Prevention and Management of Iron Deficiency/Iron-Deficiency Anemia in Women: An Asian Expert Consensus
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Expert Panel
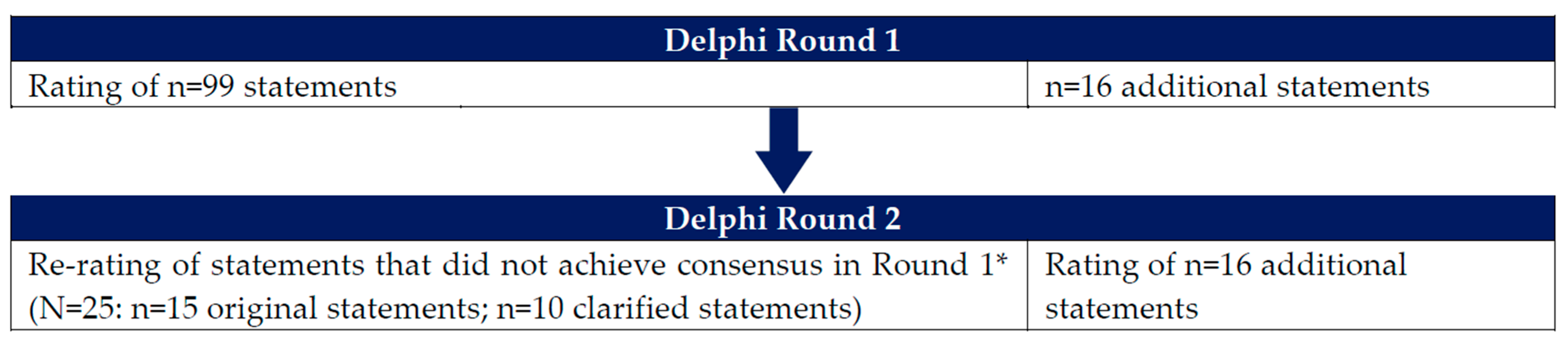
2.2. Development of Items for Round 1 of the Delphi Survey
2.3. Development of Items for Round 2 of the Delphi Survey
2.4. Grading of Statements
2.5. Development of Clinical Pathway Algorithms
3. Results
3.1. Identification of ID/IDA in Women
3.2. Diagnosis and Assessment of ID/IDA in Women
3.3. Prevention of ID/IDA in Women
3.4. Treatment of ID/IDA in Women
4. Discussion
4.1. Identification of ID/IDA in Women
4.2. Diagnosis and Assessment of ID/IDA in Women
4.3. Prevention of ID/IDA in Women
4.4. Treatment of ID/IDA in Women
4.5. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tang, C.; King, K.; Ross, B.; Hamad, N. Iron deficiency in women: Clearing the rust of silence. Lancet Haematol. 2022, 9, e247–e248. [Google Scholar] [CrossRef] [PubMed]
- Firquet, A.; Kirschner, W.; Bitzer, J. Forty to fifty-five-year-old women and iron deficiency: Clinical considerations and quality of life. Gynecol. Endocrinol. 2017, 33, 503–509. [Google Scholar] [CrossRef] [PubMed]
- Kinyoki, D.; Osgood-Zimmerman, A.E.; Bhattacharjee, N.V.; Kassebaum, N.J.; Hay, S.I. Anemia prevalence in women of reproductive age in low- and middle-income countries between 2000 and 2018. Nat. Med. 2021, 27, 1761–1782. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Jimenez, M.C.; Moreno, G.; Wright, I.; Shih, P.-C.; Vaquero, M.P.; Remacha, A.F. Iron deficiency in menstruating adult women: Much more than anemia. Womens Health Rep. 2020, 1, 26–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Safiri, S.; Kolahi, A.A.; Noori, M.; Nejadghaderi, S.A.; Karamzad, N.; Bragazzi, N.L.; Sullman, M.J.M.; Abdollahi, M.; Collins, G.S.; Kaufman, J.S.; et al. Burden of anemia and its underlying causes in 204 countries and territories, 1990–2019: Results from the Global Burden of Disease Study 2019. J. Hematol. Oncol. 2021, 14, 185. [Google Scholar] [CrossRef]
- Camaschella, C. Iron deficiency. Blood 2019, 133, 30–39. [Google Scholar]
- Mirza, F.G.; Abdul-Kadir, R.; Breymann, C.; Fraser, I.S.; Taher, A. Impact and management of iron deficiency and iron deficiency anemia in women’s health. Expert. Rev. Hematol. 2018, 11, 727–736. [Google Scholar] [CrossRef]
- Goodarzi, E.; Beiranvand, R.; Naemi, H.; Darvishi, I.; Khazaei, Z. Prevalence of iron deficiency anemia in Asian female population and human development index (HDI): An ecological study. Obstet. Gynecol. Sci. 2020, 63, 497–505. [Google Scholar] [CrossRef]
- Benson, C.S.; Shah, A.; Stanworth, S.J.; Frise, C.J.; Spiby, H.; Lax, S.J.; Murray, J.; Klein, A.A. The effect of iron deficiency and anaemia on women’s health. Anaesthesia 2021, 76 (Suppl. 4), 84–95. [Google Scholar] [CrossRef]
- Dugan, C.; MacLean, B.; Cabolis, K.; Abeysiri, S.; Khong, A.; Sajic, M.; Richards, T. The misogyny of iron deficiency. Anaesthesia 2021, 76 (Suppl. 4), 56–62. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Global Nutrition Targets 2025: Anaemia Policy Brief. Available online: https://www.who.int/publications/i/item/WHO-NMH-NHD-14.4 (accessed on 9 June 2022).
- World Health Organization (WHO). Nutritional Anaemias: Tools for Effective Prevention and Control. Available online: https://www.who.int/publications/i/item/9789241513067 (accessed on 8 May 2022).
- Gandhi, A.; Pandit, S.; Malhotra, J.; Joshi, M.; Desai, J.; Biniwale, P.; Deshmukh, V.; Shekhawat, S.S.; Sarmah, M.; Malve, V. Iron deficiency in peri-menopausal women: Clinical considerations from an expert consensus. Indian J. Obstet. Gynecol. Res. 2022, 9, 153–161. [Google Scholar]
- Mazzaferro, S.; D’Alonzo, S.; Morosetti, M. Unmet needs about iron deficiency in peritoneal dialysis: A Delphi consensus panel. BMC Nephrol. 2022, 23, 336. [Google Scholar] [CrossRef]
- Nowak, A.; Angelillo-Scherrer, A.; Betticher, D.; Dickenmann, M.; Guessous, I.; Juillerat, P.; Korte, W.; Neuner-Jehle, S.; Pfister, O.; Surbek, D.; et al. Swiss Delphi study on iron deficiency. Swiss Med. Wkly. 2019, 149, w20097. [Google Scholar] [CrossRef] [PubMed]
- Corwin, H.L.; Shander, A.; Speiss, B.; Muñoz, M.; Faraoni, D.; Calcaterra, D.; Welsby, I.; Ozawa, S.; Arnofsky, A.; Goldweit, R.S.; et al. Management of perioperative iron deficiency in cardiac surgery: A modified RAND Delphi study. Ann. Thorac. Surg. 2022, 113, 316–323. [Google Scholar] [CrossRef] [PubMed]
- Reinisch, W.; Chowers, Y.; Danese, S.; Dignass, A.; Gomollón, F.; Nielsen, O.H.; Lakatos, P.L.; Lees, C.W.; Lindgren, S.; Lukas, M.; et al. The management of iron deficiency in inflammatory bowel disease—An online tool developed by the RAND/UCLA appropriateness method. Aliment. Pharmacol. Ther. 2013, 38, 1109–1118. [Google Scholar] [CrossRef] [Green Version]
- Filler, T.; Foster, A.M.; Grace, S.L.; Stewart, D.E.; Straus, S.E.; Gagliardi, A.R. Patient-centered care for women: Delphi consensus on evidence-derived recommendations. Value Health 2020, 23, 1012–1019. [Google Scholar] [CrossRef]
- Rubino, F.; Puhl, R.M.; Cummings, D.E.; Eckel, R.H.; Ryan, D.H.; Mechanick, J.I.; Nadglowski, J.; Ramos Salas, X.; Schauer, P.R.; Twenefour, D.; et al. Joint international consensus statement for ending stigma of obesity. Nat. Med. 2020, 26, 485–497. [Google Scholar] [CrossRef] [Green Version]
- Breymann, C.; Bian, X.M.; Blanco-Capito, L.R.; Chong, C.; Mahmud, G.; Rehman, R. Expert recommendations for the diagnosis and treatment of iron-deficiency anemia during pregnancy and the postpartum period in the Asia-Pacific region. J. Perinat. Med. 2011, 39, 113–121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Q.; Lu, X.M.; Zhang, M.; Yang, C.Y.; Lv, S.Y.; Li, S.F.; Zhong, C.Y.; Geng, S.S. Adverse effects of iron deficiency anemia on pregnancy outcome and offspring development and intervention of three iron supplements. Sci. Rep. 2021, 11, 1347. [Google Scholar] [CrossRef]
- Sari, P.; Herawati, D.M.D.; Dhamayanti, M.; Hilmanto, D. Fundamental aspects of the development of a model of an integrated health care system for the prevention of iron deficiency anemia among adolescent girls: A qualitative study. Int. J. Environ. Res. Public Health 2022, 19, 13811. [Google Scholar] [CrossRef]
- World Health Organization (WHO). WHO Recommendations on Antenatal Care for a Positive Pregnancy Experience. Available online: https://www.who.int/publications/i/item/9789241549912 (accessed on 9 November 2021).
- World Health Organization (WHO). Guideline: Daily Iron Supplementation in Adult Women and Adolescent Girls. Available online: https://apps.who.int/iris/handle/10665/204761 (accessed on 9 November 2021).
- FIGO Working Group on Good Clinical Practice in Maternal-Fetal Medicine. Good clinical practice advice: Iron deficiency anemia in pregnancy. Int. J. Gynaecol. Obstet. 2019, 144, 322–324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mansour, D.; Hofmann, A.; Gemzell-Danielsson, K. A review of clinical guidelines on the management of iron deficiency and iron-deficiency anemia in women with heavy menstrual bleeding. Adv. Ther. 2021, 38, 201–225. [Google Scholar] [CrossRef]
- Jefferds, M.E.D.; Mei, Z.; Addo, Y.; Hamner, H.C.; Perrine, C.G.; Flores-Ayala, R.; Pfeiffer, C.M.; Sharma, A.J. Iron deficiency in the United States: Limitations in guidelines, data, and monitoring of disparities. Am. J. Public Health 2022, 112, S826–S835. [Google Scholar] [CrossRef]
- Pagani, A.; Nai, A.; Silvestri, L.; Camaschella, C. Hepcidin and anemia: A tight relationship. Front. Physiol. 2019, 10, 1294. [Google Scholar] [CrossRef]
- O’Brien, S.H. Evaluation and management of heavy menstrual bleeding in adolescents: The role of the hematologist. Hematol. Am. Soc. Hematol. Educ. Program 2018, 2018, 390–398. [Google Scholar] [CrossRef] [Green Version]
- Balendran, S.; Forsyth, C. Non-anaemic iron deficiency. Aust. Prescr. 2021, 44, 193–196. [Google Scholar] [CrossRef]
- Soppi, E.T. Iron deficiency without anemia—A clinical challenge. Clin. Case Rep. 2018, 6, 1082–1086. [Google Scholar] [CrossRef] [PubMed]
- Lopez, A.; Cacoub, P.; Macdougall, I.C.; Peyrin-Biroulet, L. Iron deficiency anaemia. Lancet 2016, 387, 907–916. [Google Scholar] [CrossRef] [PubMed]
- Pavord, S.; Daru, J.; Prasannan, N.; Robinson, S.; Stanworth, S.; Girling, J. UK guidelines on the management of iron deficiency in pregnancy. Br. J. Haematol. 2020, 188, 819–830. [Google Scholar] [CrossRef] [Green Version]
- Dimauro, G.; De Ruvo, S.; Di Terlizzi, F.; Ruggieri, A.; Volpe, V.; Colizzi, L.; Girardi, F. Estimate of anemia with new non-invasive systems—A moment of reflection. Electronics 2020, 9, 780. [Google Scholar] [CrossRef]
- Mannino, R.G.; Myers, D.R.; Tyburski, E.A.; Caruso, C.; Boudreaux, J.; Leong, T.; Clifford, G.D.; Lam, W.A. Smartphone app for non-invasive detection of anemia using only patient-sourced photos. Nat. Commun. 2018, 9, 4924. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manesh, R.S.; Kohlwes, R.J. Palmar Crease Pallor. J. Gen. Intern. Med. 2015, 30, 1034. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Breymann, C.; Auerbach, M. Iron deficiency in gynecology and obstetrics: Clinical implications and management. Hematol. Am. Soc. Hematol. Educ. Program 2017, 2017, 152–159. [Google Scholar] [CrossRef] [Green Version]
- Resseguier, A.S.; Guiguet-Auclair, C.; Debost-Legrand, A.; Serre-Sapin, A.F.; Gerbaud, L.; Vendittelli, F.; Ruivard, M. Prediction of iron deficiency anemia in third trimester of pregnancy based on data in the first trimester: A prospective cohort study in a high-income country. Nutrients 2022, 14, 4091. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). WHO Guideline on Use of Ferritin Concentrations to Assess Iron Status in Individuals and Populations. Available online: https://www.who.int/publications/i/item/9789240000124 (accessed on 5 November 2021).
- Daru, J.; Colman, K.; Stanworth, S.J.; De La Salle, B.; Wood, E.M.; Pasricha, S.R. Serum ferritin as an indicator of iron status: What do we need to know? Am. J. Clin. Nutr. 2017, 106, 1634s–1639s. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daru, J.; Allotey, J.; Peña-Rosas, J.P.; Khan, K.S. Serum ferritin thresholds for the diagnosis of iron deficiency in pregnancy: A systematic review. Transfus. Med. 2017, 27, 167–174. [Google Scholar] [CrossRef] [Green Version]
- Fertrin, K.Y. Diagnosis and management of iron deficiency in chronic inflammatory conditions (CIC): Is too little iron making your patient sick? Hematol. Am. Soc. Hematol. Educ. Program 2020, 2020, 478–486. [Google Scholar] [CrossRef]
- Finkelstein, J.L.; Fothergill, A.; Guetterman, H.M.; Johnson, C.B.; Bose, B.; Qi, Y.P.; Rose, C.E.; Williams, J.L.; Mehta, S.; Kuriyan, R.; et al. Iron status and inflammation in women of reproductive age: A population-based biomarker survey and clinical study. Clin. Nutr. ESPEN 2022, 49, 483–494. [Google Scholar] [CrossRef]
- Agarwal, A.M.; Rets, A. Laboratory approach to investigation of anemia in pregnancy. Int. J. Lab. Hematol. 2021, 43 (Suppl. 1), 65–70. [Google Scholar] [CrossRef]
- Grant, E.S.; Clucas, D.B.; McColl, G.; Hall, L.T.; Simpson, D.A. Re-examining ferritin-bound iron: Current and developing clinical tools. Clin. Chem. Lab. Med. 2021, 59, 459–471. [Google Scholar] [CrossRef]
- Pasricha, S.R.; Tye-Din, J.; Muckenthaler, M.U.; Swinkels, D.W. Iron deficiency. Lancet 2021, 397, 233–248. [Google Scholar] [CrossRef] [PubMed]
- Cappellini, M.D.; Musallam, K.M.; Taher, A.T. Iron deficiency anaemia revisited. J. Intern. Med. 2020, 287, 153–170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Snook, J.; Bhala, N.; Beales, I.L.P.; Cannings, D.; Kightley, C.; Logan, R.P.; Pritchard, D.M.; Sidhu, R.; Surgenor, S.; Thomas, W.; et al. British Society of Gastroenterology guidelines for the management of iron deficiency anaemia in adults. Gut 2021, 70, 2030–2051. [Google Scholar] [CrossRef]
- Zhao, J.; Zhu, X.; Dai, Q.; Hong, X.; Zhang, H.; Huang, K.; Wang, Y.; Yang, X.; Zhang, Y.; Peng, Z.; et al. The prevalence and influencing factors of anaemia among pre-pregnant women in mainland China: A large population-based, cross-sectional study. Br. J. Nutr. 2022, 127, 439–450. [Google Scholar] [CrossRef]
- Coad, J.; Pedley, K. Iron deficiency and iron deficiency anemia in women. Scand. J. Clin. Lab. Investig. Suppl. 2014, 244, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Dziembowska, I.; Kwapisz, J.; Izdebski, P.; Żekanowska, E. Mild iron deficiency may affect female endurance and behavior. Physiol. Behav. 2019, 205, 44–50. [Google Scholar] [CrossRef] [PubMed]
- McArdle, H.J.; Gambling, L.; Kennedy, C. Iron deficiency during pregnancy: The consequences for placental function and fetal outcome. Proc. Nutr. Soc. 2014, 73, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Gaxiola, A.C.; De-Regil, L.M. Intermittent iron supplementation for reducing anaemia and its associated impairments in adolescent and adult menstruating women. Cochrane Database Syst. Rev. 2019, 1, Cd009218. [Google Scholar]
- Low, M.S.; Speedy, J.; Styles, C.E.; De-Regil, L.M.; Pasricha, S.R. Daily iron supplementation for improving anaemia, iron status and health in menstruating women. Cochrane Database Syst. Rev. 2016, 4, Cd009747. [Google Scholar] [CrossRef]
- Peña-Rosas, J.P.; De-Regil, L.M.; Dowswell, T.; Viteri, F.E. Intermittent oral iron supplementation during pregnancy. Cochrane Database Syst. Rev. 2015, 2015, Cd009997. [Google Scholar] [CrossRef]
- Peña-Rosas, J.P.; De-Regil, L.M.; Garcia-Casal, M.N.; Dowswell, T. Daily oral iron supplementation during pregnancy. Cochrane Database Syst. Rev. 2015, 2015, Cd004736. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berber, I.; Diri, H.; Erkurt, M.A.; Aydogdu, I.; Kaya, E.; Kuku, I. Evaluation of ferric and ferrous iron therapies in women with iron deficiency anaemia. Adv. Hematol. 2014, 2014, 297057. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization (WHO). Global Health Observatory Data Repository: Anaemia Women of Reproductive Age, Estimates by WHO Region. Available online: https://apps.who.int/gho/data/view.main.ANAEMIAWOMENREPRODUCTIVEREGSEVv?lang=en (accessed on 3 January 2023).
- World Health Organization (WHO). Global Health Observatory Data Repository: Anaemia in Non-Pregnant Women, Estimates by WHO Region. Available online: https://apps.who.int/gho/data/node.main.ANEMIANNONPREGNANTWOMEN?lang=en (accessed on 3 January 2023).
- World Health Organization (WHO). Global Health Observatory Data Repository: Anaemia in Pregnant Women, Estimates by WHO Region. Available online: https://apps.who.int/gho/data/view.main.ANAEMIAWOMENPWREGv?lang=en (accessed on 3 January 2023).
- Stoffel, N.U.; Zeder, C.; Brittenham, G.M.; Moretti, D.; Zimmermann, M.B. Iron absorption from supplements is greater with alternate day than with consecutive day dosing in iron-deficient anemic women. Haematologica 2020, 105, 1232–1239. [Google Scholar] [CrossRef] [Green Version]
- Marshak, J.; Landy, K. Does routine iron supplementation improve anemia or health outcomes in menstruating women? Evid.-Based Pract. 2021, 24, 41. [Google Scholar] [CrossRef]
- Friedrisch, J.R.; Friedrisch, B.K. Prophylactic iron supplementation in pregnancy: A controversial issue. Biochem. Insights 2017, 10, 1178626417737738. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A.; Sharma, E.; Marley, A.; Samaan, M.A.; Brookes, M.J. Iron deficiency anaemia: Pathophysiology, assessment, practical management. BMJ Open Gastroenterol. 2022, 9, e000759. [Google Scholar] [CrossRef] [PubMed]
- Numan, S.; Kaluza, K. Systematic review of guidelines for the diagnosis and treatment of iron deficiency anemia using intravenous iron across multiple indications. Curr. Med. Res. Opin 2020, 36, 1769–1782. [Google Scholar] [CrossRef] [PubMed]
- Peyrin-Biroulet, L.; Williet, N.; Cacoub, P. Guidelines on the diagnosis and treatment of iron deficiency across indications: A systematic review. Am. J. Clin. Nutr. 2015, 102, 1585–1594. [Google Scholar] [CrossRef] [Green Version]
- Stoffel, N.U.; von Siebenthal, H.K.; Moretti, D.; Zimmermann, M.B. Oral iron supplementation in iron-deficient women: How much and how often? Mol. Aspects Med. 2020, 75, 100865. [Google Scholar] [CrossRef]
- Kron, A.; Del Giudice, M.E.; Sholzberg, M.; Callum, J.; Cserti-Gazdewich, C.; Swarup, V.; Huang, M.; Distefano, L.; Anani, W.; Skeate, R.; et al. Daily versus every other day oral iron supplementation in patients with iron deficiency anemia (DEODO): Study protocol for a phase 3 multicentered, pragmatic, open-label, pilot randomized controlled trial. Pilot Feasibility Stud. 2022, 8, 98. [Google Scholar] [CrossRef]
- Percy, L.; Mansour, D.; Fraser, I. Iron deficiency and iron deficiency anaemia in women. Best Pract. Res. Clin. Obstet. Gynaecol. 2017, 40, 55–67. [Google Scholar] [CrossRef] [PubMed]
- Govindappagari, S.; Newman, R.A.; Burwick, R.M. Iron-deficiency anemia in pregnancy and the role of intravenous iron. Contemp. OB/GYN J. 2021, 66, 20–24. [Google Scholar]
- World Health Organization (WHO). Guideline: Iron Supplementation in Postpartum Women. Available online: https://apps.who.int/iris/handle/10665/249242 (accessed on 13 November 2021).
- Kapil, U.; Kapil, R.; Gupta, A. National Iron Plus Initiative: Current status & future strategy. Indian J. Med. Res. 2019, 150, 239–247. [Google Scholar]
- Stoffel, N.U.; Cercamondi, C.I.; Brittenham, G.; Zeder, C.; Geurts-Moespot, A.J.; Swinkels, D.W.; Moretti, D.; Zimmermann, M.B. Iron absorption from oral iron supplements given on consecutive versus alternate days and as single morning doses versus twice-daily split dosing in iron-depleted women: Two open-label, randomised controlled trials. Lancet Haematol. 2017, 4, e524–e533. [Google Scholar] [CrossRef] [PubMed]
- Moretti, D.; Goede, J.S.; Zeder, C.; Jiskra, M.; Chatzinakou, V.; Tjalsma, H.; Melse-Boonstra, A.; Brittenham, G.; Swinkels, D.W.; Zimmermann, M.B. Oral iron supplements increase hepcidin and decrease iron absorption from daily or twice-daily doses in iron-depleted young women. Blood 2015, 126, 1981–1989. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Poon, M.-C.; Allan, G.M. Iron dosing frequency. Can. Fam. Phys. 2021, 67, 436. [Google Scholar] [CrossRef]
- Kaundal, R.; Bhatia, P.; Jain, A.; Jain, A.; Nampoothiri, R.V.; Mishra, K.; Jandial, A.; Goni, D.; Sandal, R.; Jindal, N.; et al. Randomized controlled trial of twice-daily versus alternate-day oral iron therapy in the treatment of iron-deficiency anemia. Ann. Hematol. 2020, 99, 57–63. [Google Scholar] [CrossRef]
- Pasupathy, E.; Kandasamy, R.; Thomas, K.; Basheer, A. Alternate day versus daily oral iron for treatment of iron deficiency anemia: A randomized controlled trial. Sci. Rep. 2023, 13, 1818. [Google Scholar] [CrossRef]
- Jimenez, K.; Kulnigg-Dabsch, S.; Gasche, C. Management of iron deficiency anemia. Gastroenterol. Hepatol. 2015, 11, 241–250. [Google Scholar]
- Moe, S.; Grill, A.K.; Allan, G.M. Newer iron supplements for anemia. Can. Fam. Phys. 2019, 65, 556. [Google Scholar]
- Muñoz, M.; Gómez-Ramírez, S. Is there a role for iron supplementation in critically ill patients? Med. Intensiv. 2019, 43, 103–107. [Google Scholar] [CrossRef]
- Cancelo-Hidalgo, M.J.; Castelo-Branco, C.; Palacios, S.; Haya-Palazuelos, J.; Ciria-Recasens, M.; Manasanch, J.; Pérez-Edo, L. Tolerability of different oral iron supplements: A systematic review. Curr. Med. Res. Opin. 2013, 29, 291–303. [Google Scholar] [CrossRef] [PubMed]
- Chan, L.N.; Mike, L.A. The science and practice of micronutrient supplementations in nutritional anemia: An evidence-based review. JPEN J. Parenter. Enter. Nutr. 2014, 38, 656–672. [Google Scholar] [CrossRef] [PubMed]
- Abdelwahid, H.A.; Zekry, O.A. The role of multiple micronutrients in treatment of iron deficient anemic children. Fam. Med. Med. Sci. Res. 2013, 1, 102. [Google Scholar] [CrossRef] [Green Version]
- Keats, E.C.; Haider, B.A.; Tam, E.; Bhutta, Z.A. Multiple-micronutrient supplementation for women during pregnancy. Cochrane Database Syst. Rev. 2019, 3, Cd004905. [Google Scholar] [CrossRef] [PubMed]
- Tuncalp, Ö.; Rogers, L.M.; Lawrie, T.A.; Barreix, M.; Peña-Rosas, J.P.; Bucagu, M.; Neilson, J.; Oladapo, O.T. WHO recommendations on antenatal nutrition: An update on multiple micronutrient supplements. BMJ Glob. Health 2020, 5, e003375. [Google Scholar] [CrossRef]
- Shand, A.W.; Bell, J.; Henry, A.; Grzeskowiak, L.E.; Kidson-Gerber, G.; Pearson, S.; Nassar, N. Rapid increase in intravenous iron therapy for women of reproductive age in Australia. Med. J. Aust. 2020, 213, 85–86. [Google Scholar] [CrossRef]
- Schaefer, B.; Meindl, E.; Wagner, S.; Tilg, H.; Zoller, H. Intravenous iron supplementation therapy. Mol. Aspects Med. 2020, 75, 100862. [Google Scholar] [CrossRef]
- Qassim, A.; Grivell, R.M.; Henry, A.; Kidson-Gerber, G.; Shand, A.; Grzeskowiak, L.E. Intravenous or oral iron for treating iron deficiency anaemia during pregnancy: Systematic review and meta-analysis. Med. J. Aust. 2019, 211, 367–373. [Google Scholar] [CrossRef]
- Neogi, S.B.; Devasenapathy, N.; Singh, R.; Bhushan, H.; Shah, D.; Divakar, H.; Zodpey, S.; Malik, S.; Nanda, S.; Mittal, P.; et al. Safety and effectiveness of intravenous iron sucrose versus standard oral iron therapy in pregnant women with moderate-to-severe anaemia in India: A multicentre, open-label, phase 3, randomised, controlled trial. Lancet Glob. Health 2019, 7, e1706–e1716. [Google Scholar] [CrossRef] [Green Version]
- Sharma, R.; Stanek, J.R.; Koch, T.L.; Grooms, L.; O’Brien, S.H. Intravenous iron therapy in non-anemic iron-deficient menstruating adolescent females with fatigue. Am. J. Hematol. 2016, 91, 973–977. [Google Scholar] [CrossRef] [PubMed]
- Krayenbuehl, P.A.; Battegay, E.; Breymann, C.; Furrer, J.; Schulthess, G. Intravenous iron for the treatment of fatigue in nonanemic, premenopausal women with low serum ferritin concentration. Blood 2011, 118, 3222–3227. [Google Scholar] [CrossRef] [Green Version]
- Gybel-Brask, M.; Seeberg, J.; Thomsen, L.L.; Johansson, P.I. Intravenous iron isomaltoside improves hemoglobin concentration and iron stores in female iron-deficient blood donors: A randomized double-blind placebo-controlled clinical trial. Transfusion 2018, 58, 974–981. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tigga, M.P.; Debbarma, A.P. A comparative study to evaluate oral iron and intravenous iron sucrose for treatment of anemia in pregnancy in a poor socioeconomic region of Northeast India. Ci Ji Yi Xue Za Zhi 2020, 32, 258–261. [Google Scholar] [CrossRef] [PubMed]
- Murugesan, S.; Sudakshina, K.; Adhimoolam, M.; Arthi, S. Comparative study on efficacy, tolerability, and cost of different iron supplements among antenatal women with iron-deficiency anemia. Natl. J. Physiol. Pharm. Pharmacol. 2023, 13, 802–808. [Google Scholar] [CrossRef]
| Domain | No. of Retained Delphi Items, n/N (%) | |
|---|---|---|
| Round 1 | Round 2 | |
| Identification of ID/IDA | 22/24 (92%) | 26/29 a (90%) |
| Diagnosis and assessment of ID/IDA | 20/37 (54%) | 28/35 b (80%) |
| Prevention of ID/IDA | 12/16 (75%) | 15/16 (94%) |
| Treatment of ID/IDA | 18/22 (82%) | 26/33 c (79%) |
| Total | 72/99 (73%) | 95/113 (84%) |
| Formulation | Preparation | Dose (mg) * | Elemental Iron (mg) * |
|---|---|---|---|
| Ferrous fumarate | Tablet | 325 | 106 |
| Ferrous gluconate | Tablet | 325 | 35 |
| Ferrous sulphate | Tablet | 325 | 65 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pai, R.D.; Chong, Y.S.; Clemente-Chua, L.R.; Irwinda, R.; Huynh, T.N.K.; Wibowo, N.; Gamilla, M.C.Z.; Mahdy, Z.A. Prevention and Management of Iron Deficiency/Iron-Deficiency Anemia in Women: An Asian Expert Consensus. Nutrients 2023, 15, 3125. https://doi.org/10.3390/nu15143125
Pai RD, Chong YS, Clemente-Chua LR, Irwinda R, Huynh TNK, Wibowo N, Gamilla MCZ, Mahdy ZA. Prevention and Management of Iron Deficiency/Iron-Deficiency Anemia in Women: An Asian Expert Consensus. Nutrients. 2023; 15(14):3125. https://doi.org/10.3390/nu15143125
Chicago/Turabian StylePai, Rishma Dhillon, Yap Seng Chong, Lyra Ruth Clemente-Chua, Rima Irwinda, Trang Nguyen Khanh Huynh, Noroyono Wibowo, Maria Corazon Zaida Gamilla, and Zaleha Abdullah Mahdy. 2023. "Prevention and Management of Iron Deficiency/Iron-Deficiency Anemia in Women: An Asian Expert Consensus" Nutrients 15, no. 14: 3125. https://doi.org/10.3390/nu15143125
APA StylePai, R. D., Chong, Y. S., Clemente-Chua, L. R., Irwinda, R., Huynh, T. N. K., Wibowo, N., Gamilla, M. C. Z., & Mahdy, Z. A. (2023). Prevention and Management of Iron Deficiency/Iron-Deficiency Anemia in Women: An Asian Expert Consensus. Nutrients, 15(14), 3125. https://doi.org/10.3390/nu15143125






