Biochemical Changes Induced by the Administration of Cannabis sativa Seeds in Diabetic Wistar Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Cannabis sativa ‘Zenit’ Variety Hemp Seed Composition
2.2. Chemical Analysis
2.2.1. Antioxidant Activity of Hemp Extracts (Methanolic and Ethanolic Extracts)
2.2.2. Extraction of Phenolic Compounds
2.2.3. Fatty Acid Analyses
2.3. Experimental Animal Protocol
Blood Biochemical Parameters
2.4. Statistical Analysis
3. Results
3.1. The Composition of Cannabis sativa ‘Zenit’, Antioxidant Activity, Identification and Quantification of Phenols and Fatty Acids from Hemp Oil
3.1.1. The Composition of Cannabis sativa ‘Zenit’, Antioxidant Activity, Identification and Quantification of Phenols and Fatty Acids from Hemp Oil
3.1.2. The Composition of Total Phenols and Total Flavonoids
3.1.3. The Antioxidant Activity
3.1.4. Phenolic Compounds
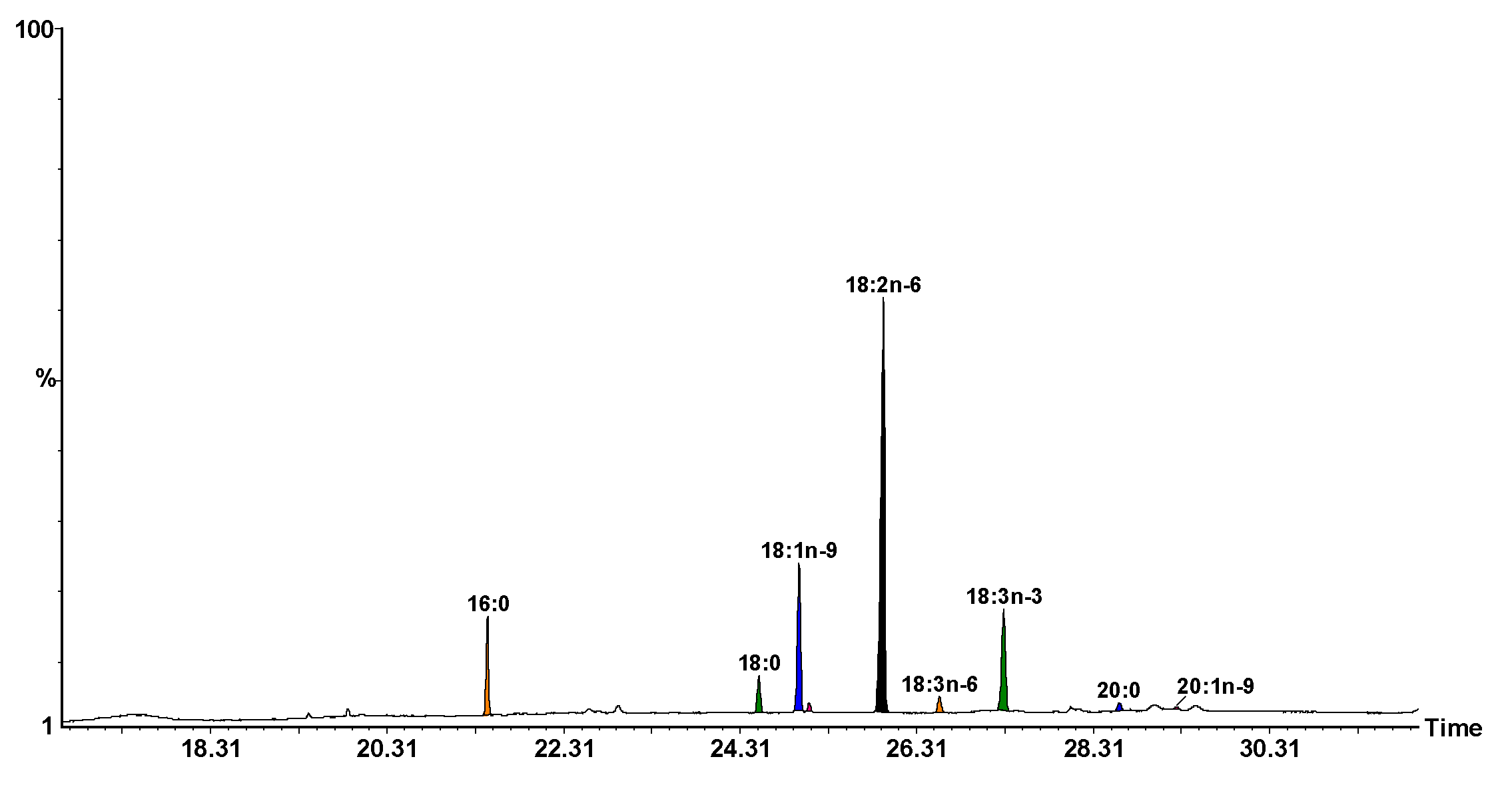
3.1.5. Fatty Acid Composition from Seed Oil
3.2. Glycemia and Weight during Diabetes Induction in Rats
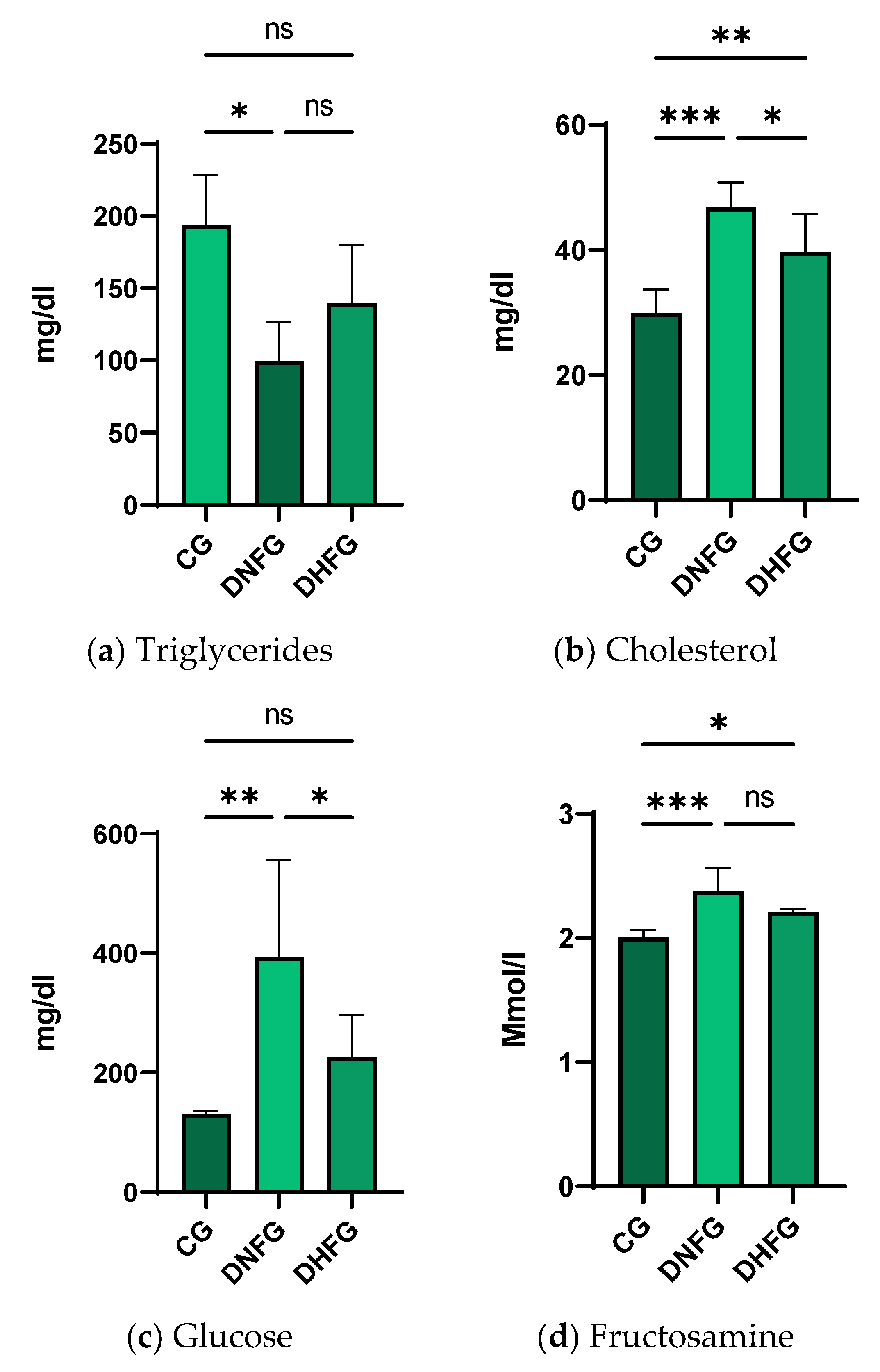
3.3. Blood Biochemical Changes after Hemp Seed Treatment in Diabetic Rats Compared to CG
4. Discussion
4.1. The Composition of Cannabis sativa ‘Zenit’, Antioxidant Activity, Identification and Quantification of Phenols and Fatty Acids from Hemp Oil
4.2. Glycemia and Weight during the Experiment
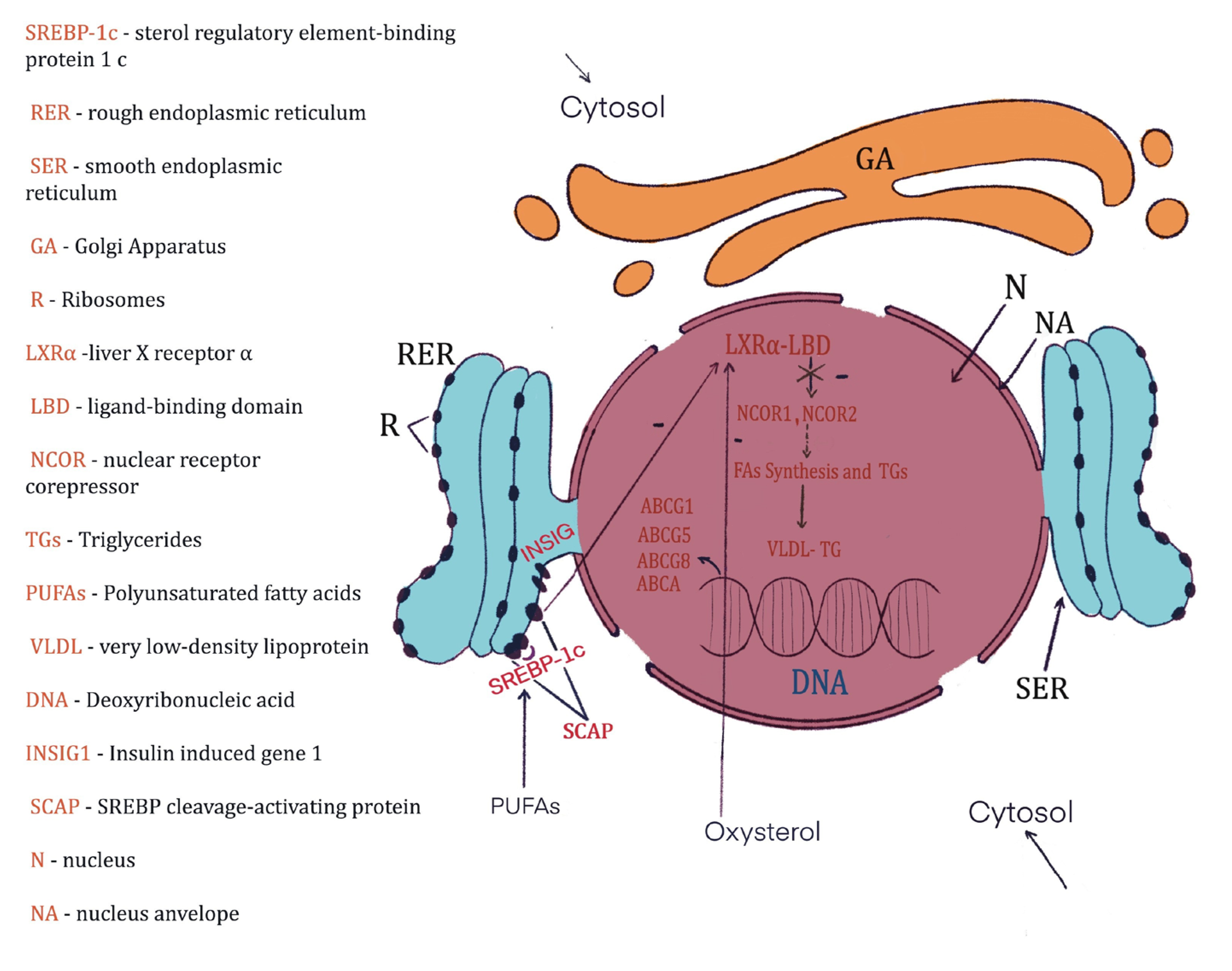
4.3. Blood Biochemical Changes after the Treatment with Hemp Seeds in Diabetic Rats
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kleinert, M.; Clemmensen, C.; Hofmann, S.M.; Moore, M.C.; Renner, S.; Woods, S.C.; Huypens, P.; Beckers, J.; de Angelis, M.H.; Schürmann, A.; et al. Animal models of obesity and diabetes mellitus. Nat. Rev. Endocrinol. 2018, 14, 140–162. [Google Scholar] [CrossRef] [PubMed]
- Kahn, S.E.; Hull, R.L.; Utzschneider, K.M. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature 2006, 444, 840–846. [Google Scholar] [CrossRef] [PubMed]
- Majewski, M.K.; Ognik, K.; Juśkiewicz, J. The interaction between resveratrol and two forms of copper as carbonate and nanoparticles on antioxidant mechanisms and vascular function in Wistar rats. Pharmacol. Rep. 2019, 71, 862–869. [Google Scholar] [CrossRef]
- Majewski, M.K.; Ognik, K.; Juśkiewicz, J. The antioxidant status, lipid profile, and modulation of vascular function by fish oil supplementation in nano-copper and copper carbonate fed Wistar rats. J. Funct. Foods 2020, 64, 103595. [Google Scholar] [CrossRef]
- Kaushal, N.; Dhadwal, S.; Kaur, P. Ameliorative effects of hempseed (Cannabis sativa) against hypercholesterolemia associated cardiovascular changes. Nutr. Metab. Cardiovasc. Dis. 2020, 30, 330–338. [Google Scholar] [CrossRef]
- IT IS. Cannabis sativa TSN 19109. 2023. Available online: https://explorer.natureserve.org/Taxon/ELEMENT_GLOBAL.2.131607/Cannabis_sativa (accessed on 23 February 2023).
- Farinon, B.; Molinari, R.; Constantini, L.; Merendino, N. The seed of industrial hemp (Cannabis sativa L.): Nutritional Quality and Potential Functionality for Human Health and Nutrition. Nutrition 2020, 12, 1935. [Google Scholar] [CrossRef]
- Placido, D.F.; Lee, C.C. Potential of industrial hemp for phytoremediation of heavy metals. Plants 2022, 11, 595. [Google Scholar] [CrossRef]
- Salentijn, E.M.J.; Zhang, Q.; Amaducci, S.; Yang, M.; Trindade, L.M. New developments in fiber hemp (Cannabis sativa L.) breeding. Ind. Crops Prod. 2015, 68, 32–41. [Google Scholar] [CrossRef]
- Zhou, Q.; Huang, F.; Zheng, C.; Guo, P.; Li, W.; Liu, C.; Wan, C. Physicochemical properties and volatile terpene of hempseed oils in Bama region. Oil Crop Sci. 2017, 1, 13–22. [Google Scholar]
- Callaway, J.C. Hempseed as a nutritional resource: An overview. Euphytica 2004, 140, 65–72. [Google Scholar] [CrossRef]
- Szlas, A.; Kurek, J.M.; Krejpcio, Z. The Potential of L-Arginine in Prevention and Treatment of Disturbed Carbohydrate and Lipid Metabolism—A Review. Nutrients 2022, 14, 961. [Google Scholar] [CrossRef]
- Rodriguez-Leyva, D.; Pierce, G.N. The cardiac and haemostatic effects of dietary hempseed. Nutr. Metab. 2010, 7, 32. [Google Scholar] [CrossRef]
- Sokoła-Wysoczańska, E.; Wysoczanski, T.; Wagner, J.; Czyz, K.; Bodkowski, R.; Lochynski, S.; Patkowska-Sokola, B. Polyunsaturated fatty acids and their potential therapeutic role in cardiovascular system disorders—A review. Nutrients 2018, 10, 1561. [Google Scholar] [CrossRef]
- Holub, B.J. Clinical nutrition: 4. Omega-3 fatty acids in cardiovascular care. Cmaj 2002, 166, 608–615. [Google Scholar]
- Lunn, J.; Theobald, H.E. The health effects of dietary unsaturated fatty acids. Nutr. Bull. 2006, 31, 178–224. [Google Scholar] [CrossRef]
- Opyd, P.M.; Jurgonski, A.; Fotschki, B.; Juskiewicz, J. Dietary hemp seeds more effectively attenuate disorders in genetically obese rats than their lipid fraction. J. Nutr. 2020, 150, 1425–1433. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.J.; Sood, S.V. Effect of gamma linolenic acid pretreatment on diabetic neuropathy in rats. Int. J. Basic Clin. Pharmacol. 2013, 2, 320–325. [Google Scholar] [CrossRef]
- Pollastro, F.; Minassi, A.; Fresu, L.G. Cannabis phenolics and their bioactivities. Curr. Med. Chem. 2018, 25, 1160–1185. [Google Scholar] [CrossRef] [PubMed]
- Radwan, M.M.; ElSohly, M.A.; Slade, D.; Ahmed, S.A.; Wilson, L.; El-Alfy, A.T.; Khan, I.A.; Ross, S.A. Non-cannabinoid constituents from a high potency Cannabis sativa variety. Phytochemistry 2008, 69, 2627–2633. [Google Scholar] [CrossRef]
- Mkpenie, V.N.; Essien, E.E.; Udoh, I.I. Effect of extraction conditions on total polyphenol contents, antioxidant and antimicrobial activities of Cannabis sativa L. Electron. J. Environ. Agric. Food Chem. 2011, 11, 300–307. [Google Scholar]
- Mandal, S.M.; Chakraborty, D.; Dey, S. Phenolic acids act as signaling molecules in plant-microbe symbioses. Plant Signal. Behav. 2010, 5, 359–368. [Google Scholar] [CrossRef] [PubMed]
- Pan, A.; Sun, J.; Chen, Y.; Ye, X.; Li, H.; Yu, Z.; Wang, Y.; Gu, W.; Zhang, X.; Chen, X.; et al. Effects of a flaxseed-derived lignan supplement in type 2 diabetic patients: A randomized, double-blind, cross-over trial. PLoS ONE 2007, 2, e1148. [Google Scholar] [CrossRef] [PubMed]
- Kasetti, R.B.; Nabi, S.A.; Swapna, S.; Apparao, C. Cinnamic acid as one of the antidiabetic active principle(s) from the seeds of Syzygium alternifolium. Food Chem. Toxicol. 2012, 50, 1425–1431. [Google Scholar] [CrossRef] [PubMed]
- Sales, P.M.; Souza, P.M.; Simeoni, L.A.; Silveira, D. α-Amylase inhibitors: A review of raw material and isolated compounds from plant source. J. Pharm. Pharm. Sci. 2012, 15, 141–183. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Liang, K.; Zhang, M.; Chen, Y.; Wu, H.; Lai, F. Identification and characterization of two novel α-glucosidase inhibitory oligopeptides from hemp (Cannabis sativa L.) seed protein. J. Funct. Foods 2016, 26, 439–450. [Google Scholar] [CrossRef]
- George, W.; Latimer, J.R. Official Methods of Analysis of AOAC, 21st ed.; AOAC International: Gaithersburg, MD, USA, 2019. [Google Scholar]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C.L.W.T. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Benkirane, C.; Moumen, A.B.; Fauconnier, M.L.; Belhaj, K.; Abid, M.; Caid, H.S.; Elamrani, A.; Mansouri, F. Bioactive compounds from hemp (Cannabis sativa L.) seeds: Optimization of phenolic antioxidant extraction using simplex lattice mixture design and HPLC-DAD/ESI-MS 2 analysis. RSC Adv. 2022, 12, 25764–25777. [Google Scholar] [CrossRef]
- Christie, W.W.; Brechany, E.Y.; Shukla, V.K.S. Analysis of seed oils containing cyclopentenyl fatty acids by combined chromatographic procedures. Lipids 1989, 24, 116–120. [Google Scholar] [CrossRef]
- Dulf, F.V.; Vodnar, D.C.; Tosa, M.I.; Dulf, E.H. Simultaneous enrichment of grape pomace with γ-linolenic acid and carotenoids by solid-state fermentation with Zygomycetes fungi and antioxidant potential of the bioprocessed substrates. Food Chem. 2020, 310, 125927. [Google Scholar] [CrossRef]
- Bindea, M.; Rusu, B.; Rusu, A.; Trif, M.; Leopold, L.F.; Dulf, F.; Vodnar, D.C. Valorification of crude glycerol for pure fractions of docosahexaenoic acid and β-carotene production by using Schizochytrium limacinum and Blakeslea trispora. Microb. Cell Factories 2018, 17, 1–13. [Google Scholar] [CrossRef]
- Lewis, C.; Barbiers, A.R. Streptozotocin, a new antibiotic. In vitro and in vivo evaluation. Antibiot. Annu. 1959, 7, 247–254. [Google Scholar] [PubMed]
- Rerup, C.C. Drugs producing diabetes through damage of the insulin secreting cells. Pharmacol. Rev. 1970, 22, 485–518. [Google Scholar]
- Furman, B.L. Streptozotocin-induced diabetic models in mice and rats. Curr. Protoc. Pharmacol. 2015, 70, 5–47. [Google Scholar] [CrossRef] [PubMed]
- National Research Coucil. Guide for the Care and Use of Laboratory Animals; The National Academies Press: Washington, DC, USA, 2010. [Google Scholar]
- Council Directive 86/609/EEC of 24 November 1986 on the approximation of laws, regulations and administrative provisions of the Member States regarding the protection of animals used for experimental and other scientific purposes. Off. J. Eur. Commun. 1986, 29, L358.
- Kohn, D.F.; Clifford, C.B. Biology and diseases of rats. In Laboratory Animal Medicine; Academic Press: Cambridge, MA, USA, 2002; pp. 121–165. [Google Scholar] [CrossRef]
- Kim, Y.S.; Kim, N.H.; Lee, S.W.; Lee, Y.M.; Jang, D.S.; Kim, S.H. Effect of protocatechualdehyde on receptor for advanced glycation end products and TGF-β1 expression in human lens epithelial cells cultured under diabetic conditions and on lens opacity in streptozotocin-diabetic rats. Eur. J. Pharmacol. 2007, 569, 171–179. [Google Scholar] [CrossRef]
- Johnson-Delaney, C.A. Exotic Companion Medicine Handbook for Veterinarians; Wingers Publishing Incorporated: Lake Worth, FL, USA, 1996. [Google Scholar]
- Baggio, G.; Donazzan, S.; Monti, D.; Mari, D.; Martini, S.; Gabelli, C.; Dalla Vestra, M.; Previato, L.; Guido, M.; Pigozzo, S.; et al. Lipoprotein(a) and lipoprotein profile in healthy centenarians: A reappraisal of vascular risk factors. FASEB J. 1998, 12, 433–437. [Google Scholar] [CrossRef] [PubMed]
- Allain, C.C.; Poon, L.S.; Chan, C.S.; Richmond, W.; Fu, P.C. Enzymatic determination of total serum cholesterol. Clin. Chem. 1974, 20, 470–475. [Google Scholar] [CrossRef] [PubMed]
- Tietz, N.W. Clinical Guide to Laboratory Tests; W.B. Saunders, Co.: Philadelphia, PA, USA, 1995; p. 1096. [Google Scholar]
- O’Brien, J.E.; Brookes, M. Determination of reference values for a novel ketoamine-specific fructosamine assay for assessment of diabetic glycemic control. Diabetes Technol. Ther. 1999, 1, 447–455. [Google Scholar] [CrossRef]
- Pietta, P.G. Flavonoids as antioxidants. J. Nat. Prod. 2000, 63, 1035–1042. [Google Scholar] [CrossRef]
- Richard, D.; Kefi, K.; Barbe, U.; Bausero, P.; Visioli, F. Polyun saturated fatty acids as antioxidants. Pharmacol. Res. 2008, 57, 451–455. [Google Scholar] [CrossRef]
- Simopoulos, A.P. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed. Pharm. 2002, 56, 365–379. [Google Scholar] [CrossRef] [PubMed]
- Rossini, A.A.; Like, A.A.; Chick, W.L.; Appel, M.C.; Cahill, G.F., Jr. Studies of streptozotocin-induced insulitis and diabetes. Proc. Natl. Acad. Sci. USA 1977, 74, 2485–2489. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Magliano, D.J.; Zimmet, P.Z. The worldwide epidemiology of type 2 diabetes mellitus—Present and future perspectives. Nat. Rev. Endocrinol. 2012, 8, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Willett, W.C.; Koplanet, J.P.; Nugent, R.; Dusenbury, C.; Puska, P.; Gaziano, T.A. Prevention of Chronic Disease By means of Diet and Lifestyle Changes. In Disease Control Priorities in Developing Countries, 2nd ed.; World Bank: Washington, DC, USA, 2006. [Google Scholar]
- Vinayagam, R.; Xu, B. Antidiabetic properties of dietary flavonoids: A cellular mechanism review. Nutr. Metab. 2015, 12, 60. [Google Scholar] [CrossRef]
- Graf, B.A.; Milbury, P.E.; Blumberg, J.B. Flavonols, flavones, flavanones, and human health: Epidemiological evidence. J. Med. Food 2005, 8, 281–290. [Google Scholar] [CrossRef]
- Flores-Sanchez, I.J.; Verpoorte, R. Secondary metabolism in cannabis. Phytochem. Rev. 2008, 7, 615–639. [Google Scholar] [CrossRef]
- Girgih, A.T.; Alashi, A.M.; He, R.; Malomo, S.A.; Raj, P.; Netticadan, T.; Aluko, R.E. A novel hemp seed meal protein hydrolysate reduces oxidative stress factors in spontaneously hypertensive rats. Nutrients 2014, 6, 5652–5666. [Google Scholar] [CrossRef]
- Peungvicha, P.; Temsiririrkkul, R.; Prasain, J.K.; Tezuka, Y.; Kadota, S.; Thirawarapan, S.S.; Watanabe, H. 4-Hydroxybenzoic acid: A hypoglycemic constituent of aqueous extract of Pandanus odorus root. J. Ethnopharmacol. 1998, 62, 79–84. [Google Scholar] [CrossRef]
- Giovino, A.; Marino, P.; Domina, G.; Rapisarda, P.; Rizza, G.; Saia, S. Fatty acid composition of the seed lipids of Chamaerops humilis L. natural populations and its relation with the environment. Plant Biosyst. 2015, 149, 767–776. [Google Scholar] [CrossRef]
- Benatti, P.; Peluso, G.; Nicolai, R.; Calvani, M. Polyunsaturated fatty acids: Biochemical, nutritional and epigenetic properties. J. Am. Coll. Nutr. 2004, 23, 281–302. [Google Scholar] [CrossRef]
- Michalak, A.; Mosińska, P.; Fichna, J. Polyunsaturated fatty acids and their derivatives: Therapeutic value for inflammatory, functional gastrointestinal disorders, and colorectal cancer. Front. Pharmacol. 2016, 7, 459. [Google Scholar] [CrossRef]
- Schroeder, H.W., Jr.; Cavacini, L. Structure and function of immunoglobulins. J. Allergy Clin. Immunol. 2010, 125, S41–S52. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Yoo, T.H.; Lee, S.H.; Kang, H.Y.; Nam, B.Y.; Kwak, S.J.; Kim, J.K.; Park, J.T.; Han, S.J.; Kang, S.W. Gamma linolenic acid exerts anti-inflammatory and anti-fibrotic effects in diabetic nephropathy. Yonsei Med. J. 2012, 536, 1165–1175. [Google Scholar] [CrossRef] [PubMed]
- Lenzen, S. The mechanisms of alloxan-and streptozotocin-induced diabetes. Diabetologia 2008, 51, 216–226. [Google Scholar] [CrossRef]
- Navale, A.M.; Paranjape, A.N. Glucose transporters: Physiological and pathological roles. Biophys. Rev. 2016, 8, 5–9. [Google Scholar] [CrossRef]
- Verma, G.K.; Yadav, Y.S.; Yadav, R.K.; Sharma, I.K.; Bharat, K.; Yadav, K.K. Study of lipid profile levels in malnourished and healthy children: A case control study. Pediatr. Rev. Int. J. Pediatr. Res. 2018, 5, 156–161. [Google Scholar] [CrossRef]
- Aloud, A.A.; Chinnadurai, V.; Govindasamy, C.; Alsaif, M.A.; Al-Numair, K.S. Galangin, a dietary flavonoid, amelio-rates hyperglycaemia and lipid abnormalities in rats with streptozotocin-induced hyperglycaemia. Pharm. Biol. 2018, 56, 302–308. [Google Scholar] [CrossRef]
- Khadke, S.; Mandave, P.; Kuvalekar, A.; Pandit, V.; Karandikar, M.; Mantri, N. Synergistic effect of omega-3 fatty acids and oral-hypoglycemic drug on lipid normalization through modulation of hepatic gene expression in high fat diet with low streptozotocin-induced diabetic rats. Nutrients 2020, 12, 3652. [Google Scholar] [CrossRef]
- Kaushal, N.; Gupta, M.; Kulshreshtha, E. Hempseed (Cannabis sativa) lipid fractions alleviate high-fat diet-induced fatty liver disease through regulation of inflammation and oxidative stress. Heliyon 2020, 6, e04422. [Google Scholar] [CrossRef]
- Testa, R.; Bonfigli, A.R.; Genovese, S.; De Nigris, V.; Ceriello, A. The possible role of flavonoids in the prevention of diabetic complications. Nutrients 2016, 8, 310. [Google Scholar] [CrossRef]
- Andallu, B.; Kumar, A.V.; Varadacharyulu, N.C. Lipid abnormalities in streptozotocin-diabetes: Amelioration by Morus indica L. cv Suguna leaves. Int. J. Diabetes Dev. Ctries. 2009, 29, 123. [Google Scholar] [CrossRef] [PubMed]
- Monnier, L.; Colette, C.; Percheron, C.; Descomps, B. Insulin, diabetes and cholesterol metabolism. C R Seances Soc. Biol. Fil. 1995, 189, 919–931. [Google Scholar] [PubMed]
- Alves-Bezerra, M.; Cohen, D.E. Triglyceride metabolism in the liver. Comprehensive Physiology 2017, 8, 1. [Google Scholar] [CrossRef] [PubMed]
- Adeyemi, W.J.; Olayaki, L.A. Calcitonin and omega–3 fatty acids exhibit antagonistic and non-additive effects in ex-perimental diabetes. Pathophysiology 2018, 25, 117–123. [Google Scholar] [CrossRef]
- Safhi, M.M.; Anwer, T.; Khan, G.; Siddiqui, R.; Sivakumar, S.M.; Alam, M.F. The combination of canagliflozin and omega-3 fatty acid ameliorates insulin resistance and cardiac biomarkers via modulation of inflammatory cytokines in type 2 diabetic rats. Korean, J. Physiol. Pharmacol. 2018, 22, 493–501. [Google Scholar] [CrossRef]
- Pan, M.; Cederbaum, A.I.; Zhang, Y.L.; Ginsberg, H.N.; Williams, K.J.; Fisher, E.A. Lipid peroxidation and oxidant stress regulate hepatic apolipoprotein B degradation and VLDL production. J. Clin. Investig. 2004, 113, 1277–1287. [Google Scholar] [CrossRef]
- Zuliani, G.; Galvani, M.; Leitersdorf, E.; Volpato, S.; Cavalieri, M.; Fellin, R. The role of polyunsaturated fatty acids (PUFA) in the treatment of dyslipidemias. Curr. Pharm. Des. 2009, 15, 4087–4093. [Google Scholar] [CrossRef]
- Yoshikawa, T.; Shimano, H.; Yahagi, N.; Ide, T.; Amemiya-Kudo, M.; Matsuzaka, T.; Nakakuki, M.; Tomita, S.; Okazaki, H.; Tamura, Y.; et al. Poly-unsaturated fatty acids suppress sterol regulatory element-binding protein 1c promoter activity by inhibition of liver X receptor (LXR) binding to LXR response elements. J. Biol. Chem. 2002, 277, 1705–1711. [Google Scholar] [CrossRef]
- Chen, W.; Chen, G.; Head, D.L.; Mangelsdorf, D.J.; Russell, D.W. Enzymatic reduction of oxysterols impairs LXR sig-naling in cultured cells and the livers of mice. Cell Metab. 2007, 5, 73–79. [Google Scholar] [CrossRef]
- Janowski, B.A.; Willy, P.J.; Devi, T.R.; Falck, J.R.; Mangelsdorf, D.J. An oxysterol signalling pathway mediated by the nuclear receptor LXRα. Nature 1996, 383, 728–731. [Google Scholar] [CrossRef]
- Akbarzadeh, A.; Norouzian, D.; Mehrabi, M.R.; Jamshidi, S.H.; Farhangi, A.; Verdi, A.A.; Mofidian, S.M.A.; Rad, B.L. Induction of diabetes by streptozotocin in rats. Indian J. Clin. Biochem. 2007, 22, 60–64. [Google Scholar] [CrossRef] [PubMed]
- Al-Ishaq, R.K.; Abotaleb, M.; Kubatka, P.; Kajo, K.; Büsselberg, D. Flavonoids and their anti-diabetic effects: Cellular mechanisms and effects to improve blood sugar levels. Biomolecules 2019, 9, 430. [Google Scholar] [CrossRef] [PubMed]
- Charles, C.; Chemais, M.; Stévigny, C.; Dubois, J.; Nachergael, A.; Duez, P. Measurement of the influence of flavonoids on DNA repair kinetics using the comet assay. Food Chem. 2012, 135, 2974–2981. [Google Scholar] [CrossRef] [PubMed]
- Costabile, A.; Corona, G.; Sarnsamak, K.; Atar-Zwillenberg, D.; Yit, C.; King, A.J.; Vauzour, D.; Barone, M.; Turroni, S.; Brigidi, P.; et al. Wholegrain fermentation affects gut microbiota composition, phenolic acid metabolism and pancreatic beta cell function in a rodent model of type 2 diabetes. Front. Microbiol. 2022, 13, 1004679. [Google Scholar] [CrossRef]
- Goodrich, A.D.; Ersek, A.; Varain, N.M.; Groza, D.; Cenariu, M.; Thain, D.S.; Almeida-Porada, G.; Porada, C.D.; Zanjani, E.D. In vivo generation of beta-cell-like cells from CD34(+) cells differentiated from human embryonic stem cells. Exp. Hematol. 2010, 38, 516–525.e4. [Google Scholar] [CrossRef]
- Neelofar, K.; Ahmad, J. A comparative analysis of fructosamine with other risk factors for kidney dysfunction in dia-betic patients with or without chronic kidney disease. Diabetes Metab. Syndr. 2019, 13, 240–244. [Google Scholar] [CrossRef]
- Pedrosa, W.; Diniz, M.D.F.H.S.; Barreto, S.M.; Vidigal, P.G. Establishing a blood fructosamine reference range for the Brazilian population based on data from ELSA–Brasil. Pract. Lab. Med. 2019, 13, e00111. [Google Scholar] [CrossRef]
- Baker, J.R.; Johnson, R.N.; Scott, D.J. Serum fructosamine concentrations in patients with type II (non-insulin-dependent) diabetes mellitus during changes in management. Br. Med. J. 1984, 288, 1484–1486. [Google Scholar] [CrossRef]
- Venos, E.; de Koning, L. Endocrine markers of diabetes and cardiovascular disease risk. In Endocrine Biomarkers; Hossein, S., Gregory, K., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 251–299. [Google Scholar] [CrossRef]
- Yoshimura, E.H.; Santos, N.W.; Machado, E.; Agustinho, B.C.; Pereira, L.M.; de Aguiar, S.C.; Sá-Nakanishi, A.B.; Mareze-da-Costa, C.E.; Zeoula, L.M. Functionality of cow milk naturally enriched with polyunsaturated fatty acids and polyphenols in diets for diabetic rats. PLoS ONE 2018, 13, e0195839. [Google Scholar] [CrossRef]
- Iggman, D.; Ärnlöv, J.; Vessby, B.; Cederholm, T.; Sjögren, P.; Risérus, U. Adipose tissue fatty acids and insulin sensitivity in elderly men. Diabetologia 2010, 53, 850–857. [Google Scholar] [CrossRef]
- Khamaisi, M.; Rudich, A.; Beeri, I.; Pessler, D.; Friger, M.; Gavrilov, V.; Tritschler, H.; Bashan, N. Metabolic Effects of γ-Linolenic Acid–α-Lipoic Acid Conjugate in Streptozotocin Diabetic Rats. Antioxid. Redox Signal. 1999, 1, 523–535. [Google Scholar] [CrossRef] [PubMed]
- Krachler, B.; Norberg, M.; Eriksson, J.W.; Hallmans, G.; Johansson, I.; Vessby, B.; Weinehall, L.; Lindahl, B. Fatty acid profile of the erythrocyte membrane preceding development of Type 2 diabetes mellitus. Nutr. Metab. Car-Diovascular Dis. 2008, 18, 503–510. [Google Scholar] [CrossRef] [PubMed]


| Analyses | Dry Matter (%) | Lipid Content (%) | Protein Content (%) | Ash Content (%) |
|---|---|---|---|---|
| Mean ± SD | 94.57 ± 0.16 | 29.17 ± 0.46 | 20.95 ± 0.21 | 4.61 ± 0.33 |
| Type of Analyses | Total Phenols | Total Flavonoids |
|---|---|---|
| UM | 2.75 ± 0.12 mg GAE/g | 106.21 ± 5.92 μg CE/g |
| Samples | uM Trolox/100 g (Mean ± SD) |
|---|---|
| Methanol extract | 112.28 ± 5.61 μmol Trolox/100 g sample |
| Ethanolic extract | 97.09 ± 4.85 μmol Trolox/100 g sample |
| Peak | Rt (min) | UV λmax (nm) | [M + H]+ (m/z) | Phenolic Compound | Subclass | Hemp Seeds Methanol (Mean ± SD) | Hemp Seeds Ethanol (Mean ± SD) |
|---|---|---|---|---|---|---|---|
| 1 | 2.78 | 270 | 123 | Benzoic acid | Benzoic acid | 93.78 ± 9.40 | 116.08 ± 11.20 |
| 2 | 2.97 | 270 | 139 | 2-Hydroxybenzoic acid | Hydroxybenzoic acid | 210.06 ± 10.20 | 271.33 ± 13.57 |
| 3 | 9.47 | 280 | 155 | Protocatechuic acid | Hydroxybenzoic acid | 294.23 ± 14.56 | 152.37 ± 7.60 |
| 4 | 10.87 | 290 | 595 | Cannabisin A | Lignanamide | 82.68 ± 7.34 | 56.11 ± 4.80 |
| 5 | 11.48 | 290 | 597 | Cannabisin B | Lignanamide | 130.05 ± 10.07 | 149.54 ± 12.46 |
| 6 | 11.78 | 320,290 | 284 | N-trans-Coumaroyltyramine | Hydroxycinnamic acid amide | 196.91 ± 9.85 | 172.56 ± 8.63 |
| 7 | 14.01 | 290 | 611 | Cannabisin C | Lignanamide | 179.65 ± 8.98 | 203.55 ± 10.15 |
| 8 | 14.62 | 290 | 597 | Cannabisin B isomer | Lignanamide | 153.96 ± 7.50 | 146.88 ± 7.23 |
| 9 | 18.20 | 322,290 | 301 | N-trans-Caffeoyltyramine | Hydroxycinnamic acid amide | 339.04 ± 16.95 | 181.42 ± 9.07 |
| 10 | 19.29 | 340,290 | 597 | Cannabisin Q | Lignanamide | 201.34 ± 10.03 | 171.67 ± 8.85 |
| 11 | 20.40 | 330,290 | 314 | N-Feruloyltyramine | Hydroxycinnamic acid amide | 278.83 ± 11.15 | 90.65 ± 8.53 |
| 12 | 22.09 | 290 | 625 | Cannabisin D | Lignanamide | 444.42 ± 17.71 | 119.87 ± 10.20 |
| 13 | 22.79 | 290 | 625 | Cannabisin F | Lignanamide | 470.99 ± 23.50 | 231.01 ± 11.55 |
| 14 | 23.39 | 320,290 | 625 | Grossamide | Lignanamide | 830.08 ± 41.50 | 421.84 ± 21.09 |
| Total phenolics | 3906.02 | 2484.88 |
| Specific Fatty Acids | Concentration (% by Area) | Standard Deviation (SD) |
|---|---|---|
| 16:0 | 7.84 | 0.27 |
| 18:0 | 3.68 | 0.15 |
| 18:1n-9 | 15.73 | 0.66 |
| 18:1n-7 | 0.71 | 0.05 |
| 18:2n-6 | 55.93 | 2.49 |
| 18:3n-6 | 1.73 | 0.08 |
| 18:3n-3 | 13.06 | 0.58 |
| 20:0 | 0.97 | 0.06 |
| 20:1n-9 | 0.35 | 0.03 |
| Fatty Acid Type | Concentration (% by Area) | Standard Deviation (SD) |
|---|---|---|
| SFA | 12.49 | 0.61 |
| MUFA | 16.78 | 0.69 |
| PUFA | 70.72 | 2.97 |
| n-3 PUFA | 13.06 | 0.59 |
| n-6 PUFA | 57.66 | 2.57 |
| n-6/n-3 | 4.41 | |
| PUFAs/SFAs | 5.66 |
| Parameters | CG | DNFG | DHFG |
|---|---|---|---|
| Glycemia (mg/dL) | 112 ± 11.9 | 422.3 ± 120.2 | 398.8 ± 98.9 |
| Weight (g) | 254.9 ± 17.9 | 246.8 ± 9.2 | 249.3 ± 17.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Munteanu, C.; Mihai, M.; Dulf, F.; Ona, A.; Muntean, L.; Ranga, F.; Urdă, C.; Pop, D.; Mihaiescu, T.; Mârza, S.M.; et al. Biochemical Changes Induced by the Administration of Cannabis sativa Seeds in Diabetic Wistar Rats. Nutrients 2023, 15, 2944. https://doi.org/10.3390/nu15132944
Munteanu C, Mihai M, Dulf F, Ona A, Muntean L, Ranga F, Urdă C, Pop D, Mihaiescu T, Mârza SM, et al. Biochemical Changes Induced by the Administration of Cannabis sativa Seeds in Diabetic Wistar Rats. Nutrients. 2023; 15(13):2944. https://doi.org/10.3390/nu15132944
Chicago/Turabian StyleMunteanu, Camelia, Mihaela Mihai, Francisc Dulf, Andreea Ona, Leon Muntean, Floricuța Ranga, Camelia Urdă, Daria Pop, Tania Mihaiescu, Sorin Marian Mârza, and et al. 2023. "Biochemical Changes Induced by the Administration of Cannabis sativa Seeds in Diabetic Wistar Rats" Nutrients 15, no. 13: 2944. https://doi.org/10.3390/nu15132944
APA StyleMunteanu, C., Mihai, M., Dulf, F., Ona, A., Muntean, L., Ranga, F., Urdă, C., Pop, D., Mihaiescu, T., Mârza, S. M., & Papuc, I. (2023). Biochemical Changes Induced by the Administration of Cannabis sativa Seeds in Diabetic Wistar Rats. Nutrients, 15(13), 2944. https://doi.org/10.3390/nu15132944










