Body Composition in Fussy-Eating Children, with and without Neurodevelopmental Disorders, and Their Parents, Following a Taste Education Intervention
Abstract
:1. Introduction
1.1. Fussy Eating and Neurodevelopmental Disorders
1.2. Weight Status of Icelandic Children and Adults
1.3. Body Composition Measurements
1.4. Relationships between Parents’ and Children’s Body Composition
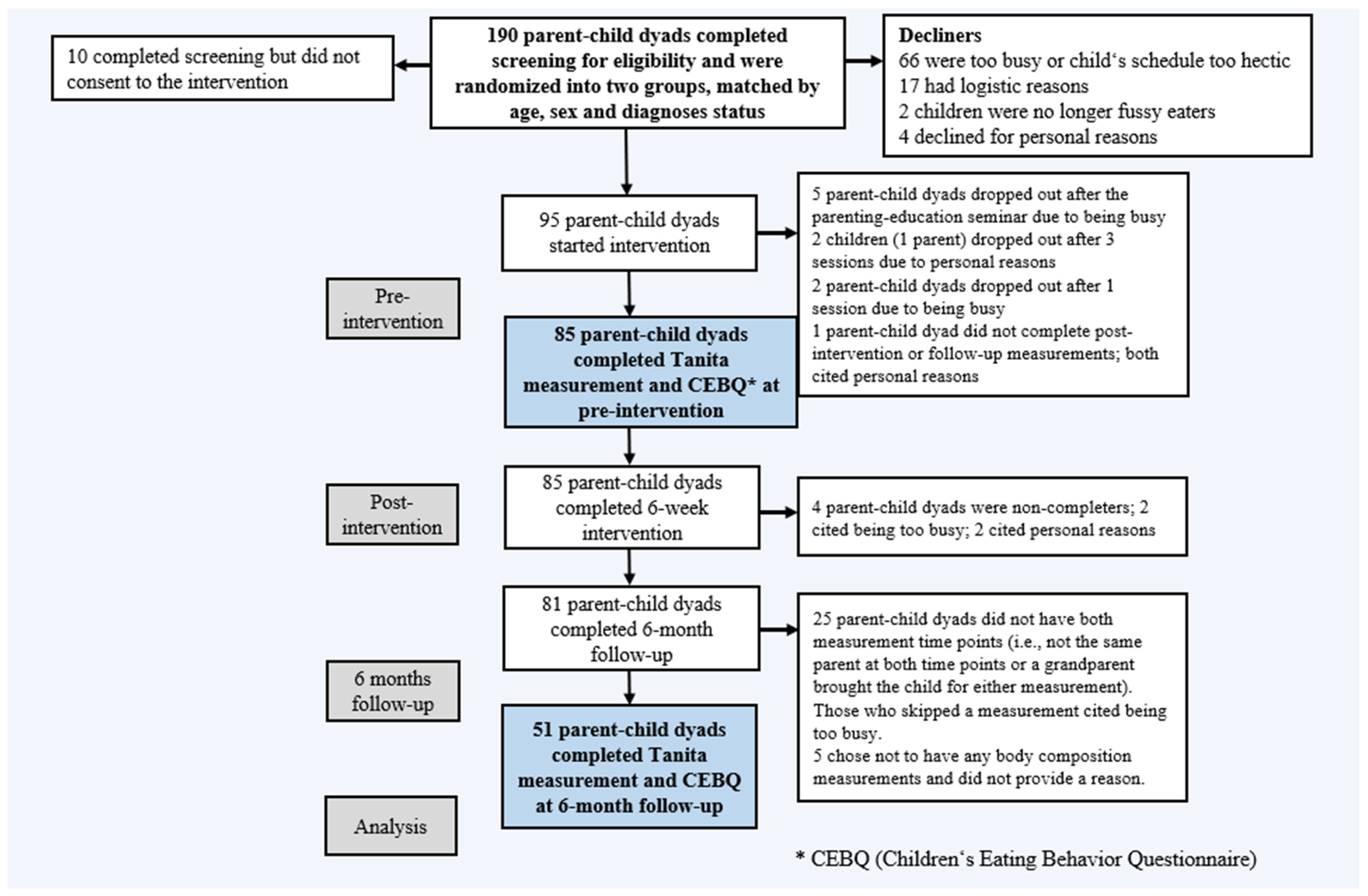
2. Materials and Methods
2.1. Measures
2.1.1. Anthropometric Measures
2.1.2. Measure of Fussy Eating
2.1.3. Tanita Body Composition Analyzer
2.2. Participants
2.3. Procedures
2.4. Statistical Analyzes
3. Results
3.1. Sample Characteristics
3.2. Body Compositions Based on Sex
3.3. Multivariable Associations between Children’s BMI or Fat Percentage (FAT%) and ND Status
3.4. Changes in Children’s BMI-SDS and Parents’ BMI and Fat Percentage (FAT%), Based on ND Status Comparing Changes between Time Points
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wabitsch, M.; Moss, A.; Kromeyer-Hauschild, K. Unexpected plateauing of childhood obesity rates in developed countries. BMC Med. 2014, 12, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weghuber, D.; Barrett, T.; Barrientos-Pérez, M.; Gies, I.; Hesse, D.; Jeppesen, O.K.; Kelly, A.S.; Mastrandrea, L.D.; Sørrig, R.; Arslanian, S. Once-Weekly Semaglutide in Adolescents with Obesity. N. Engl. J. Med. 2022, 387, 2245–2257. [Google Scholar] [CrossRef]
- Hampl, S.E.; Hassink, S.G.; Skinner, A.C.; Armstrong, S.C.; Barlow, S.E.; Bolling, C.F.; Avila Edwards, K.C.; Eneli, I.; Hamre, R.; Joseph, M.M.; et al. Clinical Practice Guideline for the Evaluation and Treatment of Children and Adolescents With Obesity. Pediatrics 2023, 151, e2022060640. [Google Scholar] [CrossRef] [PubMed]
- Abarca-Gómez, L.; Abdeen, Z.A.; Hamid, Z.A.; Abu-Rmeileh, N.M.; Acosta-Cazares, B.; Acuin, C.; Adams, R.J.; Aekplakorn, W.; Afsana, K.; Aguilar-Salinas, C.A.; et al. Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: A pooled analysis of 2416 population-based measurement studies in 128·9 million children, adolescents, and adults. Lancet 2017, 390, 2627–2642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bharti, B.; Malhi, P. Psychiatric Comorbidities in Adolescents with Obesity: A Wake-Up Call for Life Course and Multisectoral Interventions. Indian J. Pediatr. 2021, 88, 215–216. [Google Scholar] [CrossRef]
- Inchley, J.; Currie, D.; Jewell, J.; Breda, J.; Barnekow, V. Adolescent Obesity and Related Behaviours: Trends and Inequalities in the WHO European Region, 2002–2014: Observations from the Health Behaviour in School-Aged Children (HBSC) WHO Collaborative Cross-national Study; World Health Organization, Regional Office for Europe: Copenhagen, Denmark, 2017; viii + 87p. [Google Scholar]
- Chung, A.; Backholer, K.; Wong, E.; Palermo, C.; Keating, C.; Peeters, A. Trends in child and adolescent obesity prevalence in economically advanced countries according to socioeconomic position: A systematic review. Obes. Rev. 2016, 17, 276–295. [Google Scholar] [CrossRef]
- Wyse, C.; Case, L.; Walsh, Ó.; Shortall, C.; Jordan, N.; McCrea, L.; O’Malley, G. Evaluating 12 Years of Implementing a Multidisciplinary Specialist Child and Adolescent Obesity Treatment Service: Patient-Level Outcomes. Front. Nutr. 2022, 9, 895091. [Google Scholar] [CrossRef]
- Tully, L.; Arthurs, N.; Wyse, C.; Browne, S.; Case, L.; McCrea, L.; O’Connell, J.M.; O’Gorman, C.S.; Smith, S.M.; Walsh, A.; et al. Guidelines for treating child and adolescent obesity: A systematic review. Front. Nutr. 2022, 9, 902865. [Google Scholar] [CrossRef]
- Chung, Y.L.; Rhie, Y.-J. Severe Obesity in Children and Adolescents: Metabolic Effects, Assessment, and Treatment. JOMES 2021, 30, 326–335. [Google Scholar] [CrossRef]
- Patel, C.; Karasouli, E.; Shuttlewood, E.; Meyer, C. Food Parenting Practices among Parents with Overweight and Obesity: A Systematic Review. Nutrients 2018, 10, 1966. [Google Scholar] [CrossRef] [Green Version]
- Haycraft, E.; Karasouli, E.; Meyer, C. Maternal feeding practices and children’s eating behaviours: A comparison of mothers with healthy weight versus overweight/obesity. Appetite 2017, 116, 395–400. [Google Scholar] [CrossRef] [Green Version]
- Niec, L.N.; Todd, M.; Brodd, I.; Domoff, S.E. PCIT-Health: Preventing Childhood Obesity by Strengthening the Parent–Child Relationship. Cogn. Behav. Pract. 2020, 29, 335–347. [Google Scholar] [CrossRef]
- Jansen, P.W.; Derks, I.P.M.; Mou, Y.; van Rijen, E.H.M.; Gaillard, R.; Micali, N.; Voortman, T.; Hillegers, M.H.J. Associations of parents’ use of food as reward with children’s eating behaviour and BMI in a population-based cohort. Pediatr. Obes. 2020, 15, e12662. [Google Scholar] [CrossRef]
- Liang, J.; Matheson, B.E.; Rhee, K.E.; Peterson, C.B.; Rydell, S.; Boutelle, K.N. Parental control and overconsumption of snack foods in overweight and obese children. Appetite 2016, 100, 181–188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DeCosta, P.; Moller, P.; Frost, M.B.; Olsen, A. Changing children’s eating behaviour—A review of experimental research. Appetite 2017, 113, 327–357. [Google Scholar] [CrossRef]
- Taylor, C.M.; Emmett, P.M. Picky eating in children: Causes and consequences. Proc. Nutr. Soc. 2019, 78, 161–169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taylor, C.M.; Wernimont, S.M.; Northstone, K.; Emmett, P.M. Picky/fussy eating in children: Review of definitions, assessment, prevalence and dietary intakes. Appetite 2015, 95, 349–359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gibson, E.L.; Cooke, L. Understanding Food Fussiness and Its Implications for Food Choice, Health, Weight and Interventions in Young Children: The Impact of Professor Jane Wardle. Curr. Obes. Rep. 2017, 6, 46–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thorsteinsdottir, S.; Olafsdottir, A.S.; Brynjolfsdottir, B.; Bjarnason, R.; Njardvik, U. Odds of fussy eating are greater among children with obesity and anxiety. Obes. Sci. Pract. 2022, 8, 91–100. [Google Scholar] [CrossRef]
- Finistrella, V.; Manco, M.; Ferrara, A.; Rustico, C.; Presaghi, F.; Morino, G. Cross-sectional exploration of maternal reports of food neophobia and pickiness in preschooler-mother dyads. J. Am. Coll. Nutr. 2012, 31, 152–159. [Google Scholar] [CrossRef]
- Brown, C.L.; Vander Schaaf, E.B.; Cohen, G.M.; Irby, M.B.; Skelton, J.A. Association of Picky Eating and Food Neophobia with Weight: A Systematic Review. Child. Obes. 2016, 12, 247–262. [Google Scholar] [CrossRef] [Green Version]
- Carruth, B.R.; Skinner, J.D. Revisiting the picky eater phenomenon: Neophobic behaviors of young children. J. Am. Coll. Nutr. 2000, 19, 771–780. [Google Scholar] [CrossRef]
- Galloway, A.T.; Fiorito, L.; Lee, Y.; Birch, L.L. Parental pressure, dietary patterns, and weight status among girls who are “picky eaters”. J. Am. Diet. Assoc. 2005, 105, 541–548. [Google Scholar] [CrossRef] [Green Version]
- Kozioł-Kozakowska, A.; Piórecka, B.; Schlegel-Zawadzka, M. Prevalence of food neophobia in pre-school children from southern Poland and its association with eating habits, dietary intake and anthropometric parameters: A cross-sectional study. Public Health Nutr. 2018, 21, 1106–1114. [Google Scholar] [CrossRef] [Green Version]
- de Barse, L.M.; Tiemeier, H.; Leermakers, E.T.M.; Voortman, T.; Jaddoe, V.W.V.; Edelson, L.R.; Franco, O.H.; Jansen, P.W. Longitudinal association between preschool fussy eating and body composition at 6 years of age: The Generation R Study. Int. J. Behav. Nutr. Phys. Act. 2015, 12, 153. [Google Scholar] [CrossRef] [Green Version]
- Ekstein, S.; Laniado, D.; Glick, B. Does Picky Eating Affect Weight-for-Length Measurements in Young Children? Clin. Pediatr. 2009, 49, 217–220. [Google Scholar] [CrossRef]
- Xue, Y.; Lee, E.; Ning, K.; Zheng, Y.; Ma, D.; Gao, H.; Yang, B.; Bai, Y.; Wang, P.; Zhang, Y. Prevalence of picky eating behaviour in Chinese school-age children and associations with anthropometric parameters and intelligence quotient. A cross-sectional study. Appetite 2015, 91, 248–255. [Google Scholar] [CrossRef] [Green Version]
- Lim, P.S.; Balistreri, K.A.; Silverman, A.H.; Davies, W.H. Disrupted mealtime interactions are associated with stress and internalizing symptoms in caregivers of school-age children. Child. Health Care 2021, 50, 432–451. [Google Scholar] [CrossRef]
- Dovey, T.M.; Kumari, V.; Blissett, J. Eating behaviour, behavioural problems and sensory profiles of children with avoidant/restrictive food intake disorder (ARFID), autistic spectrum disorders or picky eating: Same or different? Eur. Psychiatry 2019, 61, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Ledford, J.R.; Whiteside, E.; Severini, K.E. A systematic review of interventions for feeding-related behaviors for individuals with autism spectrum disorders. Res. Autism Spectr. Disord. 2018, 52, 69–80. [Google Scholar] [CrossRef]
- Dovey, T.M.; Staples, P.A.; Gibson, E.L.; Halford, J.C. Food neophobia and ‘picky/fussy’ eating in children: A review. Appetite 2008, 50, 181–193. [Google Scholar] [CrossRef] [PubMed]
- Olsen, A. Reflections on current practice for taste learning in children. Int. J. Gastron. Food Sci. 2019, 15, 26–29. [Google Scholar] [CrossRef]
- Barnhill, K.; Gutierrez, A.; Ghossainy, M.; Marediya, Z.; Devlin, M.; Sachdev, P.; Marti, C.N.; Hewitson, L. Dietary status and nutrient intake of children with autism spectrum disorder: A case-control study. Res. Autism Spectr. Disord. 2018, 50, 51–59. [Google Scholar] [CrossRef]
- Cermak, S.A.; Curtin, C.; Bandini, L.G. Food selectivity and sensory sensitivity in children with autism spectrum disorders. J. Am. Diet. Assoc. 2010, 110, 238–246. [Google Scholar] [CrossRef] [Green Version]
- Evans, E.W.; Must, A.; Anderson, S.E.; Curtin, C.; Scampini, R.; Maslin, M.; Bandini, L. Dietary patterns and body mass index in children with autism and typically developing children. Res. Autism Spectr. Disord. 2012, 6, 399–405. [Google Scholar] [CrossRef] [Green Version]
- Hubbard, K.L.; Anderson, S.E.; Curtin, C.; Must, A.; Bandini, L.G. A comparison of food refusal related to characteristics of food in children with autism spectrum disorder and typically developing children. J. Acad. Nutr. Diet 2014, 114, 1981–1987. [Google Scholar] [CrossRef] [Green Version]
- Mayes, S.D.; Zickgraf, H. Atypical eating behaviors in children and adolescents with autism, ADHD, other disorders, and typical development. Res. Autism Spectr. Disord. 2019, 64, 76–83. [Google Scholar] [CrossRef]
- Wentz, E.; Bjork, A.; Dahlgren, J. Neurodevelopmental disorders are highly over-represented in children with obesity: A cross-sectional study. Obesity 2017, 25, 178–184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shmaya, Y.; Eilat-Adar, S.; Leitner, Y.; Reif, S.; Gabis, L. Nutritional deficiencies and overweight prevalence among children with autism spectrum disorder. Res. Dev. Disabil. 2015, 38, 1–6. [Google Scholar] [CrossRef]
- Mayes, S.D.; Calhoun, S.L.; Mayes, R.D.; Molitoris, S. Autism and ADHD: Overlapping and discriminating symptoms. Res. Autism Spectr. Disord. 2012, 6, 277–285. [Google Scholar] [CrossRef]
- Nadon, G.; Feldman, D.; Gisel, E. Feeding Issues Associated with the Autism Spectrum Disorders. In Recent Advances in Autism Spectrum Disorders; Fitzgerald, M., Ed.; IntechOpen: London, UK, 2013; Volume 1. [Google Scholar]
- Nadon, G.; Feldman, D.; Dunn, W.; Gisel, E. Mealtime problems in children with Autism Spectrum Disorder and their typically developing siblings: A comparison study. Autism Int. J. Res. Pract. 2011, 15, 98–113. [Google Scholar] [CrossRef]
- Manning-Courtney, P.; Murray, D.; Currans, K.; Johnson, H.; Bing, N.; Kroeger-Geoppinger, K.; Sorensen, R.; Bass, J.; Reinhold, J.; Johnson, A.; et al. Autism Spectrum Disorders. Curr. Probl. Pediatr. Adolesc. Health Care 2013, 43, 2–11. [Google Scholar] [CrossRef]
- de Barse, L.M.; Jansen, P.W.; Edelson-Fries, L.R.; Jaddoe, V.W.V.; Franco, O.H.; Tiemeier, H.; Steenweg-de Graaff, J. Infant feeding and child fussy eating: The Generation R Study. Appetite 2017, 114, 374–381. [Google Scholar] [CrossRef]
- Margari, L.; Marzulli, L.; Gabellone, A.; de Giambattista, C. Eating and Mealtime Behaviors in Patients with Autism Spectrum Disorder: Current Perspectives. Neuropsychiatr. Dis. Treat. 2020, 16, 2083–2102. [Google Scholar] [CrossRef]
- Sharp, W.G.; Jaquess, D.L.; Lukens, C.T. Multi-method assessment of feeding problems among children with autism spectrum disorders. Res. Autism Spectr. Disord. 2013, 7, 56–65. [Google Scholar] [CrossRef]
- Thorsteinsdottir, S.; Olsen, A.; Olafsdottir, A.S. Fussy Eating among Children and Their Parents: Associations in Parent-Child Dyads, in a Sample of Children with and without Neurodevelopmental Disorders. Nutrients 2021, 13, 2196. [Google Scholar] [CrossRef] [PubMed]
- Antoniou, E.E.; Roefs, A.; Kremers, S.P.; Jansen, A.; Gubbels, J.S.; Sleddens, E.F.; Thijs, C. Picky eating and child weight status development: A longitudinal study. J. Hum. Nutr. Diet 2016, 29, 298–307. [Google Scholar] [CrossRef] [PubMed]
- Hayes, J.F.; Altman, M.; Kolko, R.P.; Balantekin, K.N.; Holland, J.C.; Stein, R.I.; Saelens, B.E.; Welch, R.R.; Perri, M.G.; Schechtman, K.B.; et al. Decreasing Food Fussiness in Children with Obesity Leads to Greater Weight Loss in Family-Based Treatment. Obesity 2016, 24, 2158–2163. [Google Scholar] [CrossRef] [Green Version]
- Fraser, K.; Markides, B.R.; Barrett, N.; Laws, R. Fussy eating in toddlers: A content analysis of parents’ online support seeking. Matern. Child. Nutr. 2021, 17, e13171. [Google Scholar] [CrossRef] [PubMed]
- Searle, B.-R.E.; Harris, H.A.; Thorpe, K.; Jansen, E. What children bring to the table: The association of temperament and child fussy eating with maternal and paternal mealtime structure. Appetite 2020, 151, 104680. [Google Scholar] [CrossRef]
- Wolstenholme, H.; Heary, C.; Kelly, C. Fussy eating behaviours: Response patterns in families of school-aged children. Appetite 2019, 136, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Fries, L.R.; Martin, N.; van der Horst, K. Parent-child mealtime interactions associated with toddlers’ refusals of novel and familiar foods. Physiol. Behav. 2017, 176, 93–100. [Google Scholar] [CrossRef]
- Finnane, J.M.; Jansen, E.; Mallan, K.M.; Daniels, L.A. Mealtime Structure and Responsive Feeding Practices Are Associated With Less Food Fussiness and More Food Enjoyment in Children. J. Nutr. Educ. Behav. 2017, 49, 11–18.e11. [Google Scholar] [CrossRef] [Green Version]
- Curtin, C.; Hubbard, K.; Anderson, S.E.; Mick, E.; Must, A.; Bandini, L.G. Food selectivity, mealtime behavior problems, spousal stress, and family food choices in children with and without autism spectrum disorder. J. Autism Dev. Disord. 2015, 45, 3308–3315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, B.; Rogers, S.L.; Blissett, J.; Ludlow, A.K. The relationship between sensory sensitivity, food fussiness and food preferences in children with neurodevelopmental disorders. Appetite 2020, 150, 104643. [Google Scholar] [CrossRef]
- Cortese, S.; Tessari, L. Attention-Deficit/Hyperactivity Disorder (ADHD) and Obesity: Update 2016. Curr. Psychiatry Rep. 2017, 19, 4. [Google Scholar] [CrossRef] [Green Version]
- Cortese, S.; Angriman, M.; Maffeis, C.; Isnard, P.; Konofal, E.; Lecendreux, M.; Purper-Ouakil, D.; Vincenzi, B.; Bernardina, B.D.; Mouren, M.-C. Attention-Deficit/Hyperactivity Disorder (ADHD) and Obesity: A Systematic Review of the Literature. Crit. Rev. Food Sci. Nutr. 2008, 48, 524–537. [Google Scholar] [CrossRef] [PubMed]
- Nederkoorn, C.; Dassen, F.C.; Franken, L.; Resch, C.; Houben, K. Impulsivity and overeating in children in the absence and presence of hunger. Appetite 2015, 93, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Leventakou, V.; Micali, N.; Georgiou, V.; Sarri, K.; Koutra, K.; Koinaki, S.; Vassilaki, M.; Kogevinas, M.; Chatzi, L. Is there an association between eating behaviour and attention-deficit/hyperactivity disorder symptoms in preschool children? J. Child. Psychol. Psychiatry 2016, 57, 676–684. [Google Scholar] [CrossRef] [PubMed]
- Ptacek, R.; Kuzelova, H.; Stefano, G.B.; Raboch, J.; Sadkova, T.; Goetz, M.; Kream, R.M. Disruptive patterns of eating behaviors and associated lifestyles in males with ADHD. Med. Sci. Monit. 2014, 20, 608–613. [Google Scholar] [CrossRef] [Green Version]
- Altfas, J.R. Prevalence of attention deficit/hyperactivity disorder among adults in obesity treatment. BMC Psychiatry 2002, 2, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El Archi, S.; Cortese, S.; Ballon, N.; Réveillère, C.; De Luca, A.; Barrault, S.; Brunault, P. Negative Affectivity and Emotion Dysregulation as Mediators between ADHD and Disordered Eating: A Systematic Review. Nutrients 2020, 12, 3292. [Google Scholar] [CrossRef]
- Hanson, J.A.; Phillips, L.N.; Hughes, S.M.; Corson, K. Attention-deficit hyperactivity disorder symptomatology, binge eating disorder symptomatology, and body mass index among college students. J. Am. Coll. Health 2020, 68, 543–549. [Google Scholar] [CrossRef]
- Mari-Bauset, S.; Zazpe, I.; Mari-Sanchis, A.; Llopis-Gonzalez, A.; Morales-Suarez-Varela, M. Food selectivity in autism spectrum disorders: A systematic review. J. Child. Neurol. 2014, 29, 1554–1561. [Google Scholar] [CrossRef]
- Råstam, M.; Täljemark, J.; Tajnia, A.; Lundström, S.; Gustafsson, P.; Lichtenstein, P.; Gillberg, C.; Anckarsäter, H.; Kerekes, N. Eating Problems and Overlap with ADHD and Autism Spectrum Disorders in a Nationwide Twin Study of 9- and 12-Year-Old Children. Sci. World J. 2013, 2013, 7. [Google Scholar] [CrossRef] [Green Version]
- Chu, D.-T.; Minh Nguyet, N.T.; Nga, V.T.; Thai Lien, N.V.; Vo, D.D.; Lien, N.; Nhu Ngoc, V.T.; Son, L.H.; Le, D.-H.; Nga, V.B.; et al. An update on obesity: Mental consequences and psychological interventions. Diabetes Metab. Syndr.Clin. Res. Rev. 2019, 13, 155–160. [Google Scholar] [CrossRef]
- Di Cesare, M.; Sorić, M.; Bovet, P.; Miranda, J.J.; Bhutta, Z.; Stevens, G.A.; Laxmaiah, A.; Kengne, A.-P.; Bentham, J. The epidemiological burden of obesity in childhood: A worldwide epidemic requiring urgent action. BMC Med. 2019, 17, 212. [Google Scholar] [CrossRef] [Green Version]
- McGirr, J.; O’Malley, G.; Walsh, O. Identification and management of children and adolescents with obesity referred to a general paediatric outpatient department. Ir. Med. J. 2022, 114, 416. [Google Scholar]
- Eiðsdóttir, S.Þ.; Kristjánsson, Á.L.; Sigfúsdóttir, I.D.; Garber, C.E.; Allegrante, J.P. Trends in Body Mass Index among Icelandic Adolescents and Young Adults from 1992 to 2007. Int. J. Environ. Res. Public Health 2010, 7, 2191–2207. [Google Scholar] [CrossRef] [PubMed]
- DCPHI. School Health Annual Report: Overweight and Obesity 2021–2022 in Children 6–14 Years Old [Heilsuvernd Skólabarna Ársskýrsla 2021–2022]; Development Centre for Primary Healthcare in Iceland: Reykjavík, Iceland, 2023; Unpublished report. [Google Scholar]
- DCPHI. School Health Annual Report: Overweight and Obesity 2019–2020 in Children 6–14 Years Old [Heilsuvernd Skólabarna Ársskýrsla 2019–2020]; Development Centre for Primary Healthcare in Iceland: Reykjavík, Iceland, 2021; Unpublished report. [Google Scholar]
- World Health Organization. WHO European Regional Obesity Report 2022; World Health Organization: Copenhagen, Denmark, 2022. [Google Scholar]
- OECD. The Heavy Burden of Obesity; OECD: Paris, France, 2019. [Google Scholar] [CrossRef]
- Freedman, D.S.; Khan, L.K.; Serdula, M.K.; Dietz, W.H.; Srinivasan, S.R.; Berenson, G.S. The relation of childhood BMI to adult adiposity: The Bogalusa Heart Study. Pediatrics 2005, 115, 22–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCarthy, H.D. Measuring growth and obesity across childhood and adolescence. Proc. Nutr. Soc. 2014, 73, 210–217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ellis, K.J.; Shypailo, R.J.; Abrams, S.A.; Wong, W.W. The reference child and adolescent models of body composition. A contemporary comparison. Ann. N. Y. Acad. Sci. 2000, 904, 374–382. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Marín, D.; Escribano, J.; Closa-Monasterolo, R.; Ferré, N.; Venables, M.; Singh, P.; Wells, J.C.; Muñoz-Hernando, J.; Zaragoza-Jordana, M.; Gispert-Llauradó, M.; et al. Validation of bioelectrical impedance analysis for body composition assessment in children with obesity aged 8–14y. Clin. Nutr. 2021, 40, 4132–4139. [Google Scholar] [CrossRef] [PubMed]
- Wells, J. A Hattori chart analysis of body mass index in infants and children. Int. J. Obes. 2000, 24, 325–329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Talma, H.; Chinapaw, M.J.; Bakker, B.; HiraSing, R.A.; Terwee, C.B.; Altenburg, T.M. Bioelectrical impedance analysis to estimate body composition in children and adolescents: A systematic review and evidence appraisal of validity, responsiveness, reliability and measurement error. Obes. Rev. Off. J. Int. Assoc. Study Obes. 2013, 14, 895–905. [Google Scholar] [CrossRef] [PubMed]
- Phillips, S.M.; Bandini, L.G.; Compton, D.V.; Naumova, E.N.; Must, A. A longitudinal comparison of body composition by total body water and bioelectrical impedance in adolescent girls. J. Nutr. 2003, 133, 1419–1425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanita Corporation. Tanita Body Composition Analyzer: Instruction Manual; Tanita Corporation: Amsterdam, The Netherlands, 2014; pp. 1–39. [Google Scholar]
- Olafsdottir, A.S.; Torfadottir, J.E.; Arngrimsson, S.A. Health Behavior and Metabolic Risk Factors Associated with Normal Weight Obesity in Adolescents. PLoS ONE 2016, 11, e0161451. [Google Scholar] [CrossRef] [Green Version]
- Hinriksdóttir, G.; Tryggvadóttir, Á.; Ólafsdóttir, A.S.; Arngrímsson, S.Á. Fatness but Not Fitness Relative to the Fat-Free Mass Is Related to C-Reactive Protein in 18 Year-Old Adolescents. PLoS ONE 2015, 10, e0130597. [Google Scholar] [CrossRef] [PubMed]
- Arngrimsson, S.B.; Richardsson, E.E.; Jonsson, K.; Olafsdottir, A.S. Body composition, aerobic fitness, physical activity and metabolic profile among 18 year old Icelandic high-school students. Icel. J. Med. 2012, 98, 277–282. [Google Scholar] [CrossRef] [Green Version]
- Kyle, U.G.; Earthman, C.P.; Pichard, C.; Coss-Bu, J.A. Body composition during growth in children: Limitations and perspectives of bioelectrical impedance analysis. Eur. J. Clin. Nutr. 2015, 69, 1298–1305. [Google Scholar] [CrossRef] [Green Version]
- Dhawan, D.; Sharma, S. Abdominal obesity, adipokines and non-communicable diseases. J. Steroid Biochem. Mol. Biol. 2020, 203, 105737. [Google Scholar] [CrossRef]
- Haraldsdottir, A.; Steingrimsdottir, L.; Maskarinec, G.; Adami, H.-O.; Aspelund, T.; Valdimarsdottir, U.A.; Bjarnason, R.; Thorsdottir, I.; Halldorsson, T.I.; Gunnarsdottir, I.; et al. Growth Rate in Childhood and Adolescence and the Risk of Breast and Prostate Cancer: A Population-Based Study. Am. J. Epidemiol. 2021, 191, 320–330. [Google Scholar] [CrossRef]
- Kreissl, A.; Jorda, A.; Truschner, K.; Skacel, G.; Greber-Platzer, S. Clinically relevant body composition methods for obese pediatric patients. BMC Pediatr. 2019, 19, 84. [Google Scholar] [CrossRef]
- Wang, L.; Hui, S.S.-c. Validity of Four Commercial Bioelectrical Impedance Scales in Measuring Body Fat among Chinese Children and Adolescents. BioMed Res. Int. 2015, 2015, 614858. [Google Scholar] [CrossRef] [Green Version]
- Haroun, D.; Croker, H.; Viner, R.M.; Williams, J.E.; Darch, T.S.; Fewtrell, M.S.; Eaton, S.; Wells, J.C. Validation of BIA in obese children and adolescents and re-evaluation in a longitudinal study. Obesity 2009, 17, 2245–2250. [Google Scholar] [CrossRef]
- Orkin, S.; Yodoshi, T.; Romantic, E.; Hitchcock, K.; Arce-Clachar, A.C.; Bramlage, K.; Sun, Q.; Fei, L.; Xanthakos, S.A.; Trout, A.T.; et al. Body composition measured by bioelectrical impedance analysis is a viable alternative to magnetic resonance imaging in children with nonalcoholic fatty liver disease. J. Parenter. Enter. Nutr. 2022, 46, 378–384. [Google Scholar] [CrossRef]
- Chula de Castro, J.A.; Lima, T.R.d.; Silva, D.A.S. Body composition estimation in children and adolescents by bioelectrical impedance analysis: A systematic review. J. Bodyw. Mov. Ther. 2018, 22, 134–146. [Google Scholar] [CrossRef]
- Danielzik, S.; Langnase, K.; Mast, M.; Spethmann, C.; Muller, M.J. Impact of parental BMI on the manifestation of overweight 5–7 year old children. Eur. J. Nutr. 2002, 41, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Gunnarsdottir, T.; Njardvik, U.; Olafsdottir, A.S.; Craighead, L.; Bjarnason, R. Childhood obesity and co-morbid problems: Effects of Epstein’s family-based behavioural treatment in an Icelandic sample. J. Eval. Clin. Pr. 2012, 18, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Rhee, K. Childhood overweight and the relationship between parent behaviors, parenting style and family functioning. Ann. Am. Acad. Political. Soc. Sci. 2008, 615, 12–37. [Google Scholar] [CrossRef]
- Blissett, J.; Haycraft, E. Parental eating disorder symptoms and observations of mealtime interactions with children. J. Psychosom. Res. 2011, 70, 368–371. [Google Scholar] [CrossRef] [Green Version]
- Blissett, J.; Meyer, C.; Haycraft, E. Maternal and paternal controlling feeding practices with male and female children. Appetite 2006, 47, 212–219. [Google Scholar] [CrossRef] [Green Version]
- Baughcum, A.E.; Powers, S.W.; Johnson, S.B.; Chamberlin, L.A.; Deeks, C.M.; Jain, A.; Whitaker, R.C. Maternal feeding practices and beliefs and their relationships to overweight in early childhood. J. Dev. Behav. Pediatr. 2001, 22, 391–408. [Google Scholar] [CrossRef]
- Francis, L.A.; Hofer, S.M.; Birch, L.L. Predictors of maternal child-feeding style: Maternal and child characteristics. Appetite 2001, 37, 231–243. [Google Scholar] [CrossRef]
- Jansen, P.W.; Tharner, A.; van der Ende, J.; Wake, M.; Raat, H.; Hofman, A.; Verhulst, F.C.; van Ijzendoorn, M.H.; Jaddoe, V.W.; Tiemeier, H. Feeding practices and child weight: Is the association bidirectional in preschool children? Am. J. Clin. Nutr. 2014, 100, 1329–1336. [Google Scholar] [CrossRef] [Green Version]
- Thorsteinsdottir, S.; Njardvik, U.; Bjarnason, R.; Haraldsson, H.; Olafsdottir, A.S. Taste education—A food-based intervention in a school setting, focusing on children with and without neurodevelopmental disorders and their families. A randomized controlled trial. Appetite 2021, 167, 105623. [Google Scholar] [CrossRef] [PubMed]
- Lobstein, T.; Baur, L.; Uauy, R. Obesity in children and young people: A crisis in public health. Obes. Rev. 2004, 5 (Suppl. S1), 4–104. [Google Scholar] [CrossRef]
- Wikland, K.A.; Luo, Z.C.; Niklasson, A.; Karlberg, J. Swedish population-based longitudinal reference values from birth to 18 years of age for height, weight and head circumference. Acta Paediatr. 2002, 91, 739–754. [Google Scholar] [CrossRef] [PubMed]
- Wardle, J.; Guthrie, C.A.; Sanderson, S.; Rapoport, L. Development of the Children’s Eating Behaviour Questionnaire. J. Child. Psychol. Psychiatry 2001, 42, 963–970. [Google Scholar] [CrossRef] [Green Version]
- Ashcroft, J.; Semmler, C.; Carnell, S.; van Jaarsveld, C.H.; Wardle, J. Continuity and stability of eating behaviour traits in children. Eur. J. Clin. Nutr. 2008, 62, 985–990. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klar, E.K. The Psychometric Properties of the Icelandic Version of the Child Eating Behaviour Questionnaire (CEBQ); University of Iceland: Reykjavik, Iceland, 2012. [Google Scholar]
- R:CoreTeam. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023. [Google Scholar]
- CDC—National Center for Health Statistics. Defining Adult Overweight & Obesity; CDC: Atlanta, GA, USA, 2023.
- McCarthy, H.D.; Cole, T.J.; Fry, T.; Jebb, S.A.; Prentice, A.M. Body fat reference curves for children. Int. J. Obes. 2006, 30, 598–602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gallagher, D.; Heymsfield, S.B.; Heo, M.; Jebb, S.A.; Murgatroyd, P.R.; Sakamoto, Y. Healthy percentage body fat ranges: An approach for developing guidelines based on body mass index. Am. J. Clin. Nutr. 2000, 72, 694–701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakagawa, S. A farewell to Bonferroni: The problems of low statistical power and publication bias. Behav. Ecol. 2004, 15, 1044–1045. [Google Scholar] [CrossRef] [Green Version]
- Cabin, R.J.; Mitchell, R.J. To Bonferroni or not to Bonferroni: When and how are the questions. Bull. Ecol. Soc. Am. 2000, 81, 246–248. [Google Scholar]
- Jacobi, C.; Agras, W.S.; Bryson, S.; Hammer, L.D. Behavioral validation, precursors, and concomitants of picky eating in childhood. J. Am. Acad. Child. Adolesc. Psychiatry 2003, 42, 76–84. [Google Scholar] [CrossRef]
- Piazza, C.C.; Fisher, W.W.; Brown, K.A.; Shore, B.A.; Patel, M.R.; Katz, R.M.; Sevin, B.M.; Gulotta, C.S.; Blakely-Smith, A. Functional analysis of inappropriate mealtime behaviors. J. Appl. Behav. Anal. 2003, 36, 187–204. [Google Scholar] [CrossRef]
- Jansen, P.W.; de Barse, L.M.; Jaddoe, V.W.V.; Verhulst, F.C.; Franco, O.H.; Tiemeier, H. Bi-directional associations between child fussy eating and parents’ pressure to eat: Who influences whom? Physiol. Behav. 2017, 176, 101–106. [Google Scholar] [CrossRef]
- Thorsteinsdottir, S.; Njardvik, U.; Bjarnason, R.; Olafsdottir, A.S. Changes in Eating Behaviors Following Taste Education Intervention: Focusing on Children with and without Neurodevelopmental Disorders and Their Families: A Randomized Controlled Trial. Nutrients 2022, 14, 4000. [Google Scholar] [CrossRef]
- Elberg, J.; McDuffie, J.R.; Sebring, N.G.; Salaita, C.; Keil, M.; Robotham, D.; Reynolds, J.C.; Yanovski, J.A. Comparison of methods to assess change in children’s body composition. Am. J. Clin. Nutr. 2004, 80, 64–69. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Child | |
|---|---|
| Children’s age in years, Mean (SD) | 10.0 (1.44) |
| Female, n (%) | 21 (41.2) |
| Anthropomorphic measures, Mean (SD) | |
| Height (cm) | 143.0 (11.5) |
| Weight (kg) | 37.3 (12.3) |
| BMI (kg/m2) | 17.9 (3.5) |
| BMI-SDS | 0.27 (1.21) |
| Diagnoses, n (%) | |
| Without ND | 33 (64.7) |
| ND (ADHD, Autism, or both) | 18 (35.3) |
| ADHD, primarily | 4 (7.8) |
| ASD, primarily | 4 (7.8) |
| Anxiety | 10 (19.6) |
| Other | 4 (7.8) |
| Parent | |
| Parents’ age in years, Mean (SD) | 42.2 (4.94) |
| Mother, n (%) | 42 (82.3) |
| Education, n (%) | |
| No higher education | 3 (5.9) |
| Vocational education | 11 (21.6) |
| University level | 37 (72.5) |
| Single parent household, n (%) | 5 (9.8) |
| Occupational status, n (%) | |
| Full-time occupation | 34 (66.7) |
| Part-time occupation | 6 (11.8) |
| Student | 4 (7.8) |
| Other | 7 (13.7) |
| Anthropomorphic measures, Mean (SD) | |
| Height (cm) | 169.4 (7.9) |
| Weight (kg) | 76.6 (16.9) |
| BMI (kg/m2) | 26.9 (5.1) |
| Children’s body composition measurements, based on ND status | ||||
| ND Status | Without ND (n = 33) | ND (n = 18) | t-test or χ2 (1) | p |
| Measures, Mean (SD) or n (%) | ||||
| Age in years | 10.0 (1.55) | 10.2 (1.25) | −0.492 | 0.624 |
| Weight (kg) | 34.8 (9.26) | 41.9 (15.81) | −1.711 | 0.139 |
| Height (cm) | 141.2 (12.13) | 146.2 (9.63) | −1.610 | 0.053 |
| BMI (kg/m2) | 17.2 (2.39) | 19.2 (4.77) | −1.684 | 0.049 |
| BMI-SDS | −0.1 (1.05) | 0.9 (1.27) | −2.773 | 0.005 |
| Overweight/obese | 2 (6.1) | 8 (44.4) | 8.587 | 0.003 |
| Fat mass (kg) | 7.8 (3.34) | 10.8 (8.06) | −1.508 | 0.067 |
| FAT% | 21.2 (4.39) | 26.6 (6.71) | −3.074 | 0.001 |
| Overfat/obese | 3 (9.1) | 10 (55.5) | 10.906 | 0.001 |
| FFM (kg) | 27.0 (6.44) | 30.9 (8.37) | −1.742 | 0.066 |
| Bone mass (kg) * | 1.4 (0.32) | 1.7 (0.39) | −2.156 | 0.026 |
| Parents’ body composition measurements, based on children’s ND status | ||||
| ND Status | Without ND (n = 33) | ND (n = 18) | t-test or χ2 (1) | p |
| Measures, Mean (SD) or n (%) | ||||
| Age in years | 43.6 (4.97) | 39.6 (3.75) | 3.278 | 0.004 |
| Weight (kg) | 72.6 (13.60) | 84.0 (20.21) | −2.137 | 0.021 |
| Height (cm) | 169.1 (7.83) | 170.1 (8.21) | −0.435 | 0.661 |
| BMI (kg/m2) | 25.5 (3.31) | 29.9 (6.38) | −2.893 | 0.001 |
| Overweight/obese | 15 (45.5) | 14 (77.8) | 3.774 | 0.045 |
| Fat mass (kg) | 21.0 (7.28) | 29.4 (11.01) | −2.916 | 0.002 |
| FAT% | 28.7 (7.01) | 34.3 (7.07) | −2.700 | 0.009 |
| Overfat/obese | 12 (36.4) | 12 (66.7) | 3.163 | 0.046 |
| FFM (kg) | 51.6 (10.62) | 55.1 (11.55) | −1.065 | 0.280 |
| Bone mass (kg) | 2.6 (0.50) | 2.8 (0.54) | −1.100 | 0.266 |
| Multivariable Associations Using Binary Logistic Regression | |||||||
|---|---|---|---|---|---|---|---|
| (a) | |||||||
|
n |
β | Unadjusted OR (95% CI) |
p |
β | Adjusted OR (95% CI) |
p | |
| Parents’ BMI category | 51 | ||||||
| Underweight/healthy vs | 22 | ||||||
| Overweight/Obese | 29 | 2.24 | 9.45 (1.10–81.49) | 0.040 | |||
| ND status | 51 | ||||||
| Without ND vs | 33 | ||||||
| ND | 18 | 2.52 | 12.4 (2.53–68.26) | 0.003 | 2.21 | 9.08 (1.56–52.72) | 0.014 |
| (b) | |||||||
|
n |
β | Unadjusted OR (95% CI) |
p |
β | Adjusted OR (95% CI) |
p | |
| Parents’ FAT% category | 51 | ||||||
| Underfat/healthy vs | 27 | ||||||
| Overfat/Obese | 24 | 0.91 | 2.12 (0.46–81.50) | 0.334 | |||
| ND status | 51 | ||||||
| Without ND vs | 33 | ||||||
| ND | 18 | 2.53 | 12.5 (3.04–66.70) | 0.001 | 2.36 | 10.56 (2.27–49.20) | 0.003 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thorsteinsdottir, S.; Bjarnason, R.; Eliasdottir, H.G.; Olafsdottir, A.S. Body Composition in Fussy-Eating Children, with and without Neurodevelopmental Disorders, and Their Parents, Following a Taste Education Intervention. Nutrients 2023, 15, 2788. https://doi.org/10.3390/nu15122788
Thorsteinsdottir S, Bjarnason R, Eliasdottir HG, Olafsdottir AS. Body Composition in Fussy-Eating Children, with and without Neurodevelopmental Disorders, and Their Parents, Following a Taste Education Intervention. Nutrients. 2023; 15(12):2788. https://doi.org/10.3390/nu15122788
Chicago/Turabian StyleThorsteinsdottir, Sigrun, Ragnar Bjarnason, Helga G. Eliasdottir, and Anna S. Olafsdottir. 2023. "Body Composition in Fussy-Eating Children, with and without Neurodevelopmental Disorders, and Their Parents, Following a Taste Education Intervention" Nutrients 15, no. 12: 2788. https://doi.org/10.3390/nu15122788
APA StyleThorsteinsdottir, S., Bjarnason, R., Eliasdottir, H. G., & Olafsdottir, A. S. (2023). Body Composition in Fussy-Eating Children, with and without Neurodevelopmental Disorders, and Their Parents, Following a Taste Education Intervention. Nutrients, 15(12), 2788. https://doi.org/10.3390/nu15122788








