Immunol. 2021, 12, 632482. [Google Scholar] [CrossRef]
- Scheperjans, F.; Aho, V.; Pereira, P.A.B. Gut Microbiota Are Related to Parkinson’s Disease and Clinical Phenotype. Mov. Disord. 2015, 30, 350–358. [Google Scholar] [CrossRef]
- Hill-Burns, E.M.; Debelius, J.W.; Morton, J.T. Parkinson’s Disease and Parkinson’s Disease Medications Have Distinct Signatures of the Gut Microbiome. Mov. Disord. 2017, 32, 739–749. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petrov, V.A.; Saltykova, I.V.; Zhukova, I.A.; Alifirova, V.M.; Zhukova, N.G.; Dorofeeva, Y.B.; Tyakht, A.V.; Kovarsky, B.A.; Alekseev, D.G.; Kostryukova, E.S.; et al. Analysis of Gut Microbiota in Patients with Parkinson’s Disease. Bull. Exp. Biol. Med. 2017, 162, 734–737. [Google Scholar] [CrossRef] [PubMed]
- Heintz-Buschart, A.; Pandey, U.; Wicke, T.; Sixel-Döring, F.; Janzen, A.; Sittig-Wiegand, E.; Trenkwalder, C.; Oertel, W.H.; Mollenhauer, B.; Wilmes, P. The Nasal and Gut Microbiome in Parkinson’s Disease and Idiopathic Rapid Eye Movement Sleep Behavior Disorder. Mov. Disord. 2018, 33, 88–98. [Google Scholar] [CrossRef] [Green Version]
- Lin, A.; Zheng, W.; He, Y. Gut Microbiota in Patients with Parkinson’s Disease in Southern China. Park. Relat. Disord. 2018, 53, 82–88. [Google Scholar] [CrossRef]
- Aho, V.T.E.; Pereira, P.A.B.; Voutilainen, S.; Paulin, L.; Pekkonen, E.; Auvinen, P.; Scheperjans, F. Gut Microbiota in Parkinson’s Disease: Temporal Stability and Relations to Disease Progression. EBioMedicine 2019, 44, 691–707. [Google Scholar] [CrossRef] [Green Version]
- Barichella, M.; Severgnini, M.; Cilia, R.; Cassani, E.; Bolliri, C.; Caronni, S.; Ferri, V.; Cancello, R.; Ceccarani, C.; Faierman, S.; et al. Unraveling Gut Microbiota in Parkinson’s Disease and Atypical Parkinsonism. Mov. Disord. 2019, 34, 396–405. [Google Scholar] [CrossRef]
- Lin, C.H.; Chen, C.C.; Chiang, H.L. Altered Gut Microbiota and Inflammatory Cytokine Responses in Patients with Parkinson’s Disease. J. Neuroinflamm. 2019, 16, 129. [Google Scholar] [CrossRef]
- Keshavarzian, A.; Green, S.J.; Engen, P.A. Colonic Bacterial Composition in Parkinson’s Disease. Mov. Disord. 2015, 30, 1351–1360. [Google Scholar] [CrossRef]
- Bedarf, J.R.; Hildebrand, F.; Coelho, L.P.; Sunagawa, S.; Bahram, M.; Goeser, F.; Bork, P.; Wüllner, U. Functional Implications of Microbial and Viral Gut Metagenome Changes in Early Stage L-DOPA-Naïve Parkinson’s Disease Patients. Genome Med. 2017, 9, 39. [Google Scholar] [CrossRef] [Green Version]
- Tetz, G.; Brown, S.M.; Hao, Y.; Tetz, V. Parkinson’s Disease and Bacteriophages as Its Overlooked Contributors. Sci. Rep. 2018, 8, 10812. [Google Scholar] [CrossRef] [Green Version]
- Qian, Y.; Yang, X.; Xu, S. Alteration of the Fecal Microbiota in Chinese Patients with Parkinson’s Disease. Brain Behav. Immun. 2018, 70, 194–202. [Google Scholar] [CrossRef]
- Hasegawa, S.; Goto, S.; Tsuji, H.; Okuno, T.; Asahara, T.; Nomoto, K.; Shibata, A.; Fujisawa, Y.; Minato, T.; Okamoto, A.; et al. Intestinal Dysbiosis and Lowered Serum Lipopolysaccharide-Binding Protein in Parkinson’s Disease. PLoS ONE 2015, 10, e0142164. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Wu, X.; Hu, X. Structural Changes of Gut Microbiota in Parkinson’s Disease and Its Correlation with Clinical Features. Sci. China Life Sci. 2017, 60, 1223–1233. [Google Scholar] [CrossRef] [PubMed]
- Nishiwaki, H.; Ito, M.; Hamaguchi, T. Short Chain Fatty Acids-Producing and Mucin-Degrading Intestinal Bacteria Predict the Progression of Early Parkinson’s Disease. NPJ Park. Dis. 2022, 8, 65. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Zhao, D.; Ali Shah, S.Z. The Role of the Gut Microbiota in the Pathogenesis of Parkinson’s Disease. Front. Neurol. 2019, 10, 1155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Minato, T.; Maeda, T.; Fujisawa, Y. Progression of Parkinson’s Disease Is Associated with Gut Dysbiosis: Two-Year Follow-up Study. PLoS ONE 2017, 12, e0187307. [Google Scholar] [CrossRef] [Green Version]
- Santos, S.F.; Oliveira, H.L.; Yamada, E.S.; Neves, B.C.; Pereira, A. The Gut and Parkinson’s Disease—A Bidirectional Pathway. Front. Neurol. 2019, 10, 574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dalmasso, M.; Hill, C.; Ross, R.P. Exploiting Gut Bacteriophages for Human Health. Trends Microbiol. 2014, 22, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Tetz, G.; Tetz, V. Bacteriophage Infections of Microbiota Can Lead to Leaky Gut in an Experimental Rodent Model. Gut Pathog. 2016, 8, 33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asano, Y.; Hiramoto, T.; Nishino, R.; Aiba, Y.; Kimura, T.; Yoshihara, K.; Koga, Y.; Sudo, N. Critical Role of Gut Microbiota in the Production of Biologically Active, Free Catecholamines in the Gut Lumen of Mice. J. Physiol. -Gastrointest. Liver Physiol. 2012, 303, G1288–G1295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Pablo-Fernandez, E.; Gebeyehu, G.G.; Flain, L.; Slater, R.; Frau, A.; Ijaz, U.Z.; Warner, T.; Probert, C. The Faecal Metabolome and Mycobiome in Parkinson’s Disease. Park. Relat. Disord. 2022, 95, 65–69. [Google Scholar] [CrossRef]
- Margolis, K.G.; Cryan, J.F.; Mayer, E.A. The Microbiota-Gut-Brain Axis: From Motility to Mood. Gastroenterology 2021, 160, 1486–1501. [Google Scholar] [CrossRef]
- Rietdijk, C.D.; Perez-Pardo, P.; Garssen, J.; Wezel, R.J.A.; Kraneveld, A.D. Exploring Braak’s Hypothesis of Parkinson’s Disease. Front. Neurol. 2017, 8, 37. [Google Scholar] [CrossRef]
- Steiner, J.A.; Quansah, E.; Brundin, P. The Concept of Alpha-Synuclein as a Prion-like Protein: Ten Years After. Cell Tissue Res. 2018, 373, 161–173. [Google Scholar] [CrossRef]
- Dalile, B.; Van Oudenhove, L.; Vervliet, B.; Verbeke, K. The Role of Short-Chain Fatty Acids in Microbiota-Gut-Brain Communication. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 461–478. [Google Scholar] [CrossRef]
- Chen, Q.-Q.; Haikal, C.; Li, W.; Li, J.-Y. Gut Inflammation in Association With Pathogenesis of Parkinson’s Disease. Front. Mol. Neurosci. 2019, 12, 218. [Google Scholar] [CrossRef] [Green Version]
- Mou, Y.; Du, Y.; Zhou, L. Gut Microbiota Interact With the Brain Through Systemic Chronic Inflammation: Implications on Neuroinflammation, Neurodegeneration, and Aging. Front. Immunol. 2022, 13, 1046. [Google Scholar] [CrossRef]
- Miraglia, F.; Microbiome, C.E. Parkinson’s Disease and Molecular Mimicry. Cells 2019, 8, 222. [Google Scholar] [CrossRef] [Green Version]
- Sharma, S.; Awasthi, A.; Singh, S. Altered Gut Microbiota and Intestinal Permeability in Parkinson’s Disease: Pathological Highlight to Management. Neurosci. Lett. 2019, 712, 134516. [Google Scholar] [CrossRef]
- Safarpour, D.; Sharzehi, K.; Pfeiffer, R.F. Gastrointestinal Dysfunction in Parkinson’s Disease. Drugs 2022, 82, 169–197. [Google Scholar] [CrossRef]
- Stokholm, M.G.; Danielsen, E.H.; Hamilton-Dutoit, S.J.; Borghammer, P. Pathological α-synuclein in gastrointestinal tissues from pro-dromal Parkinson disease patients. Ann. Neurol. 2016, 79, 940–949. [Google Scholar] [CrossRef]
- Arumugam, M.; Raes, J.; Pelletier, E.; Le Paslier, D.; Yamada, T.; Mende, D.R.; Fernandes, G.R.; Tap, J.; Bruls, T.; Batto, J.-M.; et al. Enterotypes of the Human Gut Microbiome. Nature 2011, 473, 174–180. [Google Scholar] [CrossRef] [Green Version]
- Fu, S.-C.; Shih, L.-C.; Wu, P.-H.; Hsieh, Y.-C.; Lee, C.-H.; Lin, S.-H.; Wang, H. Exploring the Causal Effect of Constipation on Parkinson’s Disease Through Mediation Analysis of Microbial Data. Front. Cell. Infect. Microbiol. 2022, 12, 871710. [Google Scholar] [CrossRef]
- Srivastav, S.; Neupane, S.; Bhurtel, S. Probiotics Mixture Increases Butyrate, and Subsequently Rescues the Nigral Dopaminergic Neurons from MPTP and Rotenone-Induced Neurotoxicity. J. Nutr. Biochem. 2019, 69, 73–86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsieh, T.H.; Kuo, C.W.; Hsieh, K.H. Probiotics Alleviate the Progressive Deterioration of Motor Functions in a Mouse Model of Parkinson’s Disease. Brain Sci. 2020, 10, 206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, J.; Li, H.; Jin, Y. Probiotic Clostridium Butyricum Ameliorated Motor Deficits in a Mouse Model of Parkinson’s Disease via Gut Microbiota-GLP-1 Pathway. Brain Behav. Immun. 2021, 91, 703–715. [Google Scholar] [CrossRef] [PubMed]
- Cassani, E.; Privitera, G.; Pezzoli, G.; Pusani, C.; Madio, C.; Iorio, L.; Barichella, M. Use of Probiotics for the Treatment of Constipation in Parkinson’s Disease Patients. Minerva Gastroenterol. Dietol. 2011, 57, 117–121. [Google Scholar]
- Tamtaji, O.R.; Taghizadeh, M.; Daneshvar Kakhaki, R. Clinical and metabolic response to probiotic administration in people with Parkinson’s disease: A randomized, double-blind, placebo-controlled trial. Clin. Nutr. 2019, 38, 1031–1035. [Google Scholar] [CrossRef]
- Lu, C.S.; Chang, H.C.; Weng, Y.H.; Chen, C.C.; Kuo, Y.S.; Tsai, Y.C. The Add-On Effect of Lactobacillus Plantarum PS128 in Patients With Parkinson’s Disease: A Pilot Study. Front. Nutr. 2021, 8, 378. [Google Scholar] [CrossRef]
- Ibrahim, A.; Ali, R.A.R.; Manaf, M.R.A. Multi-Strain Probiotics (Hexbio) Containing MCP BCMC Strains Improved Constipation and Gut Motility in Parkinson’s Disease: A Randomised Controlled Trial. PLoS ONE 2020, 15, e0244680. [Google Scholar] [CrossRef]
- Tan, A.H.; Lim, S.Y.; Chong, K.K. Probiotics for constipation in Parkinson disease: A randomized placebo-controlled study. Neurology 2021, 96, e772–e782. [Google Scholar] [CrossRef]
- Barichella, M.; Pacchetti, C.; Bolliri, C.; Cassani, E.; Iorio, L.; Pusani, C.; Pinelli, G.; Privitera, G.; Cesari, I.; Faierman, S.A. Probiotics and Prebiotic Fiber for Constipation Associated with Parkinson Disease. Neurology 2016, 87, 1274–1280. [Google Scholar] [CrossRef] [PubMed]
- Georgescu, D.; Ancusa, O.E.; Georgescu, L.A.; Ionita, I.; Reisz, D. Nonmotor Gastrointestinal Disorders in Older Patients with Parkinson’s Disease: Is There Hope? Clin. Interv. Aging 2016, 11, 1601–1608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yin, S.; Zhu, F. Probiotics for Constipation in Parkinson’s: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Front. Cell. Infect. Microbiol 2022, 12, 1699. [Google Scholar] [CrossRef] [PubMed]
- Cammarota, G.; Ianiro, G.; Bibbò, S.; Gasbarrini, A. Fecal Microbiota Transplantation: A New Old Kid on the Block for the Management of Gut Microbiota-Related Disease. J. Clin. Gastroenterol. 2014, 48 (Suppl. 1), S80–S84. [Google Scholar] [CrossRef]
- Sun, M.F.; Zhu, Y.L.; Zhou, Z.L. Neuroprotective Effects of Fecal Microbiota Transplantation on MPTP-Induced Parkinson’s Dis-Ease Mice: Gut Microbiota, Glial Reaction and TLR4/TNF-α Signaling Pathway. Brain Behav. Immun. 2018, 70, 48–60. [Google Scholar] [CrossRef]
- Huang, H.; Xu, H.; Luo, Q. Fecal Microbiota Transplantation to Treat Parkinson’s Disease with Constipation: A Case Report. Medicine 2019, 98, e16163. [Google Scholar] [CrossRef]
- Xue, L.J.; Yang, X.Z.; Tong, Q. Fecal Microbiota Transplantation Therapy for Parkinson’s Disease: A Preliminary Study. Medicine 2020, 99, e22035. [Google Scholar] [CrossRef] [PubMed]
- Moreno, L.O.; Sánchez, P.N.; Abalo, R. Lipidomics as Tools for Finding Biomarkers of Intestinal Pathology: From Irritable Bowel Syndrome to Colorectal Cancer. Curr. Drug Targets 2022, 23, 636–655. [Google Scholar] [CrossRef]
- Bieberich, E. It’s a Lipids World. Neurochem. Res. 2013, 37, 1208–1229. [Google Scholar] [CrossRef] [Green Version]
- Holthuis, J.C.M.; Menon, A.K. Lipid Landscapes and Pipelines in Membrane Homeostasis. Nature 2014, 510, 48–57. [Google Scholar] [CrossRef]
- Xu, T.; Hu, C.; Xuan, Q.; Xu, G. Recent Advances in Analytical Strategies for Mass Spectrometry-Based Lipidomics. Anal. Chim. Acta 2020, 1137, 156–169. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Yang, K.; Gross, R.W. Multi-Dimensional Mass Spectrometry-Based Shotgun Lipidomics and Novel Strategies for Lipidomic Analyses. Mass Spectrom. Rev. 2012, 31, 134–178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fahy, E.; Subramaniam, S.; Murphy, R.C.; Nishijima, M.; Raetz, C.R.H.; Shimizu, T.; Spener, F.; Meer, G.; Wakelam, M.J.O.; Dennis, E.A. Update of the LIPID MAPS Comprehensive Classification System for Lipids. J. Lipid Res. 2009, 50, 9–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fabelo, N.; Martín, V.; Santpere, G.; Marín, R.; Torrent, L.; Ferrer, I.; Díaz, M. Severe Alterations in Lipid Composition of Frontal Cortex Lipid Rafts from Parkinson’s Disease and Incidental Parkinson’s Disease. Mol. Med. 2011, 17, 1107–1118. [Google Scholar] [CrossRef]
- Golovko, M.Y.; Faergeman, N.J.; Cole, N.B.; Castagnet, P.I.; Nussbaum, R.L.; Murphy, E.J. A-Synuclein Gene Deletion Decreases Brain Palmitate Uptake and Alters the Palmitate Metabolism in the Absence of A-Synuclein Palmitate Binding. Biochemistry 2005, 44, 8251–8259. [Google Scholar] [CrossRef]
- Kubo, S.I.; Nemani, V.M.; Chalkley, R.J.; Anthony, M.D.; Hattori, N.; Mizuno, Y.; Edwards, R.H.; Fortin, D.L. A Combinatorial Code for the Interaction of α-Synuclein with Membranes. J. Biol. Chem. 2005, 280, 31664–31672. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mori, M.A.; Delattre, A.M.; Carabelli, B.; Pudell, C.; Bortolanza, M.; Staziaki, P.V.; Visentainer, J.V.; Montanher, P.F.; Del Bel, E.A.; Ferraz, A.C. Neuroprotective Effect of Omega-3 Polyunsaturated Fatty Acids in the 6-OHDA Model of Parkinson’s Disease Is Mediated by a Reduction of Inducible Nitric Oxide Synthase. Nutr. Neurosci. 2018, 21, 341–351. [Google Scholar] [CrossRef] [PubMed]
- Dennis, E.A.; Norries, P.C. Eicosanoid Stomrm in Infection and Inflammation. Nat. Rev. Immunol. 2015, 15, 511–523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pradhan, S.; Salinas, K.; Garduno, A.; Johansson, J.; Wand, Q.; Manning-Bog, A.; Andreasson, K. Anti-Inflammatory and Neuroprotective Effects of PGE2 EP4 Signaling in Models of Parkinson’s Disease. J. Neuroimmun. Pharmacol. 2017, 12, 292–304. [Google Scholar] [CrossRef]
- Parga, J.A.; García-Garrote, M.; Martínez, S.; Raya, Á.; Labandeira-García, J.L.; Rodríguez-Pallares, J. Prostaglandin EP2 Receptors Mediate Mesenchymal Stromal Cell-Neuroprotective Effects on Dopaminergic Neurons. Mol. Neurobiol. 2018, 55, 4763–4776. [Google Scholar] [CrossRef]
- Saiki, S.; Hatano, T.; Fujimaki, M.; Ishikawa, K.I.; Mori, A.; Oji, Y.; Okuzumi, A.; Fukuhara, T.; Koinuma, T.; Imamichi, Y. Decreased Long-Chain Acylcarnitines from Insufficient β-Oxidation as Potential Early Diagnostic Markers for Parkinson’s Disease. Sci. Rep. 2017, 7, 7328. [Google Scholar] [CrossRef] [Green Version]
- Stampanoni Bassi, M.; Sancesario, A.; Morace, R.; Centonze, D.; Iezzi, E. Cannabinoids in Parkinson’s Disease. Cannabis Cannabinoid Res. 2017, 2, 21–29. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Zhang, X.; Wang, L.; Yang, C. High Performance Liquid Chromatography-Mass Spectrometry (LC-MS) Based Quantitative Lipidomics Study of Ganglioside-NANA-3 Plasma to Establish Its Association with Parkinson’s Disease Patients. Med. Sci. Monit. 2017, 23, 5345–5353. [Google Scholar] [CrossRef]
- Taguchi, Y.V.; Liu, J.; Ruan, J.; Pacheco, J.; Zhang, X.; Abbasi, J.; Keutzer, J.; Mistry, P.K.; Chandra, S.S. Glucosylsphingosine promotes α-synuclein pathology in mutant GBA-associated parkinson’s disease. J. Neurosci. 2017, 37, 9617–9631. [Google Scholar] [CrossRef] [Green Version]
- Zhao, P.; Yang, X.; Yang, L.; Li, M.; Wood, K.; Liu, Q.; Zhu, X. Neuroprotective Effects of Fingolimod in Mouse Models of Parkinson’s Disease. FASEB J. 2017, 31, 172–179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mascitelli, L.; Pezzetta, F.; Goldstein, M.R. Total Cholesterol and the Risk of Parkinson Disease. Neurology 2009, 72, 860. [Google Scholar] [CrossRef] [PubMed]
- Alecu, I.; Bennett, S.A.L. Dysregulated Lipid Metabolism and Its Role in α-Synucleinopathy in Parkinson’s Disease. Front. Neurosci. 2019, 13, 328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, D.; Jenner, A.M.; Shui, G.; Cheong, W.F.; Mitchell, T.W.; Nealon, J.R.; Kim, W.S.; McCann, H.; Wenk, M.R.; Halliday, G.M.; et al. Lipid Pathway Alterations in Parkinson’s Disease Primary Visual Cortex. PLoS ONE 2011, 6, e17299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tyurina, Y.Y.; Polimova, A.M.; Maciel, E.; Tyurin, V.A.; Kapralova, V.I.; Winnica, D.E.; Vikulina, A.S.; Domingues, M.R.M.; McCoy, J.; Sanders, L.H.; et al. LC/MS Analysis of Cardiolipins in Substantia Nigra and Plasma of Rotenone-Treated Rats: Implication for Mitochondrial Dysfunction in Parkinson’s Disease. Free Radic. Res. 2015, 49, 681–691. [Google Scholar] [CrossRef] [Green Version]
- Farmer, K.; Smith, C.A.; Hayley, S.; Smith, J. Major Alterations of Phosphatidylcholine and Lysophosphotidylcholine Lipids in the Substantia Nigra Using an Early Stage Model of Parkinson’s Disease. Int. J. Mol. Sci. 2015, 16, 18865–18877. [Google Scholar] [CrossRef] [Green Version]
- Chan, R.B.; Perotte, A.J.; Zhou, B.; Liong, C.; Shorr, E.J.; Marder, K.S.; Kang, U.J.; Waters, C.H.; Levy, O.A.; Xu, Y.; et al. Elevated GM3 Plasma Concentration in Idiopathic Parkinson’s Disease: A Lipidomic Analysis. PLoS ONE 2017, 12, e0172348. [Google Scholar] [CrossRef] [Green Version]
- Lobasso, S.; Tanzarella, P.; Vergara, D.; Maffia, M.; Cocco, T.; Corcelli, A. Lipid Profiling of Parkin-Mutant Human Skin Fibroblasts. J. Cell. Physiol. 2017, 232, 3540–3551. [Google Scholar] [CrossRef]
- Fanning, S.; Haque, A.; Imberdis, T.; Baru, V.; Inmaculada, M.; Nuber, S.; Termine, D.; Ramalingam, N.; Ho, G.P.H.; Sandoe, J.; et al. Lipidomic Analysis of α-Synuclein Neurotoxicity Identifies Stearoyl CoA Desaturase as a Target for Parkinson Treatment. Mol. Cell 2019, 73, 1001–1014. [Google Scholar] [CrossRef] [Green Version]
- Calvano, C.D.; Ventura, G.; Sardanelli, A.M.M.; Savino, L.; Losito, I.; De Michele, G.; Palmisano, F.; Cataldi, T.R.I. Searching for Potential Lipid Biomarkers of Parkinson’s Disease in Parkin-Mutant Human Skin Fibroblasts by HILIC-ESI-MS/MS: Preliminary Findings. Int. J. Mol. Sci. 2019, 20, 3341. [Google Scholar] [CrossRef] [Green Version]
- Pizarro, C.; Esteban-Díez, I.; Espinosa, M.; Rodríguez-Royo, F.; González-Sáiz, J.M. An NMR-Based Lipidomic Approach to Identify Parkinson’s Disease-Stage Specific Lipoprotein-Lipid Signatures in Plasma. Analyst 2019, 144, 1334–1344. [Google Scholar] [CrossRef]
- Xicoy, H.; Brouwers, J.F.; Kalnytska, O.; Wieringa, B.; Martens, G.J.M. Lipid Analysis of the 6-Hydroxydopamine-Treated SH-SY5Y Cell Model for Parkinson’s Disease. Mol. Neurobiol. 2020, 57, 848–859. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gill, E.L.; Koelmel, J.P.; Meke, L.; Yost, R.A.; Garrett, T.J.; Okun, M.S.; Flores, C.; Vedam-Mai, V. Ultrahigh-Performance Liquid Chromatography-High-Resolution Mass Spectrometry Metabolomics and Lipidomics Study of Stool from Transgenic Parkinson’s Disease Mice Following Immunotherapy. J. Proteome Res. 2020, 19, 424–431. [Google Scholar] [CrossRef] [PubMed]
- Xicoy, H.; Brouwers, J.F.; Wieringa, B.; Martens, G.J.M. Explorative Combined Lipid and Transcriptomic Profiling of Substantia Nigra and Putamen in Parkinson’s Disease. Cells 2020, 9, 1966. [Google Scholar] [CrossRef]
- Sinclair, E.; Trivedi, D.K.; Sarkar, D.; Walton-Doyle, C.; Milne, J.; Kunath, T.; Rijs, A.M.; de Bie, R.M.A.; Goodacre, R.; Silverdale, M.; et al. Metabolomics of Sebum Reveals Lipid Dysregulation in Parkinson’s Disease. Nat. Commun. 2021, 12, 1592. [Google Scholar] [CrossRef] [PubMed]
- Kurzawa-Akanbi, M.; Tammireddy, S.; Fabrik, I.; Gliaudelytė, L.; Doherty, M.K.; Heap, R.; Matečko-Burmann, I.; Burmann, B.M.; Trost, M.; Lucocq, J.M.; et al. Altered Ceramide Metabolism Is a Feature in the Extracellular Vesicle-Mediated Spread of Alpha-Synuclein in Lewy Body Disorders. Acta Neuropathol. 2021, 142, 961–984. [Google Scholar] [CrossRef] [PubMed]
- Tong, T.; Duan, W.; Xu, Y.; Hong, H.; Xu, J.; Fu, G.; Wang, X.; Yang, L.; Deng, P.; Zhang, J.; et al. Paraquat Exposure Induces Parkinsonism by Altering Lipid Profile and Evoking Neuroinflammation in the Midbrain. Environ. Int. 2022, 169, 107512. [Google Scholar] [CrossRef]
- Oizumi, H.; Sugimura, Y.; Totsune, T.; Kawasaki, I.; Ohshiro, S.; Baba, T.; Kimpara, T.; Sakuma, H.; Hasegawa, T.; Kawahata, I.; et al. Plasma Sphingolipid Abnormalities in Neurodegenerative Diseases. PLoS ONE 2022, 17, e0279315. [Google Scholar] [CrossRef]
- Blume, B.; Schwantes, V.; Witting, M.; Hayen, H.; Schmitt-Kopplin, P.; Helmer, P.O.; Michalke, B. Lipidomic and Metallomic Alteration of Caenorhabditis Elegans after Acute and Chronic Manganese, Iron, and Zinc Exposure with a Link to Neurodegenerative Disorders. J. Proteome Res. 2023, 22, 837–850. [Google Scholar] [CrossRef]
- Kim, S.J.; Gershov, D.; Ma, X.; Brot, N.; Elkon, K.B. I-PLA2 Activation during Apoptosis Promotes the Exposure of Membrane Lysophosphatidylcholine Leading to Binding by Natural Immunoglobulin M Antibodies and Complement Activation. J. Exp. Med. 2002, 196, 655–665. [Google Scholar] [CrossRef] [Green Version]
- Hayakawa, T.; Chang, M.C.J.; Bell, J.M.; Seeman, R.; Rapoport, S.I.; Appel, N.M. Fatty Acid Incorporation Depicts Brain Activity in a Rat Model of Parkinson’s Disease. Brain Res. 1998, 807, 177–181. [Google Scholar] [CrossRef]
- Ren, M.; Phoon, C.K.L.; Schlame, M. Metabolism and Function of Mitochondrial Cardiolipin. Prog. Lipid Res. 2014, 55, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Henchcliffe, C.; Beal, F.M. Mitochondrial Biology and Oxidative Stress in Parkinson Disease Pathogenesis. Nat. Clin. Pract. Neurol. 2008, 4, 600–609. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.P. How Mitochondria Produce Reactive Oxygen Species. Biochem. J. 2009, 417, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, G.; Wang, L.; Marcogliese, P.C.; Bellen, H.J. Sphingolipids in the Pathogenesis of Parkinson’s Disease and Parkinsonism. Trends Endocrinol. Metab. 2019, 30, 106–117. [Google Scholar] [CrossRef]
- Avisar, H.; Guardia-Laguarta, C.; Area-Gomez, E.; Surface, M.; Chan, A.K.; Alcalay, R.N.; Lerner, B. Lipidomics Prediction of Parkinson’s Disease Severity: A Machine-Learning Analysis. J. Park. Dis. 2021, 11, 1141–1155. [Google Scholar] [CrossRef]
- Chung, H.-J.; Sim, J.-H.; Min, T.-S.; Choi, H.-K. Metabolomics and Lipidomics Approaches in the Science of Probiotics: A Review. J. Med. Food 2018, 21, 1086–1095. [Google Scholar] [CrossRef]
- Chung, H.-J.; Lee, H.; Na, G.; Jung, H.; Kim, D.-G.; Shin, S.-I.; Jung, S.-E.; Choi, I.-D.; Lee, J.-H.; Sim, J.-H.; et al. Metabolic and Lipidomic Profiling of Vegetable Juices Fermented with Various Probiotics. Biomolecules 2020, 10, 725. [Google Scholar] [CrossRef]
- Schifano, E.; Cicalini, I.; Pieragostino, D.; Heipieper, H.J.; Boccio, P.D.; Uccelletti, D. In Vitro and in Vivo Lipidomics as a Tool for Probiotics Evaluation. Appl. Microbiol. 2020, 104, 8937–8948. [Google Scholar] [CrossRef]
- de Vos, W.M.; Tilg, H.; Van Hul, M.; Cani, P.D. Gut Microbiome and Health: Mechanistic Insights. Gut 2022, 71, 1020–1032. [Google Scholar] [CrossRef]
- Naudin, C.R.; Maner-Smith, K.; Owens, J.A.; Wynn, G.M.; Robinson, B.S.; Matthews, J.D.; Reedy, A.R.; Luo, L.; Wolfarth, A.A.; Darby, T.M.; et al. Lactococcus Lactis Subspecies Cremoris Elicits Protection Against Metabolic Changes Induced by a Western-Style Diet. Gastroenterology 2020, 159, 639–651. [Google Scholar] [CrossRef]
- Stancu, C.S.; Sanda, G.M.; Deleanu, M.; Sima, A.V. Probiotics Determine Hypolipidemic and Antioxidant Effects in Hyperlipidemic Hamsters. Mol. Nutr. Food Res. 2014, 58, 559–568. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, L.D.; Torrez Lamberti, M.F.; DeBose-Scarlett, E.; Bahadiroglu, E.; Garrett, T.J.; Gardner, C.L.; Meyer, J.L.; Lorca, G.L.; Gonzalez, C.F. Lactobacillus Johnsonii N6.2 and Blueberry Phytophenols Affect Lipidome and Gut Microbiota Composition of Rats Under High-Fat Diet. Front. Nutr. 2021, 8, 757256. [Google Scholar] [CrossRef] [PubMed]
- Bonfili, L.; Cuccioloni, M.; Gong, C.; Cecarini, V.; Spina, M.; Zheng, Y.; Angeletti, M.; Eleuteri, A.M. Gut Microbiota Modulation in Alzheimer’s Disease: Focus on Lipid Metabolism. Clin. Nutr. 2022, 41, 698–708. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Ren, H.; Zhong, H.; Zhao, X.; Li, C.; Ma, J.; Gu, X.; Xue, Y.; Huang, S.; Yang, J.; et al. Combined Berberine and Probiotic Treatment as an Effective Regimen for Improving Postprandial Hyperlipidemia in Type 2 Diabetes Patients: A Double Blinded Placebo Controlled Randomized Study. Gut Microbes 2022, 14, 2003176. [Google Scholar] [CrossRef]

| Neurotoxin | Pathogenesis | PD Symptoms | Advantages | Disadvantages |
|---|---|---|---|---|
| 6-OHDA | Loss of DA innervation | Rotational motor behavior Sensory motor deficits Akinesia (with bilateral administration) | Activation of glial cells Economical Different variants depending on site and dose administered | Needs intracerebral administration Does not mimic the multisystem pathology of PD Does not induce a progressive nigrostriatal degeneration Does not induce LB formation nor synuclein aggregation |
| MPTP | Dose-dependent loss of DA neurons Reduced DA levels in striatum and midbrain DA neuron loss | Dose-dependent locomotor alterations Sensorimotor deficits | Translatable to human disease Easy to administer | Not toxic in rats Does not induce LBs Does not mimic the multisystem pathology of PD Variability in behavioral and biochemical results High mortality |
| Rotenone | DA neuron loss in SNpc Nigrostriatal dopaminergic denervation | Motor disturbances Sensory-motor deficits | Easy to administer Induces most motor symptoms of PD | Toxicity in other organs Causes nonspecific cerebral damage High mortality High interindividual variability |
| Paraquat | Controversy across studies Recent studies describe a reduction in dopaminergic neurons in the SNpc | Decreased locomotor activity and sensory-motor function | Easy to administer Translatable to human disease Increased synuclein immunoreactivity and LB-like structures in DA neurons of the SNpc Increased synuclein immunoreactivity in enteric neurons | Controversial results Important toxic effects, which can difficult the interpretation of behavioral tests High mortality (if using high doses) |
| Level | Group | Change | References |
|---|---|---|---|
| Phylum | Actinobacteria Bacteroidetes Firmicutes Lentisphaera Synergistetes Verrucomicrobia Proteobacteria | ↑ | [118,143,144] |
| Firmicutes | ↓ | [143] | |
| Family | Acidaminococcaceae Akkermansiaceae Barnesiellaceae Bifidobacteriaceae Bradyrizobiaceae Christensenellaceae Clostridiales Family XIII. Incertae Sedis Clostridialesvadin BB60 group Corpobacillaceae Corynebacteriaceae Coriobacteriaceae Desulfovibrionaceae Enterobacteriacea Enterococcaceae Erysipelotrichaceae Eubacteraceae Lachnopsiraceae NK4A Lactobacillaceae Porphyromodaceae Odoribacteriaceae Oscillospiraceae Porphyromonadaceae Prevotelaceae Rikenellaceae Ruminococacceae Streptococcaceae Synergistaceae Tissierellaceae Thermoanaerobacterales Family IV. Incertae Sedis Unclassified Victivallales Veillonellaceae Verrucomicrobiaceae | ↑ | [118,132,134,136,137,139,141,143,144,145,146,147,148,149,150,151,152] |
| Corpobacillaceae Enterococcaceae Erysipelotrichaceae Hungateiclostridiaceae Lactobacillace Muribaculaceae Pasteurellaceae Peptostreptococcaceae Porphyromonadaceae Puniceicoccaceae Prevotellaceae Rikenellaceae Ruminococcaceae Streptococcaceae Uricibacteraceae Verrucomicrobiaceae | No change | [118,129,132,136,139,140,141,143,146,149,150,151,152,153,154,155] |
| Bacterial Group | Change | References |
|---|---|---|
| Acidaminococcus | ↑ | [118,139] |
| Akkermansia | ↑ | [118,146,148,151,153,154] |
| Alistipes | ↑ | [118,136,144] |
| Alistipes shahii | ↑ | [154] |
| Anaerofustis | ↑ | [139] |
| Anaerotruncus | ↑ | [139,156] |
| Aquabacterium | ↑ | [156] |
| Bacteroides | ↓ | [137,139,147] |
| Bacteroides fragilis | ↓ | [157] |
| Bacteroidetes spp. | ↑ | [153] |
| ↓ | [132] | |
| Bifidobactium spp. | ↑ | [118,132,143,144,146,150] |
| Bilophila spp. | ↑ | [129,144] |
| Blautia spp. | ↓ | [139,150,153,158] |
| Butyricicoccus spp. | ↑ | [156] |
| ↓ | [129,140] | |
| Butyricimonas | ↑ | [118,136] |
| Butyrivibrio | ↓ | [118] |
| Campylobacter | ↑ | [139] |
| Catabacter spp. | ↑ | [147] |
| Citrobacter | ↑ | [139] |
| Cloacibacillus | ↑ | [118] |
| Coprococcus spp. | ↓ | [153] |
| Clostridiales incertae sedis IV | ↓ | [145] |
| Clostridium IV | ↑ | [156] |
| Clostridium XVIII | ↑ | [156] |
| Clostridium XIVa | ↓ | [150] |
| Clostridium coccides | ↓ | [157] |
| Clostridium leptum | ↓ | [157] |
| Clostridium saccharolyticum | ↓ | [154] |
| Collinsella | ↑ | [118,144] |
| Dehalobacterium | ↑ | [139] |
| Desulfovibrio | ↑ | [118,144] |
| Dorea spp. | ↓ | [139,147,153] |
| Enterococcus | ↑ | [158] |
| Escherichia | ↑ | [118,158] |
| Eubacterium | ↓ | [154] |
| Eubacterium biforme | ↓ | [154] |
| Faecalibacterium | ↓ | [118,139,143,147,158] |
| Faecalibacterium prusnitzii | ↓ | [132] |
| Finegoldia | ↑ | [139] |
| Fusicatenibacter | ↓ | [140] |
| Fusobacteriales (unclassified) | ↑ | [118] |
| Hallomonas | ↑ | [139] |
| Holdemania | ↑ | [156] |
| Hungatella | ↑ | [118] |
| Hydrogenonaerobacterium | ↑ | [137] |
| Kliebsiella | ↑ | [141] |
| Lactobacillus (nomenclaure updated to Lacticaseibacillus) | ↓ | [118,155,156] |
| ↑ | [143,144,146,147,150,152,157] | |
| Lactococcus | ↓ | [155] |
| ↑ | [141] | |
| Mahella | ↑ | [118] |
| Megasphaera | ↑ | [118] |
| Methanobrevibacter | ↑ | [139] |
| Methanomassiliicoccus | ↑ | [139] |
| Mogibacterium | ↑ | [118] |
| Mucispirillum | ↑ | [152] |
| Oscillibacter | ↑ | [144] |
| Oscillospira | ↑ | [147,151,153] |
| Parabacteroides | ↑ | [136,152] |
| Peptoniphilus | ↑ | [139] |
| Phascolarctobacterium | ↓ | [139] |
| Porphyromionas | ↑ | [152] |
| Prevotella | ↓ | [118,132,136,137,139,145,147,150,152,154,155,157] |
| ↑ | [143,148] | |
| Prevotella copri | ↓ | [154] |
| Proteus spp. | ↑ | [129,158] |
| Pseudoramibacter_Eubacterium | ↑ | [139] |
| Roseburia spp. | ↓ | [118,139,141,144,150,151,153] |
| ↑ | [129] | |
| Ruminococcus | ↓ | [139,151,158] |
| Sediminibacterium | ↓ | [156] |
| Sphingomonas | ↑ | [156] |
| Streptococcus | ↑ | [118,158] |
| Sunergistes | ↑ | [139] |
| Sutterella | ↓ | [139] |
| Turicibacter | ↑ | [139] |
| Varibaculum | ↑ | [139] |
| Veillonella | ↑ | [118] |
| Victivallales (unclassified) | ↑ | [118] |
| Type | Definition | Example | Abbreviation | Number of C Atoms |
|---|---|---|---|---|
| Saturated | No C-to-C double bounds | Palmitic acid | 16:0 | 16 C atoms and 0 C-to-C double bound |
| Unsaturated | C-to-C double bounds | |||
| Monounsaturated (MUFA) | One C-to-C double bound | Oleic acid | 18:1 | 18 C atoms and one C-to-C double bound |
| Polyunsaturated (PUFA) | Two or more C-to-C double bounds | Linoleic acid (omega-6) | 18:2 | 18 C atoms and two C-to-C double bounds |
| Linolenic acid (omega-3) | 18:3 | 18 C atoms and three C-to-C double bounds |
| Author and Year of Publication | Lipidomic Approach | Biological Samples | Conclusions |
|---|---|---|---|
| Cheng et al. 2011 [214] | LC-MS | 10 PD and 10 HC. Primary visual cortex, amygdale, and anterior cingulated cortex tissues. | Changes in lipid metabolism occur in PD visual cortex in the absence of obvious pathology. Lipid metabolism in the visual cortex may represent a novel target for treatment of non-motor symptoms. |
| Tyurina et al. 2015 [215] | LC-MS | Rats exposed to rotenone | Elevated levels of PUFA cardiolipins in plasma. |
| Farmer et al. 2015 [216] | HPLC-ESI-MS/MS | Rats treated with 6-OHDA | Upregulation of lysophosphatidylcholine, important for neuroinflammatory signaling. |
| Zhang et al. 2017 [209] | HPLC-MS | Human plasma (170 PD; 120 HC) | Elevated concentration of ganglioside-NANA-3 is associated with PD. |
| Chan et al. 2017 [217] | LC-MS | Plasma: 150 idiopathic PD and 100 HC | Elevated GM3 levels in plasma might be associated with PD. |
| Lobasso et al. 2017 [218] | MALDI-TOF-MS | Primary skin fibroblasts | Phospholipids and glycosphingolipids were altered in parkin mutants’ fibroblasts and lysophosphatidylcholine were increased, pointing to this molecule as a marker of neuroinflammatory state. |
| Fanning et al. 2019 [219] | LC-MS | Rat cortical neurons Human iPS-derived neurons Yeast cells | Monounsaturated fatty acid metabolism induces neurotoxicity increasing α-synuclein. |
| Calvano et al. 2019 [220] | HILIC/ESI-MS MS | Primary skin fibroblasts (5 PD and HC) | Abnormal PLs metabolism and plasmalogens in PD could be associated with neurodegeneration. |
| Pizarro et al. 2019 [221] | NMR | Plasma (38 early-stage PD, 10 PD-related dementia, 23 Alzheimer’s dementia, and 23 HC9) | 30 chemical shift buckets enabled differentiation between PD patients, Alzheimer patients, and controls, demonstrating that this approach is a good diagnostic tool for PD. |
| Xicoy et al. 2020 [222] | LC-MS | Neuronal cell line SH-SY5Y treated with the neurotoxin 6-hydroxydopamine. | Changes in phosphatidylcholine, phosphatidylglycerol, phosphatidylinositol, phosphatididylserine, sphingomyelin, and total cholesterol in 6-OHDA-treated cells. |
| Gill et al. 2020 [223] | UHP-LC-MS. Metabolomics and lipidomics. | Stool samples (3 PD mice and 3 vaccinated (T-cell vaccination) PD mice) | L-carnitine seems to act as neuroprotector. Diacylglycerols and triacylglycerols were upregulated in the PD mice. Targeting the adaptative immune system in PD might have potential therapeutic value. |
| Xicoy et al. 2020 [224] | LC-MS and RNAseq. Lipidomics and transcriptomics. | Susbtantia nigra and putamen from post-mortem samples (10 PD and 10 HC) | Transcriptome changes leading to differences in the lipid profile could be a mechanism of neurodegeneration in PD. Some of the changes in the PD brain lipid profile were gender dependent. |
| Sinclair et al. 2021 [225] | LC-MS | Skin sebum (80 drug naïve PD patients, 138 medicated PD patients and 56 HC) | Alterations in carnitine shuttle, SP, arachidonic acid, and FA metabolism in PD. Skin sebum may work as a good biological sample to identify biomarkers of PD. |
| Kurzawa-Akanbi et al. 2021 [226] | UHP-LC-MS | 59 samples of post-mortem frontal cortex and 48 samples of cingulate cortex from matched LBD and controls (with and without GBA mutations); 15 post-mortem CSF samples | Increase in ceramides in LBD post-mortem tissue and CSF, irrespective of GBA mutation status (although GBA mutations might increase risk). Marked parallel elevation of ceramide levels in EV, probably associated with ER stress, a general loss of endolysosomal homeostasis, and lysosomal degradation capacity. This might cause abnormal pathogenic α-synuclein to be associated with EV. |
| Tong et al. 2022 [227] | UHP-LC-MS | Midbrain of mice exposed to paraquat | Increase in proinflammatory lipids in midbrain, including ceramide, LPC, LPS, and LPI, and a decrease in SM. |
| Oizumi et al. 2022 [228] | LC-MS | Plasma samples (49 healthy controls, 58 idiopathic PD patients, 28 DLB patients, 13 MSA patients, 13 AD patients, and 16 PSP patients) | Compared with healthy controls, the plasma levels of S1P decreased, whereas those of monohexylceramide and lactosylceramide increased in patients with the neurodegenerative disease studied. Abnormal SP metabolism is key in neurodegeneration. |
| Blume et al. 2022 [229] | UHP-LC-MS. Lipidomics and metallomics | C. elegans exposed to iron, manganese, zinc … | Altered metallostasis due to metal treatment caused lipidomic alterations affecting phospholipid composition in plasma membrane and cardiolipins in the inner mitochondrial membrane. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ortega Moreno, L.; Bagues, A.; Martínez, V.; Abalo, R. New Pieces for an Old Puzzle: Approaching Parkinson’s Disease from Translatable Animal Models, Gut Microbiota Modulation, and Lipidomics. Nutrients 2023, 15, 2775. https://doi.org/10.3390/nu15122775
Ortega Moreno L, Bagues A, Martínez V, Abalo R. New Pieces for an Old Puzzle: Approaching Parkinson’s Disease from Translatable Animal Models, Gut Microbiota Modulation, and Lipidomics. Nutrients. 2023; 15(12):2775. https://doi.org/10.3390/nu15122775
Chicago/Turabian StyleOrtega Moreno, Lorena, Ana Bagues, Vicente Martínez, and Raquel Abalo. 2023. "New Pieces for an Old Puzzle: Approaching Parkinson’s Disease from Translatable Animal Models, Gut Microbiota Modulation, and Lipidomics" Nutrients 15, no. 12: 2775. https://doi.org/10.3390/nu15122775





