Nutraceuticals Prepared with Specific Strains of Probiotics for Supplementing Gut Microbiota in Hosts Allergic to Certain Foods or Their Additives
Abstract
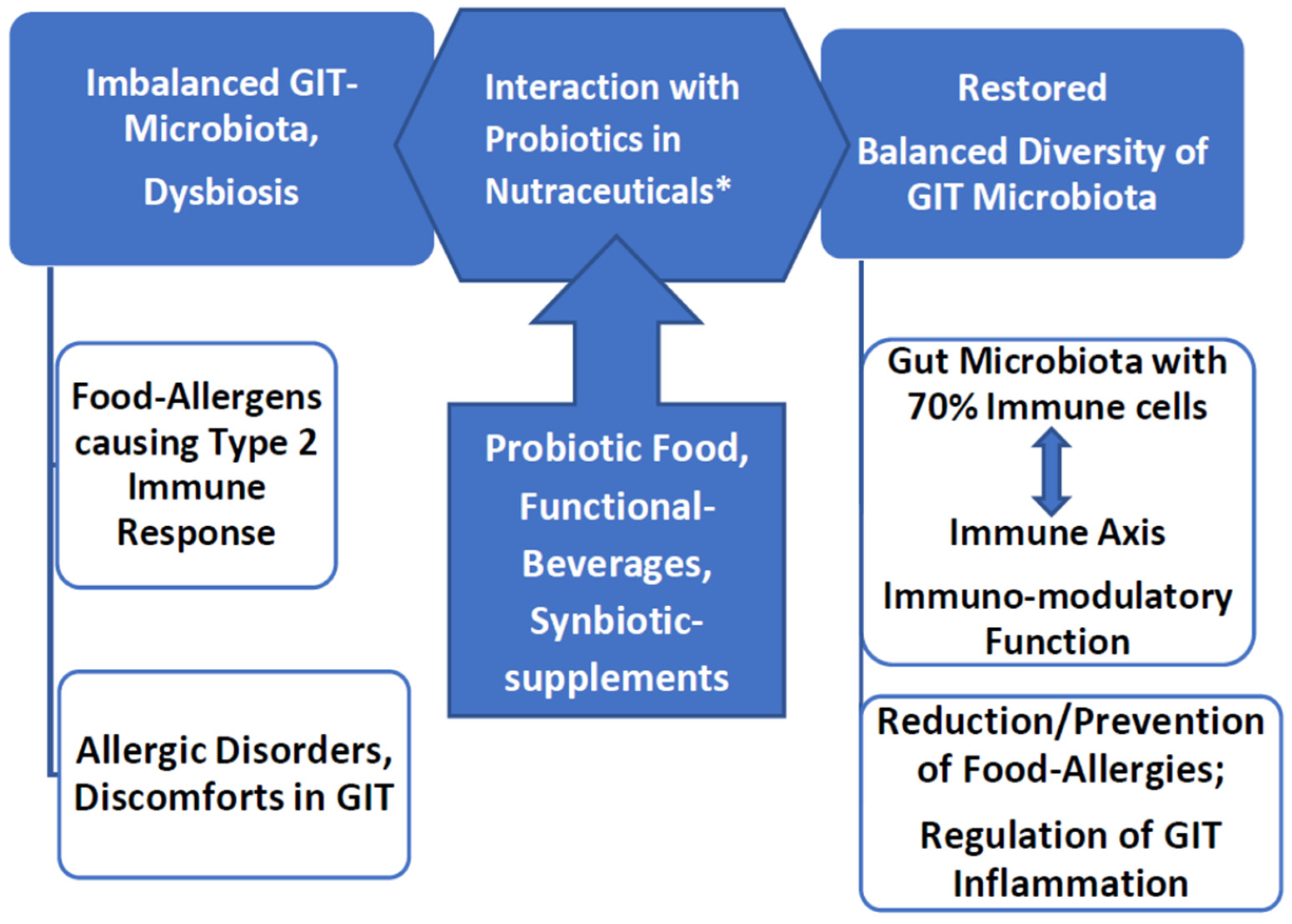
:1. Introduction
2. Foods Causing Intolerance Reactions
2.1. Symptoms of Allergies and Discomforts Caused by Food
2.2. Imbalanced Gut Microbiota Cause of Food Discomforts and Allergies
3. Role of Probiotics in Restoring Gut Health
3.1. Probiotic Strains as Biotherapeutic Agents
3.2. Specific Probiotic Strains Used to Prepare Functional Food
4. Probiotic Nutraceutical Products
Lactose-Free Products for Dairy-Allergic Population
5. Intervention of Probiotics for Normalizing Food Discomforts
6. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Warren, C.M.; Jiang, J.; Gupta, R.S. Epidemiology and burden of food allergy. Curr. Allergy Asthma Rep. 2020, 20, 6. [Google Scholar] [CrossRef] [PubMed]
- Leung, A.S.Y.; Wong, G.W.K.; Tang, M.L.K. Food allergy in the developing world. J. Allergy Clin. Immunol. 2018, 141, 76–78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brough, H.A.; Nadeau, K.C.; Sindher, S.B.; Alkotob, S.S.; Chan, S.; Bahnson, H.T.; Leung, D.Y.M.; Lack, G. Epicutaneous sensitization in the development of food allergy: What is the evidence and how can this be prevented? Allergy 2020, 75, 2185–2205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sicherer, S.H.; Sampson, H.A. Food allergy: A review and update on epidemiology, pathogenesis, diagnosis, prevention, and management. J. Allergy Clin. Immunol. 2018, 141, 41–58. [Google Scholar] [CrossRef] [Green Version]
- Tedner, S.G.; Asarnoj, A.; Thulin, H.; Westman, M.; Konradsen, J.R.; Nilsson, C. Food allergy and hypersensitivity reactions in children and adults—A review. J. Intern. Med. 2022, 291, 283–302. [Google Scholar] [CrossRef]
- Lee, A.J.; Thalayasingam, M.; Lee, B.W. Food allergy in Asia: How does it compare? Asia Pac. Allergy 2013, 3, 3–14. [Google Scholar] [CrossRef] [Green Version]
- Renz, H.; Allen, K.J.; Sicherer, S.H.; Sampson, H.A.; Lack, G.; Beyer, K.; Oettgen, H.C. Food allergy. Nat. Rev. Dis. Primers 2018, 4, 17098. [Google Scholar] [CrossRef]
- NHS. Allergies. Available online: https://www.nhs.uk/conditions/allergies/ (accessed on 6 February 2023).
- Sicherer, S.H.; Warren, C.M.; Dant, C.; Gupta, R.S.; Nadeau, K.C. Food allergy from infancy through adulthood. J. Allergy Clin. Immunol. Pract. 2020, 8, 1854–1864. [Google Scholar] [CrossRef]
- NHS. Food Allergies. Available online: https://www.nhsinform.scot/illnesses-and-conditions/nutritional/food-allergy (accessed on 6 February 2023).
- Konradsen, J.R.; Borres, M.P.; Nilsson, C. Unusual and unexpected allergic reactions can be unraveled by molecular allergy diagnostics. Int. Arch. Allergy Immunol. 2021, 182, 904–916. [Google Scholar] [CrossRef]
- Reyes-Pavon, D.; Jimenez, M.; Salinas, E. Physiopathology of food allergies. Rev. Alerg. Mex. 2020, 67, 34–53. [Google Scholar]
- Dahiya, D.; Nigam, P.S. Antibiotic-Therapy-Induced Gut Dysbiosis Affecting Gut Microbiota—Brain Axis and Cognition: Restoration by Intake of Probiotics and Synbiotics. Int. J. Mol. Sci. 2023, 24, 3074. [Google Scholar] [CrossRef]
- Nowak-Wegrzyn, A.; Sampson, H.A.; Sicherer, S.H. Food allergy and adverse reactions to foods. In Nelson Textbook of Pediatrics, 20th ed.; Kliegman, R.M., Stanton, B.F., St Geme, J.W., Schor, N.F., Eds.; Elsevier: Philadelphia, PA, USA, 2016; pp. 1137–1143. [Google Scholar]
- Yu, W.; Freeland, D.M.H.; Nadeau, K.C. Food allergy: Immune mechanisms, diagnosis and immunotherapy. Nat. Rev. Immunol. 2016, 16, 751–765. [Google Scholar] [CrossRef]
- Rutgers University. Beneficial Bacteria Can Be Restored to C-Section Babies at Birth. ScienceDaily 17 June 2021. Available online: www.sciencedaily.com/releases/2021/06/210617163655.htm (accessed on 6 February 2023).
- Burks, A.W.; Harthoorn, L.F.; Van Ampting, M.T.; Oude Nijhuis, M.M.; Langford, J.E.; Wopereis, H.; Goldberg, S.B.; Ong, P.Y.; Essink, B.J.; Scott, R.B.; et al. Synbiotics-supplemented amino acid-based formula supports adequate growth in cow’s milk allergic infants. Pediatr. Allergy Immunol. 2015, 26, 316–322. [Google Scholar] [CrossRef]
- Dissanayake, E.; Tani, Y.; Nagai, K.; Sahara, M.; Mitsuishi, C.; Togawa, Y.; Suzuki, Y.; Nakano, T.; Yamaide, F.; Ohno, H.; et al. Skincare and synbiotics for prevention of atopic dermatitis or food allergy in newborn infants: A 2 × 2 factorial, randomized, non-treatment controlled trial. Int. Arch. Allergy Immunol. 2019, 180, 202–211. [Google Scholar] [CrossRef]
- Hua, X.; Song, L.; Yu, G.; Vogtmann, E.; Goedert, J.J.; Abnet, C.C.; Landi, M.T.; Shi, J. MicrobiomeGWAS: A Tool for identi-fying Host Genetic Variants Associated with Microbiome Composition. Genes 2022, 13, 1224. [Google Scholar] [CrossRef]
- Patil, S.U.; Bunyavanich, S.; Berin, M.C. Emerging food allergy biomarkers. J. Allergy Clin. Immunol. Pract. 2020, 8, 2516–2524. [Google Scholar] [CrossRef]
- Zhao, W.; Ho, H.E.; Bunyavanich, S. The gut microbiome in food allergy. Ann. Allergy Asthma Immunol. 2019, 122, 276–282. [Google Scholar] [CrossRef] [Green Version]
- Ali, A.; Tan, H.; Kaiko, G.E. Role of the intestinal epithelium and its interaction with the microbiota in food allergy. Front. Immunol. 2020, 11, 604054. [Google Scholar] [CrossRef]
- Satitsuksanoa, P.; van de Veen, W.; Akdis, M. B cells in food allergy. J. Allergy Clin. Immunol. 2021, 147, 49–51. [Google Scholar] [CrossRef]
- Satitsuksanoa, P.; Daanje, M.; Akdis, M.; Boyd, S.D.; van de Veen, W. Biology and dynamics of B cells in the context of IgE-mediated food allergy. Allergy 2021, 76, 1707–1717. [Google Scholar] [CrossRef]
- Montalban-Arques, A.; De Schryver, P.; Bossier, P.; Gorkiewicz, G.; Mulero, V.; Gatlin, D.M., 3rd; Galindo-Villegas, J. Selective Manipulation of the Gut Microbiota Improves Immune Status in Vertebrates. Front. Immunol. 2015, 6, 512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marco, M.L.; Sanders, M.E.; Gänzle, M.; Arrieta, M.C.; Cotter, P.D.; De Vuyst, L.; Hill, C.; Holzapfel, W.; Lebeer, S.; Merenstein, D.; et al. The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on fermented foods Nature Reviews. Gastroenterol. Hepatol. 2021, 18, 196–208. [Google Scholar] [CrossRef]
- Deng, Y.; Misselwitz, B.; Dai, N.; Fox, M. Lactose intolerance in adults: Biological mechanism and dietary management. Nutrients 2015, 7, 8020–8035. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cormier, H.; Thifault, É.; Garneau, V.; Tremblay, A.; Drapeau, V.; Pérusse, L.; Vohl, M.-C. Association between yogurt consumption, dietary patterns, and cardio-metabolic risk factors. Eur. J. Nutr. 2016, 55, 577–587. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Givens, D.I.; Astrup, A.; Bakker, S.J.L.; Goossens, G.H.; Kratz, M.; Marette, A.; Pijl, H.; Soedamah-Muthu, S.S. The impact of dairy products in the development of type 2 diabetes: Where does the evidence stand in 2019? Adv. Nutr. 2019, 10, 1066–1075. [Google Scholar] [CrossRef] [Green Version]
- Fernandez, M.A.; Panahi, S.; Daniel, N.; Tremblay, A.; Marette, A. Yogurt and cardiometabolic diseases: A critical review of potential mechanisms. Adv. Nutr. 2017, 8, 812–829. [Google Scholar] [CrossRef] [Green Version]
- Pražnikar, Z.J.; Kenig, S.; Vardjan, T.; Bizjak, M.; Petelin, A. Effects of kefir or milk supplementation on zonulin in overweight subjects. J. Dairy. Sci. 2020, 103, 3961–3970. [Google Scholar] [CrossRef]
- Kim, H.-Y.; Park, K.-Y. Clinical trials of kimchi intakes on the regulation of metabolic parameters and colon health in healthy Korean young adults. J. Funct. Foods 2018, 47, 325–333. [Google Scholar] [CrossRef]
- Nielsen, E.S.; Garnås, E.; Jensen, K.J.; Hansen, L.H.; Olsen, P.S.; Ritz, C.; Krych, L.; Nielsen, D.S. Lacto-fermented sauerkraut improves symptoms in IBS patients independent of product pasteurisation—A pilot study. Food Funct. 2018, 9, 5323–5335. [Google Scholar] [CrossRef]
- Araki, R.; Fujie, K.; Yuine, N.; Watabe, Y.; Maruo, K.; Suzuki, H.; Hashimoto, K. The possibility of suppression of increased postprandial blood glucose levels by gamma-polyglutamic acid-rich natto in the early phase after eating: A randomized crossover pilot study. Nutrients 2020, 12, 915. [Google Scholar] [CrossRef] [Green Version]
- Rizzello, C.G.; Portincasa, P.; Montemurro, M.; Di Palo, D.M.; Lorusso, M.P.; De Angelis, M.; Bonfrate, L.; Genot, B.; Gobbetti, M. Sourdough fermented breads are more digestible than those started with baker’s yeast alone: An in vivo challenge dissecting distinct gastrointestinal response. Nutrients 2019, 11, 2954. [Google Scholar] [CrossRef] [Green Version]
- Nicklaus, S.; Divaret-Chauveau, A.; Chardon, M.-L.; Roduit, C.; Kaulek, V.; Ksiazek, E.; Dalphin, M.-L.; Karvonen, A.M.; Kirjavainen, P.; Pekkanen, J.; et al. The protective effect of cheese consumption at 18 months on allergic diseases in the first 6 years. Allergy 2019, 74, 788–798. [Google Scholar] [CrossRef]
- Ouwehand, A.C.; Nermes, M.; Collado, M.C.; Rautonen, N.; Salminen, S.; Isolauri, E. Specific probiotics alleviate allergic rhinitis during the birch pollen season. World J. Gastroenterol. 2009, 15, 3261–3268. [Google Scholar] [CrossRef]
- Tang, M.L.K.; Ponsonby, A.-L.; Orsini, F.; Tey, D.; Robinson, M.; Su, E.L.; Licciardi, P.; Burks, W.; Donath, S. Administration of a probiotic with peanut oral immunotherapy: A randomized trial. J. Allergy Clin. Immunol. 2015, 135, 737–744.e8. [Google Scholar] [CrossRef]
- Probiotics Database. Exploring the World’s Most Researched Probiotic Strains. Available online: https://www.optibacprobiotics.com/uk/professionals/probiotics-database (accessed on 8 February 2023).
- Ringel-Kulka, T.; Palsson, O.S.; Maier, D.; Carroll, I.; Galanko, J.A.; Leyer, G.; Ringel, Y. Probiotic bacteria Lactobacillus acidophilus NCFM and Bifidobacterium lactis Bi-07 versus placebo for the symptoms of bloating in patients with functional bowel disorders: A double-blind study. J. Clin. Gastroenterol. 2011, 45, 518–525. [Google Scholar] [CrossRef]
- Magro, D.O.; de Oliveira, L.M.; Bernasconi, I.; Ruela Mde, S.; Credidio, L.; Barcelos, I.K.; Leal, R.F.; Ayrizono Mde, L.; Fagundes, J.J.; Teixeira Lde, B.; et al. Effect of yogurt containing polydextrose, Lactobacillus acidophilus NCFM and Bifidobacterium lactis HN019: A randomized, double-blind, controlled study in chronic constipation. Nutr. J. 2014, 13, 75. [Google Scholar] [CrossRef] [Green Version]
- Kabeerdoss, J.; Devi, R.S.; Mary, R.R.; Prabhavathi, D.; Vidya, R.; Mechenro, J.; Mahendri, N.V.; Pugazhendhi, S.; Ramakrishna, B.S. Effect of yoghurt containing Bifidobacterium lactis Bb12® on faecal excretion of secretory immunoglobulin A and human beta-defensin 2 in healthy adult volunteers. Nutr. J. 2011, 10, 138. [Google Scholar] [CrossRef] [Green Version]
- Ibarra, A.; Latreille-Barbier, M.; Donazzolo, Y.; Pelletier, X.; Ouwehand, A.C. Effects of 28-day Bifidobacterium animalis subsp. lactis HN019 supplementation on colonic transit time and gastrointestinal symptoms in adults with functional constipation: A double-blind, randomized, placebo-controlled, and dose-ranging trial. Gut Microbes 2018, 9, 236–251. [Google Scholar] [CrossRef] [Green Version]
- Waller, P.A.; Gopal, P.K.; Leyer, G.J.; Ouwehand, A.C.; Reifer, C.; Stewart, M.E.; Miller, L.E. Dose-response effect of Bifidobacterium lactis HN019 on whole gut transit time and functional gastrointestinal symptoms in adults. Scand. J. Gastroenterol. 2011, 46, 1057–1064. [Google Scholar] [CrossRef] [Green Version]
- Tan-Lim, C.S.C.; Esteban-Ipac, N.A.R. Probiotics as treatment for food allergies among pediatric patients: A meta-analysis. World Allergy Organ. J. 2018, 11, 25. [Google Scholar] [CrossRef] [Green Version]
- Rizzardini, G.; Eskesen, D.; Calder, P.C.; Capetti, A.; Jespersen, L.; Clerici, M. Evaluation of the immune benefits of two probiotic strains Bifidobacterium animalis ssp. lactis, BB-12® and Lactobacillus paracasei ssp. paracasei, L. casei 431® in an influenza vaccination model: A randomised, double-blind, placebo-controlled study. Br. J. Nutr. 2012, 107, 876–884. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eskesen, D.; Jespersen, L.; Michelsen, B.; Whorwell, P.J.; Müller-Lissner, S.; Morberg, C.M. Effect of the probiotic strain Bifidobacterium animalis subsp. lactis, BB-12®, on defecation frequency in healthy subjects with low defecation frequency and abdominal discomfort: A randomised, double-blind, placebo-controlled, parallel-group trial. Br. J. Nutr. 2015, 114, 1638–1646. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Batatinha, H.; Tavares-Silva, E.; Leite, G.S.F.; Resende, A.S.; Albuquerque, J.A.T.; Arslanian, C.; Fock, R.A.; Lancha, A.H., Jr.; Lira, F.S.; Krüger, K.; et al. Probiotic supplementation in marathonists and its impact on lymphocyte population and function after a marathon: A randomized placebo-controlled double-blind study. Sci. Rep. 2020, 10, 18777. [Google Scholar] [CrossRef] [PubMed]
- Cazzola, M.; Pham-Thi, N.; Kerihuel, J.C.; Durand, H.; Bohbot, S. Efficacy of a synbiotic supplementation in the prevention of common winter diseases in children: A randomized, double-blind, placebo-controlled pilot study. Ther. Adv. Respir. Dis. 2010, 4, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Madempudi, R.S.; Ahire, J.J.; Neelamraju, J.; Tripathi, A.; Nanal, S. Randomized clinical trial: The effect of probiotic Bacillus coagulans Unique IS2 vs. placebo on the symptoms management of irritable bowel syndrome in adults. Sci. Rep. 2019, 9, 12210. [Google Scholar] [CrossRef] [Green Version]
- Berni Canani, R.; Di Costanzo, M.; Bedogni, G.; Amoroso, A.; Cosenza, L.; Di Scala, C.; Granata, V.; Nocerino, R. Extensively hydrolyzed casein formula containing lactobacillus rhamnosus GG reduces the occurrence of other allergic manifestations in children with cow’s milk allergy: 3-year randomized controlled trial. J. Allergy Clin. Immunol. 2017, 139, 1906–1913. [Google Scholar] [CrossRef] [Green Version]
- Ducrotté, P.; Sawant, P.; Jayanthi, V. Clinical trial: Lactobacillus plantarum 299v (DSM 9843) improves symptoms of irritable bowel syndrome. World J. Gastroenterol. 2012, 18, 4012–4018. [Google Scholar] [CrossRef]
- Das, S.; Gupta, P.K.; Das, R.R. Efficacy and Safety of Saccharomyces boulardii in Acute Rotavirus Diarrhea: Double Blind Ran-domized Controlled Trial from a Developing Country. J. Trop. Pediatr. 2016, 62, 464–470. [Google Scholar] [CrossRef] [Green Version]
- Tung, J.M.; Dolovich, L.R.; Lee, C.H. Prevention of Clostridium difficile infection with Saccharomyces boulardii: A systematic review. Can. J. Gastroenterol. 2009, 23, 817–821. [Google Scholar] [CrossRef] [Green Version]
- Moré, M.I.; Vandenplas, Y. Saccharomyces boulardii CNCM I-745 Improves Intestinal Enzyme Function: A Trophic Effects Review. Clin. Med. Insights Gastroenterol. 2018, 11, 1179552217752679. [Google Scholar] [CrossRef] [Green Version]
- Kajandar, K.; Myllyluoma, E.; Rajlic-Stojanovic, M.; Kyronpalo, S.S.; Rasmussen, M.; Jar-venpaa, S.S.; Zoetendal, E.G.; Vos, W.M.; de Vapaatalo, H.; Korpela, R. Clinical trial: Multi-species probiotic supplementation alleviates the symptoms of irritable bowel syndrome and stabilizes intestinal microbiota. Aliment. Pharmacol. Ther. 2008, 27, 48–57. [Google Scholar] [CrossRef]
- Omar, J.M.; Chan, Y.-M.; Jones, M.L.; Prakash, S.; Jones, P.J.H. Lactobacillus fermentum and Lactobacillus amylovorus as probiotics alter body adiposity and gut microflora in health persons. J. Funct. Foods 2013, 116–123. [Google Scholar] [CrossRef]
- Linwood, Natural Plant Goodness. Available online: https://linwoodshealthfoods.com/product/flaxseed-bio-cultures-vitamin-d/ (accessed on 8 February 2023).
- Scalabrin, D.M.F.; Harris, C.; Johnston, W.H.; Berseth, C.L. Long-term safety assessment in children who received hydrolyzed protein formulas with Lactobacillus rhamnosus GG: A 5-year follow-up. Eur. J. Pediatr. 2017, 176, 217–224. [Google Scholar] [CrossRef] [Green Version]
- Knol, E.F.; de Jong, N.W.; Ulfman, L.H.; Tiemessen, M.M. Management of cow’s milk allergy from an immunological perspective: What are the options? Nutrients 2019, 11, 2734. [Google Scholar] [CrossRef] [Green Version]
- Dahiya, D.; Nigam, P.S. Biotherapy Using Probiotics as Therapeutic Agents to Restore the Gut Microbiota to Relieve Gastrointestinal Tract Inflammation, IBD, IBS and Prevent Induction of Cancer. Int. J. Mol. Sci. 2023, 24, 5748. [Google Scholar] [CrossRef]
- Salinas, E.; Reyes-Pavón, D.; Cortes-Perez, N.G.; Torres-Maravilla, E.; Bitzer-Quintero, O.K.; Langella, P.; Bermúdez-Humarán, L.G. Bioactive Compounds in Food as a Current Therapeutic Approach to Maintain a Healthy Intestinal Epithelium. Microorganisms 2021, 9, 1634. [Google Scholar] [CrossRef]
- Craig, W.J.; Fresán, U. International analysis of the nutritional content and a review of health benefits of non-dairy plant-based beverages. Nutrients 2021, 13, 842. [Google Scholar] [CrossRef]
- Terpou, A.; Nigam, P.; Bosnea, L.; Kanellaki, M. Evaluation of Chios mastic gum as antimicrobial agent and matrix-forming material targeting probiotic cell encapsulation for functional fermented milk production. LWT 2018, 97, 109–116. [Google Scholar] [CrossRef]
- Dahiya, D.; Nigam, P.S. The Gut Microbiota Influenced by the Intake of Probiotics and Functional Foods with Prebiotics Can Sustain Wellness and Alleviate Certain Ailments like Gut-Inflammation and Colon-Cancer. Microorganisms 2022, 10, 665. [Google Scholar] [CrossRef]
- Panghal, A.; Janghu, S.; Virkar, K.; Gat, Y.; Kumar, V.; Chhikara, N. Potential Non-Dairy Probiotic Products-A Healthy Approach. Food Biosci. 2018, 21, 80–89. [Google Scholar] [CrossRef]
- Plamada, D.; Teleky, B.-E.; Nemes, S.A.; Mitrea, L.; Szabo, K.; Călinoiu, L.-F.; Pascuta, M.S.; Varvara, R.-A.; Ciont, C.; Martău, G.A.; et al. Plant-Based Dairy Alternatives—A Future Direction to the Milky Way. Foods 2023, 12, 1883. [Google Scholar] [CrossRef] [PubMed]
- Rasika, D.M.; Vidanarachchi, J.K.; Rocha, R.S.; Balthazar, C.F.; Cruz, A.G.; Sant’Ana, A.S.; Ranadheera, C.S. Plant-based milk substitutes as emerging probiotic carriers. Curr. Opin. Food Sci. 2021, 38, 8–20. [Google Scholar] [CrossRef]
- Grasso, N.; Alonso-Miravalles, L.; O’mahony, J.A. Composition, physicochemical and sensorial properties of commercial plant-based yogurts. Foods 2020, 9, 252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harper, A.R.; Dobson, R.C.J.; Morris, V.K.; Moggré, G. Fermentation of plant-based dairy alternatives by lactic acid bacteria. Microb. Biotechnol. 2022, 15, 1404–1421. [Google Scholar] [CrossRef] [PubMed]
- Dahiya, D.; Nigam, P.S. Probiotics, Prebiotics, Synbiotics, and Fermented Foods as potential biotics in Nutrition Improving Health via Microbiome-Gut-Brain Axis. Fermentation 2022, 8, 303. [Google Scholar] [CrossRef]
- Dahiya, D.; Nigam, P.S. Clinical Potential of Microbial Strains, Used in Fermentation for Probiotic Food, Beverages and in Synbiotic Supplements, as Psychobiotics for Cognitive Treatment through Gut-Brain Signaling. Microorganisms 2022, 10, 1687. [Google Scholar] [CrossRef]
- Biotiful Gut Health. Available online: https://biotifulguthealth.com/ (accessed on 10 February 2023).
- Lancashire Farm. Available online: https://lancashirefarm.com/ (accessed on 10 February 2023).
- Dahiya, D.; Nigam, P.S. Nutrition and Health through the Use of Probiotic Strains in Fermentation to Produce Non-Dairy Functional Beverage Products Supporting Gut Microbiota. Foods 2022, 11, 2760. [Google Scholar] [CrossRef]
- Dahiya, D.; Nigam, P. Use of Characterized Microorganisms in Fermentation of Non-Dairy-Based Substrates to Produce Probiotic Food for Gut-Health and Nutrition. Fermentation 2023, 9, 1. [Google Scholar] [CrossRef]
- Satwong, N.; Promsai, S. Feasibility study on co-culture Bacillus coagulans and Lacticaseibacillus rhamnosus formulated in probiotic-supplemented pigmented rice products. J. Food Proc. Preserv. 2022, 46, e16893. [Google Scholar] [CrossRef]
- Chumphon, T.; Sriprasertsak, P.; Promsai, S. Development of rice as potential carriers for probiotic Lactobacillus amylovorus. Int. J. Food Sci. Technol. 2016, 51, 1260–1267. [Google Scholar] [CrossRef]
- Williams, M.; Sharareh, H. Lactobacillus rhamnosus GR-1 in fermented rice pudding supplemented with short Chain Inulin, Long Chain Inulin, and Oat as a novel functional food. Fermentation. 2017, 3, 55. [Google Scholar] [CrossRef] [Green Version]
- Pino, A.; Nicosia, F.D.; Agolino, G.; Timpanaro, N.; Barbagallo, I.; Ronsisvalle, S.; Caggia, C.; Randazzo, C.L. Formulation of germinated brown rice fermented products functionalized by probiotics. Innov. Food Sci. Emerg. Technol. 2022, 80, 103076. [Google Scholar] [CrossRef]
- Güney, D.; Güngörmüşler, M. Development and Comparative Evaluation of a Novel Fermented Juice Mixture with Probiotic Strains of Lactic Acid Bacteria and Bifidobacteria. Probiotics Antimicrob. Proteins 2020, 13, 495–505. [Google Scholar] [CrossRef]
- Salmerón, I.; Thomas, K.; Pandiella, S.S. Effect of substrate composition and inoculum on the fermentation kinetics and flavour compound profiles of potentially non-dairy probiotic formulations. LWT 2013, 55, 240–247. [Google Scholar] [CrossRef]
- Menezes, A.G.T.; Ramos, C.L.; Dias, D.R.; Schwan, R.F. Combination of probiotic yeast and lactic acid bacteria as starter culture to produce maize-based beverages. Food Res. Int. 2018, 111, 187–197. [Google Scholar] [CrossRef]
- Freire, A.L.; Ramos, C.L.; Souza, P.N.D.C.; Cardoso, M.G.B.; Schwan, R.F. Nondairy beverage produced by controlled fermentation with potential probiotic starter cultures of lactic acid bacteria and yeast. Int. J. Food Microbiol. 2017, 248, 39–46. [Google Scholar] [CrossRef]
- Freire, A.L.; Ramos, C.L.; Schwan, R.F. Microbiological and chemical parameters during cassava based-substrate fermentation using potential starter cultures of lactic acid bacteria and yeast. Food Res. Int. 2015, 76, 787–795. [Google Scholar] [CrossRef] [Green Version]
- Freire, A.L.; Ramos, C.; Schwan, R.F. Effect of symbiotic interaction between a fructooligosaccharide and probiotic on the kinetic fermentation and chemical profile of maize blended rice beverages. Food Res. Int. 2017, 100, 698–707. [Google Scholar] [CrossRef]
- Giri, S.S.; Sen, S.S.; Saha, S.; Sukumaran, V.; Park, S.C. Use of a Potential Probiotic, Lactobacillus plantarum L7, for the Preparation of a Rice-Based Fermented Beverage. Front. Microbiol. 2018, 9, 473. [Google Scholar] [CrossRef]
- Ghosh, K.; Ray, M.; Adak, A.; Halder, S.K.; Das, A.; Jana, A.; Mondal, S.P.; Vágvölgyi, C.; Das Mohapatra, P.K.; Pati, B.R.; et al. Role of probiotic Lactobacillus fermentum KKL1 in the preparation of a rice based fermented beverage. Bioresour. Technol. 2015, 188, 161–168. [Google Scholar] [CrossRef]
- Paz, P.C.; Janny, R.J.; Håkansson, Å. Safeguarding of quinoa beverage production by fermentation with Lactobacillus plantarum DSM 9843. Int. J. Food Microbiol. 2020, 324, 108630. [Google Scholar] [CrossRef]
- Iraporda, C.; Rubel, I.A.; Managó, N.; Manrique, G.D.; Garrote, G.L.; Abraham, A.G. Inulin addition improved probiotic survival in soy-based fermented beverage. World J. Microbiol. Biotechnol. 2022, 38, 133. [Google Scholar] [CrossRef] [PubMed]
- Dahiya, D.; Nigam, P. Therapeutic and Dietary support for Gastrointestinal tract using kefir a nutraceutical beverage: Dairy-milk based or plant-sourced probiotic products for vegans and lactose-intolerants. Fermentation 2023, 9, 388. [Google Scholar] [CrossRef]
- Canon, F.; Mariadassou, M.; Maillard, M.-B.; Falentin, H.; Parayre, S.; Madec, M.-N.; Valence, F.; Henry, G.; Laroute, V.; Daveran-Mingot, M.-L.; et al. Function-driven design of lactic acid bacteria co-cultures to produce new fermented food associating milk and lupin. Front. Microbiol. 2020, 11, 584163. [Google Scholar] [CrossRef] [PubMed]
- Canon, F.; Nidelet, T.; Guédon, E.; Thierry, A.; Gagnaire, V. Understanding the mechanisms of positive microbial interactions that benefit lactic acid bacteria co-cultures. Front. Microbiol. 2020, 11, 2088. [Google Scholar] [CrossRef]
- Dahiya, D.; Manuel, V.; Nigam, P.S. An Overview of Bioprocesses Employing Specifically Selected Microbial Catalysts for γ-Aminobutyric Acid Production. Microorganisms 2021, 9, 2457. [Google Scholar] [CrossRef]
- Licona-Limón, P.; Kim, L.K.; Palm, N.; A Flavell, R. TH2, allergy and group 2 innate lymphoid cells. Nat. Immunol. 2013, 14, 536–542. [Google Scholar] [CrossRef]
- Klatka, M.; Grywalska, E.; Partyka, M.; Charytanowicz, M.; Kiszczak-Bochynska, E.; Rolinski, J. Th17 and Treg cells in adolescents with Graves’ disease. Impact of treatment with methimazole on these cell subsets. Autoimmunity 2014, 47, 201–211. [Google Scholar] [CrossRef]
- Liu, Q.; Jing, W.; Wang, W. Bifidobacterium lactis Ameliorates the Risk of Food Allergy in Chinese Children by Affecting Relative Percentage of Treg and Th17 Cells. Can. J. Infect. Dis. Med. Microbiol. 2018, 2018, 4561038. [Google Scholar] [CrossRef] [Green Version]
- Marrs, T.; Jo, J.-H.; Perkin, M.R.; Rivett, D.W.; Witney, A.A.; Bruce, K.D.; Logan, K.; Craven, J.; Radulovic, S.; Versteeg, S.A.; et al. Gut microbiota development during infancy: Impact of introducing allergenic foods. Food Allergy Gastrointest. Dis. 2021, 147, 613–621.e9. [Google Scholar] [CrossRef]
- Perkin, M.R.; Logan, K.; Tseng, A.; Raji, B.; Ayis, S.; Peacock, J.; Brough, H.; Marrs, T.; Radulovic, S.; Craven, J.; et al. Randomized trial of introduction of allergenic foods in breast-fed infants. N. Engl. J. Med. 2016, 374, 1733–1743. [Google Scholar] [CrossRef] [Green Version]
- Dahiya, D.; Chettri, R.; Nigam, P. Biosynthesis of polyglutamic acid (γ-PGA), a biodegradable and economical polyamide biopolymer for industrial applications. In Microbial and Natural Macromolecules: Synthesis and Applications; Academic Press: Cambridge, MA, USA, 2021; Volume 1, pp. 681–688. [Google Scholar] [CrossRef]
- Bontzolis, C.; Plioni, I.; Dimitrellou, D.; Boura, K.; Kanellaki, M.; Nigam, P.S.; Koutinas, A. Isolation of antimicrobial compounds from aniseed and techno-economic feasibility report for industrial-scale application. Int. J. Food Sci. Technol. 2022, 57, 5155–5163. [Google Scholar] [CrossRef]
- Dahiya, D.; Terpou, A.; Dasenaki, M.; Nigam, P. Current status and future prospects of bioactive molecules delivered through sustainable encapsulation techniques for food fortification. Sustain. Food Technol. R. Soc. Chem. 2023. [Google Scholar] [CrossRef]
| Category of foods, Ingredients, Additives | Items as Potential Allergens |
|---|---|
| Plant-based products | Gluten-containing cereals, Nuts (peanuts, walnuts, almonds, hazelnuts, pecans, cashews, pistachios and Brazil nut), mustard, sesame seeds items, soybeans, food products prepared from lupini beans (pasta, noodles, sauces, bread, pastries, pies) |
| Food sourced from sea | Crustaceans (prawns, crabs, lobsters, shrimp, and krill etc.), Molluscs (clams, scallops, oysters, octopus and squid etc.) |
| Dairy-based Products | Lactose-containing dairy milk, Products prepared using dairy milk |
| Preservatives, chemicals added in food for antimicrobial activity to extend shelf-life | Benzoic acid (E210), and its sodium, potassium and calcium salts (E211–213); Parabens, Sulphites, Nitrites, Nitrates, Acetic Acid BHA (butylated hydroxyanisole), BHT (butylated hydroxytoluene) |
| Flavor Enhancers, Sweetener-Additives | Monosodium Glutamate (MSG), Hydrolyzed vegetable protein, Aspartame, High Fructose Syrup |
| Colorings used in processed food, beverages, snacks and candies | Yellow-5 (Tartrazine), Yellow-6, Annatto, Blue-1, Red 40, Carmine (cochineal extract or natural red-4) |
| Genus | Species | Strain | Reference |
|---|---|---|---|
| Bifidobacterium | infantis | 35624; Rossell-33 | [39] |
| Bifidobacterium | lactis | Bi-07@; Bl-04@; HN019; BB-12@ | [40,41,42,43,44] |
| Bifidobacterium | breve | M-16V@; Bbi99 | [39,45] |
| Bifidobacterium | animalis ssp. lactis | BB-12® | [46,47,48] |
| Bifidobacterium | bifidum | Rosell-71 | [49] |
| Escherichia strain | coli | Nissle 1917 | [45] |
| Bacillus | coagulans | Unique IS-2; BC30TM | [39,50] |
| Lactobacillus | reuteri | Protectis@; RC-14@ | [39] |
| Lactobacillus | rhamnosus | LGG@; HN001; GR-1@; Rosell-11; LC705 | [39,51] |
| Lactobacillus | plantarum | DSM9843; LP299v@ (LP299v@) | [39,52] |
| Lactobacillus | casei | DN-114001@; Shirota@ DN001 | [39] |
| Lactobacillus | paracasei ssp. paracasei | CASEI 431@; Lpc-37@ | [46] |
| Lactobacillus | acidophilus | Rosell-52; NCFM@; LA05 | [39,40,41,48] |
| Saccharomyces | cerevisiae | boulardii | [53,54,55] |
| Propionibacterium | freudenreichii | ssp. shermanii JS | [56] |
| Lactobacillus | amylovorus; fermentum | [57] | |
| Enterococcus | faecium | SF68 | [45] |
| Lactobacillus | casei | LOCK 0900; LOCK 0908; | [45] |
| Lactobacillus | paracasei | LOCK 0919 | [45] |
| Bacillus | Coagulans (Ganeden BC30) | GBI-30, 6086 | [58] |
| Substrates/Product | Probiotic Culture | Strain | Reference |
|---|---|---|---|
| Cow’s milk/commercial dairy-based Probiotic beverage Kefir | Bifidobacterium + Lactobacillus acidophilus + Lactobacillus casei + Lactobacillus rhamnosus + Lactobacillus plantarum | Not disclosed | [73] |
| Oat, Coconut Cream, Rice Flour, Stabilisers (Tapioca Starch, Pectin)/commercial non-dairy-based Probiotic beverage Kefir | Live Vegan Kefir Cultures Bifidobacterium, Lactobacillus acidophilus, Lactobacillus bulgaricus, Lactobacillus rhamnosus | Not disclosed | [73] |
| Free Range Milk/Natural Bio-Yogurt | Bifidobacterium animalis, Streptococcus thermophilus, Lactobacillus acidophillus | BB12 is a particular strain of the B. animalis | [74] |
| Cow’s milk/commercial dairy-based Kefir Yogurt | Bifidobacterium, Streptococcus thermophilus, Lactobacillus bulgaricus, Lactobacillus acidophilus, Lactobacillus casei | Not disclosed | [73] |
| whole oats/a synbiotic food | Lactobacillus plantarum; Bifidobacterium animalis | TK9; subsp. lactis V9 | [75,76] |
| Thai-pigmented rice/Novel probiotic products | Bacillus coagulans; Lacti-caseibacillus rhamnosus | KPS-TF02; KPS-VE9 | [77] |
| Glutinous Rice/probiotic product | Lactobacillus amylovorus | TISTR1110 | [78] |
| Rice, Oats and Inulin/Functional fermented food | Lactobacillus rhamnosus | GR-1 | [79] |
| Formulations of germinated brown rice/fermented products functionalized by probiotics, with enhanced GABA, oryzanol and neutralized phytic acid | Bifidobacterium longum; Bifidobacterium bifidum; Lacticaseibacillus rhamnosus; Streptococcus thermophilus; S. thermophilus + Lactobacillus del-brueckii ssp. Bulgaricus; Thermophilic LAB | BB536; Bb-12; GG (ATCC 52103); Cryofast SST 31; Lyofast SY 1; YoFlex®YF-L02DA | [80] |
| Synbiotic Blend of Probiotic with Flaxseed | Bacillus coagulans (Ganeden BC30) | GBI-30, 6086 | [58] |
| Functional fermented juice of a mixture of pineapple, spinach, cucumber, pumpkin, and Jerusalem artichoke juices | Lacticaseibacillus rhamnosus; Lacticaseibacillus paracasei subsp. paracasei; Lactobacillus acidophilus; Bifidobacterium animalis subsp. lactis; Lactiplantibacillus plantarum | n.a. | [81] |
| Oats, Barley and Malt/functional probiotic beverages | Lactobacillus acidophilus; Lactobacillus plantarum; Lactobacillus reuteri | NCIMB 8821 NCIMB 8826 NCIMB 11951 | [82] |
| Corn-based/Functional beverage | Lactobacillus paracasei; Saccharomyces cerevisiae; S. cerevisiae; Pichia kluyveri | LBC-81; CCMA 0731; CCMA 0732; CCMA 0615 | [83] |
| Cassava (Manihot esculenta Crantz) and rice-based/beverage with functional properties | Lactobacillus plantarum; Torulaspora delbrueckii; Lactobacillus acidophilus | CCMA 0743; CCMA 0235; LAC-04 | [84] |
| Cassava and rice-based/beverage with functional properties | Lactobacillus fermentum; Torulaspora delbrueckii; Pichia caribbica; Saccharomyces cerevisiae | CCMA 0215; CCMA 0234,0235; CCMA 0198; CCMA 0232, 0233 | [85] |
| Maize blended with rice/Functional beverages | Lactobacillus acidophilus; Lactobacillus plantarum; Torulaspora delbrueckii | LACA; CCMA 0743; CCMA 0235 | [86] |
| Rice-based fermented beverage “Bhaati Jaanr” | Lactobacillus plantarum | L7 | [87] |
| Rice-based fermented beverage | Lactobacillus fermentum | KKL1 | [88] |
| Quinoa beverage | Lactobacillus plantarum | DSM 9843 | [89] |
| Soya-based fermented beverage | Lactiplantibacillus plantarum; Lacticaseibacillus paracasei | CIDCA 8327; BGP1 | [90] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dahiya, D.; Nigam, P.S. Nutraceuticals Prepared with Specific Strains of Probiotics for Supplementing Gut Microbiota in Hosts Allergic to Certain Foods or Their Additives. Nutrients 2023, 15, 2979. https://doi.org/10.3390/nu15132979
Dahiya D, Nigam PS. Nutraceuticals Prepared with Specific Strains of Probiotics for Supplementing Gut Microbiota in Hosts Allergic to Certain Foods or Their Additives. Nutrients. 2023; 15(13):2979. https://doi.org/10.3390/nu15132979
Chicago/Turabian StyleDahiya, Divakar, and Poonam Singh Nigam. 2023. "Nutraceuticals Prepared with Specific Strains of Probiotics for Supplementing Gut Microbiota in Hosts Allergic to Certain Foods or Their Additives" Nutrients 15, no. 13: 2979. https://doi.org/10.3390/nu15132979
APA StyleDahiya, D., & Nigam, P. S. (2023). Nutraceuticals Prepared with Specific Strains of Probiotics for Supplementing Gut Microbiota in Hosts Allergic to Certain Foods or Their Additives. Nutrients, 15(13), 2979. https://doi.org/10.3390/nu15132979







