Ketogenic-Mimicking Diet as a Therapeutic Modality for Bipolar Disorder: Biomechanistic Rationale and Protocol for a Pilot Clinical Trial
Abstract
:1. Introduction
2. Biomechanistic Rationale
3. Methods
3.1. Study Design: Overview
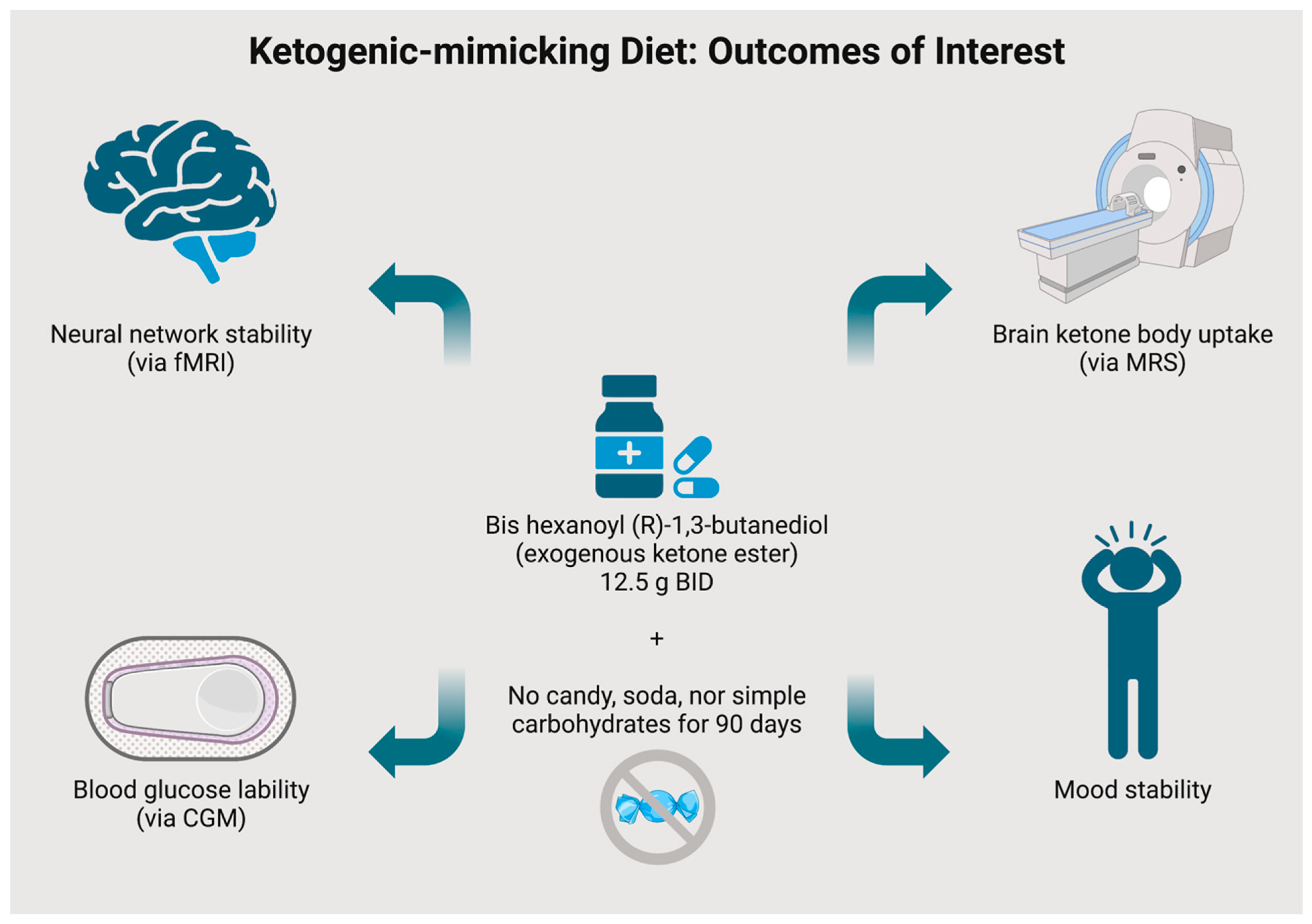
3.2. Experimental Intervention
3.3. Outcome Measures
- Neural network stability as ascertained via functional magnetic resonance imaging (fMRI)
- Neural uptake of ketone bodies as ascertained via magnetic resonance spectroscopy (MRS)
- Clinical assessments and global functioning metrics (see Table 1)
- Laboratory assessments (see Table 2)
- Continuous glucose monitoring (CGM) glycemic trends
- ß-hydroxybutyrate (ß-HB) blood level (as ascertained via Keto-Mojo™ monitoring device)
- Sleep quality (as ascertained via Oura™ biometric ring)
- Not consuming soda for the duration of the intervention period.
- Not consuming candy/sweets for the duration of the intervention period.
- Not consuming white grains/rice for the duration of the intervention period, instead replacing these with complex carbohydrates such as whole grains and brown rice.
- If consuming fruit with a meal, eating the fruit at the end of the meal to minimize glycemic spiking.
- Adults 18 and older, able to provide informed consent
- Diagnosis of bipolar disorder (any subtype)
- History of traumatic brain injury
- Evidence of large vessel stroke or mass lesion on MRI
- History of significant gastrointestinal disease or malabsorptive disorder
- Pregnancy or breastfeeding
- Uncontrolled diabetes mellitus
- History of mitochondrial disorder and/or significant uncontrolled metabolic/medical disorder
- Active/current substance dependence (and/or consumption of two or more alcoholic beverages per day)
- Subjects with contra-indications to MR imaging, including pacemakers or severe claustrophobia
- Active suicidal or homicidal ideation
| Outcome Metric | Pre-Intervention Imaging * | Baseline Clinical Assessment * | Daily | Mid-Intervention | Post-Intervention Imaging * | Post-Intervention Clinical Assessment * |
|---|---|---|---|---|---|---|
| fMRI/MRS | X | X | ||||
| CGM | X | X | X | X | ||
| Oura™ | X | X | X | X | X | X |
| YMRS | X | X | X | |||
| BDI | X | X | X | |||
| PHQ-9 | X | X | X | |||
| Mood Surveys | X | X | X | X | ||
| CGI | X | X | X | |||
| GAF | X | X | X | |||
| LFQ | X | X | X | |||
| SDS | X | X | X | |||
| STAI | X | X | X | |||
| SF-36 | X | X | X | |||
| Blood ketones | X | X | X | |||
| Blood glucose | X | X | X | |||
| Lab assays (see Table 2) | X | X |
4. Statistical Analysis
5. Discussion
6. Limitations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Arvilommi, P.; Suominen, K.; Mantere, O.; Valtonen, H.; Leppämäki, S.; Isometsä, E. Predictors of long-term work disability among patients with type I and II bipolar disorder: A prospective 18-month follow-up study. Bipolar Disord. 2015, 17, 821–835. [Google Scholar] [CrossRef]
- Ferrari, A.J.; Stockings, E.; Khoo, J.P.; Erskine, H.E.; Degenhardt, L.; Vos, T.; Whiteford, H.A. The prevalence and burden of bipolar disorder: Findings from the Global Burden of Disease Study 2013. Bipolar Disord. 2016, 18, 440–450. [Google Scholar] [CrossRef] [PubMed]
- Judd, L.L.; Akiskal, H.S. The prevalence and disability of bipolar spectrum disorders in the US population: Re-analysis of the ECA database taking into account subthreshold cases. J. Affect. Disord. 2003, 73, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Post, R.M. Treatment of Bipolar Depression: Evolving Recommendations. Psychiatr. Clin. N. Am. 2016, 39, 11–33. [Google Scholar] [CrossRef]
- McIntyre, R.S.; Berk, M.; Brietzke, E.; Goldstein, B.I.; Lopez-Jaramillo, C.; Kessing, L.V.; Malhi, G.S.; Nierenberg, A.A.; Rosenblat, J.D.; Majeed, A.; et al. Bipolar disorders. Lancet 2020, 396, 1841–1856. [Google Scholar] [CrossRef] [PubMed]
- Baldessarini, R.J.; Vazquez, G.H.; Tondo, L. Bipolar depression: A major unsolved challenge. Int. J. Bipolar Disord. 2020, 8, 1. [Google Scholar] [CrossRef]
- Li, C.T.; Bai, Y.M.; Huang, Y.L.; Chen, Y.S.; Chen, T.J.; Cheng, J.Y.; Su, T.P. Association between antidepressant resistance in unipolar depression and subsequent bipolar disorder: Cohort study. Br. J. Psychiatry 2012, 200, 45–51. [Google Scholar] [CrossRef]
- McElroy, S.L.; Kotwal, R.; Kaneria, R.; Keck, P.E., Jr. Antidepressants and suicidal behavior in bipolar disorder. Bipolar Disord. 2006, 8, 596–617. [Google Scholar] [CrossRef]
- Chakrabarti, S. Treatment-adherence in bipolar disorder: A patient-centred approach. World J. Psychiatry 2016, 6, 399–409. [Google Scholar] [CrossRef]
- Montes, J.M.; Maurino, J.; de Dios, C.; Medina, E. Suboptimal treatment adherence in bipolar disorder: Impact on clinical outcomes and functioning. Patient Prefer. Adherence 2013, 7, 89–94. [Google Scholar] [CrossRef]
- Tohen, M.; Zarate, C.A., Jr.; Hennen, J.; Khalsa, H.M.; Strakowski, S.M.; Gebre-Medhin, P.; Salvatore, P.; Baldessarini, R.J. The McLean-Harvard First-Episode Mania Study: Prediction of recovery and first recurrence. Am. J. Psychiatry 2003, 160, 2099–2107. [Google Scholar] [CrossRef] [PubMed]
- Kishi, T.; Ikuta, T.; Matsuda, Y.; Sakuma, K.; Okuya, M.; Mishima, K.; Iwata, N. Mood stabilizers and/or antipsychotics for bipolar disorder in the maintenance phase: A systematic review and network meta-analysis of randomized controlled trials. Mol. Psychiatry 2021, 26, 4146–4157. [Google Scholar] [CrossRef] [PubMed]
- Nath, M.; Gupta, V. Mood Stabilizers; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Gould, T.D.; Chen, G.; Manji, H.K. Mood stabilizer psychopharmacology. Clin. Neurosci. Res. 2002, 2, 193–212. [Google Scholar] [CrossRef] [PubMed]
- Post, R.M.; Denicoff, K.D.; Frye, M.A.; Dunn, R.T.; Leverich, G.S.; Osuch, E.; Speer, A. A history of the use of anticonvulsants as mood stabilizers in the last two decades of the 20th century. Neuropsychobiology 1998, 38, 152–166. [Google Scholar] [CrossRef]
- Cunningham, M.O.; Jones, R.S. The anticonvulsant, lamotrigine decreases spontaneous glutamate release but increases spontaneous GABA release in the rat entorhinal cortex in vitro. Neuropharmacology 2000, 39, 2139–2146. [Google Scholar] [CrossRef]
- Bailey, E.E.; Pfeifer, H.H.; Thiele, E.A. The use of diet in the treatment of epilepsy. Epilepsy Behav. 2005, 6, 4–8. [Google Scholar] [CrossRef]
- Christofferson, T. Ketones, The Fourth Fuel: Warburg to Krebs to Veech; Independently Published; Amazon Digital Services LLC: Seattle, WA, USA, 2020. [Google Scholar]
- Phelps, J.R.; Siemers, S.V.; El-Mallakh, R.S. The ketogenic diet for type II bipolar disorder. Neurocase 2013, 19, 423–426. [Google Scholar] [CrossRef]
- Campbell, I.H.; Campbell, H. Ketosis and bipolar disorder: Controlled analytic study of online reports. BJPsych Open 2019, 5, e58. [Google Scholar] [CrossRef]
- Danan, A.; Westman, E.C.; Saslow, L.R.; Ede, G. The Ketogenic Diet for Refractory Mental Illness: A Retrospective Analysis of 31 Inpatients. Front. Psychiatry 2022, 13, 951376. [Google Scholar] [CrossRef]
- Kim, Y.; Santos, R.; Gage, F.H.; Marchetto, M.C. Molecular Mechanisms of Bipolar Disorder: Progress Made and Future Challenges. Front. Cell. Neurosci. 2017, 11, 30. [Google Scholar] [CrossRef]
- Andreazza, A.C.; Shao, L.; Wang, J.F.; Young, L.T. Mitochondrial complex I activity and oxidative damage to mitochondrial proteins in the prefrontal cortex of patients with bipolar disorder. Arch. Gen. Psychiatry 2010, 67, 360–368. [Google Scholar] [CrossRef]
- Das, S.C.; Hjelm, B.E.; Rollins, B.L.; Sequeira, A.; Morgan, L.; Omidsalar, A.A.; Schatzberg, A.F.; Barchas, J.D.; Lee, F.S.; Myers, R.M.; et al. Mitochondria DNA copy number, mitochondria DNA total somatic deletions, Complex I activity, synapse number, and synaptic mitochondria number are altered in schizophrenia and bipolar disorder. Transl. Psychiatry 2022, 12, 353. [Google Scholar] [CrossRef] [PubMed]
- Folbergrová, J.; Kunz, W.S. Mitochondrial dysfunction in epilepsy. Mitochondrion 2012, 12, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Hou, Q.; Wang, M.; Lin, A.; Jarzylo, L.; Navis, A.; Raissi, A.; Liu, F.; Man, H.Y. Na,K-ATPase activity regulates AMPA receptor turnover through proteasome-mediated proteolysis. J. Neurosci. 2009, 29, 4498–4511. [Google Scholar] [CrossRef] [PubMed]
- El-Mallakh, R.S.; Barrett, J.L.; Jed Wyatt, R. The Na,K-ATPase Hypothesis for Bipolar Disorder: Implications of Normal Development. J. Child Adolesc. Psychopharmacol. 1993, 3, 37–52. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Lei, Z.; El-Mallakh, R.S. Lithium normalizes elevated intracellular sodium. Bipolar Disord. 2007, 9, 298–300. [Google Scholar] [CrossRef]
- El-Mallakh, R.S.; Huff, M.O. Mood stabilizers and ion regulation. Harv. Rev. Psychiatry 2001, 9, 23–32. [Google Scholar] [CrossRef]
- Roberts, R.J.; Repass, R.; El-Mallakh, R.S. Effect of dopamine on intracellular sodium: A common pathway for pharmacological mechanism of action in bipolar illness. World J. Biol. Psychiatry 2010, 11, 181–187. [Google Scholar] [CrossRef]
- Gano, L.B.; Patel, M.; Rho, J.M. Ketogenic diets, mitochondria, and neurological diseases. J. Lipid Res. 2014, 55, 2211–2228. [Google Scholar] [CrossRef]
- Miller, V.J.; Villamena, F.A.; Volek, J.S. Nutritional Ketosis and Mitohormesis: Potential Implications for Mitochondrial Function and Human Health. J. Nutr. Metab. 2018, 2018, 5157645. [Google Scholar] [CrossRef]
- Bostock, E.C.; Kirkby, K.C.; Taylor, B.V. The Current Status of the Ketogenic Diet in Psychiatry. Front. Psychiatry 2017, 8, 43. [Google Scholar] [CrossRef] [PubMed]
- Sinning, A.; Hubner, C.A. Minireview: pH and synaptic transmission. FEBS Lett. 2013, 587, 1923–1928. [Google Scholar] [CrossRef] [PubMed]
- Olson, C.A.; Vuong, H.E.; Yano, J.M.; Liang, Q.Y.; Nusbaum, D.J.; Hsiao, E.Y. The Gut Microbiota Mediates the Anti-Seizure Effects of the Ketogenic Diet. Cell 2018, 173, 1728–1741.e13. [Google Scholar] [CrossRef] [PubMed]
- Lutas, A.; Yellen, G. The ketogenic diet: Metabolic influences on brain excitability and epilepsy. Trends Neurosci. 2013, 36, 32–40. [Google Scholar] [CrossRef]
- Yudkoff, M.; Daikhin, Y.; Nissim, I.; Lazarow, A.; Nissim, I. Ketogenic diet, brain glutamate metabolism and seizure control. Prostaglandins Leukot. Essent. Fatty Acids 2004, 70, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Machado-Vieira, R.; Khairova, R. Synaptic plasticity in the pathophysiology and treatment of bipolar disorder. Curr. Top. Behav. Neurosci. 2011, 5, 167–185. [Google Scholar] [CrossRef]
- Valenzuela, P.L.; Castillo-García, A.; Morales, J.S.; Lucia, A. Perspective: Ketone Supplementation in Sports-Does It Work? Adv. Nutr. 2021, 12, 305–315. [Google Scholar] [CrossRef]
- Hu, E.; Du, H.; Zhu, X.; Wang, L.; Shang, S.; Wu, X.; Lu, H.; Lu, X. Beta-hydroxybutyrate Promotes the Expression of BDNF in Hippocampal Neurons under Adequate Glucose Supply. Neuroscience 2018, 386, 315–325. [Google Scholar] [CrossRef]
- Norgren, J.; Daniilidou, M.; Kåreholt, I.; Sindi, S.; Akenine, U.; Nordin, K.; Rosenborg, S.; Ngandu, T.; Kivipelto, M.; Sandebring-Matton, A. Serum proBDNF Is Associated With Changes in the Ketone Body β-Hydroxybutyrate and Shows Superior Repeatability Over Mature BDNF: Secondary Outcomes From a Cross-Over Trial in Healthy Older Adults. Front. Aging Neurosci. 2021, 13, 716594. [Google Scholar] [CrossRef]
- Sleiman, S.F.; Henry, J.; Al-Haddad, R.; El Hayek, L.; Abou Haidar, E.; Stringer, T.; Ulja, D.; Karuppagounder, S.S.; Holson, E.B.; Ratan, R.R.; et al. Exercise promotes the expression of brain derived neurotrophic factor (BDNF) through the action of the ketone body β-hydroxybutyrate. Elife 2016, 5, e15092. [Google Scholar] [CrossRef]
- Walsh, J.J.; Myette-Côté, É.; Little, J.P. The Effect of Exogenous Ketone Monoester Ingestion on Plasma BDNF during an Oral Glucose Tolerance Test. Front. Physiol. 2020, 11, 1094. [Google Scholar] [CrossRef] [PubMed]
- Chase, H.W.; Phillips, M.L. Elucidating neural network functional connectivity abnormalities in bipolar disorder: Toward a harmonized methodological approach. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2016, 1, 288–298. [Google Scholar] [CrossRef] [PubMed]
- Mujica-Parodi, L.R.; Amgalan, A.; Sultan, S.F.; Antal, B.; Sun, X.; Skiena, S.; Lithen, A.; Adra, N.; Ratai, E.M.; Weistuch, C.; et al. Diet modulates brain network stability, a biomarker for brain aging, in young adults. Proc. Natl. Acad. Sci. USA 2020, 117, 6170–6177. [Google Scholar] [CrossRef] [PubMed]
- Mertens, J.; Wang, Q.W.; Kim, Y.; Yu, D.X.; Pham, S.; Yang, B.; Zheng, Y.; Diffenderfer, K.E.; Zhang, J.; Soltani, S.; et al. Differential responses to lithium in hyperexcitable neurons from patients with bipolar disorder. Nature 2015, 527, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, Z.; D’Agostino, D.P.; Diamond, D.; Kindy, M.S.; Rogers, C.; Ari, C. Therapeutic Potential of Exogenous Ketone Supplement Induced Ketosis in the Treatment of Psychiatric Disorders: Review of Current Literature. Front. Psychiatry 2019, 10, 363. [Google Scholar] [CrossRef]
- Jensen, N.J.; Wodschow, H.Z.; Nilsson, M.; Rungby, J. Effects of Ketone Bodies on Brain Metabolism and Function in Neurodegenerative Diseases. Int. J. Mol. Sci. 2020, 21, 8767. [Google Scholar] [CrossRef]
- Youm, Y.H.; Nguyen, K.Y.; Grant, R.W.; Goldberg, E.L.; Bodogai, M.; Kim, D.; D’Agostino, D.; Planavsky, N.; Lupfer, C.; Kanneganti, T.D.; et al. The ketone metabolite beta-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nat. Med. 2015, 21, 263–269. [Google Scholar] [CrossRef]
- Rao, J.S.; Harry, G.J.; Rapoport, S.I.; Kim, H.W. Increased excitotoxicity and neuroinflammatory markers in postmortem frontal cortex from bipolar disorder patients. Mol. Psychiatry 2010, 15, 384–392. [Google Scholar] [CrossRef]
- Fu, S.P.; Li, S.N.; Wang, J.F.; Li, Y.; Xie, S.S.; Xue, W.J.; Liu, H.M.; Huang, B.X.; Lv, Q.K.; Lei, L.C.; et al. BHBA suppresses LPS-induced inflammation in BV-2 cells by inhibiting NF-kappaB activation. Mediators Inflamm. 2014, 2014, 983401. [Google Scholar] [CrossRef]
- Goldberg, E.L.; Asher, J.L.; Molony, R.D.; Shaw, A.C.; Zeiss, C.J.; Wang, C.; Morozova-Roche, L.A.; Herzog, R.I.; Iwasaki, A.; Dixit, V.D. beta-Hydroxybutyrate Deactivates Neutrophil NLRP3 Inflammasome to Relieve Gout Flares. Cell Rep. 2017, 18, 2077–2087. [Google Scholar] [CrossRef]
- Newman, J.C.; Verdin, E. Ketone bodies as signaling metabolites. Trends Endocrinol. Metab. 2014, 25, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Elamin, M.; Ruskin, D.N.; Masino, S.A.; Sacchetti, P. Ketogenic Diet Modulates NAD(+)-Dependent Enzymes and Reduces DNA Damage in Hippocampus. Front. Cell. Neurosci. 2018, 12, 263. [Google Scholar] [CrossRef]
- Kolb, H.; Kempf, K.; Rohling, M.; Lenzen-Schulte, M.; Schloot, N.C.; Martin, S. Ketone bodies: From enemy to friend and guardian angel. BMC Med. 2021, 19, 313. [Google Scholar] [CrossRef] [PubMed]
- Bosch-Presegue, L.; Vaquero, A. Sirtuins in stress response: Guardians of the genome. Oncogene 2014, 33, 3764–3775. [Google Scholar] [CrossRef]
- Coello, K.; Vinberg, M.; Knop, F.K.; Pedersen, B.K.; McIntyre, R.S.; Kessing, L.V.; Munkholm, K. Metabolic profile in patients with newly diagnosed bipolar disorder and their unaffected first-degree relatives. Int. J. Bipolar Disord. 2019, 7, 8. [Google Scholar] [CrossRef]
- Shi, X.F.; Carlson, P.J.; Sung, Y.H.; Fiedler, K.K.; Forrest, L.N.; Hellem, T.L.; Huber, R.S.; Kim, S.E.; Zuo, C.; Jeong, E.K.; et al. Decreased brain PME/PDE ratio in bipolar disorder: A preliminary (31) P magnetic resonance spectroscopy study. Bipolar Disord. 2015, 17, 743–752. [Google Scholar] [CrossRef]
- Pivovarov, A.S.; Calahorro, F.; Walker, R.J. Na(+)/K(+)-pump and neurotransmitter membrane receptors. Invert. Neurosci. 2018, 19, 1. [Google Scholar] [CrossRef]
- Palmer, C. Brain Energy: A Revolutionary Breakthrough in Understanding Mental Health–and Improving Treatment for Anxiety, Depression, OCD, PTSD, and More; BenBella Books: Dallas, TX, USA, 2022. [Google Scholar]
- Daniels, T.E.; Olsen, E.M.; Tyrka, A.R. Stress and Psychiatric Disorders: The Role of Mitochondria. Annu. Rev. Clin. Psychol. 2020, 16, 165–186. [Google Scholar] [CrossRef] [PubMed]
- Brooks, J.O., 3rd; Hoblyn, J.C.; Woodard, S.A.; Rosen, A.C.; Ketter, T.A. Corticolimbic metabolic dysregulation in euthymic older adults with bipolar disorder. J. Psychiatr. Res. 2009, 43, 497–502. [Google Scholar] [CrossRef]
- Brooks, J.O., 3rd; Wang, P.W.; Bonner, J.C.; Rosen, A.C.; Hoblyn, J.C.; Hill, S.J.; Ketter, T.A. Decreased prefrontal, anterior cingulate, insula, and ventral striatal metabolism in medication-free depressed outpatients with bipolar disorder. J. Psychiatr. Res. 2009, 43, 181–188. [Google Scholar] [CrossRef]
- Bohnen, J.L.B.; Albin, R.L.; Bohnen, N.I. Ketogenic interventions in mild cognitive impairment, Alzheimer’s disease, and Parkinson’s disease: A systematic review and critical appraisal. Front. Neurol. 2023, 14, 1123290. [Google Scholar] [CrossRef] [PubMed]
- Rho, J.M.; Shao, L.R.; Stafstrom, C.E. 2-Deoxyglucose and Beta-Hydroxybutyrate: Metabolic Agents for Seizure Control. Front. Cell. Neurosci. 2019, 13, 172. [Google Scholar] [CrossRef]
- Mattson, M.P. Involvement of GABAergic interneuron dysfunction and neuronal network hyperexcitability in Alzheimer’s disease: Amelioration by metabolic switching. Int. Rev. Neurobiol. 2020, 154, 191–205. [Google Scholar] [CrossRef] [PubMed]
- Mujica-Parodi, L.R.; Amgalan, A.; Sultan, S.F.; Antal, B.; Skiena, S.; Lithen, A.; Adra, N.; Ratai, E.-M.; Weistuch, C.; Govindarajan, S.T.; et al. Protecting the Aging Brain—Diet-Study. Openneuro 2021. [Google Scholar] [CrossRef]
- Raichle, M.E.; Snyder, A.Z. A default mode of brain function: A brief history of an evolving idea. NeuroImage 2007, 37, 1083–1090. [Google Scholar] [CrossRef]
- Bi, B.; Che, D.; Bai, Y. Neural network of bipolar disorder: Toward integration of neuroimaging and neurocircuit-based treatment strategies. Transl. Psychiatry 2022, 12, 143. [Google Scholar] [CrossRef]




| Clinical Outcome | Metric |
|---|---|
| Mania Symptomatology | Young Mania Rating Scale (YMRS) |
| Depression Symptomatology | Beck Depression Inventory (BDI) Patient Health Questionnaire-9 (PHQ-9) |
| Mood Stability | Brief daily mood surveys delivered via CareEvolution platform |
| Global Functioning | Clinical Global Impression (CGI) Global Assessment of Functioning (GAF) Life Functioning Questionnaire (LFQ) Sheehan Disability Scale (SDS) |
| State and Trait Anxiety | Spielberger State-Trait Anxiety Inventory (STAI) |
| Quality of Life | 36-Item Short Form Health Survey (SF-36) |
| High-sensitivity C-reactive protein (hs-CRP) |
| Comprehensive metabolic panel (CMP) |
| Peripheral mitochondrial function assessment (e.g., serum lactate, WBC mitochondrial DNA copy number, mitochondrial haplotype, or Seahorse assay) |
| Urine drug screen (UDS) |
| 1. | Not consuming soda for the duration of the intervention period. |
| 2. | Not consuming candy/sweets for the duration of the intervention period. |
| 3. | Not consuming white grains/rice for the duration of the intervention period, instead replacing these with complex carbohydrates such as whole grains and brown rice. |
| 4. | If consuming fruit with a meal, eating the fruit at the end of the meal to minimize glycemic spiking. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bohnen, J.L.B.; Wigstrom, T.P.; Griggs, A.M.; Roytman, S.; Paalanen, N.; Andrews, H.A.; Bohnen, N.I.; Franklin, J.J.H.; McInnis, M.G. Ketogenic-Mimicking Diet as a Therapeutic Modality for Bipolar Disorder: Biomechanistic Rationale and Protocol for a Pilot Clinical Trial. Nutrients 2023, 15, 3068. https://doi.org/10.3390/nu15133068
Bohnen JLB, Wigstrom TP, Griggs AM, Roytman S, Paalanen N, Andrews HA, Bohnen NI, Franklin JJH, McInnis MG. Ketogenic-Mimicking Diet as a Therapeutic Modality for Bipolar Disorder: Biomechanistic Rationale and Protocol for a Pilot Clinical Trial. Nutrients. 2023; 15(13):3068. https://doi.org/10.3390/nu15133068
Chicago/Turabian StyleBohnen, Jeffrey L. B., Travis P. Wigstrom, Alexis M. Griggs, Stiven Roytman, Noah Paalanen, Hailemicael A. Andrews, Nicolaas I. Bohnen, Jacob J. H. Franklin, and Melvin G. McInnis. 2023. "Ketogenic-Mimicking Diet as a Therapeutic Modality for Bipolar Disorder: Biomechanistic Rationale and Protocol for a Pilot Clinical Trial" Nutrients 15, no. 13: 3068. https://doi.org/10.3390/nu15133068
APA StyleBohnen, J. L. B., Wigstrom, T. P., Griggs, A. M., Roytman, S., Paalanen, N., Andrews, H. A., Bohnen, N. I., Franklin, J. J. H., & McInnis, M. G. (2023). Ketogenic-Mimicking Diet as a Therapeutic Modality for Bipolar Disorder: Biomechanistic Rationale and Protocol for a Pilot Clinical Trial. Nutrients, 15(13), 3068. https://doi.org/10.3390/nu15133068










