Stingless Bee (Heterotrigona Itama) Honey and Its Phenolic-Rich Extract Ameliorate Oxidant–Antioxidant Balance via KEAP1-NRF2 Signalling Pathway
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemical and Reagent
2.2. Preparation of Stingless Bee Honey (SBH) and Phenolic-Rich Extract (PRE)
2.3. Experimental Animal
2.4. Induction of Diabetes
2.5. Biochemical Analysis
Kidney and Liver Function
2.6. Antioxidant Analysis
2.6.1. Total Antioxidant Status (TAS)
2.6.2. Oxygen Radical Absorbance Capacity (ORAC)
2.6.3. Thiobarbituric Acid Reactive Substances Assay (TBARS)
2.6.4. Low Molecular Weight Advanced Glycation End Products (LMW-AGEs)
2.6.5. Antioxidant Enzymes and Oxidative Stress Markers
2.7. Gene Expression Analysis
2.7.1. RNA Extraction
2.7.2. cDNA Synthesis
2.7.3. Quantitative Transcription Polymerase Chain Reaction (qPCR)
2.8. Western Blotting
2.9. Statistical Analysis
3. Results and Discussion
3.1. Biochemical Analysis
Liver and Kidney Profile Function
3.2. Antioxidant Analysis
3.2.1. Overall Antioxidant Defense
3.2.2. Systemic Antioxidant Defence against Diabetes-Induced Oxidative Stress
3.3. Tissue-Targeted Antioxidant Defence
3.4. Gene Expressions on Selected Tissue
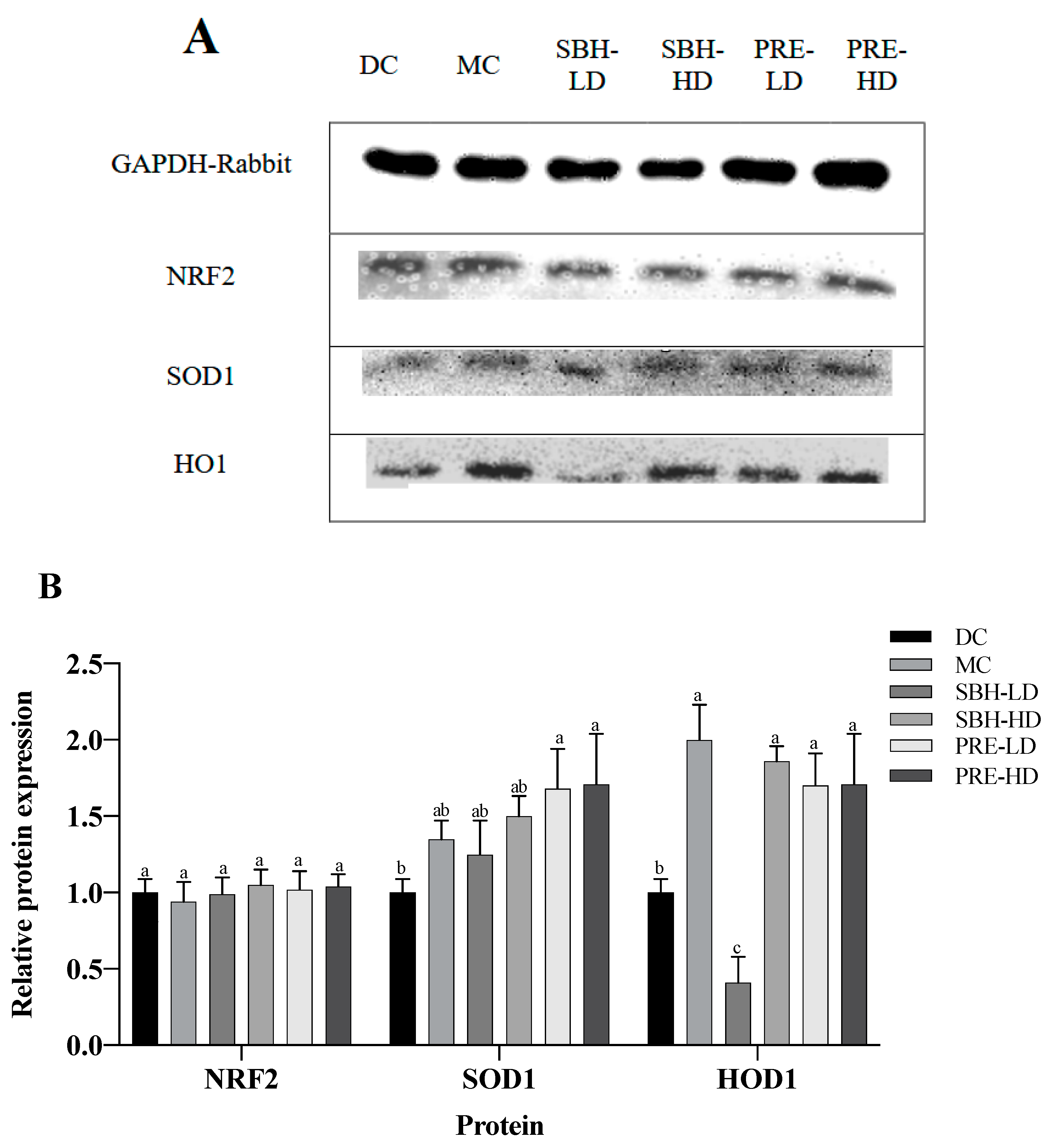
3.5. Protein Translation on Hepatic Tissue
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shin, J.W.; Chun, K.-S.; Kim, D.-H.; Kim, S.-J.; Kim, S.H.; Cho, N.-C.; Na, H.-K.; Surh, Y.-J. Curcumin induces stabilization of Nrf2 protein through Keap1 cysteine modification. Biochem. Pharmacol. 2020, 173, 113820. [Google Scholar] [CrossRef] [PubMed]
- Stefan, N.; Cusi, K. A global view of the interplay between non-alcoholic fatty liver disease and diabetes. Lancet Diabetes Endocrinol. 2022, 10, 284–296. [Google Scholar] [CrossRef] [PubMed]
- Charlton, A.; Garzarella, J.; Jandeleit-Dahm, K.A.M.; Jha, J.C. Oxidative stress and inflammation in renal and cardiovascular complications of diabetes. Biology 2020, 10, 18. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Ambigaipalan, P. Phenolics and polyphenolics in foods, beverages and spices: Antioxidant activity and health effects—A review. J. Funct. Foods 2015, 18, 820–897. [Google Scholar] [CrossRef]
- Martemucci, G.; Costagliola, C.; Mariano, M.; D’andrea, L.; Napolitano, P.; D’alessandro, A.G. Free radical properties, source and targets, antioxidant consumption and health. Oxygen 2022, 2, 48–78. [Google Scholar] [CrossRef]
- Saddique, M.; Kausar, A.; Iqra, I.; Akhter, N.; Mujahid, N.; Parveen, A.; Zaman, Q.; Hussain, S. Amino acids application alleviated salinity stress in spinach (Spinacia oleracea L.) by improving oxidative defense, osmolyte accumulation, and nutrient balance. Turk. J. Agric. For. 2022, 46, 875–887. [Google Scholar] [CrossRef]
- Tamer, C.E.; Temel, Ş.G.; Suna, S.; Karabacak, A.Ö.; Özcan, T.; Ersan, L.Y.; Çopur, Ö.U. Evaluation of bioaccessibility and functional properties of kombucha beverages fortified with different medicinal plant extracts. Turk. J. Agric. For. 2021, 45, 13–32. [Google Scholar] [CrossRef]
- Dunaway, S.; Odin, R.; Zhou, L.; Ji, L.; Zhang, Y.; Kadekaro, A.L. Natu-ral antioxidants: Multiple mechanisms to protect skin from solar radiation. Front. Pharmacol. 2018, 9, 392. [Google Scholar] [CrossRef]
- Li, D.; Zhang, Y.; Liu, Y.; Sun, R.; Xia, M. Purified anthocyanin supplementation reduces dyslipidemia, enhances antioxidant capacity, and prevents insulin resistance in diabetic patients. J. Nutr. 2015, 145, 742–748. [Google Scholar] [CrossRef]
- Phoboo, S.; Shetty, K.; ElObeid, T. In-vitro assays of antidiabetic and anti-hypertensive potential of some traditional edible plants of Qatar. J. Med. Act. Plants 2015, 4, 22–29. [Google Scholar] [CrossRef]
- Francenia Santos Sánchez, N.; Salas-Coronado, R.; Villanueva-Cañongo, C.; Hernández-Carlos, B. Antioxidant compounds and their antioxidant mechanism. Antioxidants 2019, 10, 1–29. Available online: https://www.intechopen.com/chapters/66259 (accessed on 6 November 2019).
- Li, R.; Jia, Z.; Zhu, H. Regulation of Nrf2 signalling. React. Oxyg. Species 2019, 8, 312. Available online: https://pubmed.ncbi.nlm.nih.gov/31692987/ (accessed on 8 November 2019).
- Canning, P.; Sorrell, F.J.; Bullock, A.N. Structural basis of Keap1 interactions with Nrf2. Free Radic. Biol. Med. (Apex NC) 2015, 88, 101–107. [Google Scholar] [CrossRef]
- Majid, M.; Ellulu, M.S.; Abu Bakar, M.F. Melissopalynological study, phenolic compounds, and antioxidant properties of Heterotrigona itama honey from Johor, Malaysia. Scientifica 2020, 1, 6–22. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.Z.S.Z.; Ismail, M.; Chan, K.W.; Ooi, D.J.; Ismail, N.; Zawawi, N.; Mohd Esa, N. Comparison of sugar content, mineral elements and antioxidant properties of Heterotrigona itama honey from suburban and forest in Malaysia. Malays. J. Med. Health Sci. 2019, 15, 104–112. Available online: https://medic.upm.edu.my/upload/dokumen/2019041008261915_MJMHS_Vol_15_SP1.pdf (accessed on 19 April 2019).
- Cheng, M.Z.S.Z.; Ishak, A.; Chan, K.W.; Zawawi, N.; Mohd Esa, N. In-Vitro Investigation of Antioxidant and Antidiabetic Properties of Phenolic-Rich Extract from Stingless Bee Honey (Heterotrigona itama) Honey. Malays. J. Med. Health Sci. 2023. (Submitt). [Google Scholar]
- Moniruzzaman, M.; Yung An, C.; Rao, P.V.; Hawlader, M.N.I.; Azlan, S.A.B.M.; Sulaiman, S.A.; Gan, S.H. Identification of phenolic acids and flavonoids in monofloral honey from Bangladesh by high performance liquid chromatography: Determination of antioxidant capacity. BioMed Res. Int. 2014, 2014, 737490. [Google Scholar] [CrossRef]
- Ooi, D.J.; Chan, K.W.; Ismail, N.; Imam, M.U.; Ismail, M. Polyphenol-rich ethyl acetate fraction of Molineria latifolia rhizome restores oxidant-antioxidant balance by possible engagement of KEAP1-NRF2 and PKC/NF-κB signalling pathways. J. Funct. Foods 2018, 42, 111–121. [Google Scholar] [CrossRef]
- Xu, J.; Chen, L.; Tang, L.; Chang, L.; Liu, S.; Tan, J.; Chen, Y.; Ren, Y.; Liang, F.; Cui, J. Electroacupuncture inhibits weight gain in diet-induced obese rats by activating hypothalamicLKB1-AMPK signaling. BMC Complement. Altern. Med. 2015, 15, 147. [Google Scholar] [CrossRef]
- Chan, K.W.; Iqbal, S.; Khong, N.M.; Ooi, D.-J.; Ismail, M. Antioxidant activity of phenolics–saponins rich fraction prepared from defatted kenaf seed meal. LWT-Food Sci. Technol. 2014, 56, 181–186. [Google Scholar] [CrossRef]
- Yida, Z.; Imam, M.U.; Ismail, M. In vitro bioaccessibility and antioxidant properties of edible bird’s nest following simulated human gastro-intestinal digestion. BMC Complement. Altern. Med. 2014, 14, 468. [Google Scholar] [CrossRef]
- Ooi, D.J.; Adamu, H.A.; Imam, M.U.; Ithnin, H.; Ismail, M. Polyphenol-rich ethyl acetate fraction isolated from Molineria latifolia ameliorates insulin resistance in experimental diabetic rats via IRS1/AKT activation. Biomed. Pharmacother. 2018, 98, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Balamash, K.; Albar, O.; Wang, Q.; Ahmed, N. Effect of Kyolic® aged garlic extract on glycaemia, lipidaemia and oxidative stress in patients with type 2 diabetes mellitus. J. Diabetes Res. Clin. Metab. 2012, 1, 18. [Google Scholar] [CrossRef]
- Yazdi, H.B.; Hojati, V.; Shiravi, A.; Hosseinian, S.; Vaezi, G.; Hadjzadeh, M.-A. Liver Dysfunction and Oxidative stress in streptozotocin-induced diabetic rats: Protective role of Artemisia turanica. J. Pharmacopunct. 2019, 22, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Zhao, D.; Zhang, Y.; Chen, Y.; Zhang, S.; Li, Q.; Yang, X. Rosiglitazone ameliorates bile duct ligation-induced liver fibrosis by down-regulating NF-κB-TNF-α signalling pathway in a PPARγ-dependent manner. Biochem. Biophys. Res. Commun. 2019, 519, 854–860. [Google Scholar] [CrossRef] [PubMed]
- Erejuwa, O.O.; Sulaiman, S.A.; Wahab, M.S. Honey-a novel antidiabetic agent. Int. J. Biol. Sci. 2012, 8, 913. Available online: https://www.ijbs.com/v08p0913.htm (accessed on 7 July 2012). [CrossRef]
- Uyar, A.; Abdulrahman, N.T. A histopathological, immunohistochemical and biochemical investigation of the antidiabetic effects of the Pistacia terebinthus in diabetic rats. Biotech. Histochem. 2020, 95, 92–104. [Google Scholar] [CrossRef]
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive oxygen species in inflammation and tissue injury. Antioxid. Redox Signal. 2014, 20, 1126–1167. [Google Scholar] [CrossRef]
- Kaneto, H.; Katakami, N.; Matsuhisa, M.; Matsuoka, T.-A. Role of reactive oxygen species in the progression of type 2 diabetes and atherosclerosis. Mediat. Inflamm. 2010, 2010, 453892. [Google Scholar] [CrossRef]
- Vilela, D.D.; Peixoto, L.G.; Teixeira, R.R.; Baptista, N.B.; Caixeta, D.C.; de Souza, A.V.; Machado, H.L.; Pereira, M.N.; Sabino-Silva, R.; Espindola, F.S. The role of metformin in controlling oxidative stress in muscle of diabetic rats. Oxidative Med. Cell. Longev. 2016, 2016, 6978625. [Google Scholar] [CrossRef]
- Erejuwa, O.O.; Sulaiman, S.A.; Ab Wahab, M.S.; Salam, S.K.N.; Salleh, S.M.; Gurtu, S. Comparison of antioxidant effects of honey, glibenclamide, metformin, and their combinations in the kidneys of streptozotocin-induced diabetic rats. Int. J. Mol. Sci. 2011, 12, 829–843. [Google Scholar] [CrossRef]
- Erejuwa, O.O.; Sulaiman, S.A.; Wahab, M.S.A.; Sirajudeen, K.N.S.; Salleh, S.M.; Gurtu, S. Antioxidant protective effect of glibenclamide and metformin in combination with honey in pancreas of streptozotocin-induced diabetic rats. Int. J. Mol. Sci. 2010, 11, 2056–2066. [Google Scholar] [CrossRef] [PubMed]
- Erejuwa, O.O.; Sulaiman, S.A.; Ab Wahab, M.S.; Sirajudeen, K.N.S.; Salzihan, M.S. Effects of Malaysian tualang honey supplementation on glycemia, free radical scavenging enzymes and markers of oxidative stress in kidneys of normal and streptozotocin-induced diabetic rats. Int. J. Cardiol. 2009, 137, S45. [Google Scholar] [CrossRef]
- Edge, R.; Truscott, T.G. The reactive oxygen species singlet oxygen, hydroxy radicals, and the superoxide radical ani-on—Examples of their roles in biology and medicine. Oxygen 2021, 1, 77–95. [Google Scholar] [CrossRef]
- Zeni, A.L.B.; Moreira, T.D.; Dalmagro, A.P.; Camargo, A.; Bini, L.A.; Simionatto, E.L.; Scharf, D.R. Evaluation of phenolic compounds and lipid-lowering effect of Morus nigra leaves extract. An. Da Acad. Bras. De Ciências 2017, 89, 2805–2815. [Google Scholar] [CrossRef]
- Hacışevki, A.; Baba, B. An overview of melatonin as an antioxidant molecule: A biochemical approach. Melatonin Mol. Biol. Clin. Pharm. Approaches 2018, 5, 59–85. [Google Scholar] [CrossRef]
- Sahhugi, Z.; Hasenan, S.M.; Jubri, Z. Protective effects of gelam honey against oxidative damage in young and aged rats. Oxidative Med. Cell. Longev. 2014, 2014, 673628. [Google Scholar] [CrossRef]
- Busserolles, J.; Gueux, E.; Rock, E.; Mazur, A.; Rayssiguier, Y. Substituting honey for refined carbohydrates protects rats from hypertriglyceridemic and prooxidative effects of fructose. J. Nutr. 2002, 132, 3379–3382. [Google Scholar] [CrossRef] [PubMed]
- Twarda-Clapa, A.; Olczak, A.; Białkowska, A.M.; Koziołkiewicz, M. Advanced Glycation End-Products (AGEs): Formation, Chemistry, Classification, Receptors, and Diseases Related to AGEs. Cells 2022, 11, 1312. [Google Scholar] [CrossRef]
- Baynes, J.W. Role of oxidative stress in development of complications in diabetes. Diabetes 1991, 40, 405–412. [Google Scholar] [CrossRef]
- Tangvarasittichai, S. Oxidative stress, insulin resistance, dyslipidemia and type 2 diabetes mellitus. World J. Diabetes 2015, 6, 456–480. [Google Scholar] [CrossRef]
- Hirano, T. Pathophysiology of diabetic dyslipidemia. J. Atheroscler. Thromb. 2018, 25, 771–782. [Google Scholar] [CrossRef] [PubMed]
- Huh, J.Y.; Jung, I.; Piao, L.; Ha, H.; Chung, M.-H. 8-Hydroxy-2-deoxyguanosine ameliorates high-fat diet-induced insulin resistance and adipocyte dysfunction in mice. Biochem. Biophys. Res. Commun. 2017, 491, 890–896. [Google Scholar] [CrossRef] [PubMed]
- McMurray, F.; Patten, D.A.; Harper, M.E. Reactive oxygen species and oxidative stress in obesity—Recent findings and empirical approaches. Obesity 2016, 24, 2301–2310. [Google Scholar] [CrossRef]
- Gao, Y.; Bielohuby, M.; Fleming, T.; Grabner, G.F.; Foppen, E.; Bernhard, W.; Yi, C.X. Dietary sugars, not lipids, drive hypo-thalamic inflammation. Mol. Metab. 2017, 6, 897–908. [Google Scholar] [CrossRef]
- Chadt, A.; Al-Hasani, H. Glucose transporters in adipose tissue, liver, and skeletal muscle in metabolic health and disease. Pflügers Arch. -Eur. J. Physiol. 2020, 472, 1273–1298. [Google Scholar] [CrossRef]
- Gholami, M.; Hemmati, M.; Taheri-Ghahfarokhi, A.; Hoshyar, R.; Moossavi, M. Expression of glucokinase, glucose 6-phosphatase, and stress protein in streptozotocin-induced diabetic rats treated with natural honey. Int. J. Diabetes Dev. Ctries. 2015, 36, 125–131. [Google Scholar] [CrossRef]
- Hemmati, M.; Karamian, M.; Malekaneh, M. Anti-atherogenic potential of natural honey: Antidiabetic and antioxidant ap-proaches. J. Pharm. Pharmacol. 2015, 3, 278–284. [Google Scholar] [CrossRef]
- Gustafson, B.; Hedjazifar, S.; Gogg, S.; Hammarstedt, A.; Smith, U. Insulin resistance and impaired adipogenesis. Trends Endocrinol. Metab. 2015, 26, 193–200. [Google Scholar] [CrossRef]
- Satta, S.; Mahmoud, A.M.; Wilkinson, F.L.; Yvonne Alexander, M.; White, S.J. The role of Nrf2 in cardiovascular function and disease. Oxid. Med. Cell. Longev. 2017, 2017, 9237263. [Google Scholar] [CrossRef]
- Hussein, O.E.; Hozayen, W.G.; Bin-Jumah, M.N.; Germoush, M.O.; El-Twab, A.; Sanaa, M.; Mahmoud, A.M. Chicoric acid prevents methotrexate hepatotoxicity via attenuation of oxidative stress and inflammation and up-regulation of PPARγ and Nrf2/HO-1 signalling. Environ. Sci. Pollut. Res. 2020, 27, 20725–20735. Available online: https://link.springer.com/article/10.1007/s11356-020-08557-y (accessed on 3 April 2020). [CrossRef]
- Mo, C.; Wang, L.; Zhang, J.; Numazawa, S.; Tang, H.; Tang, X.; Han, X.J.; Li, J.; Yang, M.; Wang, Z.; et al. The crosstalk between Nrf2 and AMPK signal pathways is important for the anti-inflammatory effect of berberine in LPS-stimulated macrophages and endotoxin-shocked mice. Antioxid. Redox Signal. 2014, 20, 574–588. [Google Scholar] [CrossRef] [PubMed]
- Baird, L.; Yamamoto, M. The molecular mechanisms regulating the KEAP1-NRF2 pathway. Mol. Cell. Biol. 2020, 40, e00099-20. [Google Scholar] [CrossRef] [PubMed]
- Annie-Mathew, A.; Prem-Santhosh, S.; Jayasuriya, R.; Ganesh, G.; Ramkumar, K.M.; Sarada, D. The pivotal role of Nrf2 activators in adipocyte biology. Pharmacol. Res. 2021, 173, 105853. [Google Scholar] [CrossRef]
- David, J.A.; Rifkin, W.J.; Rabbani, P.S.; Ceradini, D.J. The Nrf2/Keap1/ARE pathway and oxidative stress as a therapeutic target in type II diabetes mellitus. J. Diabetes Res. 2017, 2017, 4826724. [Google Scholar] [CrossRef] [PubMed]




| Animal | Description | Treatment |
|---|---|---|
| Group | ||
| NC | Normal control (non-diabetic) | Distilled water |
| Normal pellet diet-fed | ||
| DC | Diabetes control without medications | Distilled water |
| High fat diet-fed | ||
| STZ-NAM-induced diabetes | ||
| MC | Metformin-treated diabetes control | Metformin |
| High-fat diet-fed | (10.0 mg/kg body weight) | |
| STZ-NAM-induced diabetes | ||
| SBH-LD | Diabetic test rats | Stingless bee honey (low dose) |
| High-fat diet-fed | (0.25 g/kg body weight) | |
| STZ-NAM-induced diabetes | ||
| SBH-HD | Diabetic test rats | Stingless bee honey (high dose) |
| High-fat diet-fed | (0.5 g/kg body weight) | |
| STZ-NAM-induced diabetes | ||
| PRE-LD | Diabetic test rats | Phenolic-rich extract (low dose) |
| High-fat diet-fed | (12.5 mg/kg body weight) | |
| STZ-NAM-induced diabetes | ||
| PRE-HD | Diabetic test rats | Phenolic-rich extract (high dose) |
| High-fat diet-fed | (25.0 mg/kg body weight) | |
| STZ-NAM-induced diabetes |
| Treatment | ||||||||
|---|---|---|---|---|---|---|---|---|
| Unit | NC | DC | MC | SBH-LD | SBH-HD | PRE-LD | PRE-HD | |
| Biochemical Parameter (Serum) | ||||||||
| AST | U/L | 268.01 ± 26.00 c | 328.82 ± 38.11 a | 296.67 ± 26.12 b | 311.11 ± 31.02 ab | 307.54 ± 33.79 b | 301.24 ± 29.08 b | 299.37 ± 19.19 b |
| ALP | U/L | 164.50 ± 22.11 c | 279.44 ± 22.62 a | 200.01 ± 12.23 b | 241.20 ± 12.29 ab | 216.34 ± 14.71 b | 216.06 ± 20.11 b | 172.29 ± 11.92 c |
| ALT | U/L | 94.94 ± 16.72 c | 156.81 ± 29.11 a | 96.62 ± 15.74 c | 130.10 ± 15.77 b | 120.51 ± 11.14 b | 109.33 ± 13.26 c | 93.43 ± 10.07 c |
| GGT | U/L | 11.83 ± 2.53 a | 12.11 ± 2.32 a | 12.45 ± 1.42 a | 12.19 ± 1.73 a | 11.99 ± 3.26 a | 12.87 ± 2.15 a | 12.64 ± 1.74 a |
| Urea | mmol/L | 4.42 ± 1.49 a | 5.61 ± 0.64 a | 5.07 ± 0.60 a | 5.29 ± 1.12 a | 5.41 ± 1.17 a | 5.48 ± 1.33 a | 5.01 ± 1.18 a |
| Creatinine | μmol/L | 39.50 ± 3.38 b | 53.81 ± 1.42 a | 47.86 ± 2.12 a | 49.84 ± 2.82 a | 47.85 ± 3.90 a | 47.19 ± 8.12 a | 42.72 ± 7.43 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, M.Z.S.Z.; Amin, F.A.Z.; Zawawi, N.; Chan, K.W.; Ismail, N.; Ishak, N.A.; Esa, N.M. Stingless Bee (Heterotrigona Itama) Honey and Its Phenolic-Rich Extract Ameliorate Oxidant–Antioxidant Balance via KEAP1-NRF2 Signalling Pathway. Nutrients 2023, 15, 2835. https://doi.org/10.3390/nu15132835
Cheng MZSZ, Amin FAZ, Zawawi N, Chan KW, Ismail N, Ishak NA, Esa NM. Stingless Bee (Heterotrigona Itama) Honey and Its Phenolic-Rich Extract Ameliorate Oxidant–Antioxidant Balance via KEAP1-NRF2 Signalling Pathway. Nutrients. 2023; 15(13):2835. https://doi.org/10.3390/nu15132835
Chicago/Turabian StyleCheng, Mohamad Zulhafiz Shafiq Zulhilmi, Fatin Aina Zulkhairi Amin, Norhasnida Zawawi, Kim Wei Chan, Norsharina Ismail, Nur Akmal Ishak, and Norhaizan Mohd Esa. 2023. "Stingless Bee (Heterotrigona Itama) Honey and Its Phenolic-Rich Extract Ameliorate Oxidant–Antioxidant Balance via KEAP1-NRF2 Signalling Pathway" Nutrients 15, no. 13: 2835. https://doi.org/10.3390/nu15132835
APA StyleCheng, M. Z. S. Z., Amin, F. A. Z., Zawawi, N., Chan, K. W., Ismail, N., Ishak, N. A., & Esa, N. M. (2023). Stingless Bee (Heterotrigona Itama) Honey and Its Phenolic-Rich Extract Ameliorate Oxidant–Antioxidant Balance via KEAP1-NRF2 Signalling Pathway. Nutrients, 15(13), 2835. https://doi.org/10.3390/nu15132835









