Negative Association between Serum Vitamin D Levels and Depression in a Young Adult US Population: A Cross-Sectional Study of NHANES 2007–2018 †
Abstract
1. Introduction
2. Materials and Methods
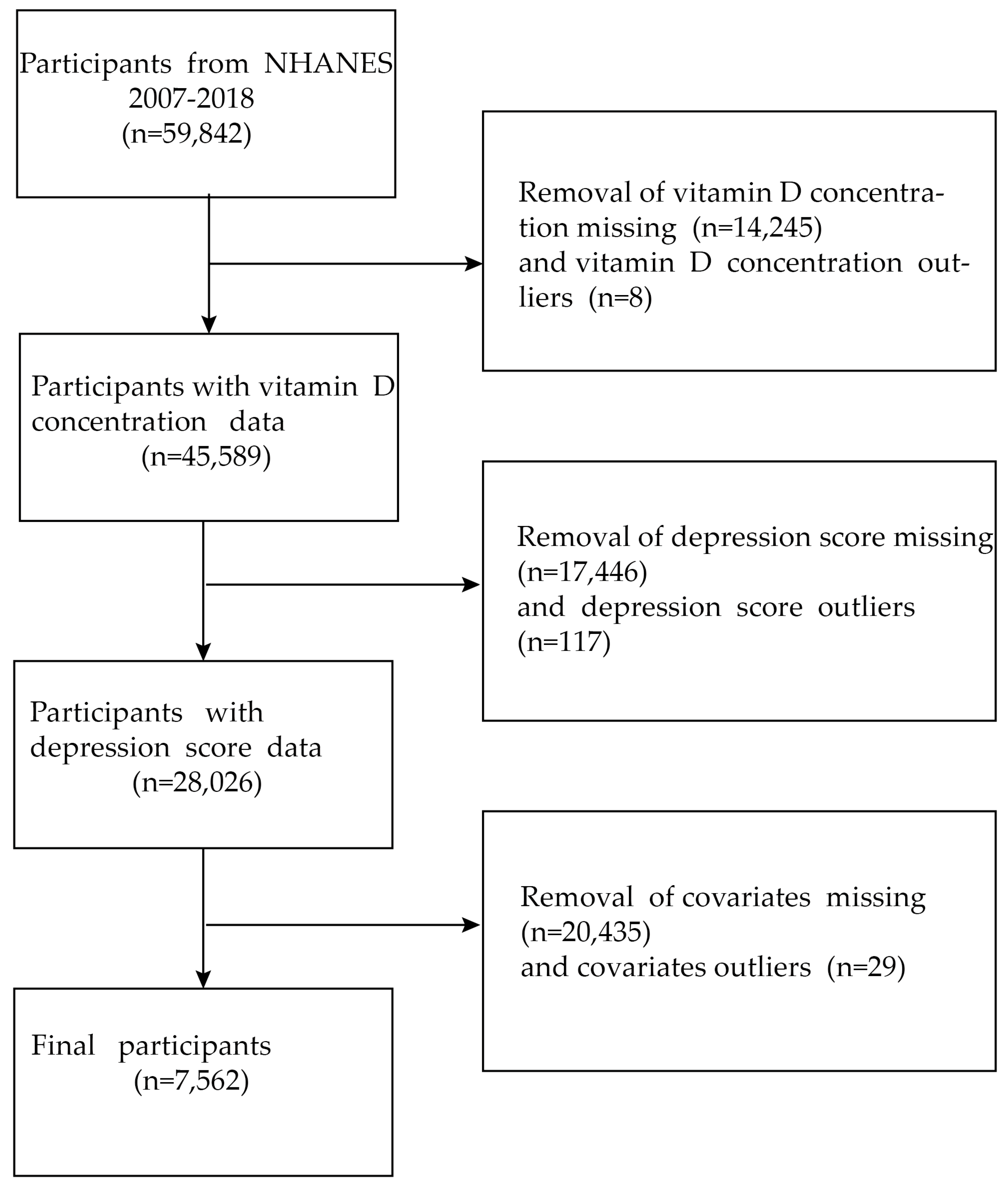
2.1. Data Sources and Study Population
2.2. Determination of Serum Vitamin D Concentration
2.3. Assessment of Depressive Symptoms
2.4. Covariates
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics of the Study Populations
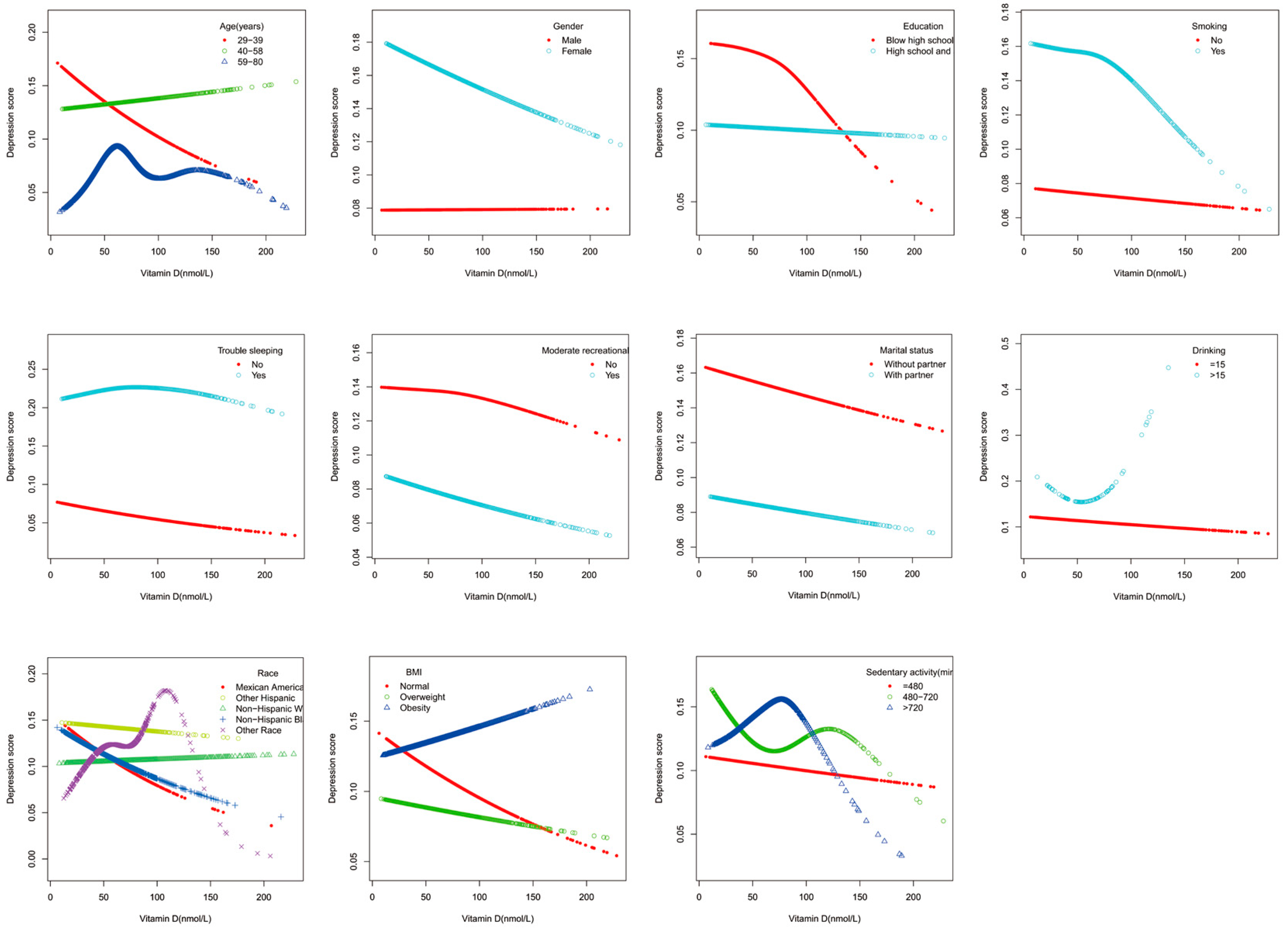
3.2. Association between Serum Vitamin D Concentrations and Depressive Symptoms
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pearce, M.; Garcia, L.; Abbas, A.; Strain, T.; Schuch, F.B.; Golubic, R.; Kelly, P.; Khan, S.; Utukuri, M.; Laird, Y.; et al. Association Between Physical Activity and Risk of Depression: A Systematic Review and Meta-analysis. JAMA Psychiatry 2022, 79, 550–559. [Google Scholar] [CrossRef] [PubMed]
- Anglin, R.E.; Samaan, Z.; Walter, S.D.; McDonald, S.D. Vitamin D deficiency and depression in adults: Systematic review and meta-analysis. Br. J. Psychiatry J. Ment. Sci. 2013, 202, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Singhal, G.; Baune, B.T. Microglia: An Interface between the Loss of Neuroplasticity and Depression. Front. Cell. Neurosci. 2017, 11, 270. [Google Scholar] [CrossRef] [PubMed]
- Benasi, G.; Fava, G.A.; Guidi, J. Prodromal Symptoms in Depression: A Systematic Review. Psychother. Psychosom. 2021, 90, 365–372. [Google Scholar] [CrossRef]
- Bigman, G. Vitamin D metabolites, D(3) and D(2), and their independent associations with depression symptoms among adults in the United States. Nutr. Neurosci. 2022, 25, 648–656. [Google Scholar] [CrossRef] [PubMed]
- Boulkrane, M.S.; Fedotova, J.; Kolodyaznaya, V.; Micale, V.; Drago, F.; van den Tol, A.J.M.; Baranenko, D. Vitamin D and Depression in Women: A Mini-review. Curr. Neuropharmacol. 2020, 18, 288–300. [Google Scholar] [CrossRef]
- Ganji, V.; Milone, C.; Cody, M.M.; McCarty, F.; Wang, Y.T. Serum vitamin D concentrations are related to depression in young adult US population: The Third National Health and Nutrition Examination Survey. Int. Arch. Med. 2010, 3, 29. [Google Scholar] [CrossRef] [PubMed]
- Ferrillo, M.; Migliario, M.; Marotta, N.; Lippi, L.; Antonelli, A.; Calafiore, D.; Ammendolia, V.; Fortunato, L.; Renò, F.; Giudice, A.; et al. Oral Health in Breast Cancer Women with Vitamin D Deficiency: A Machine Learning Study. J. Clin. Med. 2022, 11, 4662. [Google Scholar] [CrossRef]
- Pludowski, P. COVID-19 and Other Pleiotropic Actions of Vitamin D: Proceedings from the Fifth International Conference “Vitamin D-Minimum, Maximum, Optimum” under the Auspices of the European Vitamin D Association (EVIDAS). Nutrients 2023, 15, 2530. [Google Scholar] [CrossRef]
- Iolascon, G.; Mauro, G.L.; Fiore, P.; Cisari, C.; Benedetti, M.G.; Panella, L.; De Sire, A.; Calafiore, D.; Moretti, A.; Gimigliano, F. Can vitamin D deficiency influence muscle performance in postmenopausal women? A multicenter retrospective study. Eur. J. Phys. Rehabil. Med. 2018, 54, 676–682. [Google Scholar] [CrossRef]
- Jorde, R.; Sneve, M.; Figenschau, Y.; Svartberg, J.; Waterloo, K. Effects of vitamin D supplementation on symptoms of depression in overweight and obese subjects: Randomized double blind trial. J. Intern. Med. 2008, 264, 599–609. [Google Scholar] [CrossRef] [PubMed]
- de Koning, E.J.; Lips, P.; Penninx, B.; Elders, P.J.M.; Heijboer, A.C.; den Heijer, M.; Bet, P.M.; van Marwijk, H.W.J.; van Schoor, N.M. Vitamin D supplementation for the prevention of depression and poor physical function in older persons: The D-Vitaal study, a randomized clinical trial. Am. J. Clin. Nutr. 2019, 110, 1119–1130. [Google Scholar] [CrossRef]
- Okereke, O.I.; Reynolds, C.F., 3rd; Mischoulon, D.; Chang, G.; Vyas, C.M.; Cook, N.R.; Weinberg, A.; Bubes, V.; Copeland, T.; Friedenberg, G.; et al. Effect of Long-term Vitamin D3 Supplementation vs Placebo on Risk of Depression or Clinically Relevant Depressive Symptoms and on Change in Mood Scores: A Randomized Clinical Trial. JAMA 2020, 324, 471–480. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Zhao, Y.; Fan, X.; Wang, J.; Lu, W.; Zheng, X. Independent Associations of Serum 25-hydroxyvitamin D(3) and D(2) with Depressive Symptoms in Females. J. Affect. Disord. 2022, 296, 342–349. [Google Scholar] [CrossRef] [PubMed]
- Curtin, L.R.; Mohadjer, L.K.; Dohrmann, S.M.; Montaquila, J.M.; Kruszan-Moran, D.; Mirel, L.B.; Carroll, M.D.; Hirsch, R.; Schober, S.; Johnson, C.L. The National Health and Nutrition Examination Survey: Sample Design, 1999–2006. Vital Health Stat. Ser. 2 Data Eval. Methods Res. 2012, 155, 1–39. [Google Scholar]
- Curtin, L.R.; Mohadjer, L.K.; Dohrmann, S.M.; Kruszon-Moran, D.; Mirel, L.B.; Carroll, M.D.; Hirsch, R.; Burt, V.L.; Johnson, C.L. National Health and Nutrition Examination Survey: Sample design, 2007–2010. Vital Health Stat. Ser. 2 Data Eval. Methods Res. 2013, 160, 1–23. [Google Scholar]
- Mirel, L.B.; Mohadjer, L.K.; Dohrmann, S.M.; Clark, J.; Burt, V.L.; Johnson, C.L.; Curtin, L.R. National Health and Nutrition Examination Survey: Estimation procedures, 2007–2010. Vital Health Stat. Ser. 2 Data Eval. Methods Res. 2013, 159, 1–17. [Google Scholar]
- Johnson, C.L.; Dohrmann, S.M.; Burt, V.L.; Mohadjer, L.K. National health and nutrition examination survey: Sample design, 2011–2014. Vital Health Stat. Ser. 2 Data Eval. Methods Res. 2014, 162, 1–33. [Google Scholar]
- Tuckey, R.C.; Cheng, C.Y.S.; Slominski, A.T. The serum vitamin D metabolome: What we know and what is still to discover. J. Steroid Biochem. Mol. Biol. 2019, 186, 4–21. [Google Scholar] [CrossRef]
- Al-Sabah, R.; Al-Taiar, A.; Shaban, L.; Albatineh, A.N.; Sharaf Alddin, R.; Durgampudi, P.K. Vitamin D level in relation to depression symptoms during adolescence. Child Adolesc. Psychiatry Ment. Health 2022, 16, 53. [Google Scholar] [CrossRef]
- Kroenke, K.; Spitzer, R.L.; Williams, J.B. The PHQ-9: Validity of a brief depression severity measure. J. Gen. Intern. Med. 2001, 16, 606–613. [Google Scholar] [CrossRef] [PubMed]
- Rhee, S.J.; Lee, H.; Ahn, Y.M. Serum Vitamin D Concentrations Are Associated with Depressive Symptoms in Men: The Sixth Korea National Health and Nutrition Examination Survey 2014. Front. Psychiatry 2020, 11, 756. [Google Scholar] [CrossRef] [PubMed]
- Hayley, S.; Hakim, A.M.; Albert, P.R. Depression, dementia and immune dysregulation. Brain A J. Neurol. 2021, 144, 746–760. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Gu, X.; Liu, Y.Y.; Yang, L.; Zheng, M.; Jiang, L. Association between dietary calcium and depression among American adults: National health and nutrition examination survey. Front. Nutr. 2023, 10, 1042522. [Google Scholar] [CrossRef]
- Annweiler, C.; Rastmanesh, R.; Richard-Devantoy, S.; Beauchet, O. The role of vitamin D in depression: From a curious idea to a therapeutic option. J. Clin. Psychiatry 2013, 74, 1121–1122. [Google Scholar] [CrossRef]
- Sahasrabudhe, N.; Lee, J.S.; Scott, T.M.; Punnett, L.; Tucker, K.L.; Palacios, N. Serum Vitamin D and Depressive Symptomatology among Boston-Area Puerto Ricans. J. Nutr. 2020, 150, 3231–3240. [Google Scholar] [CrossRef]
- Musazadeh, V.; Keramati, M.; Ghalichi, F.; Kavyani, Z.; Ghoreishi, Z.; Alras, K.A.; Albadawi, N.; Salem, A.; Albadawi, M.I.; Salem, R.; et al. Vitamin D protects against depression: Evidence from an umbrella meta-analysis on interventional and observational meta-analyses. Pharmacol. Res. 2023, 187, 106605. [Google Scholar] [CrossRef]
- Krivoy, A.; Onn, R.; Vilner, Y.; Hochman, E.; Weizman, S.; Paz, A.; Hess, S.; Sagy, R.; Kimhi-Nesher, S.; Kalter, E.; et al. Vitamin D Supplementation in Chronic Schizophrenia Patients Treated with Clozapine: A Randomized, Double-Blind, Placebo-controlled Clinical Trial. EBioMedicine 2017, 26, 138–145. [Google Scholar] [CrossRef]
- Zhu, C.; Zhang, Y.; Wang, T.; Lin, Y.; Yu, J.; Xia, Q.; Zhu, P.; Zhu, D.M. Vitamin D supplementation improves anxiety but not depression symptoms in patients with vitamin D deficiency. Brain Behav. 2020, 10, e01760. [Google Scholar] [CrossRef]
- Mason, C.; de Dieu Tapsoba, J.; Duggan, C.; Wang, C.Y.; Korde, L.; McTiernan, A. Repletion of vitamin D associated with deterioration of sleep quality among postmenopausal women. Prev. Med. 2016, 93, 166–170. [Google Scholar] [CrossRef]
- Kaviani, M.; Nikooyeh, B.; Etesam, F.; Behnagh, S.J.; Kangarani, H.M.; Arefi, M.; Yaghmaei, P.; Neyestani, T.R. Effects of vitamin D supplementation on depression and some selected pro-inflammatory biomarkers: A double-blind randomized clinical trial. BMC Psychiatry 2022, 22, 694. [Google Scholar] [CrossRef] [PubMed]
- Nagy, E.E.; Frigy, A.; Szász, J.A.; Horváth, E. Neuroinflammation and microglia/macrophage phenotype modulate the molecular background of post-stroke depression: A literature review. Exp. Ther. Med. 2020, 20, 2510–2523. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.; Li, Y.; Lan, T.; Wang, W.; Long, Y.; Yu, S.Y. Microglia secrete miR-146a-5p-containing exosomes to regulate neurogenesis in depression. Mol. Ther. J. Am. Soc. Gene Ther. 2022, 30, 1300–1314. [Google Scholar] [CrossRef] [PubMed]
- Bivona, G.; Gambino, C.M.; Lo Sasso, B.; Scazzone, C.; Giglio, R.V.; Agnello, L.; Ciaccio, M. Serum Vitamin D as a Biomarker in Autoimmune, Psychiatric and Neurodegenerative Diseases. Diagnostics 2022, 12, 130. [Google Scholar] [CrossRef] [PubMed]
- Djukic, M.; Onken, M.L.; Schütze, S.; Redlich, S.; Götz, A.; Hanisch, U.K.; Bertsch, T.; Ribes, S.; Hanenberg, A.; Schneider, S.; et al. Vitamin d deficiency reduces the immune response, phagocytosis rate, and intracellular killing rate of microglial cells. Infect. Immunity 2014, 82, 2585–2594. [Google Scholar] [CrossRef]
- Wang, H.; He, Y.; Sun, Z.; Ren, S.; Liu, M.; Wang, G.; Yang, J. Microglia in depression: An overview of microglia in the pathogenesis and treatment of depression. J. Neuroinflamm. 2022, 19, 132. [Google Scholar] [CrossRef]
- Cui, P.; Lu, W.; Wang, J.; Wang, F.; Zhang, X.; Hou, X.; Xu, F.; Liang, Y.; Chai, G.; Hao, J. Microglia/macrophages require vitamin D signaling to restrain neuroinflammation and brain injury in a murine ischemic stroke model. J. Neuroinflamm. 2023, 20, 63. [Google Scholar] [CrossRef]
- Wang, Y.; Zhong, W.; Zhao, A.; Szeto, I.M.; Lan, H.; Zhang, J.; Li, P.; Ren, Z.; Mao, S.; Jiang, H.; et al. Perinatal depression and serum vitamin D status: A cross-sectional study in urban China. J. Affect. Disord. 2023, 322, 214–220. [Google Scholar] [CrossRef]
- Duan, N.; Zhang, Y.; Tan, S.; Sun, J.; Ye, M.; Gao, H.; Pu, K.; Wu, M.; Wang, Q.; Zhai, Q. Therapeutic targeting of STING-TBK1-IRF3 signalling ameliorates chronic stress induced depression-like behaviours by modulating neuroinflammation and microglia phagocytosis. Neurobiol. Dis. 2022, 169, 105739. [Google Scholar] [CrossRef]
- Alessio, N.; Belardo, C.; Trotta, M.C.; Paino, S.; Boccella, S.; Gargano, F.; Pieretti, G.; Ricciardi, F.; Marabese, I.; Luongo, L.; et al. Vitamin D Deficiency Induces Chronic Pain and Microglial Phenotypic Changes in Mice. Int. J. Mol. Sci. 2021, 22, 3604. [Google Scholar] [CrossRef]
| Serum Vitamin D Groups | <30 | ≥30, <50 | ≥50, <125 | ≥125 | p Value |
|---|---|---|---|---|---|
| Age (years) | 43.21 ± 14.51 | 44.61 ± 15.60 | 47.86 ± 16.35 | 55.29 ± 14.50 | <0.0001 |
| Ratio of family income to poverty | 2.11 ± 1.46 | 2.50 ± 1.63 | 3.07 ± 1.63 | 3.74 ± 1.52 | <0.0001 |
| Energy (kcal) | 2288.62 ± 1188.03 | 2330.99 ± 1014.87 | 2309.18 ± 1013.64 | 2056.00 ± 864.00 | 0.0002 |
| Protein (gm) | 80.79 ± 48.61 | 85.01 ± 43.30 | 86.71 ± 42.63 | 81.01 ± 39.12 | 0.0070 |
| Total sugars (gm) | 111.66 ± 84.87 | 118.55 ± 82.18 | 115.88 ± 82.11 | 97.15 ± 66.11 | 0.0004 |
| Total polyunsaturated fatty acids (gm) | 18.23 ± 12.50 | 20.04 ± 12.70 | 20.23 ± 13.21 | 19.23 ± 12.43 | 0.0188 |
| Vitamin E (mg) | 7.22 ± 5.48 | 8.36 ± 5.98 | 9.10 ± 6.77 | 9.65 ± 6.20 | <0.0001 |
| Vitamin A (mcg) | 472.08 ± 700.59 | 531.69 ± 486.83 | 653.67 ± 664.54 | 658.86 ± 421.46 | <0.0001 |
| Vitamin B1 (mg) | 1.51 ± 0.97 | 1.60 ± 0.90 | 1.68 ± 0.93 | 1.49 ± 0.90 | <0.0001 |
| Vitamin B2 (mg) | 1.84 ± 1.27 | 2.14 ± 1.41 | 2.38 ± 1.40 | 2.22 ± 1.31 | <0.0001 |
| Vitamin B6 (mg) | 2.03 ± 1.59 | 2.18 ± 1.82 | 2.27 ± 1.69 | 2.16 ± 1.36 | 0.0236 |
| Total folate (mcg) | 369.93 ± 254.14 | 397.05 ± 254.41 | 417.56 ± 271.01 | 412.75 ± 253.15 | 0.0011 |
| Vitamin C (mg) | 67.45 ± 83.20 | 70.24 ± 87.64 | 73.61 ± 83.39 | 81.80 ± 79.89 | 0.0797 |
| Waist circumference (cm) | 103.21 ± 19.86 | 104.13 ± 18.41 | 99.95 ± 15.65 | 95.74 ± 14.40 | <0.0001 |
| Alcohol (gm) | 27.48 ± 58.12 | 20.57 ± 38.12 | 19.86 ± 37.06 | 23.23 ± 32.36 | 0.0011 |
| Gender | <0.0001 | ||||
| Male | 52.14 | 60.99 | 57.72 | 29.45 | |
| Female | 47.86 | 39.01 | 42.28 | 70.55 | |
| Race | <0.0001 | ||||
| Mexican American | 9.78 | 13.83 | 5.27 | 1.06 | |
| Other Hispanic | 6.07 | 6.23 | 4.01 | 1.76 | |
| Non-Hispanic White | 33.13 | 51.86 | 81.17 | 89.83 | |
| Non-Hispanic Black | 41.90 | 20.33 | 4.20 | 3.78 | |
| Other Race | 9.11 | 7.75 | 5.35 | 3.58 | |
| Education | <0.0001 | ||||
| Below high school | 24.02 | 20.78 | 14.10 | 10.84 | |
| High school and above | 75.98 | 79.22 | 85.90 | 89.16 | |
| Marital status | <0.0001 | ||||
| Without partner | 53.36 | 42.82 | 35.62 | 30.98 | |
| With partner | 46.64 | 57.18 | 64.38 | 69.02 | |
| BMI | <0.0001 | ||||
| Normal | 27.58 | 23.69 | 29.48 | 35.72 | |
| Overweight | 25.89 | 27.44 | 35.26 | 38.29 | |
| Obesity | 46.53 | 48.87 | 35.26 | 25.99 | |
| Moderate recreational activities | <0.0001 | ||||
| No | 71.60 | 62.73 | 51.30 | 42.45 | |
| Yes | 28.40 | 37.27 | 48.70 | 57.55 | |
| Sedentary activity (min) | <0.0001 | ||||
| ≤480 | 58.82 | 58.40 | 65.66 | 63.09 | |
| 480–720 | 30.81 | 31.74 | 25.84 | 29.23 | |
| >720 | 10.37 | 9.86 | 8.50 | 7.69 | |
| Trouble sleeping | 0.0263 | ||||
| No | 69.68 | 69.01 | 66.09 | 61.46 | |
| Yes | 30.32 | 30.99 | 33.91 | 38.54 | |
| Smoking | <0.0001 | ||||
| No | 30.55 | 43.64 | 58.41 | 74.94 | |
| Yes | 69.45 | 56.36 | 41.59 | 25.06 | |
| Depression score | 0.0016 | ||||
| <10 | 87.12 | 87.72 | 90.46 | 93.07 | |
| ≥10 | 12.88 | 12.28 | 9.54 | 6.93 | |
| Drinking | 0.0327 | ||||
| ≤15 | 97.93 | 98.78 | 99.11 | 99.93 | |
| >15 | 2.07 | 1.22 | 0.89 | 0.07 |
| Depression Score | <10 | ≥10 | p-Value |
|---|---|---|---|
| N | 6717 | 845 | |
| Age (years) | 49.65 ± 17.04 | 45.79 ± 15.07 | <0.001 |
| Gender | <0.001 | ||
| Male | 4186 (62.32%) | 359 (42.49%) | |
| Female | 2531 (37.68%) | 486 (57.51%) | |
| Race | 0.107 | ||
| Mexican American | 842 (12.54%) | 101 (11.95%) | |
| Other Hispanic | 557 (8.29%) | 92 (10.89%) | |
| Non-Hispanic White | 3460 (51.51%) | 411 (48.64%) | |
| Non-Hispanic Black | 1283 (19.10%) | 164 (19.41%) | |
| Other Race | 575 (8.56%) | 77 (9.11%) | |
| Education | <0.001 | ||
| Below high school | 1443 (21.48%) | 251 (29.70%) | |
| High school and above | 5274 (78.52%) | 594 (70.30%) | |
| Marital status | <0.001 | ||
| Without partner | 2625 (39.08%) | 475 (56.21%) | |
| With partner | 4092 (60.92%) | 370 (43.79%) | |
| The ratio of family income to poverty | 2.56 ± 1.63 | 1.68 ± 1.38 | <0.001 |
| Energy (kcal) | 2287.43 ± 1043.37 | 2169.70 ± 1050.51 | <0.001 |
| Protein (gm) | 85.88 ± 44.10 | 75.90 ± 45.61 | <0.001 |
| Total sugars (gm) | 115.09 ± 81.90 | 127.91 ± 94.98 | <0.001 |
| Total polyunsaturated fatty acids (gm) | 19.86 ± 13.11 | 17.99 ± 13.12 | <0.001 |
| Vitamin E (mg) | 8.64 ± 6.46 | 7.76 ± 6.61 | <0.001 |
| Vitamin A (mcg) | 605.53 ± 629.37 | 509.24 ± 506.97 | <0.001 |
| Vitamin B1 (mg) | 1.65 ± 0.93 | 1.48 ± 0.88 | <0.001 |
| Vitamin B2 (mg) | 2.22 ± 1.37 | 2.08 ± 1.73 | <0.001 |
| Vitamin B6 (mg) | 2.20 ± 1.62 | 1.99 ± 1.94 | <0.001 |
| Total folate (mcg) | 408.48 ± 259.37 | 363.64 ± 245.67 | <0.001 |
| Serum Vitamin D (nmol/L) | 66.07 ± 27.52 | 61.66 ± 26.10 | <0.001 |
| Vitamin C (mg) | 76.69 ± 88.53 | 66.79 ± 91.94 | <0.001 |
| Alcohol (gm) | 19.13 ± 36.98 | 16.03 ± 38.90 | <0.001 |
| Waist circumference (cm) | 100.40 ± 16.02 | 102.67 ± 18.74 | 0.002 |
| BMI | <0.001 | ||
| Normal | 1907 (28.39%) | 233 (27.57%) | |
| Overweight | 2338 (34.81%) | 220 (26.04%) | |
| Obesity | 2472 (36.80%) | 392 (46.39%) | |
| Moderate recreational activities | <0.001 | ||
| No | 3848 (57.29%) | 609 (72.07%) | |
| Yes | 2869 (42.71%) | 236 (27.93%) | |
| Sedentary activity (min) | 0.004 | ||
| ≤480 | 4531 (67.46%) | 524 (62.01%) | |
| 480–720 | 1655 (24.64%) | 235 (27.81%) | |
| >720 | 531 (7.91%) | 86 (10.18%) | |
| Trouble sleeping | <0.001 | ||
| No | 4909 (73.08%) | 325 (38.46%) | |
| Yes | 1808 (26.92%) | 520 (61.54%) | |
| Smoking | <0.001 | ||
| No | 3627 (54.00%) | 286 (33.85%) | |
| Yes | 3090 (46.00%) | 559 (66.15%) | |
| Drinking | 0.057 | ||
| ≤15 | 6647 (98.96%) | 830 (98.22%) | |
| >15 | 70 (1.04%) | 15 (1.78%) |
| Exposure | Non-Adjusted | Adjust I | Adjust II |
|---|---|---|---|
| OR (95% CI) p-Value | OR (95% CI) p-Value | OR (95% CI) p-Value | |
| Serum Vitamin D (nmol/L) | 0.99 (0.99, 1.00) <0.0001 | 0.99 (0.99, 1.00) 0.0002 | 1.00 (1.00, 1.00) 0.4140 |
| Male | 0.68 (0.46, 1.01) 0.0542 | 0.65 (0.42, 1.01) 0.0576 | 0.90 (0.56, 1.44) 0.6550 |
| Female | 0.46 (0.34, 0.64) <0.0001 | 0.57 (0.40, 0.82) 0.0026 | 0.95 (0.64, 1.42) 0.8022 |
| Age (29–39) | 0.52 (0.34, 0.81) 0.0041 | 0.44 (0.26, 0.74) 0.0018 | 0.54 (0.31, 0.95) 0.0316 |
| Age (40–58) | 0.79 (0.53, 1.18) 0.2550 | 0.68 (0.44, 1.06) 0.0863 | 1.16 (0.72, 1.89) 0.5422 |
| Age (59–80) | 0.71 (0.43, 1.18) 0.1911 | 0.75 (0.44, 1.28) 0.2846 | 1.13 (0.63, 2.03) 0.6842 |
| Mexican American | 0.39 (0.16, 0.94) 0.0351 | 0.41 (0.17, 0.99) 0.0477 | 0.46 (0.18, 1.19) 0.1094 |
| Other Hispanic | 0.78 (0.34, 1.80) 0.5565 | 0.63 (0.27, 1.49) 0.2942 | 0.78 (0.30, 2.06) 0.6220 |
| Non-Hispanic White | 0.51 (0.35, 0.75) 0.0007 | 0.56 (0.38, 0.84) 0.0043 | 1.23 (0.79, 1.92) 0.3524 |
| Non-Hispanic Black | 0.40 (0.21, 0.77) 0.0057 | 0.58 (0.29, 1.15) 0.1183 | 0.71 (0.33, 1.50) 0.3674 |
| Other Race | 1.28 (0.56, 2.93) 0.5605 | 1.28 (0.54, 3.05) 0.5797 | 1.56 (0.59, 4.12) 0.3674 |
| Vitamin D (nmol/L) group | |||
| <30 | 1.0 | 1.0 | 1.0 |
| ≥30, <50 | 0.87 (0.67, 1.14) 0.3176 | 0.95 (0.72, 1.25) 0.7106 | 1.04 (0.77, 1.40) 0.8006 |
| ≥50, <125 | 0.71 (0.56, 0.90) 0.0056 | 0.78 (0.60, 1.01) 0.0588 | 1.01 (0.76, 1.34) 0.9586 |
| ≥125 | 0.45 (0.25, 0.79) 0.0058 | 0.45 (0.25, 0.81) 0.0075 | 0.78 (0.42, 1.45) 0.4346 |
| Subgroup | N | OR (95% CI) | p Value | p (Interaction) |
|---|---|---|---|---|
| Gender | 0.6639 | |||
| Male | 4545 | 1.00 (0.99, 1.00) | 0.5797 | |
| Female | 3017 | 1.00 (0.99, 1.00) | 0.1662 | |
| Age (years) | 0.0335 * | |||
| 29–39 | 2507 | 0.99 (0.99, 1.00) | 0.0213 * | |
| 40–58 | 2521 | 1.00 (1.00, 1.01) | 0.3275 | |
| 59–80 | 2534 | 1.00 (1.00, 1.01) | 0.4810 | |
| Race | 0.3928 | |||
| Mexican American | 943 | 0.99 (0.98, 1.00) | 0.1488 | |
| Other Hispanic | 649 | 1.00 (0.99, 1.01) | 0.7461 | |
| Non-Hispanic White | 3871 | 1.00 (0.99, 1.00) | 0.7181 | |
| Non-Hispanic Black | 1447 | 0.99 (0.99, 1.00) | 0.1295 | |
| Other Race | 652 | 1.00 (0.99, 1.01) | 0.3996 | |
| BMI | 0.2811 | |||
| Normal | 2140 | 1.00 (0.99, 1.00) | 0.1487 | |
| Overweight | 2558 | 1.00 (0.99, 1.00) | 0.5226 | |
| Obesity | 2864 | 1.00 (1.00, 1.01) | 0.4757 | |
| Sedentary activity (min) | 0.8208 | |||
| ≤480 | 5055 | 1.00 (0.99, 1.00) | 0.3741 | |
| 480–720 | 1890 | 1.00 (0.99, 1.00) | 0.6005 | |
| >720 | 617 | 1.00 (0.99, 1.01) | 0.7524 | |
| Moderate recreational activities | 0.6966 | |||
| No | 4457 | 1.00 (1.00, 1.00) | 0.6090 | |
| Yes | 3105 | 1.00 (0.99, 1.00) | 0.4277 | |
| Education | 0.3304 | |||
| Below high school | 1694 | 1.00 (0.99, 1.00) | 0.1643 | |
| High school and above | 5868 | 1.00 (1.00, 1.00) | 0.6394 | |
| Marital status | 0.4464 | |||
| Without partner | 3100 | 1.00 (1.00, 1.00) | 0.9027 | |
| With partner | 4462 | 1.00 (0.99, 1.00) | 0.2687 | |
| Trouble sleeping | 0.0517 | |||
| No | 5234 | 0.99 (0.99, 1.00) | 0.0012 ** | |
| Yes | 2328 | 1.00 (0.99, 1.00) | 0.3067 | |
| Smoking | 0.6026 | |||
| No | 3913 | 1.00 (1.00, 1.01) | 0.7082 | |
| Yes | 3649 | 1.00 (1.00, 1.00) | 0.7144 | |
| Drinking | 0.4411 | |||
| ≤15 | 7477 | 1.00 (1.00, 1.00) | 0.1919 | |
| >15 | 85 | 1.01 (0.98, 1.04) | 0.5222 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, J.; Li, K. Negative Association between Serum Vitamin D Levels and Depression in a Young Adult US Population: A Cross-Sectional Study of NHANES 2007–2018. Nutrients 2023, 15, 2947. https://doi.org/10.3390/nu15132947
Ma J, Li K. Negative Association between Serum Vitamin D Levels and Depression in a Young Adult US Population: A Cross-Sectional Study of NHANES 2007–2018. Nutrients. 2023; 15(13):2947. https://doi.org/10.3390/nu15132947
Chicago/Turabian StyleMa, Jiwen, and Ka Li. 2023. "Negative Association between Serum Vitamin D Levels and Depression in a Young Adult US Population: A Cross-Sectional Study of NHANES 2007–2018" Nutrients 15, no. 13: 2947. https://doi.org/10.3390/nu15132947
APA StyleMa, J., & Li, K. (2023). Negative Association between Serum Vitamin D Levels and Depression in a Young Adult US Population: A Cross-Sectional Study of NHANES 2007–2018. Nutrients, 15(13), 2947. https://doi.org/10.3390/nu15132947







