Assessment of the Concentration of 51 Elements in the Liver and in Various Parts of the Human Brain—Profiling of the Mineral Status
Abstract
:1. Introduction
2. Materials and Methods
2.1. Population
2.2. Sample Collection Procedure
2.3. Sample Preparation
2.4. ICP-MS Measurements
2.5. Statistics
3. Results
3.1. Descriptive Statistics for ICP-MS/MS Elemental Measurements
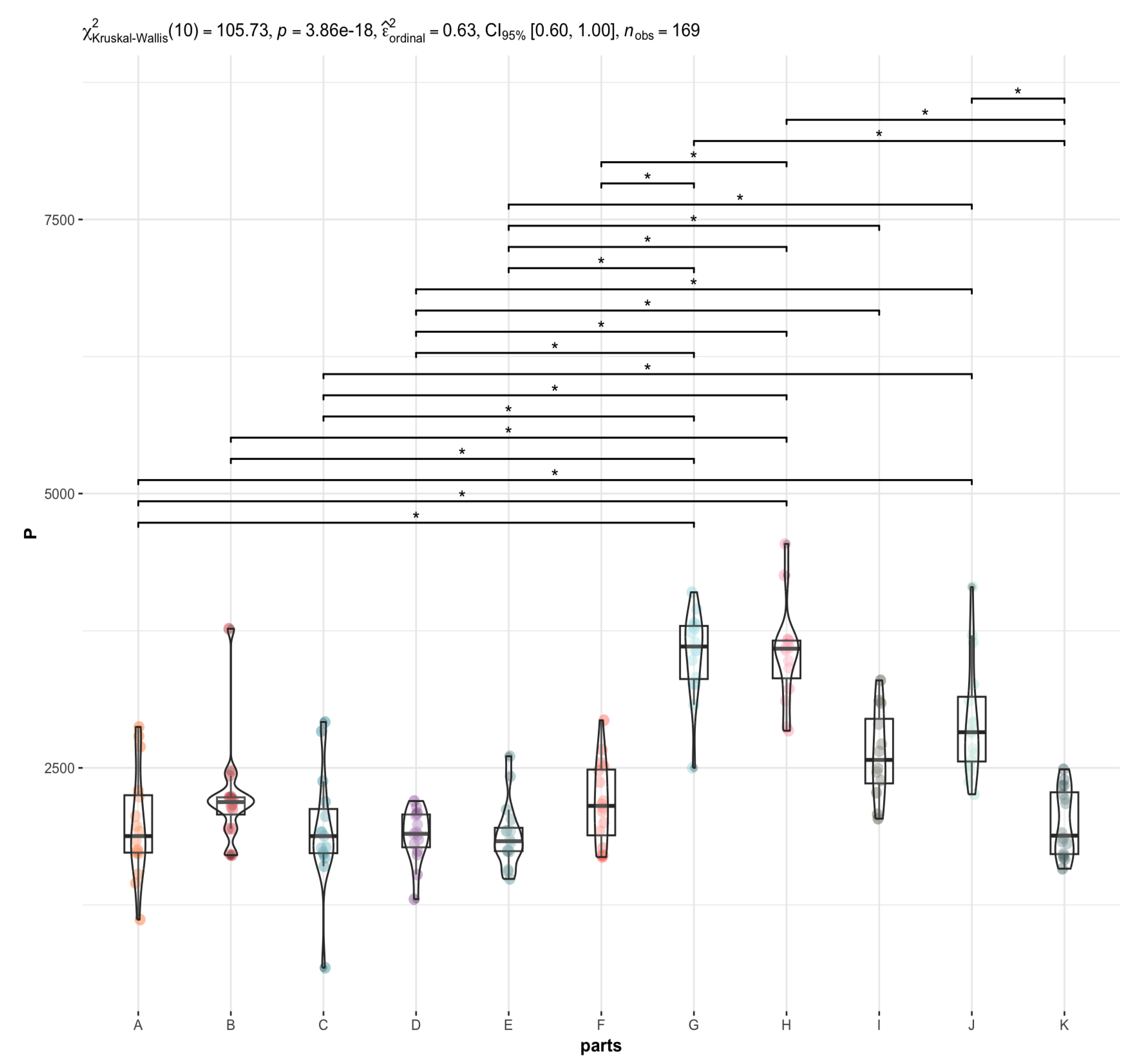
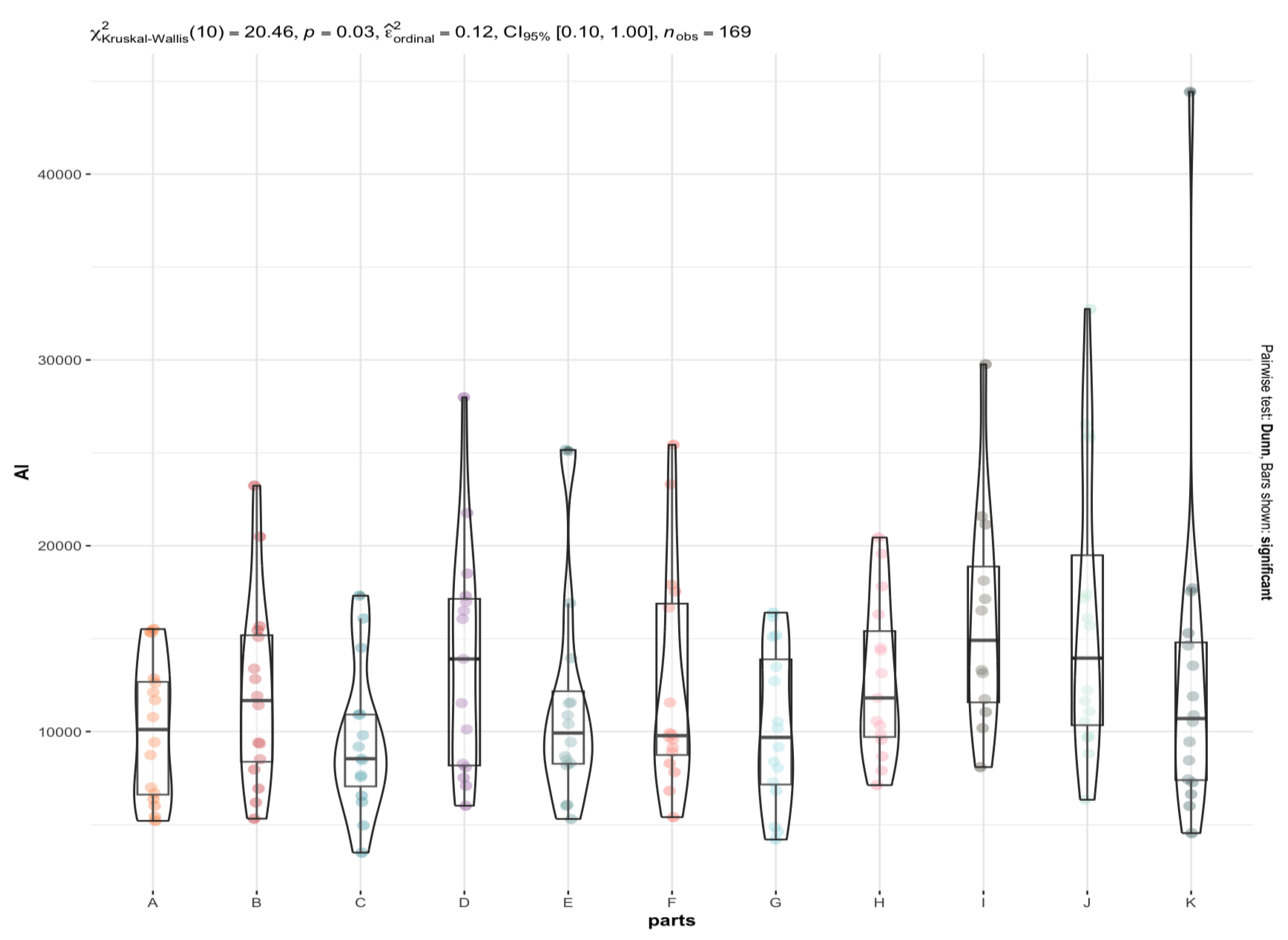
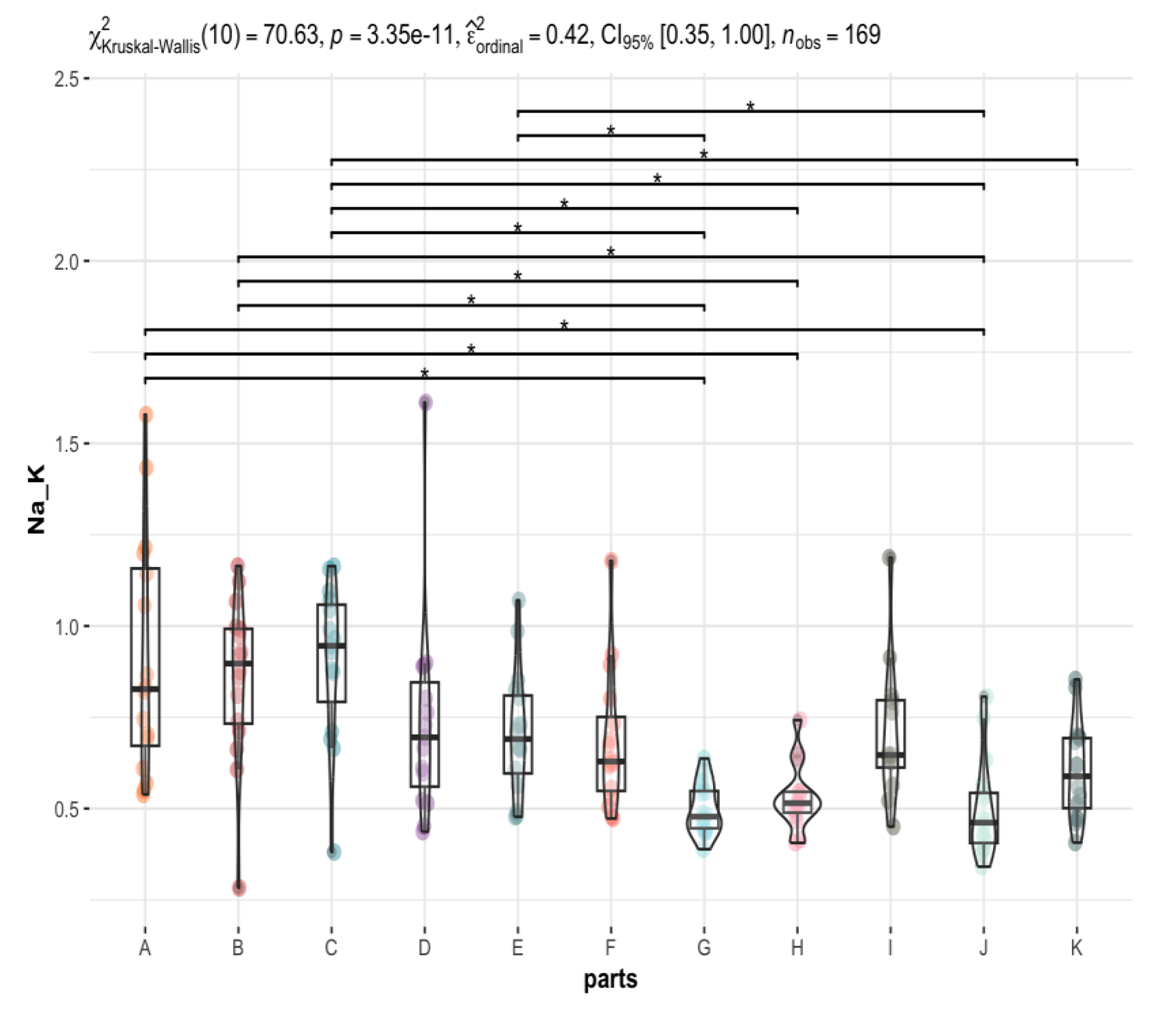
3.2. Are There Any Statistically Significant Differences in the Levels of the Individual Elements between the Brain Areas under Investigation (A to K)?
3.3. Are Elemental Levels in Different Areas of the Brain Different for Men and Women?
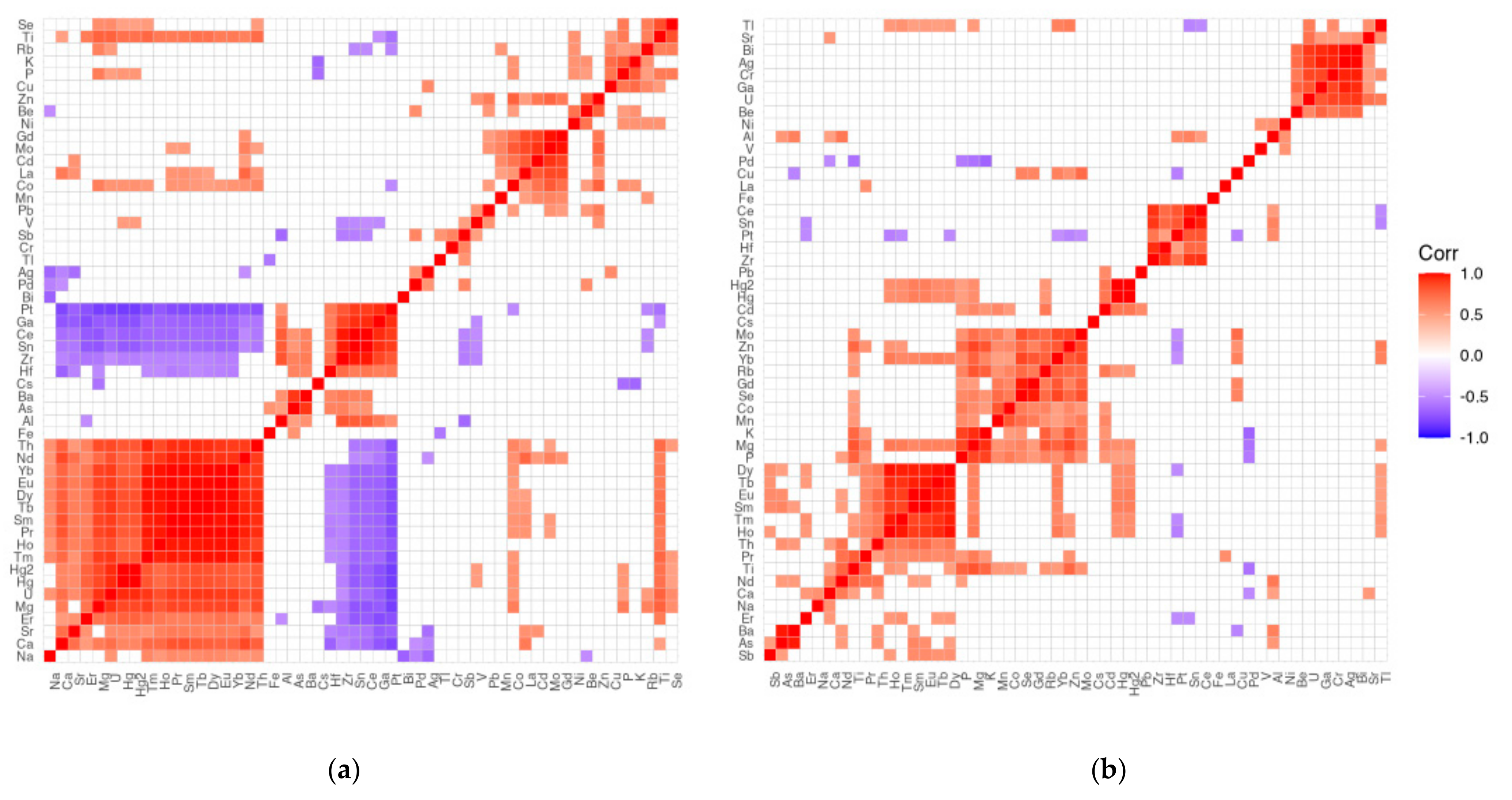
3.4. What Are the Correlations between the Content of the Different Elements in the Liver (L) and the Different Brain Areas (A to K)?
4. Discussion
4.1. P in the Brain vs. Liver
4.2. Xenobiotic Toxic Metals
4.3. The Rare Earth Metal Content
4.4. The Strongest Positive Intercorrelation between Brain and Liver for Se
4.5. The Strongest Negative Intercorrelation between Brain and Liver for Mn and La
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tao, C.; Huang, Y.; Huang, X.; Li, Z.; Fan, Y.; Zhang, Y.; Wan, T.; Lu, L.; Xu, Q.; Wu, W.; et al. Association between Blood Manganese Levels and Visceral Adipose Tissue in the United States: A Population-Based Study. Nutrients 2022, 14, 4770. [Google Scholar] [CrossRef] [PubMed]
- Underwood, E.J. Trace Elements in Human and Animal Nutrition; Academic Press: New York, NY, USA, 1977. [Google Scholar]
- Levander, O.A.; Cheng, L. Micronutreient Interactions, Vitamins, Minerals, and Hazardous Elements; New York Academy of Sciences: New York, NY, USA, 1980; Volume 355. [Google Scholar]
- Skalny, A.V.; Timashev, P.S.; Aschner, M.; Aaseth, J.; Chernova, L.N.; Belyaev, V.E.; Grabeklis, A.R.; Notova, S.V.; Lobinski, R.; Tsatsakis, A.; et al. Serum Zinc, Copper, and Other Biometals Are Associated with COVID-19 Severity Markers. Metabolites 2021, 11, 244. [Google Scholar] [CrossRef] [PubMed]
- Foulquier, F.; Legrand, D. Biometals and glycosylation in humans: Congenital disorders of glycosylation shed lights into the crucial role of Golgi manganese homeostasis. Biochim. Biophys. Acta Gen. Subj. 2020, 1864, 129674. [Google Scholar] [CrossRef] [PubMed]
- Maciejewski, R.; Radzikowska-Büchner, E.; Flieger, W.; Kulczycka, K.; Baj, J.; Forma, A.; Flieger, J. An Overview of Essential Microelements and Common Metallic Nanoparticles and Their Effects on Male Fertility. Int. J. Environ. Res. Public Health 2022, 19, 11066. [Google Scholar] [CrossRef] [PubMed]
- Momčilović, B.; Prejac, J.; Brundić, S.; Morović, S.; Skalny, A.; Mimica, N.; Stipe, D. An essay on human and elements, multielement profiles, and depression. Transl. Neurosci. 2010, 1, 322. [Google Scholar] [CrossRef] [Green Version]
- Krzywoszyńska, K.; Witkowska, D.; Świątek-Kozłowska, J.; Szebesczyk, A.; Kozłowski, H. General Aspects of Metal Ions as Signaling Agents in Health and Disease. Biomolecules 2020, 10, 1417. [Google Scholar] [CrossRef]
- Zhang, Y.; Zheng, J. Bioinformatics of Metalloproteins and Metalloproteomes. Molecules 2020, 25, 3366. [Google Scholar] [CrossRef]
- Shimamura, T.; Iijima, S.; Hirayama, M.; Iwashita, M.; Akiyama, S.; Takaku, Y.; Yumoto, S. Age-related effects of major and trace element concentrations in rat liver and their mutual relationships. J. Trace Elem. Med. Biol. 2013, 27, 286–294. [Google Scholar] [CrossRef]
- Skrajnowska, D.; Jagielska, A.; Ruszczyńska, A.; Wagner, B.; Bielecki, W.; Bobrowska-Korczak, B. Title Changes in the Mineral Composition of Rat Femoral Bones Induced by Implantation of LNCaP Prostate Cancer Cells and Dietary Supplementation. Nutrients 2021, 13, 100. [Google Scholar] [CrossRef]
- Ye, Q.; Park, J.E.; Gugnani, K.; Betharia, S.; Pino-Figueroa, A.; Kim, J. Influence of iron metabolism on manganese transport and toxicity. Metallomics 2017, 9, 1028–1046. [Google Scholar] [CrossRef]
- Chen, J. An original discovery: Selenium deficiency and Keshan disease (an endemic heart disease). Asia Pac. J. Clin. Nutr. 2012, 21, 320–326. [Google Scholar]
- Biban, B.G.; Lichiardopol, C. Iodine Deficiency, Still a Global Problem? Curr. Health Sci. J. 2017, 43, 103–111. [Google Scholar] [CrossRef]
- Hussain, I.; Ur. Rehman, H.; Itai, T.; Ali Khattak, J.; Farooqi, A. Geographic distribution of arsenic contamination in the Himalayan Rivers flowing through Pakistan: Implications for its natural source and effects of anthropogenic activities. Intern. J. Sediment Res. 2023, in press. [Google Scholar] [CrossRef]
- Chakraborti, D.; Rahman, M.M.; Chatterjee, A.; Das, D.; Das, B.; Nayak, B.; Pal, A.; Chowdhury, U.K.; Ahmed, S.; Biswas, B.K.; et al. Fate of over 480 million inhabitants living in arsenic and fluoride endemic Indian districts: Magnitude, health, socio-economic effects and mitigation approaches. J. Trace Elem. Med. Biol. 2016, 38, 33–45. [Google Scholar] [CrossRef]
- Saeed, M.; Rehman, M.Y.A.; Farooqi, A.; Malik, R.N. Arsenic and fluoride co-exposure through drinking water and their impacts on intelligence and oxidative stress among rural school-aged children of Lahore and Kasur districts, Pakistan. Environ. Geochem. Health. 2022, 44, 3929–3951. [Google Scholar] [CrossRef]
- Heidari, S.; Mostafaei, S.; Razazian, N.; Rajati, M.; Saeedi, A.; Rajati, F. The effect of lead exposure on IQ test scores in children under 12 years: A systematic review and meta-analysis of case-control studies. Syst. Rev. 2022, 11, 106. [Google Scholar] [CrossRef]
- Lewińska-Preis, L.; Jabłońska, M.; Fabiańska, M.J.; Kita, A. Bioelements and mineral matter in human livers from the highly industrialized region of the Upper Silesia Coal Basin (Poland). Environ. Geochem. Health 2011, 33, 595–611. [Google Scholar] [CrossRef]
- Varga, I.; Szebeni, A.; Szoboszlai, N.; Kovács, B. Determination of trace elements in human liver biopsy samples by ICP-MS and TXRF: Hepatic steatosis and nickel accumulation. Anal. Bioanal. Chem. 2005, 383, 476–482. [Google Scholar] [CrossRef]
- Ivanenko, N.; Ivanenko, A.A.; Solovyev, N.D.; Zeimal, A.E.; Navolotskii, D.V.; Drobyshev, E.J. Biomonitoring of 20 trace elements in blood and urine of occupationally exposed workers by sector field inductively coupled plasma mass spectrometry. Talanta 2013, 116, 764–769. [Google Scholar] [CrossRef]
- Katoh, Y.; Sato, T.; Yamamoto, Y. Determination of multielement concentrations in normal human organs from the Japanese. Biol. Trace Elem. Res. 2002, 90, 57. [Google Scholar] [CrossRef]
- El Hosry, L.; Sok, N.; Richa, R.; Al Mashtoub, L.; Cayot, P.; Bou-Maroun, E. Sample Preparation and Analytical Techniques in the Determination of Trace Elements in Food: A Review. Foods 2023, 12, 895. [Google Scholar] [CrossRef] [PubMed]
- Planeta, K.; Kubala-Kukus, A.; Drozdz, A.; Matusiak, K.; Setkowicz, Z.; Chwiej, J. The assessment of the usability of selected instrumental techniques for the elemental analysis of biomedical samples. Sci. Rep. 2021, 11, 3704. [Google Scholar] [CrossRef] [PubMed]
- Stewart, T.J. Across the spectrum: Integrating multidimensional metal analytics for in situ metallomic imaging. Metallomics 2019, 11, 29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanaka, Y.K.; Yajima, N.; Okada, M.; Matsumoto, T.; Higuchi, Y.; Miyazaki, S.; Yamato, H.; Hirata, T. The effect of Mg and Sr on the crystallinity of bones evaluated through Raman spectroscopy and laser ablation-ICP-MS analysis. Analyst 2017, 142, 4265–4278. [Google Scholar] [CrossRef] [PubMed]
- Johnson-Davis, K.L.; Farnsworth, C.; Law, C.; Parker, R. Method validation for a multi-element panel in serum by inductively coupled plasma mass spectrometry (ICP-MS). Clin. Biochem. 2020, 82, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Rahil-Khazen, R.; Bolann, B.J.; Myking, A.O.; Ulvik, R.J. Multi-element analysis of trace element levels in human autopsy tissues by using inductively coupled atomic emission spectrometry technique (ICP-AES). J. Trace Elem. Med. Biol. 2002, 16, 15–25. [Google Scholar] [CrossRef]
- Taylor, H.E. Inductively Coupled Plasma-Mass Spectrometry. Practices and Techniques; Academic Press: New York, NY, USA, 2001. [Google Scholar]
- Baj, J.; Teresiński, G.; Forma, A.; Flieger, M.; Proch, J.; Niedzielski, P.; Grochowski, C.; Blicharska, E.; Buszewicz, G.; Bogucki, J.; et al. Chronic Alcohol Abuse Alters Hepatic Trace Element Concentrations-Metallomic Study of Hepatic Elemental Composition by Means of ICP-OES. Nutrients 2022, 14, 546. [Google Scholar] [CrossRef]
- Grochowski, C.; Blicharska, E.; Bogucki, J.; Proch, J.; Mierzwińska, A.; Baj, J.; Litak, J.; Podkowiński, A.; Flieger, J.; Teresiński, G.; et al. Increased Aluminum Content in Certain Brain Structures is Correlated with Higher Silicon Concentration in Alcoholic Use Disorder. Molecules 2019, 24, 1721. [Google Scholar] [CrossRef] [Green Version]
- Baj, J.; Teresiński, G.; Kowalska, B.; Krajka, T.; Buszewicz, G.; Forma, A.; Flieger, W.; Karakuła, K.H.; Kędzierawski, P.; Cywka, T.; et al. ICP-MS Multi-Elemental Analysis of the Human Meninges Collected from Sudden Death Victims in South-Eastern Poland. Molecules 2022, 27, 1911. [Google Scholar] [CrossRef]
- Baj, J.; Forma, A.; Kowalska, B.; Teresiński, G.; Buszewicz, G.; Majerek, D.; Flieger, W.; Maciejewski, R.; Karakuła, K.; Flieger, M.; et al. Multi-Elemental Analysis of Human Optic Chiasm—A New Perspective to Reveal the Pathomechanism of Nerve Fibers’ Degeneration. Int. J. Environ. Res. Public Health 2022, 19, 4420. [Google Scholar] [CrossRef]
- Dolar-Szczasny, J.; Flieger, J.; Kowalska, B.; Majerek, D.; Tatarczak-Michalewska, M.; Zakrocka, I.; Załuska, W.; Rejdak, R. Hemodialysis Effect on the Composition of the Eye Fluid of Cataract Patients. J. Clin. Med. 2021, 10, 5485. [Google Scholar] [CrossRef]
- Dolar-Szczasny, J.; Święch, A.; Flieger, J.; Tatarczak-Michalewska, M.; Niedzielski, P.; Proch, J.; Majerek, D.; Kawka, J.; Mackiewicz, J. Levels of Trace Elements in the Aqueous Humor of Cataract Patients Measured by the Inductively Coupled Plasma Optical Emission Spectrometry. Molecules 2019, 24, 4127. [Google Scholar] [CrossRef] [Green Version]
- Flieger, J.; Dolar-Szczasny, J.; Rejdak, R.; Majerek, D.; Tatarczak-Michalewska, M.; Proch, J.; Blicharska, E.; Flieger, W.; Baj, J.; Niedzielski, P. The Multi-Elemental Composition of the Aqueous Humor of Patients Undergoing Cataract Surgery, Suffering from Coexisting Diabetes, Hypertension, or Diabetic Retinopathy. Int. J. Mol. Sci. 2021, 22, 9413. [Google Scholar] [CrossRef]
- Krebs, N.; Langkammer, C.; Goessler, W.; Ropele, S.; Fazekas, F.; Yen, K.; Scheurer, E. Assessment of trace elements in human brain using inductively coupled plasma mass spectrometry. J. Trace Elem. Med. Biol. 2014, 28, 1–7. [Google Scholar] [CrossRef]
- McDonald, R.J.; McDonald, J.S.; Kallmes, D.F.; Jentoft, M.E.; Paolini, M.A.; Murray, D.L.; Williamson, E.E.; Eckel, L.J. Gadolinium Deposition in Human Brain Tissues after Contrast-enhanced MR Imaging in Adult Patients without Intracranial Abnormalities. Radiology 2017, 285, 546–554. [Google Scholar] [CrossRef]
- Panayi, A.E.; Spyrou, N.M.; Iversen, B.S.; White, M.A.; Part, P. Determination of cadmium and zinc in Alzheimer’s brain tissue using inductively coupled plasma mass spectrometry. J. Neurol. Sci. 2002, 195, 1–10. [Google Scholar] [CrossRef]
- Wilschefski, S.C.; Baxter, M.R. Inductively Coupled Plasma Mass Spectrometry: Introduction to Analytical Aspects. Clin. Biochem. Rev. 2019, 40, 115–133. [Google Scholar] [CrossRef]
- Manikandan, S. Measures of Dispersion. J. Pharmacol. Pharmacother. 2011, 2, 315–316. [Google Scholar] [CrossRef]
- Manikandan, S. Measures of Central Tendency: Median and Mode. J. Pharmacol. Pharmacother. 2011, 2, 214–215. [Google Scholar] [CrossRef] [Green Version]
- Armstrong, R.A.; Hilton, A.C. Nonparametric Analysis of Variance. In Statistical Analysis in Microbiology: Statnotes, 1st ed.; Armstrong, R.A., Hilton, A.C., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2011; Volume 24, pp. 154–196. [Google Scholar]
- Field, A. Discovering Statistics Using IBM SPSS Statistics, 4th ed.; SAGE: Sussex, UK, 2013; Available online: https://in.sagepub.com/en-in/sas/discovering-statistics-using-ibm-spss-statistics/book238032 (accessed on 4 March 2020).
- Dunn, O.J. Multiple Comparisons Using Rank Sums. Technometrics 1964, 6, 241–252. [Google Scholar] [CrossRef]
- Holland, B.S.; Diponzio Copenhaver, M. An Improved Sequentially Rejective Bonferroni Test Procedure. Biometrics 1987, 43, 737. [Google Scholar] [CrossRef]
- Fay, M.P.; Proschan, M.A. Wilcoxon-Mann-Whitney or t-Test? On Assumptions for Hypothesis Tests and Multiple Interpretations of Decision Rules. Statist. Surv. 2010, 4, 1–39. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing, Versión, 4.3.0; R Foundation for Statistical Computing: Indianapolis, IN, USA, 2023; Available online: https://www.r-project.org/ (accessed on 21 April 2023).
- Wickham, H.; Averick, M.; Bryan, J.; Chang, W.; D’Agostino McGowan, L.; François, R.; Grolemund, G.; Hayes, A.; Henry, L.; Hester, J.; et al. Welcome to the Tidyverse. J. Open Source Softw. 2019, 4, 1686. [Google Scholar] [CrossRef] [Green Version]
- Kassambara, A.; Patil, I. Ggcorrplot: Visualization of a Correlation Matrix Using ’Ggplot2’. Available online: https://CRAN.R-project.org/package=ggcorrplot (accessed on 27 October 2022).
- Lüdecke, D.; Ben-Shachar, M.S.; Patil, I.; Wiernik, B.M.; Makowski, D.; Easystats: Framework for Easy Statistical Modeling, Visualization, and Reporting. CRAN 2022. Available online: https://easystats.github.io/easystats/ (accessed on 29 November 2022).
- Sjoberg, D.D.; Whiting, K.; Curry, M.; Lavery, J.A.; Larmarange, J. Reproducible Summary Tables with the Gtsummary Package. R J. 2021, 13, 570–580. [Google Scholar] [CrossRef]
- Holm, S. A Simple Sequentially Rejective Multiple Test Procedure. Scand. J. Statist. 1979, 6, 65–70. [Google Scholar]
- Janelle, F.; Iorio-Morin, C.; D’amour, S.; Fortin, D. Superior Longitudinal Fasciculus: A Review of the Anatomical Descriptions with Functional Correlates. Front. Neurol. 2022, 13, 794618. [Google Scholar] [CrossRef]
- Kaszewski, B.M. Air Pollution Research in Lublin. Ann. Univ. Mariae Curie-Skłodowska Lub. Pol. 2020, 75, 69–86. [Google Scholar]
- Bryliński, Ł.; Kostelecka, K.; Woliński, F.; Duda, P.; Góra, J.; Granat, M.; Flieger, J.; Teresiński, G.; Buszewicz, G.; Sitarz, R.; et al. Aluminium in the Human Brain: Routes of Penetration, Toxicity, and Resulting Complications. Int. J. Mol. Sci. 2023, 24, 7228. [Google Scholar] [CrossRef]
- Exley, C.; Clarkson, E. Aluminium in human brain tissue from donors without neurodegenerative disease: A comparison with Alzheimer’s disease, multiple sclerosis and autism. Sci. Rep. 2020, 10, 7770. [Google Scholar] [CrossRef]
- Mold, M.; Cottle, J.; King, A.; Exley, C. Intracellular Aluminium in Inflammatory and Glial Cells in Cerebral Amyloid Angiopathy: A Case Report. Int. J. Environ. Res. Public Health 2019, 16, 1459. [Google Scholar] [CrossRef] [Green Version]
- Sánchez-Muniz, F.J.; Macho-González, A.; Garcimartín, A.; Santos-López, J.A.; Benedí, J.; Bastida, S.; González-Muñoz, M.J. The Nutritional Components of Beer and Its Relationship with Neurodegeneration and Alzheimer’s Disease. Nutrients 2019, 11, 1558. [Google Scholar] [CrossRef] [Green Version]
- Blanco, C.A.; Sancho, D.; Caballero, I. Aluminium Content in Beers and Silicon Sequestering Effects. Food Res. Int. 2010, 43, 2432–2436. [Google Scholar] [CrossRef]
- Exley, C.; House, E.R. Aluminium in the human brain. Monatsh. Chem. 2011, 142, 357–363. [Google Scholar] [CrossRef]
- Stojsavljević, A.; Rovčanin, M.; Rovčanin, B.; Miković, Ž.; Jeremić, A.; Perović, M.; Manojlović, D. Human biomonitoring of essential, nonessential, rare earth, and noble elements in placental tissues. Chemosphere 2021, 285, 131518. [Google Scholar] [CrossRef]
- Pałasz, A.; Segovia, Y.; Skowronek, R.; Worthington, J.J. Molecular neurochemistry of the lanthanides. Synapse 2019, 73, e22119. [Google Scholar] [CrossRef]
- MacFarquhar, J.K.; Broussard, D.L.; Melstrom, P.; Hutchinson, R.; Wolkin, A.; Martin, C.; Burk, R.F.; Dunn, J.R.; Green, A.L.; Hammond, R.; et al. Acute selenium toxicity associated with a dietary supplement. Arch. Intern. Med. 2010, 170, 256–261. [Google Scholar] [CrossRef] [Green Version]
- Buckman, T.D.; Sutphin, M.S.; Eckhert, C.D. A comparison of the effects of dietary selenium on selenoprotein expression in rat brain and liver. Biochim. Biophys. Acta 1993, 1163, 176–184. [Google Scholar] [CrossRef]
- Aschner, J.L.; Aschner, M. Nutritional aspects of manganese homeostasis. Mol. Aspects Med. 2005, 26, 353–362. [Google Scholar] [CrossRef]
- Li, L.; Yang, X. The Essential Element Manganese, Oxidative Stress, and Metabolic Diseases: Links and Interactions. Oxid. Med. Cell Longev. 2018, 2018, 7580707. [Google Scholar] [CrossRef] [Green Version]
- Nasr, P.; Ignatova, S.; Lundberg, P.; Kechagias, S.; Ekstedt, M. Low hepatic manganese concentrations in patients with hepatic steatosis–A cohort study of copper, iron and manganese in liver biopsies. J. Trace Elem. Med. Biol. 2021, 67, 126772. [Google Scholar] [CrossRef]
- Martins, A.C., Jr.; Morcillo, P.; Ijomone, O.M.; Venkataramani, V.; Harrison, F.E.; Lee, E.; Bowman, A.B.; Aschner, M. New Insights on the Role of Manganese in Alzheimer’s Disease and Parkinson’s Disease. Int. J. Environ. Res. Public Health 2019, 16, 3546. [Google Scholar] [CrossRef] [PubMed]
- Deskin, R.; Bursain, S.J.; Edens, F.W. Neurochemical alterations induced by manganese chloride in neonatal rats. Neurotoxicology 1980, 2, 65–73. [Google Scholar]




| Population | Gender | % | BMI 1 | Min-Max Age | Median Age | Mean Age ± SD |
|---|---|---|---|---|---|---|
| n = 15 | Female n = 4 | 26.67 | 21.85 ± 4.54 | 20–41 | 33.0 | 31.33 ± 10.59 |
| Male n = 11 | 73.33 | 27.29 ± 6.98 | 49–86 | 68.5 | 66.38 ± 11.90 |
| A N = 16 | B N = 16 | C N = 15 | D N = 15 | E N = 16 | F N = 16 | G N = 16 | H N = 15 | I N = 12 | J N = 16 | K N = 16 | p-Value |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.83 (0.67, 1.16) | 0.90 (0.73, 0.99) | 0.95 (0.79, 1.06) | 0.70 (0.56, 0.85) | 0.69 (0.60, 0.81) | 0.63 (0.55, 0.75) | 0.48 (0.45, 0.55) | 0.51 (0.49, 0.55) | 0.65 (0.61, 0.80) | 0.46 (0.41, 0.54) | 0.59 (0.50, 0.69) | <0.001 |
| Area of the Brain | Alkali and Alkaline Earth Metals | Transition Metals | Rare Earth Elements | Actinides | p-Block Metals | Metalloids | Non-Metals |
|---|---|---|---|---|---|---|---|
| A | Na | Ti, Cr | Nd, Sm, Eu, Tb, Dy, Er, Tm, Yb | - | Bi | - | - |
| B | Be | Ti | Sm, Eu, Tb, Dy, Ho, Er, Tm, Yb | - | Bi | - | - |
| C | Cr | Pr, Nd, Sm, Eu, Tb, Dy, Ho, Er, Tm, Yb, | U | - | - | - | |
| D | Cs | Pt | Pr | Th | - | - | - |
| E | Na | Pr, Sm, Eu, Tb, Dy, Ho, Er, Tm, Yb | Th | - | Sb | - | |
| F | Na, Sr | Sm, Eu, Tb, Dy, Er, Tm, Yb | U | - | - | - | |
| G | Na | Ni, Zr, Hg, Hf | Pr, Sm, Eu, Gd, Tb, Dy, Er, Yb | U | Sn, Ga | - | - |
| H | Na | - | - | - | - | - | Se |
| I | - | Mn, Zr, Mo, Pt | Ga, Ce, Gd | U | Sn | - | - |
| J | - | Hg | Eu, Dy, Ho, Er, Tm, Yb | - | - | - | - |
| K | Na, Mg | Ni | Pr, Nd, Sm, Eu, Tb, Dy, Ho, Er, Tm, Yb | Th, U | - | - | - |
| L | - | Ni, Mn, V | - | U | - | - | - |
| Parameter 1 | Parameter 2 | r | 95% CI | t(50) | p |
|---|---|---|---|---|---|
| brain | liver | 0.99 | [0.98, 0.99] | 44.33 | < 0.001 *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baj, J.; Kowalska, B.; Flieger, W.; Radzikowska-Büchner, E.; Forma, A.; Czeczelewski, M.; Kędzierawski, P.; Karakuła, K.; Flieger, M.; Majerek, D.; et al. Assessment of the Concentration of 51 Elements in the Liver and in Various Parts of the Human Brain—Profiling of the Mineral Status. Nutrients 2023, 15, 2799. https://doi.org/10.3390/nu15122799
Baj J, Kowalska B, Flieger W, Radzikowska-Büchner E, Forma A, Czeczelewski M, Kędzierawski P, Karakuła K, Flieger M, Majerek D, et al. Assessment of the Concentration of 51 Elements in the Liver and in Various Parts of the Human Brain—Profiling of the Mineral Status. Nutrients. 2023; 15(12):2799. https://doi.org/10.3390/nu15122799
Chicago/Turabian StyleBaj, Jacek, Beata Kowalska, Wojciech Flieger, Elżbieta Radzikowska-Büchner, Alicja Forma, Marcin Czeczelewski, Paweł Kędzierawski, Kaja Karakuła, Michał Flieger, Dariusz Majerek, and et al. 2023. "Assessment of the Concentration of 51 Elements in the Liver and in Various Parts of the Human Brain—Profiling of the Mineral Status" Nutrients 15, no. 12: 2799. https://doi.org/10.3390/nu15122799






