Prevalence of Micronutrient Deficiency among People Living with HIV in Selected Rural Districts of the Eastern Cape Province of South Africa
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design, Setting, and Eligibility Criteria
2.2. Sampling and Study Population
2.3. Data Collection
Blood Collection for Micronutrient Analysis
2.4. Data Management and Analysis
2.4.1. Micronutrient Analysis and Quantification
2.4.2. Geospatial Analysis of Micronutrient Deficiencies and Viral Load
3. Results
3.1. Sociodemographic and Clinical Characteristics
3.2. Micronutrient Deficiency Patterns
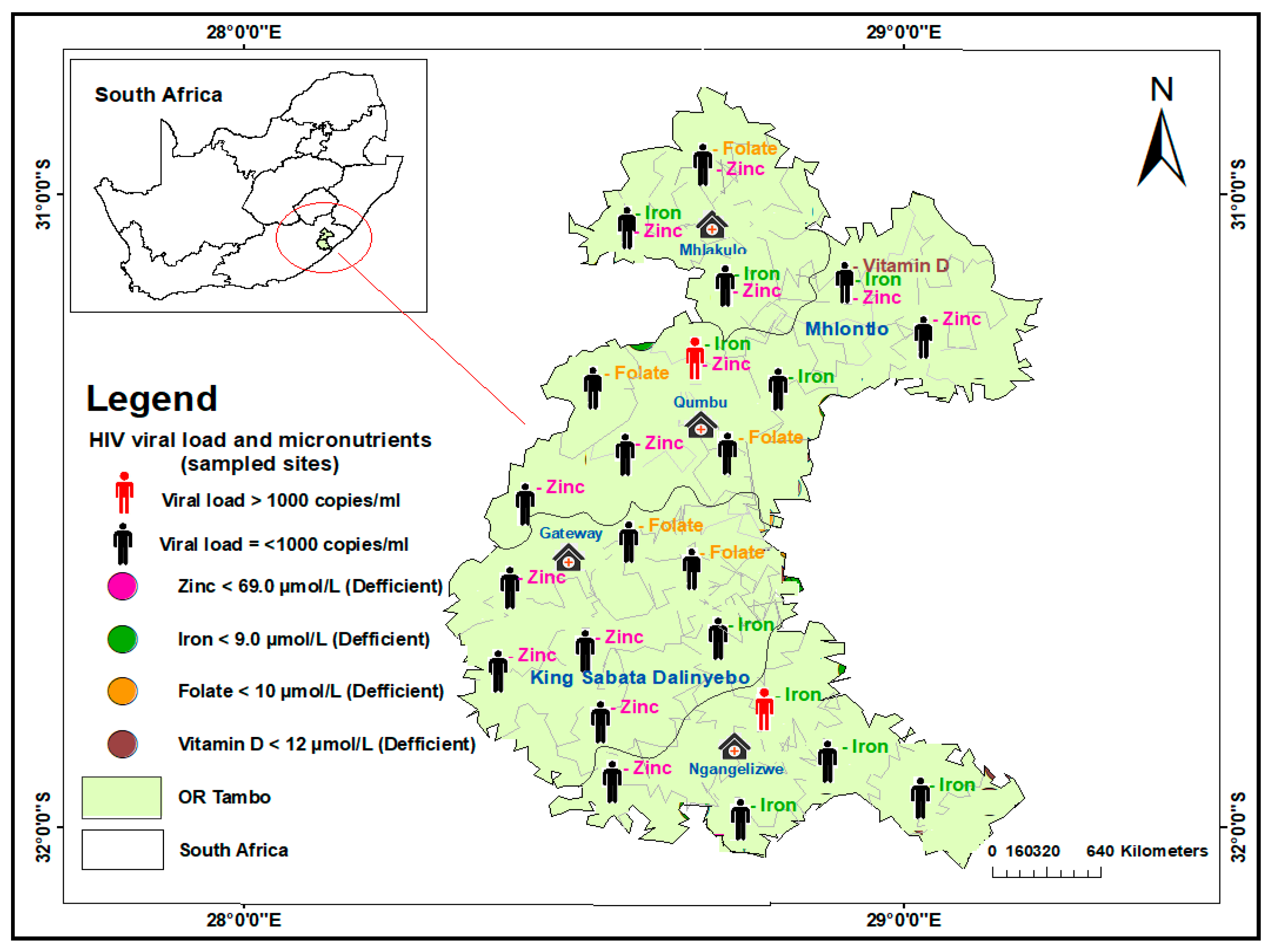
3.3. Geospatial Distribution of Micronutrient Deficiency
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gona, P.N.; Gona, C.M.; Ballout, S.; Rao, S.R.; Kimokoti, R.; Mapoma, C.C.; Mokdad, A.H. Burden and changes in HIV/AIDS morbidity and mortality in Southern Africa Development Community Countries, 1990–2017. BMC Public Health 2020, 20, 867. [Google Scholar] [CrossRef] [PubMed]
- UNAIDS Fact Sheet 2022. Available online: www.unaids.org/sites/default/files/media_asset/UNAIDS_FactSheet_en.pdf (accessed on 30 July 2022).
- WHO HIV/AIDS Data and Statistics 2022. Available online: https://www.who.int/teams/global-hiv-hepatitis-and-stis-programmes/hiv/strategic-information/hiv-data-and-statistics (accessed on 30 July 2022).
- UNAIDS Data 2021. Available online: https://www.unaids.org/en/resources/documents/2021/2021_unaids_data (accessed on 15 August 2022).
- Isabirye, N.; Ezeamama, A.E.; Kyeyune-Bakyayita, R.; Bagenda, D.; Fawzi, W.W.; Guwatudde, D. Dietary Micronutrients and Gender, Body Mass Index and Viral Suppression Among HIV-Infected Patients in Kampala, Uganda. Int. J. MCH AIDS 2020, 9, 337–349. [Google Scholar] [CrossRef] [PubMed]
- FAO. Food and Agriculture Organization of the United Nations: Rome, Italy. FAOSTAT Database. Available online: https://www.fao.org/food-agriculture-statistics/en/ (accessed on 15 May 2020).
- WHO; UNICEF. Report of the Fourth Meeting of the WHO UNICEF Technical Expert Advisory Group on Nutrition Monitoring (TEAM); WHO: Geneva, Switzerland, 2017. [Google Scholar]
- Lawrence, M.; Wingrove, K.; Naude, C.; Durao, S. Evidence Synthesis and Translation for Nutrition Interventions to Combat Micronutrient Deficiencies with Particular Focus on Food Fortification. Nutrients 2016, 8, 555. [Google Scholar] [CrossRef] [PubMed]
- Galani, Y.J.H.; Orfila, C.; Gong, Y.Y. A review of micronutrient deficiencies and analysis of maize contribution to nutrient requirements of women and children in Eastern and Southern Africa. Crit. Rev. Food Sci. Nutr. 2020, 62, 1568–1591. [Google Scholar] [CrossRef]
- Bouis, H.E.; Saltzman, A. Improving nutrition through biofortification: A review of evidence from Harvest Plus, 2003 through 2016. Glob. Food Secur. 2017, 2, 49–58. [Google Scholar] [CrossRef]
- Eshetu, A.; Tsegaye, A.; Petros, B. Selected micronutrient levels and response to highly active antiretroviral therapy (HAART) among HIV/AIDS patients attending a teaching Hospital in Addis Ababa, Ethiopia. Biol. Trace Elem. Res. 2014, 162, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Thimmapuram, R.; Lanka, S.; Esswein, A.; Dall, L. Correlation of Nutrition with Immune Status in Human Immunodeficiency Virus Outpatients. Mo. Med. 2019, 116, 336–339. [Google Scholar] [PubMed]
- Duggal, S.; Chugh, T.D.; Duggal, A.K. HIV and Malnutrition: Effects on Immune System. Clin. Dev. Immunol. 2012, 2012, 1–8. [Google Scholar] [CrossRef]
- Thapa, R.; Amatya, A.; Pahari, D.P.; Bam, K.; Newman, M.S. Nutritional status and its association with quality of life among people living with HIV attending public anti-retroviral therapy sites of Kathmandu Valley, Nepal. AIDS Res. Ther. 2015, 12, 14. [Google Scholar] [CrossRef]
- Hailemariam, S.; Bune, G.T.; Ayele, H.T. Malnutrition: Prevalence and its associated factors in People living with HIV/AIDS, in Dilla University Referral Hospital. Arch. Public Health 2013, 71, 13. [Google Scholar] [CrossRef]
- Alebel, A.; Demant, D.; Petrucka, P.; Sibbritt, D. Effects of undernutrition on opportunistic infections among adults living with HIV on ART in Northwest Ethiopia: Using inverse-probability weighting. PLoS ONE 2022, 17, e0264843. [Google Scholar] [CrossRef]
- Gebremichael, D.Y.; Hadush, K.T.; Kebede, E.M.; Zegeye, R.T. Food Insecurity, Nutritional Status, and Factors Associated with Malnutrition among People Living with HIV/AIDS Attending Antiretroviral Therapy at Public Health Facilities in West Shewa Zone, Central Ethiopia. Biomed. Res Int. 2018, 2018, 1913534. [Google Scholar] [CrossRef]
- Benzekri, N.A.; Sambou, J.; Diaw, B.; Sall, E.H.I.; Sall, F.; Niang, A.; Ba, S.; Gueye, N.F.N.; Diallo, M.B.; Hawes, S.; et al. High prevalence of severe food insecurity and malnutrition among HIV-infected adults in Senegal, West Africa. PLoS ONE 2015, 10, e0141819. [Google Scholar] [CrossRef]
- Sicotte, M.; Langlois, É.V.; Aho, J.; Ziegler, D.; Zunzunegui, M.V. Association between nutritional status and the immune response in HIV+ patients under HAART: Protocol for a systematic review. Syst. Rev. 2014, 3, 9. [Google Scholar] [CrossRef] [PubMed]
- Abba, A.; Fokam, J.; Kamgaing, R.S.; Yimga, J.F.; Ka’e, A.C.; Nka, A.D.; Tchouaket, M.C.T.; Chenwi, C.A.; Semengue, E.N.J.; Ndjolo, A.; et al. Correlation between the immuno-virological response and the nutritional profile of treatment-experienced HIV-infected patients in the East region of Cameroon. PLoS ONE 2021, 16, e0229550. [Google Scholar] [CrossRef]
- Piwoz, E.; Greble, E. HIV/AIDS and Nutrition: A Review of the Literature and Recommendations for Nutritional Care and Support in Sub—Saharan Africa; Academy for Educational Development: Washington, DC, USA, 2000. [Google Scholar]
- Maertens, J.A. Barriers to Nutrition Management among People Living with HIV on Antiretroviral Therapy. Ph.D. Thesis, Colorado State University, Fort Collins, CO, USA, 2011. Available online: https://mountainscholar.org/bitstream/handle/10217/46751/Maertens_colostate_0053A_10627.pdf (accessed on 15 December 2022).
- PEPFAR. Report on Food and Nutrition for People Living with HIV/AIDS; PEPFAR: Washington, DC, USA, 2006. Available online: https://pdf.usaid.gov/pdf_docs/PCAAB509.pdf (accessed on 25 January 2023).
- Olson, R.; Gavin-Smith, B.; Ferraboschi, C.; Kraemer, K. Food Fortification: The Advantages, Disadvantages and Lessons from Sight and Life Programs. Nutrients 2021, 13, 1118. [Google Scholar] [CrossRef] [PubMed]
- van Tonder, E.; Gardner, l.; Cressey, S.; Tydeman-Edwards, R.; Gerber, K. Adult malnutrition: Prevalence and use of nutrition-related quality indicators in South African public-sector hospitals. South Afr. J. Clin. Nutr. 2019, 32, 1–7. [Google Scholar] [CrossRef]
- Mchiza, Z.J.; Steyn, N.P.; Hill, J.; Kruger, A.; Schönfeldt, H.; Nel, J.; Wentzel-Viljoen, E. A Review of Dietary Surveys in the Adult South African Population from 2000 to 2015. Nutrients 2015, 7, 8227–8250. [Google Scholar] [CrossRef] [PubMed]
- The WHO STEPwise Approach to Chronic Disease Risk Factor Surveillance (STEPS). STEPSInstrument. Available online: https://www.who.int/teams/noncommunicable-diseases/surveillance/systems-tools/steps (accessed on 17 January 2021).
- Simbayi, L.C.; Zuma, K.; Zungu, N.; Moyo, S.; Marinda, E.; Jooste, S.; Mabaso, M.; Ramlagan, S.; North, A.; van Zyl, J.; et al. South African National HIV Prevalence, Incidence, Behaviour and Communication Survey, 2017; HSRC Press: Cape Town, South Africa, 2019. [Google Scholar]
- Adhikari, P.M.; Chowta, M.N.; Ramapuram, J.T.; Rao, S.; Udupa, K.; Acharya, S.D. Prevalence of Vitamin B12 and folic acid deficiency in HIV-positive patients and its association with neuropsychiatric symptoms and immunological response. Indian J. Sex. Transm. Dis. AIDS 2016, 37, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Agwu, A.L.; Fleishman, J.A.; Korthuis, P.T.; Siberry, G.K.; Ellen, J.M.; Gaur, A.; Rutstein, R.; Gebo, K. Disparities in antiretroviral treatment: A comparison of behaviorally HIV-infected youth and adults in the HIV Research Network. J. Acquir. Immune Defic. Syndr. 2011, 58, 100–107. [Google Scholar] [CrossRef]
- Palmer, A.; Gabler, K.; Rachlis, B.; Ding, E.; Chia, J.; Bacani, N.; Bayoumi, A.M.; Closson, K.; Klein, M.; Cooper, C.; et al. Viral suppression and viral rebound among young adults living with HIV in Canada. Medicine 2018, 97, e10562. [Google Scholar] [CrossRef]
- Ryscavage, P.; Anderson, E.J.; Sutton, S.H.; Reddy, S.; Taiwo, B. Clinical outcomes of adolescents and young adults in adult HIV care. J. Acquir. Immune Defic. Syndr. 2011, 58, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Zanoni, B.C.; Mayer, K.H. The adolescent and young adult HIV cascade of care in the United States: Exaggerated health disparities. AIDS Patient Care STDS 2014, 28, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Cooperative Governance and Traditional Affairs—PROFILE: OR Tambo District Municipality. Available online: https://www.cogta.gov.za/ddm/wp-content/uploads/2020/11/ORTamnco-September-2020.pdf (accessed on 3 May 2023).
- Baum, M.K.; Campa, A.; Lai, S.; Sales Martinez, S.; Tsalaile, L.; Burns, P.; Farahani, M.; Li, Y.; van Widenfelt, E.; Page, J.B.; et al. Effect of micronutrient supplementation on disease progression in asymptomatic, antiretroviral-naive, HIV-infected adults in Botswana: A randomized clinical trial. JAMA 2013, 310, 2154–2163. [Google Scholar] [CrossRef] [PubMed]
- HRSC—Fact Sheet 2: South African National Health and Nutrition Examination Survey (SANHANES). Available online: https://hsrc.ac.za/uploads/pageContent/257/FactSheetSANHANES_final.pdf (accessed on 26 January 2023).
- Shisana, O.; Labadarios, D.; Rehle, T.; Simbayi, L.; Zuma, K.; Dhansay, A.; Reddy, P.; Parker, W.; Hoosain, E.; Naidoo, P.; et al. South African National Health and Nutrition Examination Survey (SANHANES-1); HSRC Press: Cape Town, South Africa, 2013. [Google Scholar]
- Academy of Science of South Africa. Consensus Study on Improved Nutritional Assessment of Micronutrients. 2013. Available online: https://www.assaf.org.za/files/2017%20reports/Improved%20Nutritional%20Assessment%20Full%20Consensus%20Report1.pdf (accessed on 26 January 2023).
- Zanoni, B.C.; Archary, M.; Buchan, S.; Katz, I.T.; Haberer, J.E. Systematic review and meta-analysis of the adolescent HIV continuum of care in South Africa: The Cresting Wave. BMJ Glob. Health 2016, 1, e000004. [Google Scholar] [CrossRef]


| Sociodemographic Characteristics | VL < 1000 (n = 43) | VL ≥ 1000 (n = 7) | Total (n = 50) | p-Value |
|---|---|---|---|---|
| Age a | 42.9 ± 8.9 | 32 ± 6.3 | 41.4 ± 9.3 | 0.003 c |
| District b | ||||
| Alfred Nzo | 3 (7.0) | 1 (14.3) | 4 (8.0) | 0.240 d |
| Joe Gqabi | 6 (14.0) | 2 (28.6) | 8 (16.0) | |
| Oliver R Tambo | 34 (79.1) | 4 (57.1) | 38 (76.0) | |
| Sub-district b | ||||
| KSD | 17 (39.5) | 3 (42.9) | 20 (40.0) | 0.348 d |
| Elundini | 6 (14.0) | 2 (28.6) | 8 (16.0) | |
| Mhlontlo | 17 (39.5) | 1 (14.3) | 18 (36.0) | |
| Mzimvubu | 3 (7.0) | 1 (14.3) | 4 (8.0) | |
| Employment b | ||||
| Employed | 22 (55.0) | 4 (66.7) | 26 (56.5) | 0.684 d |
| Unemployed | 18 (45.0) | 2 (33.3) | 20 (43.5) | |
| Income b | ||||
| <R5500 | 32 (80.0) | 5 (83.3) | 37 (80.4) | >0.999 d |
| ≥R5500 | 8 (20.0) | 1 (16.7) | 9 (19.6) | |
| Gender b | ||||
| Female | 42 (97.7) | 7 (100.0) | 49 (98.0) | >0.999 d |
| Male | 1 (2.3) | 0 (0.0) | 1 (2.0) |
| Clinical Characteristics | VL < 1000 (n = 43) | VL ≥ 1000 (n = 7) | Total (n = 50) | p-Value |
|---|---|---|---|---|
| BMI a | 31.6 ± 7.5 | 26.7 ± 6.2 | 31.0 ± 7.5 | 0.135 c |
| BMI categorised b | ||||
| Underweight | 0 (0.0) | 1 (16.7) | 1 (2.3) | 0.081 d |
| Normal weight | 4 (10.8) | 1 (16.7) | 5 (11.6) | |
| Overweight | 14 (37.8) | 3 (50.0) | 17 (39.5) | |
| Obesity | 19 (51.4) | 1 (16.7) | 20 (46.5) | |
| Dyslipidaemia a | ||||
| Triglycerides | 1.39 ± 0.68 | 1.12 ± 0.23 | 1.35 ± 0.64 | 0.339 c |
| HDL | 1.39 ± 0.36 | 1.06 ± 0.21 | 1.34 ± 0.36 | 0.036 c |
| LDL | 2.18 ± 0.97 | 1.69 ± 0.47 | 2.11 ± 0.92 | 0.237 c |
| CD4 a | 620.7 ± 236.4 | 301.7 ± 362.4 | 576 ± 276.5 | 0.004 c |
| CD4 categorised b | ||||
| <200 | 1 (2.3) | 4 (57.1) | 5 (10.0) | 0.000 d |
| 200–350 | 4 (9.3) | 1 (14.3) | 5 (10.0) | |
| >350 | 38 (88.4) | 2 (28.6) | 40 (80.0) | |
| Medical history b | ||||
| Diabetes | ||||
| Yes | 2 (8.3) | 0 (0.0) | 2 (7.7) | >0.999 d |
| No | 22 (91.7) | 2 (100.0) | 24 (92.3) | |
| Hypertension | ||||
| Yes | 11 (28.2) | 0 (0.0) | 11 (24.4) | 0.311 d |
| No | 28 (71.8) | 6 (100.0) | 34 (75.6) | |
| Co-infection with hepatitis | ||||
| Positive | 4 (10.0) | 0 (0.0) | 4 (8.9) | >0.999 d |
| Negative | 36 (90.0) | 5 (100.0) | 41 (91.1) |
| Micronutrients | VL < 1000 (n = 43) | VL ≥ 1000 (n = 7) | Total (n = 50) | p-Value |
|---|---|---|---|---|
| Vitamin D a | 25.8 ± 8.5 | 23.6 ± 11.1 | 25.5 ± 8.8 | 0.608 c |
| Vitamin D categorised b | ||||
| <12 ng/mL | 1 (2.9) | 0 (0.0) | 1 (2.6) | 0.629 d |
| 12–19 ng/mL | 7 (20.6) | 2 (40.0) | 9 (23.1) | |
| ≥20 ng/mL | 26 (76.5) | 3 (60.0) | 29 (74.4) | |
| Folate a | 16.5 ± 10.2 | 14.5 ± 8.1 | 16.2 ± 9.9 | 0.653 c |
| Folate categorised b | ||||
| <10 nmol/L | 9 (22.0) | 2 (33.3) | 11 (23.4) | 0.614 d |
| ≥10 nmol/L | 32 (78.0) | 4 (66.7) | 36 (76.6) | |
| Iron a | 14.8 ± 6.1 | 8.1 ± 1.6 | 14.0 ± 6.1 | 0.000 c |
| Iron categorised b | ||||
| <9 | 10 (25.0) | 4 (66.7) | 14 (30.4) | 0.060 d |
| 9–30 | 30 (75.0) | 2 (33.3) | 32 (69.6) | |
| Zinc a | 76.9 ± 30.8 | 62.7 ± 16.4 | 75.0 ± 29.5 | 0.241 c |
| Zinc categorised b | ||||
| <69 µg/L | 18 (41.9) | 4 (57.1) | 22 (44.0) | 0.805 d |
| 69–149 µg/L | 22 (51.2) | 3 (42.9) | 25 (50.0) | |
| >149 µg/L | 3 (7.0) | 0 (0.0) | 3 (6.0) |
| Micronutrients | OR Tambo | Joe Gqabi | Alfred Nzo | p-Value |
|---|---|---|---|---|
| Vitamin D a | 24.9 ± 8.5 | 28.8 ± 11.0 | 25.0 ± 8.6 | 0.614 c |
| Vitamin D categorised b | ||||
| <12 ng/mL | 1 (3.4) | 0 (0.0) | 0 (0.0) | >0.999 d |
| 12–19 ng/mL | 7 (24.1) | 1 (16.7) | 1 (25.0) | |
| ≥20 ng/mL | 21 (72.4) | 5 (83.3) | 3 (75.0) | |
| Folate a | 18.1 ± 10.3 | 10.3 ± 6.4 | 9.3 ± 2.7 | 0.053 c |
| Folate categorised b | ||||
| <10 nmol/L | 5 (13.9) | 4 (57.1) | 2 (50.0) | 0.015 d |
| ≥10 nmol/L | 31 (86.1) | 3 (42.9) | 2 (50.0) | |
| Iron a | 13.2 ± 5.4 | 18.9 ± 8.9 | 13.8 ± 6.0 | 0.101 c |
| Iron categorised b | ||||
| <9 | 10 (27.8) | 2 (33.3) | 2 (50.0) | 0.724 d |
| 9–30 | 26 (72.2) | 4 (66.7) | 2 (50.0) | |
| Zinc a | 79.7 ± 32.1 | 59.0 ± 11.1 | 61.8 ± 7.7 | 0.128 c |
| Zinc categorised b | ||||
| <69 µg/L | 13 (34.2) | 6 (75.0) | 3 (75.0) | 0.207 d |
| 69–149 µg/L | 22 (57.9) | 2 (25.0) | 1 (25.0) | |
| >149 µg/L | 3 (7.9) | 0 (0.0) | 0 (0.0) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nxasana, N.; Oladimeji, K.E.; Pulido-Estrada, G.-A.; Apalata, T.R. Prevalence of Micronutrient Deficiency among People Living with HIV in Selected Rural Districts of the Eastern Cape Province of South Africa. Nutrients 2023, 15, 3017. https://doi.org/10.3390/nu15133017
Nxasana N, Oladimeji KE, Pulido-Estrada G-A, Apalata TR. Prevalence of Micronutrient Deficiency among People Living with HIV in Selected Rural Districts of the Eastern Cape Province of South Africa. Nutrients. 2023; 15(13):3017. https://doi.org/10.3390/nu15133017
Chicago/Turabian StyleNxasana, Ntombizodumo, Kelechi Elizabeth Oladimeji, Guillermo-Alfredo Pulido-Estrada, and Teke Ruffin Apalata. 2023. "Prevalence of Micronutrient Deficiency among People Living with HIV in Selected Rural Districts of the Eastern Cape Province of South Africa" Nutrients 15, no. 13: 3017. https://doi.org/10.3390/nu15133017
APA StyleNxasana, N., Oladimeji, K. E., Pulido-Estrada, G.-A., & Apalata, T. R. (2023). Prevalence of Micronutrient Deficiency among People Living with HIV in Selected Rural Districts of the Eastern Cape Province of South Africa. Nutrients, 15(13), 3017. https://doi.org/10.3390/nu15133017






