Associations between Ileal Juice Bile Acids and Colorectal Advanced Adenoma
Abstract
1. Introduction
2. Methods
2.1. Study Population
2.2. Metabolomic Assay
2.3. Statistical Analysis
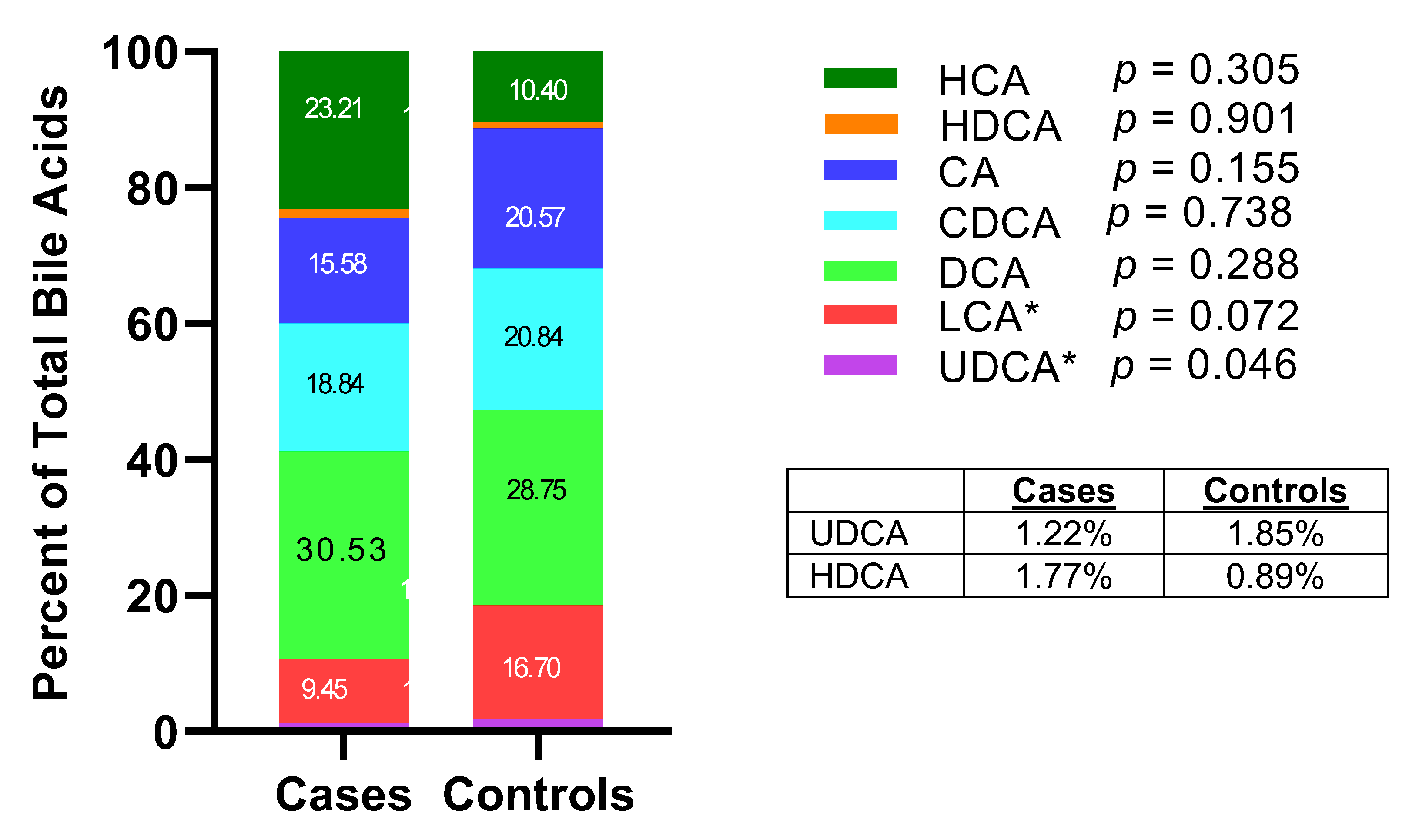
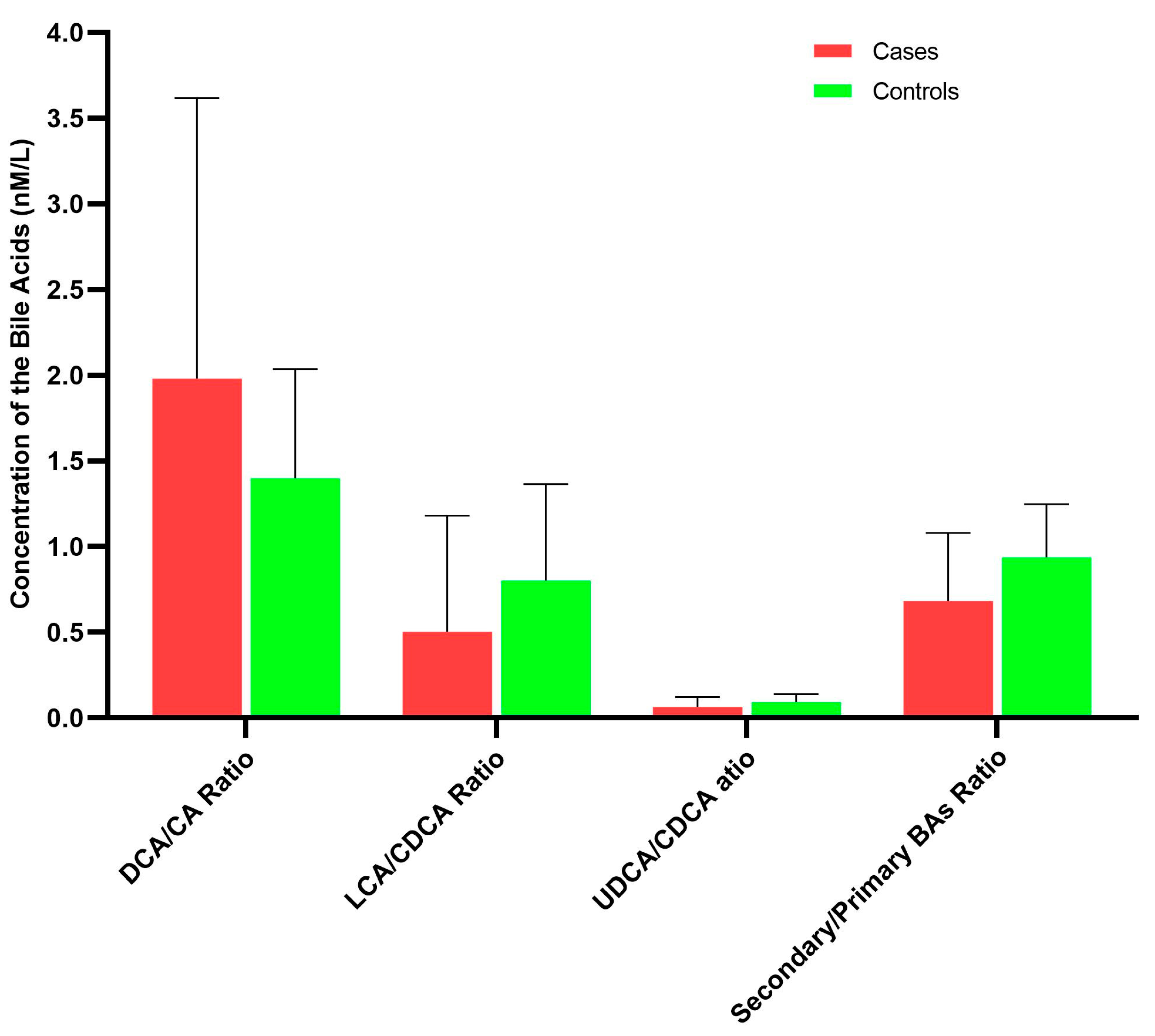
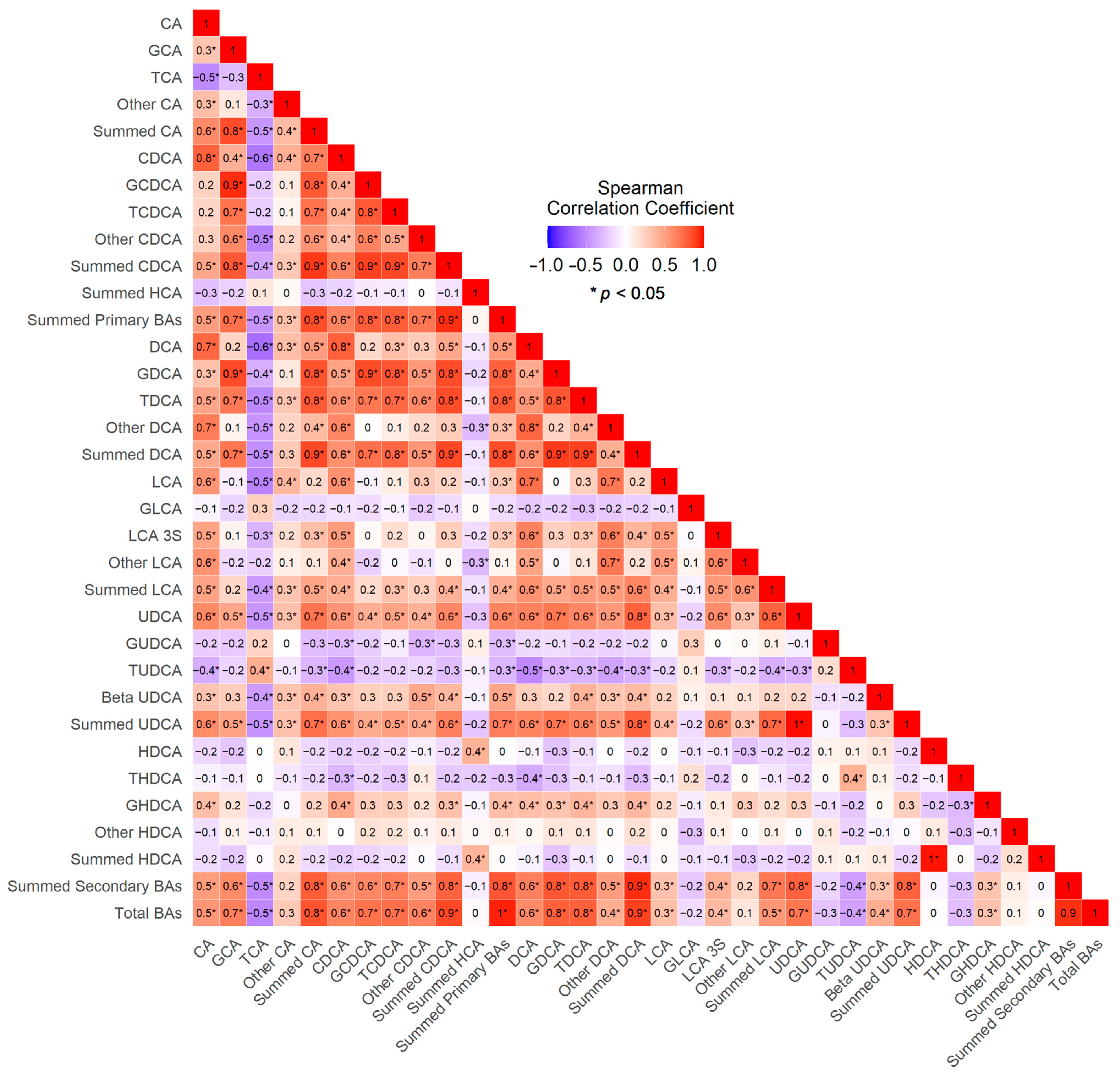
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kuipers, E.J.; Grady, W.M.; Lieberman, D.; Seufferlein, T.; Sung, J.J.; Boelens, P.G.; van de Velde, C.J.H.; Watanabe, T. Colorectal cancer. Nat. Rev. Dis. Primers 2015, 1, 15065. [Google Scholar] [CrossRef] [PubMed]
- Ries, L.A.G.; Eisner, M.P.; Kosary, C.L.; Hankey, B.F.; Miller, B.A.; Clegg, L.; Mariotto, A.; Feuer, E.J.; Edwards, B.K. SEER Cancer Statistics Review: 1975–2004; National Cancer Institute: Bethesda, MD, USA, 2004. [Google Scholar]
- Mathers, C.D.; Loncar, D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006, 3, e442. [Google Scholar] [CrossRef]
- Jass, J.R. Classification of colorectal cancer based on correlation of clinical, morphological and molecular features. Histopathology 2007, 50, 113–130. [Google Scholar] [CrossRef]
- Winawer, S.J.; Zauber, A.G.; O’Brien, M.J.; Gottlieb, L.S.; Sternberg, S.S.; Stewart, E.T.; Bond, J.H.; Schapiro, M.; Panish, J.F.; Waye, J.D. The National Polyp Study. Design, methods, and characteristics of patients with newly diagnosed polyps. The National Polyp Study Workgroup. Cancer 1992, 70, 1236–1245. [Google Scholar] [CrossRef]
- Click, B.; Pinsky, P.F.; Hickey, T.; Doroudi, M.; Schoen, R.E. Association of Colonoscopy Adenoma Findings with Long-term Colorectal Cancer Incidence. JAMA 2018, 319, 2021–2031. [Google Scholar] [CrossRef] [PubMed]
- Rex, D.K.; Lehman, G.A.; Ulbright, T.M.; Smith, J.J.; Pound, D.C.; Hawes, R.H.; Helper, D.J.; Wiersema, M.J.; Langefeld, C.D.; Li, W. Colonic neoplasia in asymptomatic persons with negative fecal occult blood tests: Influence of age, gender, and family history. Am. J. Gastroenterol. 1993, 88, 825–831. [Google Scholar] [PubMed]
- Siegel, R.L.; Fedewa, S.A.; Anderson, W.F.; Miller, K.D.; Ma, J.; Rosenberg, P.S.; Jemal, A. Colorectal Cancer Incidence Patterns in the United States, 1974–2013. J. Natl. Cancer Inst. 2017, 109, djw322. [Google Scholar] [CrossRef] [PubMed]
- Mudaliar, S.; Henry, R.R.; Sanyal, A.J.; Morrow, L.; Marschall, H.-U.; Kipnes, M.; Adorini, L.; Sciacca, C.I.; Clopton, P.; Castelloe, E.; et al. Efficacy and safety of the farnesoid X receptor agonist obeticholic acid in patients with type 2 diabetes and nonalcoholic fatty liver disease. Gastroenterology 2013, 145, 574.e1–582.e1. [Google Scholar] [CrossRef]
- Kuipers, F.; Bloks, V.W.; Groen, A.K. Beyond intestinal soap--bile acids in metabolic control. Nat. Rev. Endocrinol. 2014, 10, 488–498. [Google Scholar] [CrossRef]
- Dawson, P.A.; Karpen, S.J. Intestinal transport and metabolism of bile acids. J. Lipid. Res. 2015, 56, 1085–1099. [Google Scholar] [CrossRef]
- Chiang, J.Y.L. Bile acid metabolism and signaling. Compr. Physiol. 2013, 3, 1191–1212. [Google Scholar] [PubMed]
- Chiang, J.Y.L. Bile acids: Regulation of synthesis. J. Lipid. Res. 2009, 50, 1955–1966. [Google Scholar] [CrossRef] [PubMed]
- Ridlon, J.M.; Harris, S.C.; Bhowmik, S.; Kang, D.-J.; Hylemon, P.B. Consequences of bile salt biotransformations by intestinal bacteria. Gut. Microbes. 2016, 7, 22–39. [Google Scholar] [CrossRef]
- Hofmann, A.F.; Hagey, L.R. Key discoveries in bile acid chemistry and biology and their clinical applications: History of the last eight decades. J. Lipid. Res. 2014, 55, 1553–1595. [Google Scholar] [CrossRef] [PubMed]
- Jia, W.; Xie, G.; Jia, W. Bile acid-microbiota crosstalk in gastrointestinal inflammation and carcinogenesis. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 111–128. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Ung, T.T.; Kim, N.H.; Jung, Y.D. Role of bile acids in colon carcinogenesis. World J. Clin. Cases 2018, 6, 577–588. [Google Scholar] [CrossRef]
- Nagengast, F.M.; Grubben, M.J.; van Munster, I.P. Role of bile acids in colorectal carcinogenesis. Eur. J. Cancer. 1995, 31A, 1067–1070. [Google Scholar] [CrossRef]
- Hofmann, A.F.; Cravetto, C.; Molino, G.; Belforte, G.; Bona, B. Simulation of the metabolism and enterohepatic circulation of endogenous deoxycholic acid in humans using a physiologic pharmacokinetic model for bile acid metabolism. Gastroenterology 1987, 93, 693–709. [Google Scholar] [CrossRef]
- Ajouz, H.; Mukherji, D.; Shamseddine, A. Secondary bile acids: An underrecognized cause of colon cancer. World J. Surg. Oncol. 2014, 12, 164. [Google Scholar] [CrossRef]
- Hylemon, P.B.; Zhou, H.; Pandak, W.M.; Ren, S.; Gil, G.; Dent, P. Bile acids as regulatory molecules. J. Lipid. Res. 2009, 50, 1509–1520. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, H.; Bernstein, C.; Payne, C.M.; Dvorakova, K.; Garewal, H. Bile acids as carcinogens in human gastrointestinal cancers. Mutat. Res. 2005, 589, 47–65. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, C.; Holubec, H.; Bhattacharyya, A.K.; Nguyen, H.; Payne, C.M.; Zaitlin, B.; Bernstein, H. Carcinogenicity of deoxycholate, a secondary bile acid. Arch. Toxicol. 2011, 85, 863–871. [Google Scholar] [CrossRef] [PubMed]
- Imray, C.H.; Radley, S.; Davis, A.; Barker, G.; Hendrickse, C.W.; Donovan, I.A.; Lawson, A.M.; Baker, P.R.; Neoptolemos, J.P. Faecal unconjugated bile acids in patients with colorectal cancer or polyps. Gut 1992, 33, 1239–1245. [Google Scholar] [CrossRef] [PubMed]
- Weir, T.L.; Manter, D.K.; Sheflin, A.M.; Barnett, B.A.; Heuberger, A.L.; Ryan, E.P. Stool microbiome and metabolome differences between colorectal cancer patients and healthy adults. PLoS ONE 2013, 8, e70803. [Google Scholar] [CrossRef] [PubMed]
- Eckburg, P.B.; Bik, E.M.; Bernstein, C.N.; Purdom, E.; Dethlefsen, L.; Sargent, M.; Gill, S.R.; Nelson, K.E.; Relman, D.A. Diversity of the human intestinal microbial flora. Science 2005, 308, 1635–1638. [Google Scholar] [CrossRef]
- Ou, J.; Carbonero, F.; Zoetendal, E.G.; DeLany, J.P.; Wang, M.; Newton, K.; Gaskins, H.R.; O’Keefe, S.J.D. Diet, microbiota, and microbial metabolites in colon cancer risk in rural Africans and African Americans. Am. J. Clin. Nutr. 2013, 98, 111–120. [Google Scholar] [CrossRef]
- Kühn, T.; Stepien, M.; López-Nogueroles, M.; Damms-Machado, A.; Sookthai, D.; Johnson, T.; Roca, M.; Hüsing, A.; Maldonado, S.G.; Cross, A.J.; et al. Prediagnostic Plasma Bile Acid Levels and Colon Cancer Risk: A Prospective Study. J. Natl. Cancer Inst. 2020, 112, 516–524. [Google Scholar] [CrossRef]
- Aguirre de Cárcer, D.; Cuív, P.O.; Wang, T.; Kang, S.; Worthley, D.; Whitehall, V.; Gordon, I.; McSweeney, C.; Leggett, B.; Morrison, M. Numerical ecology validates a biogeographical distribution and gender-based effect on mucosa-associated bacteria along the human colon. ISME J. 2011, 5, 801–809. [Google Scholar] [CrossRef]
- Tong, M.; Jacobs, J.P.; McHardy, I.H.; Braun, J. Sampling of intestinal microbiota and targeted amplification of bacterial 16S rRNA genes for microbial ecologic analysis. Curr. Protoc. Immunol. 2014, 107, 7–41. [Google Scholar] [CrossRef]
- Harris, P.A.; Taylor, R.; Thielke, R.; Payne, J.; Gonzalez, N.; Conde, J.G. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 2009, 42, 377–381. [Google Scholar] [CrossRef]
- Ferslew, B.C.; Xie, G.; Johnston, C.K.; Su, M.; Stewart, P.W.; Jia, W.; Brouwer, K.L.R.; Barritt, A.S. Altered Bile Acid Metabolome in Patients with Nonalcoholic Steatohepatitis. Dig. Dis. Sci. 2015, 60, 3318–3328. [Google Scholar] [CrossRef]
- Yu, H.; Ni, Y.; Bao, Y.; Zhang, P.; Zhao, A.; Chen, T.; Xie, G.; Tu, Y.; Zhang, L.; Su, M.; et al. Chenodeoxycholic Acid as a Potential Prognostic Marker for Roux-en-Y Gastric Bypass in Chinese Obese Patients. J. Clin. Endocrinol. Metab. 2015, 100, 4222–4230. [Google Scholar] [CrossRef] [PubMed]
- Xie, G.; Wang, Y.; Wang, X.; Zhao, A.; Chen, T.; Ni, Y.; Wong, L.; Zhang, H.; Zhang, J.; Liu, C.; et al. Profiling of serum bile acids in a healthy Chinese population using UPLC-MS/MS. J. Proteome. Res. 2015, 14, 850–859. [Google Scholar] [CrossRef]
- Hofmann, A.F.; Hagey, L.R.; Krasowski, M.D. Bile salts of vertebrates: Structural variation and possible evolutionary significance. J. Lipid. Res. 2010, 51, 226–246. [Google Scholar] [CrossRef]
- Deo, A.K.; Bandiera, S.M. Identification of human hepatic cytochrome p450 enzymes involved in the biotransformation of cholic and chenodeoxycholic acid. Drug. Metab. Dispos. 2008, 36, 1983–1991. [Google Scholar] [CrossRef]
- Mahowald, T.A.; Matschiner, J.T.; Hsia, S.L.; Doisy, E.A.; Elliott, W.H.; Doisy, E.A. Bile acids. III. Acid I; the principal bile acid in urine of surgically jaundiced rats. J. Biol. Chem. 1957, 225, 795–802. [Google Scholar] [CrossRef]
- Hsla, S.L.; Matschiner, J.T.; Mahowald, T.A.; Elliott, W.H.; Doisy, E.A.; Thayer, S.A.; Doisy, E.A. Bile acids. VI. The structure and synthesis of acid II. J. Biol. Chem. 1957, 226, 667–671. [Google Scholar]
- Takahashi, S.; Fukami, T.; Masuo, Y.; Brocker, C.N.; Xie, C.; Krausz, K.W.; Wolf, C.R.; Henderson, C.J.; Gonzalez, F.J. Cyp2c70 is responsible for the species difference in bile acid metabolism between mice and humans. J. Lipid. Res. 2016, 57, 2130–2137. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.-K.; Hsu, H.-C.; Liu, J.-R.; Yang, T.-S.; Chen, J.-S.; Chang, J.W.-C.; Lin, Y.-C.; Yu, K.-H.; Kuo, C.-F.; See, L.-C. The Association of Ursodeoxycholic Acid Use with Colorectal Cancer Risk: A Nationwide Cohort Study. Medicine 2016, 95, e2980. [Google Scholar] [CrossRef] [PubMed]
- Ridlon, J.M.; Kang, D.-J.; Hylemon, P.B. Bile salt biotransformations by human intestinal bacteria. J. Lipid. Res. 2006, 47, 241–259. [Google Scholar] [CrossRef] [PubMed]
- Kotb, M.A. Molecular mechanisms of ursodeoxycholic acid toxicity & side effects: Ursodeoxycholic acid freezes regeneration & induces hibernation mode. Int. J. Mol. Sci. 2012, 13, 8882–8914. [Google Scholar]
- Yoshimoto, T.; Higashi, H.; Kanatani, A.; Lin, X.S.; Nagai, H.; Oyama, H.; Kurazono, K.; Tsuru, D. Cloning and sequencing of the 7 alpha-hydroxysteroid dehydrogenase gene from Escherichia coli HB101 and characterization of the expressed enzyme. J. Bacteriol. 1991, 173, 2173–2179. [Google Scholar] [CrossRef] [PubMed]
- Coleman, J.P.; Hudson, L.L.; Adams, M.J. Characterization and regulation of the NADP-linked 7 alpha-hydroxysteroid dehydrogenase gene from Clostridium sordellii. J. Bacteriol. 1994, 176, 4865–4874. [Google Scholar] [CrossRef]
- Baron, S.F.; Franklund, C.V.; Hylemon, P.B. Cloning, sequencing, and expression of the gene coding for bile acid 7 alpha-hydroxysteroid dehydrogenase from Eubacterium sp. strain VPI 12708. J. Bacteriol. 1991, 173, 4558–4569. [Google Scholar] [CrossRef] [PubMed]
- Bennett, M.J.; McKnight, S.L.; Coleman, J.P. Cloning and characterization of the NAD-dependent 7alpha-Hydroxysteroid dehydrogenase from Bacteroides fragilis. Curr. Microbiol. 2003, 47, 475–484. [Google Scholar] [CrossRef] [PubMed]
- Macdonald, I.A.; Hutchison, D.M.; Forrest, T.P. Formation of urso- and ursodeoxy-cholic acids from primary bile acids by Clostridium absonum. J. Lipid. Res. 1981, 22, 458–466. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, J.D.; Holdeman, L.V.; Williams, C.N.; Macdonald, I.A. Formation of urso- and ursodeoxy-cholic acids from primary bile acids by a Clostridium limosum soil isolate. J. Lipid. Res. 1984, 25, 1084–1089. [Google Scholar] [CrossRef]
- Dean, M.; Fantin, G.; Fogagnolo, M.; Medici, A.; Pedrini, P.; Poli, S. Microbial 7-OH Epimerisation of Bile Acids. Chem. Lett. 1999, 28, 693–694. [Google Scholar] [CrossRef]
- Wei, M.; Huang, F.; Zhao, L.; Zhang, Y.; Yang, W.; Wang, S.; Li, M.; Han, X.; Ge, K.; Qu, C.; et al. A dysregulated bile acid-gut microbiota axis contributes to obesity susceptibility. EBioMedicine 2020, 55, 102766. [Google Scholar] [CrossRef]
- Suraweera, D.; Rahal, H.; Jimenez, M.; Viramontes, M.; Choi, G.; Saab, S. Treatment of primary biliary cholangitis ursodeoxycholic acid non-responders: A systematic review. Liver Int. 2017, 37, 1877–1886. [Google Scholar] [CrossRef]
- Hyun, J.J.; Lee, H.S.; Kim, C.D.; Dong, S.H.; Lee, S.-O.; Ryu, J.K.; Lee, D.H.; Jeong, S.; Kim, T.N.; Lee, J.; et al. Efficacy of Magnesium Trihydrate of Ursodeoxycholic Acid and Chenodeoxycholic Acid for Gallstone Dissolution: A Prospective Multicenter Trial. Gut Liver 2015, 9, 547–555. [Google Scholar] [CrossRef] [PubMed]
- Guarino, M.P.L.; Cocca, S.; Altomare, A.; Emerenziani, S.; Cicala, M. Ursodeoxycholic acid therapy in gallbladder disease, a story not yet completed. World J. Gastroenterol. 2013, 19, 5029–5034. [Google Scholar] [CrossRef] [PubMed]
- Alberts, D.S.; Martínez, M.E.; Hess, L.M.; Einspahr, J.G.; Green, S.B.; Bhattacharyya, A.K.; Guillen, J.; Krutzsch, M.; Batta, A.K.; Salen, G.; et al. Phase III trial of ursodeoxycholic acid to prevent colorectal adenoma recurrence. J. Natl. Cancer Inst. 2005, 97, 846–853. [Google Scholar] [CrossRef]
- Pearson, T.; Caporaso, J.G.; Yellowhair, M.; Bokulich, N.A.; Padi, M.; Roe, D.J.; Wertheim, B.C.; Linhart, M.; Martinez, J.A.; Bilagody, C.; et al. Effects of ursodeoxycholic acid on the gut microbiome and colorectal adenoma development. Cancer Med. 2019, 8, 617–628. [Google Scholar] [CrossRef]
- Barlow, G.M.; Yu, A.; Mathur, R. Role of the Gut Microbiome in Obesity and Diabetes Mellitus. Nutr. Clin. Pract. 2015, 30, 787–797. [Google Scholar] [CrossRef]
- Quagliariello, A.; Aloisio, I.; Bozzi Cionci, N.; Luiselli, D.; D’Auria, G.; Martinez-Priego, L.; Pérez-Villarroya, D.; Langerholc, T.; Primec, M.; Mičetić-Turk, D.; et al. Effect of Bifidobacterium breve on the Intestinal Microbiota of Coeliac Children on a Gluten Free Diet: A Pilot Study. Nutrients 2016, 8, 660. [Google Scholar] [CrossRef] [PubMed]
- Walker, A.W.; Sanderson, J.D.; Churcher, C.; Parkes, G.C.; Hudspith, B.N.; Rayment, N.; Brostoff, J.; Parkhill, J.; Dougan, G.; Petrovska, L. High-throughput clone library analysis of the mucosa-associated microbiota reveals dysbiosis and differences between inflamed and non-inflamed regions of the intestine in inflammatory bowel disease. BMC Microbiol. 2011, 11, 7. [Google Scholar] [CrossRef] [PubMed]
- Gevers, D.; Kugathasan, S.; Denson, L.A.; Vázquez-Baeza, Y.; Van Treuren, W.; Ren, B.; Schwager, E.; Knights, D.; Song, S.J.; Yassour, M.; et al. The treatment-naive microbiome in new-onset Crohn’s disease. Cell Host. Microbe 2014, 15, 382–392. [Google Scholar] [CrossRef]

| Cases (n, %) | Controls (n, %) | p-Value | |
|---|---|---|---|
| No. of subjects | 14 | 32 | |
| Age (Mean ± SD) | 62.79 ± 8.05 | 61.06 ± 10.53 | 0.71 |
| Gender | |||
| Male | 12 (85.7) | 12 (37.5) | 0.003 |
| Female | 2 (14.3) | 20 (62.5) | |
| BMI (Mean ± SD) | 22.3 ± 2.8 | 22.6 ± 2.7 | 0.71 |
| Smoking status | |||
| Never | 7 (50.0) | 23 (71.9) | 0.31 |
| Former smoker | 3 (21.4) | 5 (15.6) | |
| Current smoker | 4 (20.6) | 4 (12.5) | |
| Current alcohol use a | |||
| No | 4 (28.6) | 24 (75.0) | 0.007 |
| Yes | 10 (71.4) | 8 (25.0) | |
| History of diabetes | |||
| Yes | 12 (85.7) | 26 (81.2) | 0.71 |
| No | 2 (14.3) | 6 (18.7) | |
| Medication usage | |||
| Antibiotic, past 6 m | 6 (42.9) | 13 (40.6) | 0.89 |
| Metformin | 1 (7.1) | 3 (9.4) | 0.80 |
| Statin | 3 (21.4) | 0 (0.0) | 0.79 |
| Aspirin | 1 (7.1) | 1 (3.1) | 0.53 |
| Other NSAIDs | 0 (0.0) | 1 (3.1) | 0.50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luu, H.N.; Tran, C.T.-D.; Wang, R.; Nguyen, M.V.-T.; Tran, M.T.; Tuong, T.T.-V.; Tran, Q.H.; Le, L.C.; Pham, H.T.-T.; Vu, H.H.; et al. Associations between Ileal Juice Bile Acids and Colorectal Advanced Adenoma. Nutrients 2023, 15, 2930. https://doi.org/10.3390/nu15132930
Luu HN, Tran CT-D, Wang R, Nguyen MV-T, Tran MT, Tuong TT-V, Tran QH, Le LC, Pham HT-T, Vu HH, et al. Associations between Ileal Juice Bile Acids and Colorectal Advanced Adenoma. Nutrients. 2023; 15(13):2930. https://doi.org/10.3390/nu15132930
Chicago/Turabian StyleLuu, Hung N., Chi Thi-Du Tran, Renwei Wang, Mai Vu-Tuyet Nguyen, Mo Thi Tran, Thuy Thi-Van Tuong, Quang Hong Tran, Linh Cu Le, Huong Thi-Thu Pham, Hien Huy Vu, and et al. 2023. "Associations between Ileal Juice Bile Acids and Colorectal Advanced Adenoma" Nutrients 15, no. 13: 2930. https://doi.org/10.3390/nu15132930
APA StyleLuu, H. N., Tran, C. T.-D., Wang, R., Nguyen, M. V.-T., Tran, M. T., Tuong, T. T.-V., Tran, Q. H., Le, L. C., Pham, H. T.-T., Vu, H. H., Bui, N. C., Ha, H. T.-T., Trinh, D. T., Thomas, C. E., Adams-Haduch, J., Velikokhatnaya, L., Schoen, R. E., Xie, G., Jia, W., ... Yuan, J.-M. (2023). Associations between Ileal Juice Bile Acids and Colorectal Advanced Adenoma. Nutrients, 15(13), 2930. https://doi.org/10.3390/nu15132930








