Circulating Levels of Cathelicidin Antimicrobial Peptide (CAMP) Are Affected by Oral Lipid Ingestion
Abstract
:1. Introduction
2. Materials and Methods
2.1. SGBS Adipocyte Cell Culture and Stimulation Experiments
2.2. Gene Expression Analysis in SGBS Adipocytes
- Human CAMP:
- 5′-TAGATGGCATCAACCAGCGG-3′/5′-CTGGGTCCCCATCCATCGT -3′
- Human DPP4:
- 5′-TTCTGCTGAACAAAGGCAATGA-3′/5′-CTGTTCTCCAAGAAAACTGAGCTG-3′
- Human FABP4:
- 5′-ATGGGGGTGTCCTGGTACAT-3′/5′-CTTTCATGACGCATTCCACCA-3′
- Human GAPDH:
- 5′-GAGTCCACTGGCGTCTTCAC-3′/5′- CCAGGGGTGCTAAGCAGTT-3′
- All oligonucleotides used were purchased from Metabion, Martinsried, Germany.
2.3. Study Cohort
2.4. Measurement of Systemic Adipokine Concentrations
2.5. Measurement of Metabolic and Anthropometric Parameters
2.6. Statistical Analysis
3. Results
3.1. Characteristics of the Study Cohort
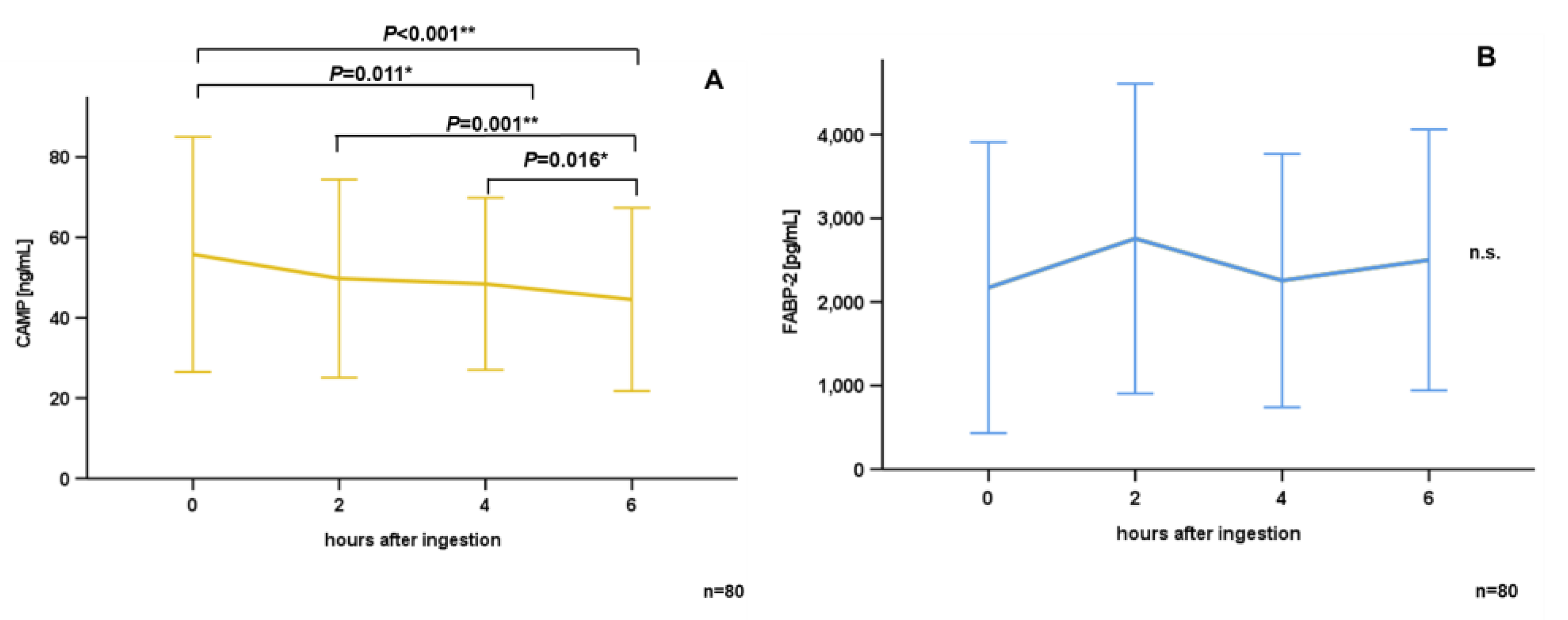
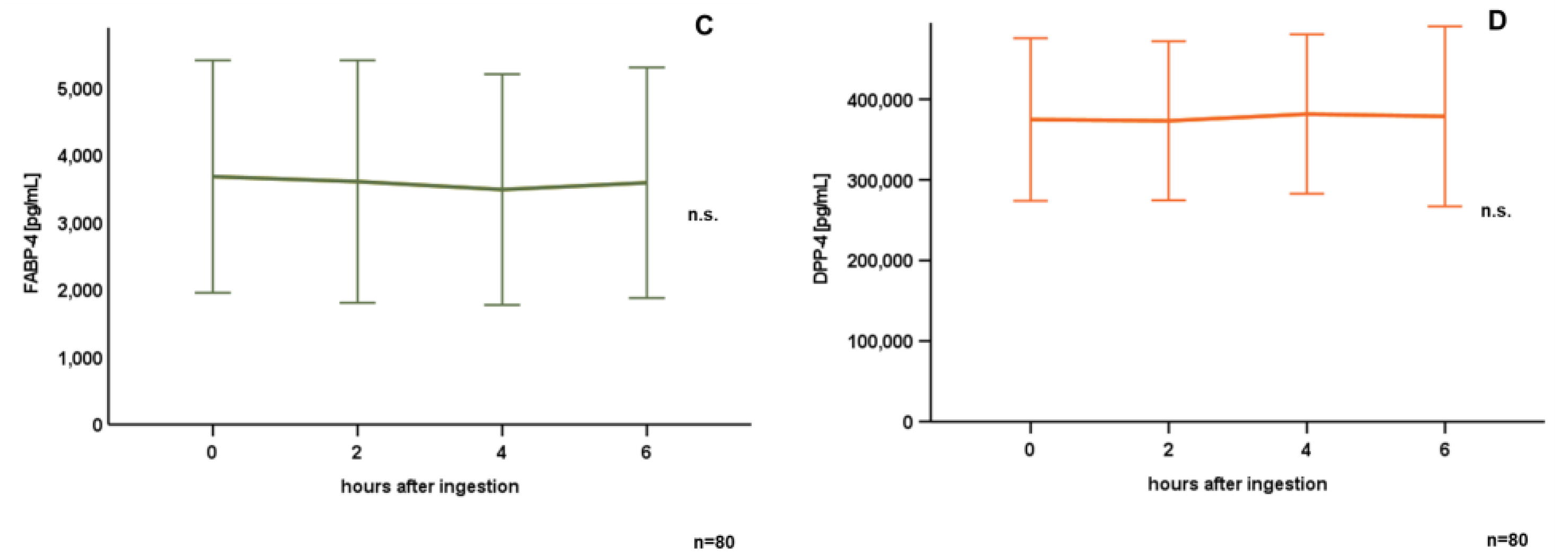
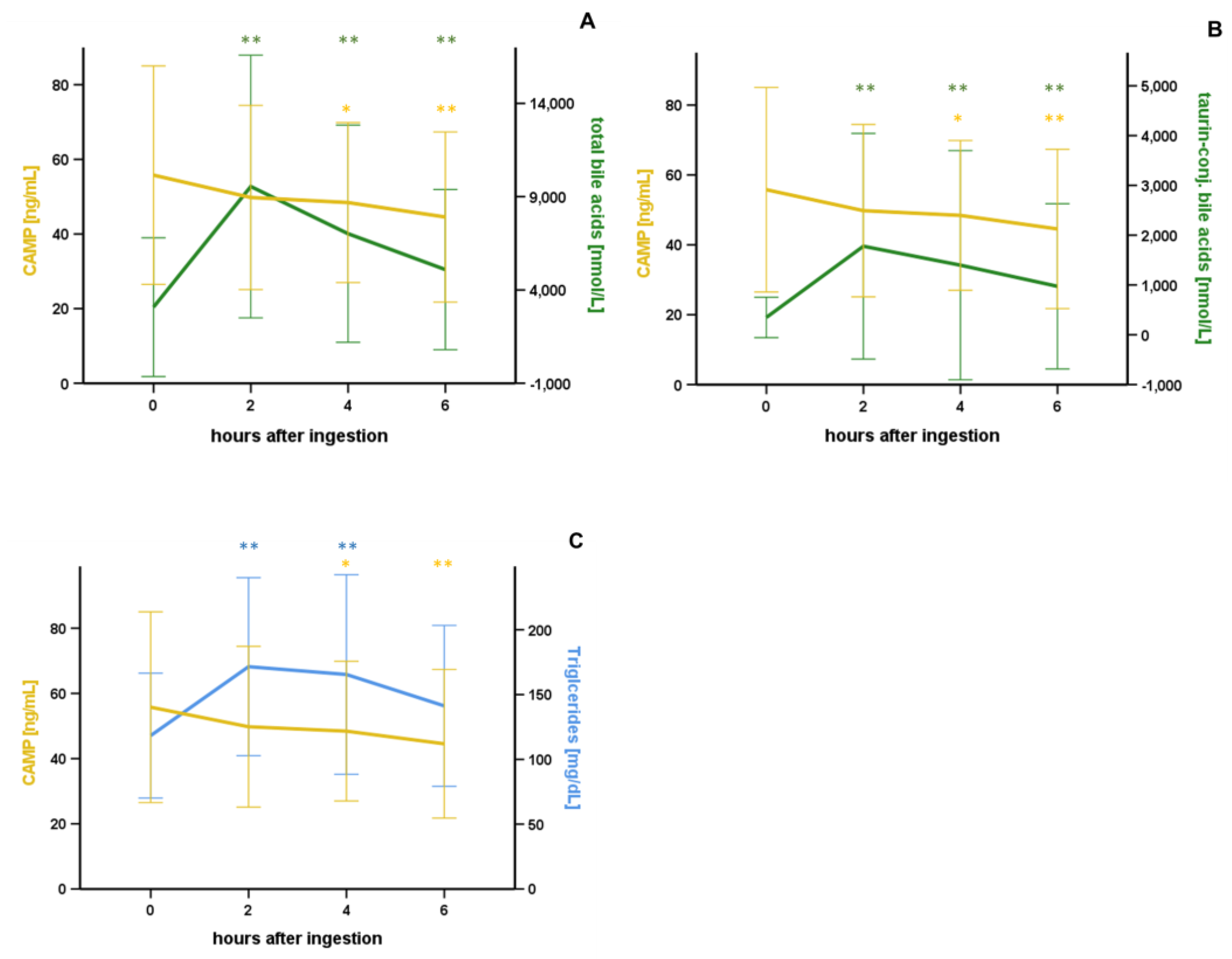
3.2. Oral Lipid Ingestion Reduces CAMP but Does Not Affect FABP-2, FABP-4, and DPP-4 Serum Concentrations
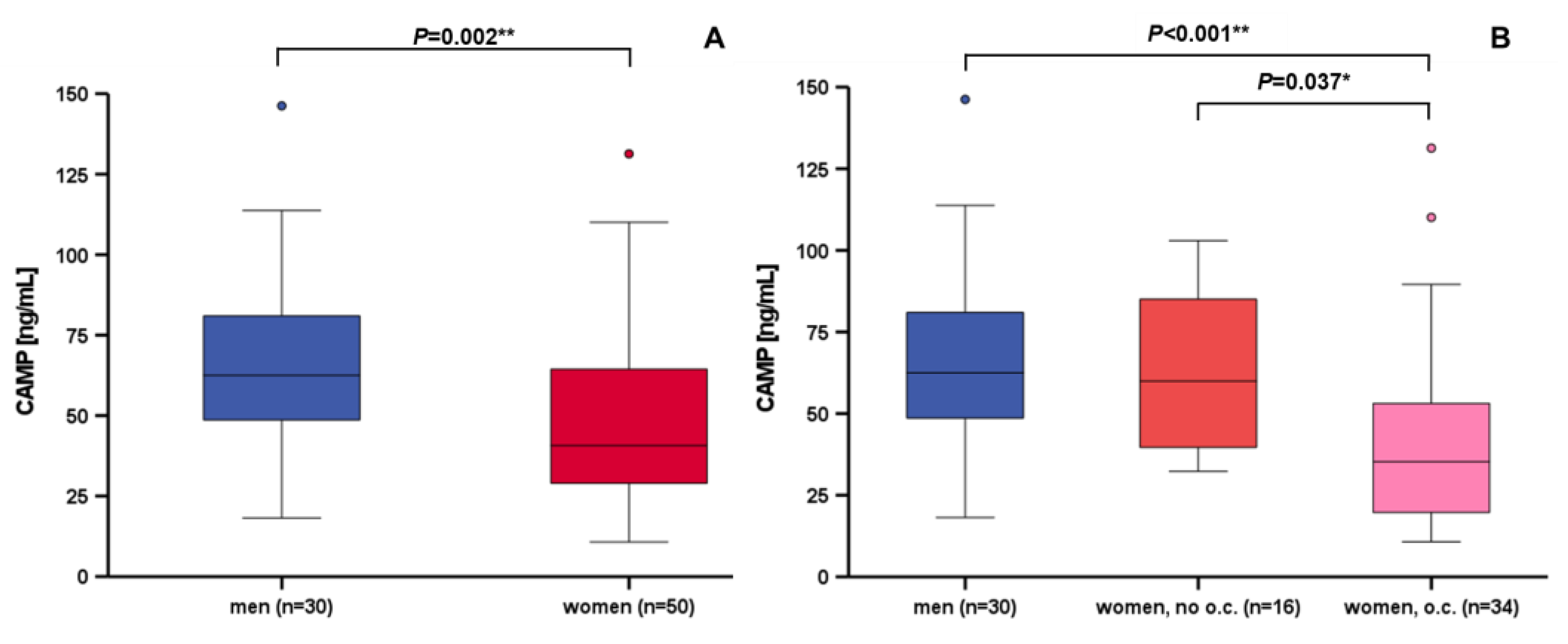
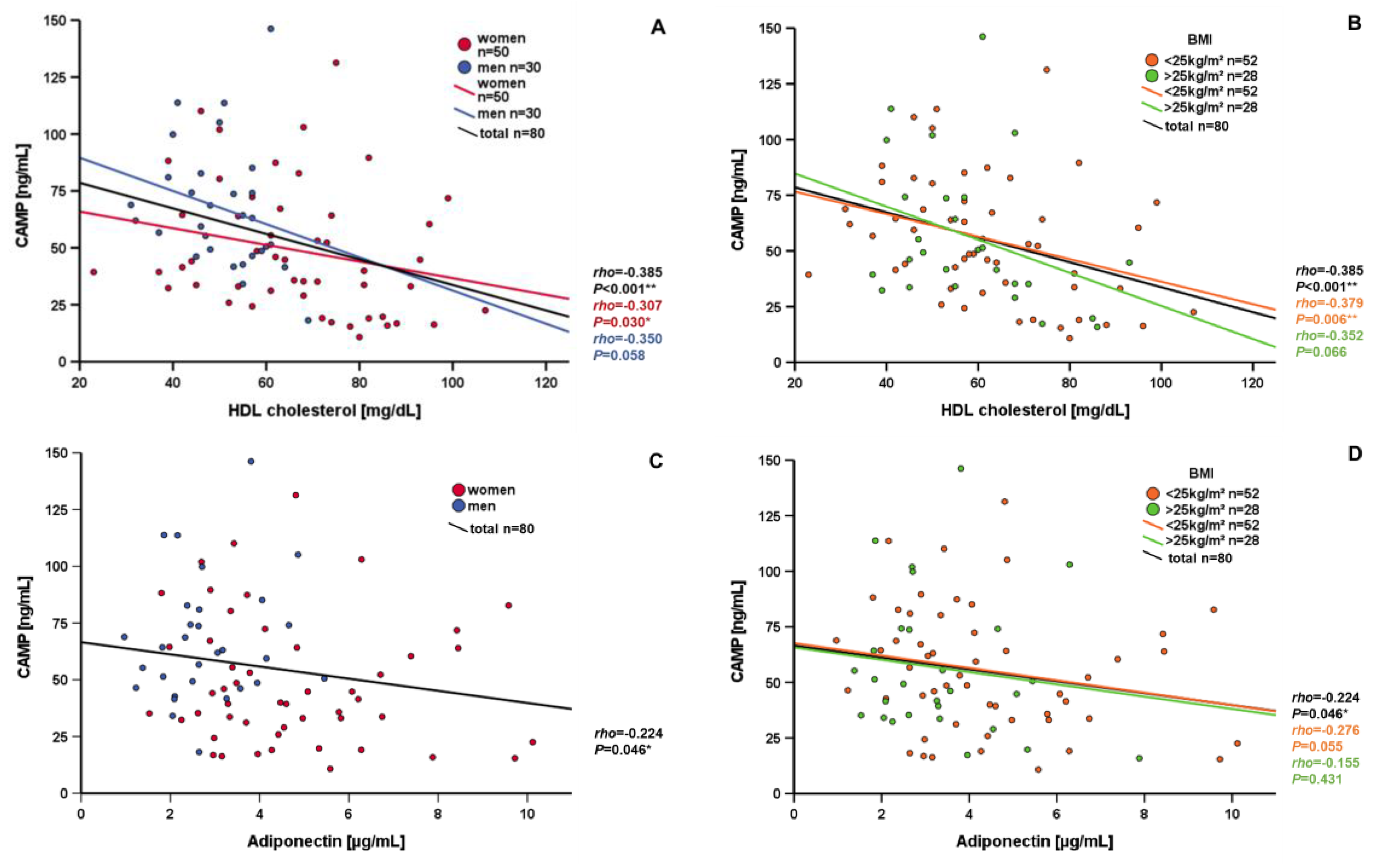
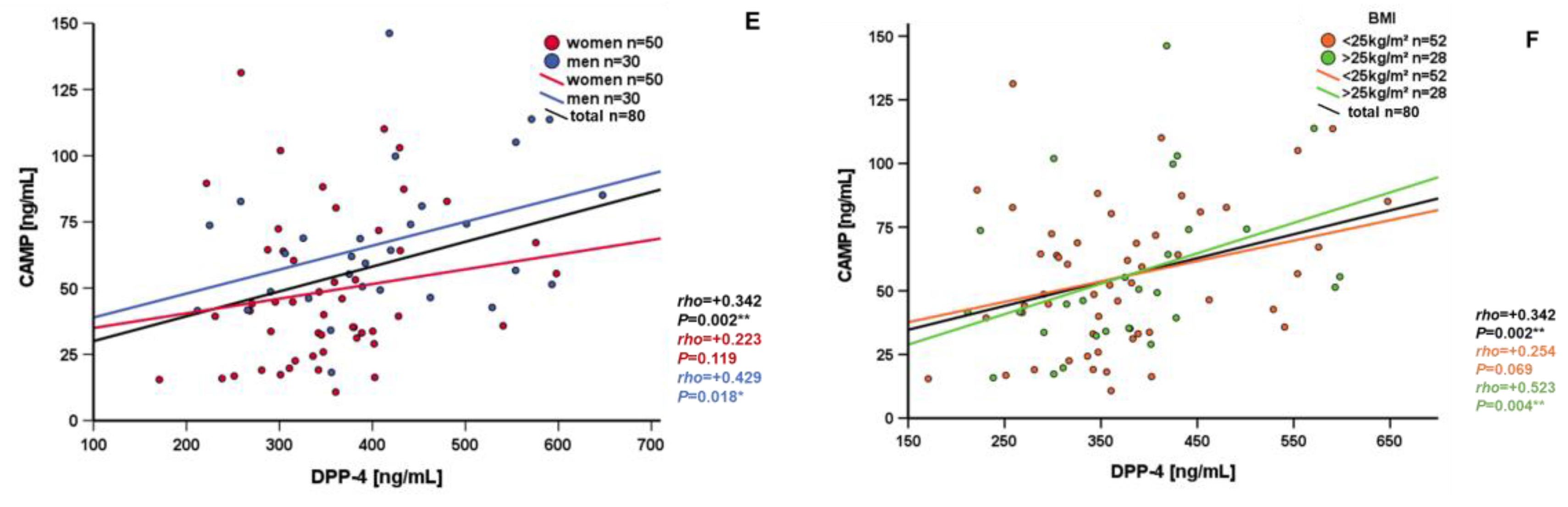
3.3. Correlation and Subgroup Analysis of Systemic CAMP Levels
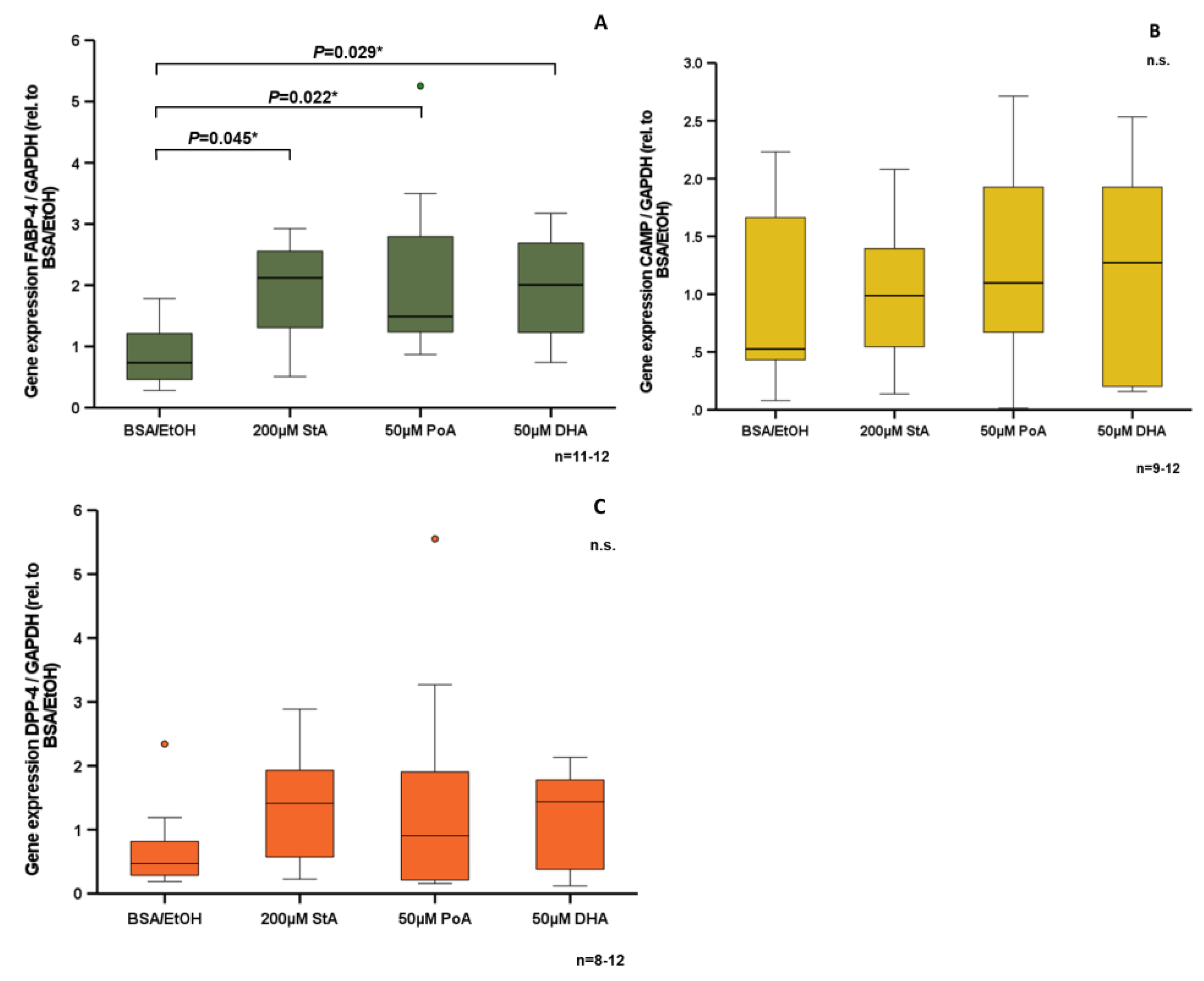
3.4. Effect of Free Fatty Acids on CAMP Expression in Human SGBS Adipocytes In Vitro
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Obesity and Overweight 2021; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- Engin, A. The Definition and Prevalence of Obesity and Metabolic Syndrome. Adv. Exp. Med. Biol. 2017, 960, 1–17. [Google Scholar] [CrossRef]
- Bornstein, S.R.; Abu-Asab, M.; Glasow, A.; Path, G.; Hauner, H.; Tsokos, M.; Chrousos, G.P.; Scherbaum, W.A. Immunohistochemical and ultrastructural localization of leptin and leptin receptor in human white adipose tissue and differentiating human adipose cells in primary culture. Diabetes 2000, 49, 532–538. [Google Scholar] [CrossRef] [Green Version]
- Ouchi, N.; Parker, J.L.; Lugus, J.J.; Walsh, K. Adipokines in inflammation and metabolic disease. Nat. Rev. Immunol. 2011, 11, 85–97. [Google Scholar] [CrossRef]
- Li, Y.; Yun, K.; Mu, R. A review on the biology and properties of adipose tissue macrophages involved in adipose tissue physiological and pathophysiological processes. Lipids Health Dis. 2020, 19, 164. [Google Scholar] [CrossRef]
- Fabbrini, E.; Cella, M.; McCartney, S.A.; Fuchs, A.; Abumrad, N.A.; Pietka, T.A.; Chen, Z.; Finck, B.N.; Han, D.H.; Magkos, F.; et al. Association between specific adipose tissue CD4+ T-cell populations and insulin resistance in obese individuals. Gastroenterology 2013, 145, 366–374.e3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernø, J.; Strand, K.; Mellgren, G.; Stiglund, N.; Björkström, N.K. Natural Killer Cells as Sensors of Adipose Tissue Stress. Trends Endocrinol. Metab. 2020, 31, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Fasshauer, M.; Blüher, M. Adipokines in health and disease. Trends Pharmacol. Sci. 2015, 36, 461–470. [Google Scholar] [CrossRef]
- Cao, H. Adipocytokines in obesity and metabolic disease. J. Endocrinol. 2014, 220, T47–T59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taylor, E.B. The complex role of adipokines in obesity, inflammation, and autoimmunity. Clin. Sci. 2021, 135, 731–752. [Google Scholar] [CrossRef]
- Santos-Alvarez, J.; Goberna, R.; Sánchez-Margalet, V. Human leptin stimulates proliferation and activation of human circulating monocytes. Cell Immunol. 1999, 194, 6–11. [Google Scholar] [CrossRef]
- Bowdish, D.M.E.; Davidson, D.J.; Hancock, R.E.W. Immunomodulatory properties of defensins and cathelicidins. Curr. Top. Microbiol. Immunol. 2006, 306, 27–66. [Google Scholar] [CrossRef]
- Zhang, L.-J.; Guerrero-Juarez, C.F.; Hata, T.; Bapat, S.P.; Ramos, R.; Plikus, M.V.; Gallo, R.L. Dermal adipocytes protect against invasive Staphylococcus aureus skin infection. Science 2015, 347, 67–71. [Google Scholar] [CrossRef] [Green Version]
- Alcorn, J.F.; Kolls, J.K. Physiology. Killer fat. Science 2015, 347, 26–27. [Google Scholar] [CrossRef] [Green Version]
- Höpfinger, A.; Karrasch, T.; Schäffler, A.; Schmid, A. Regulation of CAMP (cathelicidin antimicrobial peptide) expression in adipocytes by TLR 2 and 4. Innate Immun. 2021, 27, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Hochberg, A.; Patz, M.; Karrasch, T.; Schäffler, A.; Schmid, A. Serum Levels and Adipose Tissue Gene Expression of Cathelicidin Antimicrobial Peptide (CAMP) in Obesity and During Weight Loss. Horm. Metab. Res. 2021, 53, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Wabitsch, M.; Brenner, R.E.; Melzner, I.; Braun, M.; Möller, P.; Heinze, E.; Debatin, K.M.; Hauner, H. Characterization of a human preadipocyte cell strain with high capacity for adipose differentiation. Int. J. Obes. Relat. Metab. Disord. 2001, 25, 8–15. [Google Scholar] [CrossRef] [Green Version]
- Schmid, A.; Berghoff, M.; Hochberg, A.; Schäffler, A.; Karrasch, T. CTRP-3 is permeable to the blood-brain barrier and is not regulated by glucose or lipids in vivo. Eur. J. Clin. Invest. 2017, 47, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Karrasch, T.; Leszczak, S.; Bala, M.; Ober, I.; Martin, J.; Schmid, A.; Kopp, A.; Schaffler, A. Short-term regulation of Visfatin release in vivo by oral lipid ingestion and in vitro by fatty acid stimulation. Exp. Clin. Endocrinol. Diabetes 2014, 122, 126–134. [Google Scholar] [CrossRef]
- Schmid, A.; Leszczak, S.; Ober, I.; Karrasch, T.; Schäffler, A. Short-term Regulation of Resistin in vivo by Oral Lipid Ingestion and in vitro by Fatty Acid Stimulation. Exp. Clin. Endocrinol. Diabetes 2015, 123, 553–560. [Google Scholar] [CrossRef]
- Schmid, A.; Neumann, H.; Karrasch, T.; Liebisch, G.; Schäffler, A. Bile Acid Metabolome after an Oral Lipid Tolerance Test by Liquid Chromatography-Tandem Mass Spectrometry (LC-MS/MS). PLoS ONE 2016, 11, e0148869. [Google Scholar] [CrossRef]
- Jokinen, E. Obesity and cardiovascular disease. Minerva Pediatr. 2015, 67, 25–32. [Google Scholar]
- Mihailovic, P.M.; Lio, W.M.; Yano, J.; Zhao, X.; Zhou, J.; Chyu, K.-Y.; Shah, P.K.; Cercek, B.; Dimayuga, P.C. The cathelicidin protein CRAMP is a potential atherosclerosis self-antigen in ApoE(−/−) mice. PLoS ONE 2017, 12, e0187432. [Google Scholar] [CrossRef]
- Burkes, R.M.; Astemborski, J.; Lambert, A.A.; Brown, T.T.; Wise, R.A.; Kirk, G.D.; Drummond, M.B. Plasma cathelicidin and longitudinal lung function in current and former smokers. PLoS ONE 2019, 14, e0212628. [Google Scholar] [CrossRef]
- Starke, A.A. The influence of diet and physical activity on insulin sensitivity. Wien. Klin. Wochenschr. 1994, 106, 768–773. [Google Scholar]
- Borghouts, L.B.; Keizer, H.A. Exercise and insulin sensitivity: A review. Int. J. Sports Med. 2000, 21, 1–12. [Google Scholar] [CrossRef]
- Small, L.; Brandon, A.E.; Turner, N.; Cooney, G.J. Modeling insulin resistance in rodents by alterations in diet: What have high-fat and high-calorie diets revealed? Am. J. Physiol. Endocrinol. Metab. 2018, 314, E251–E265. [Google Scholar] [CrossRef]
- Coe, N.R.; Bernlohr, D.A. Physiological properties and functions of intracellular fatty acid-binding proteins. Biochim. Biophys. Acta 1998, 1391, 287–306. [Google Scholar] [CrossRef]
- Boord, J.B.; Fazio, S.; Linton, M.F. Cytoplasmic fatty acid-binding proteins: Emerging roles in metabolism and atherosclerosis. Curr. Opin. Lipidol. 2002, 13, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Schmid, A.; Leszczak, S.; Ober, I.; Schäffler, A.; Karrasch, T. Serum progranulin concentrations are not responsive during oral lipid tolerance test and oral glucose tolerance test. Horm. Metab. Res. 2015, 47, 571–576. [Google Scholar] [CrossRef]
- Schmid, A.; Schlegel, J.; Thomalla, M.; Karrasch, T.; Schäffler, A. Evidence of functional bile acid signaling pathways in adipocytes. Mol. Cell. Endocrinol. 2019, 483, 1–10. [Google Scholar] [CrossRef]
- D’Aldebert, E.; Biyeyeme Bi Mve, M.-J.; Mergey, M.; Wendum, D.; Firrincieli, D.; Coilly, A.; Fouassier, L.; Corpechot, C.; Poupon, R.; Housset, C.; et al. Bile salts control the antimicrobial peptide cathelicidin through nuclear receptors in the human biliary epithelium. Gastroenterology 2009, 136, 1435–1443. [Google Scholar] [CrossRef]
| Study Cohort (t = 0) | |
|---|---|
| Gender (men/women) | 30/50 |
| Age [y] | 7.53 (18–54) |
| BMI [kg/m²] | 5.06 (14.80–46.10) |
| WHR | 0.09 (0.69–1.07) |
| HOMA Index | 1.43 (0.18-8.47) |
| Glucose [mg/dL] | 13.73 (19–110) |
| Total cholesterol [mg/dL] | 38.01 (52–297) |
| Triglycerides [mg/dL] | 48.17 (24–299) |
| HDL cholesterol [mg/dL] | 17.45 (23–107) |
| LDL cholesterol [mg/dL] | 31.33 (26–196) |
| CAMP [ng/mL] | 29.26 (10.77–146.24) |
| A | ||
| Metabolic Parameters (t = 0), n = 80 | CAMP [ng/mL] (t = 0) | |
| rho | p | |
| Skinfold [mm] | −0.384 | <0.001 ** |
| HDL cholesterol [mg/dL] | −0.385 | <0.001 ** |
| DPP-4 [pg/mL] | +0.342 | 0.002 ** |
| Metabolic Parameters (t = 0), n = 80 | ΔCAMP [ng/mL] (t = (t = 6)–(t = 0)) | |
| DPP-4 [pg/mL] | −0.380 | <0.001 ** |
| B | ||
| Metabolic Parameters BMI < 25kg/m² (t = 0), n = 52 | CAMP [ng/mL] (t = 0) | |
| HDL cholesterol [mg/dL] | −0.379 | 0.006 ** |
| LDL cholesterol [mg/dL] | +0.342 | 0.013 * |
| Triglycerides [mg/dL] | +0.321 | 0.020 * |
| Metabolic Parameters BMI > 25kg/m² (t = 0), n = 28 | CAMP [ng/mL] (t = 0) | |
| Skinfold [mm] | −0.667 | <0.001 ** |
| Insulin [mU/L] | −0.375 | 0.049 * |
| DPP-4 [pg/mL] | +0.523 | 0.004 ** |
| Bile acids (t = 0), n = 80 | CAMP [ng/mL] (t = 0) | |
| rho | p | |
| Taurine-conj. Bile acids [nmol/L] | −0.276 | 0.013 * |
| bile acids (t = 0), n = 80 | ΔCAMP [ng/mL] (t = t6 – t0) | |
| Taurine-conj. Bile acids [nmol/L] | +0.358 | 0.001 ** |
| bile acids (t = 0), n = 80 | ΔCAMP [ng/mL] (t = t2 – t0) | |
| Taurine-conj. Bile acids [nmol/L] | +0.422 | <0.001 ** |
| bile acids, BMI > 25 kg/m² (t = 0), n = 28 | CAMP [ng/mL] (t = 0) | |
| Taurine-conj. Bile acids [nmol/L] | −0.572 | 0.001 ** |
| Glycine-conj. Bile acids [nmol/L] | −0.383 | 0.044 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Höpfinger, A.; Karrasch, T.; Schäffler, A.; Schmid, A. Circulating Levels of Cathelicidin Antimicrobial Peptide (CAMP) Are Affected by Oral Lipid Ingestion. Nutrients 2023, 15, 3021. https://doi.org/10.3390/nu15133021
Höpfinger A, Karrasch T, Schäffler A, Schmid A. Circulating Levels of Cathelicidin Antimicrobial Peptide (CAMP) Are Affected by Oral Lipid Ingestion. Nutrients. 2023; 15(13):3021. https://doi.org/10.3390/nu15133021
Chicago/Turabian StyleHöpfinger, Alexandra, Thomas Karrasch, Andreas Schäffler, and Andreas Schmid. 2023. "Circulating Levels of Cathelicidin Antimicrobial Peptide (CAMP) Are Affected by Oral Lipid Ingestion" Nutrients 15, no. 13: 3021. https://doi.org/10.3390/nu15133021
APA StyleHöpfinger, A., Karrasch, T., Schäffler, A., & Schmid, A. (2023). Circulating Levels of Cathelicidin Antimicrobial Peptide (CAMP) Are Affected by Oral Lipid Ingestion. Nutrients, 15(13), 3021. https://doi.org/10.3390/nu15133021






