Low Muscle Mass Is Associated with Poorer Glycemic Control and Higher Oxidative Stress in Older Patients with Type 2 Diabetes
Abstract
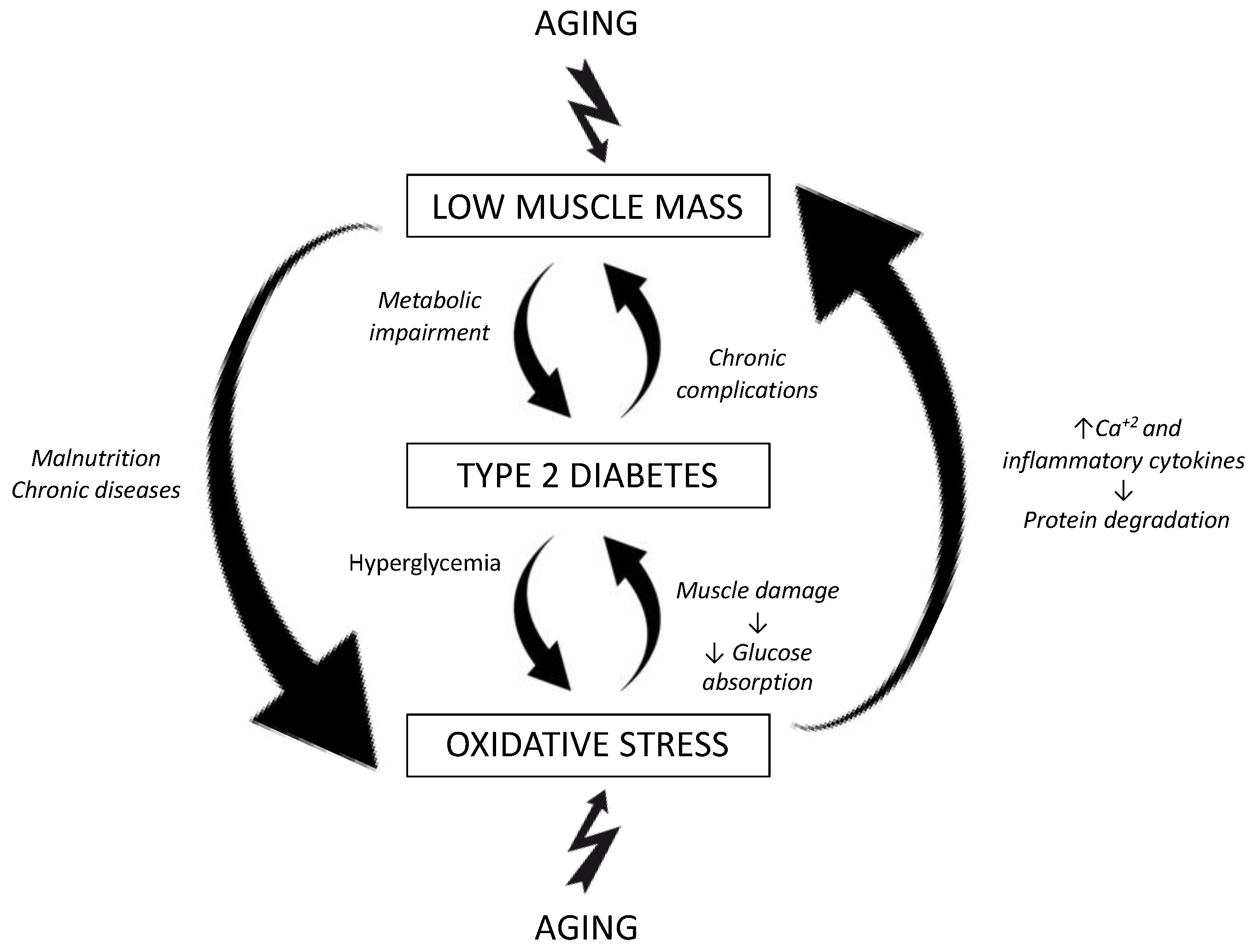
:1. Introduction
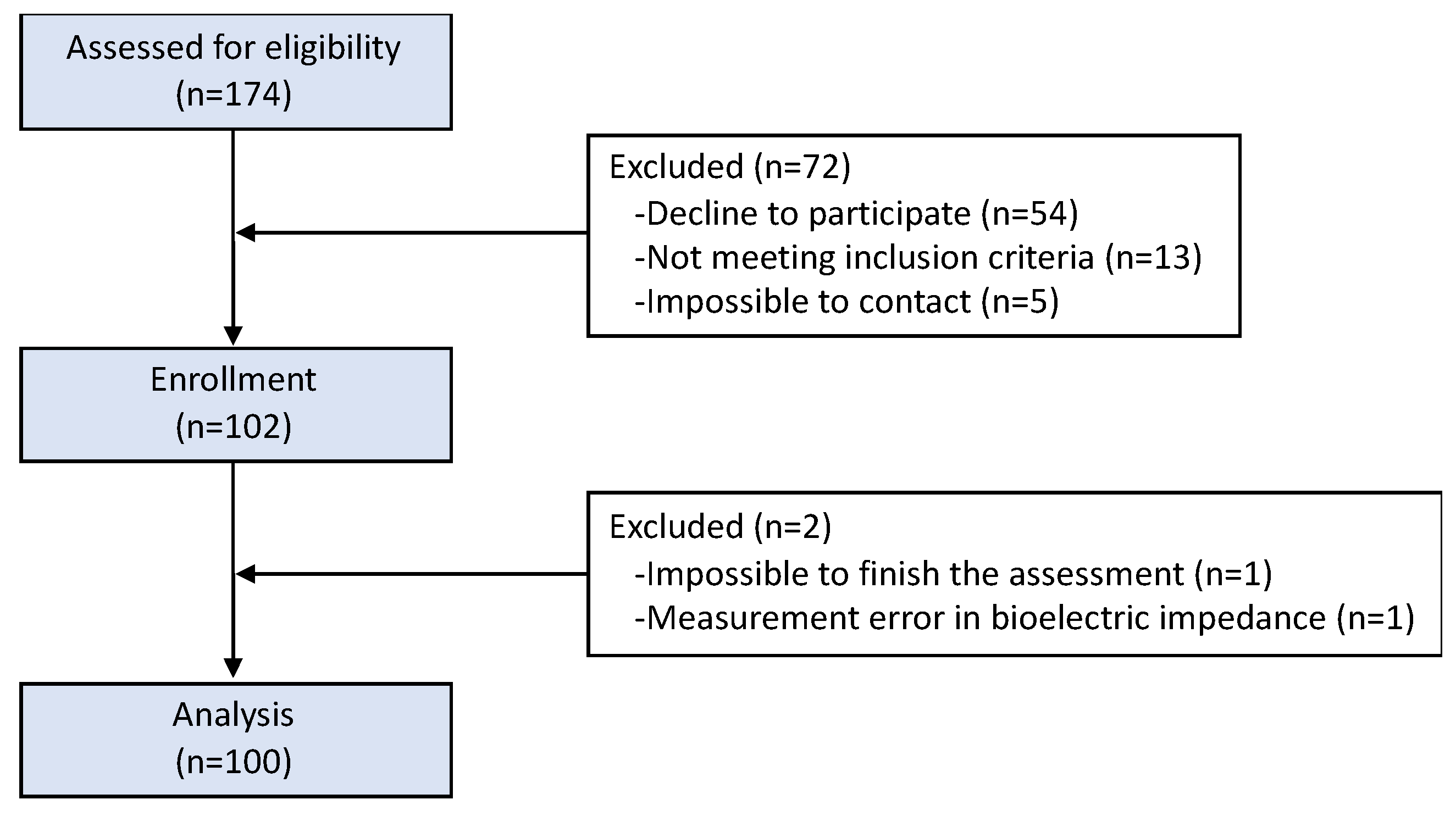
2. Materials and Methods
2.1. Participants
2.2. Clinical and Anthropometric Parameters
2.3. Body Composition and Functionality Parameters
2.4. Biochemical Parameters
2.5. Oxidative Stress
2.6. Statistical Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The hallmarks of aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef] [Green Version]
- Fernando, R.; Drescher, C.; Nowotny, K.; Grune, T.; Castro, J.P. Impaired proteostasis during skeletal muscle aging. Free Radic. Biol. Med. 2019, 132, 58–66. [Google Scholar] [CrossRef]
- Angulo, J.; El Assar, M.; Rodríguez-Mañas, L. Frailty and sarcopenia as the basis for the phenotypic manifestation of chronic diseases in older adults. Mol. Asp. Med. 2016, 50, 1–32. [Google Scholar] [CrossRef] [PubMed]
- Hajek, A.; Buczak-Stec, E.; van den Bussche, H.; Eisele, M.; Oey, A.; Wiese, B.; Weyerer, S.; Werle, J.; Fuchs, A.; Pentzek, M.; et al. Factors leading to institutionalization among the oldest old: Longitudinal findings from the AgeCoDe-AgeQualiDe study. Gerontology 2022, 68, 894–902. [Google Scholar] [CrossRef]
- Bossola, M.; Marino, C.; Di Napoli, A.; Agabiti, N.; Tazza, L.; Davoli, M. Functional impairment and risk of mortality in patients on chronic hemodialysis: Results of the Lazio dialysis registry. J. Nephrol. 2018, 31, 593–602. [Google Scholar] [CrossRef]
- Hajek, A.; König, H.H. What factors are associated with functional impairment among the oldest old? Front. Med. 2022, 9, 1092775. [Google Scholar] [CrossRef]
- Abellan van Kan, G. Epidemiology and consequences of sarcopenia. J. Nutr. Health Aging 2009, 13, 708–712. [Google Scholar] [CrossRef] [PubMed]
- Jungert, A.; Eichner, G.; Neuhäuser-Berthold, M. Trajectories of Body Composition during Advanced Aging in Consideration of Diet and Physical Activity: A 20-Year Longitudinal Study. Nutrients 2020, 12, 3626. [Google Scholar] [CrossRef] [PubMed]
- Tyrovolas, S.; Haro, J.M.; Mariolis, A.; Piscopo, S.; Valacchi, G.; Bountziouka, V.; Anastasiou, F.; Zeimbekis, A.; Tyrovola, D.; Foscolou, A.; et al. Skeletal muscle mass and body fat in relation to successful ageing of older adults: The multi-national MEDIS study. Arch. Gerontol. Geriatr. 2016, 66, 95–101. [Google Scholar] [CrossRef]
- Al-Sofiani, M.E.; Ganji, S.S.; Kalyani, R.R. Body composition changes in diabetes and aging. J. Diabetes Complicat. 2019, 33, 451–459. [Google Scholar] [CrossRef]
- He, Q.; Wang, X.; Yang, C.; Zhuang, X.; Yue, Y.; Jing, H.; Hu, J.; Sun, M.; Guo, L. Metabolic and Nutritional Characteristics in Middle-Aged and Elderly Sarcopenia Patients with Type 2 Diabetes. J. Diabetes Res. 2020, 2020, 6973469. [Google Scholar] [CrossRef] [PubMed]
- Viña, J.; Borras, C.; Gomez-Cabrera, M.C. A free radical theory of frailty. Free Radic. Biol. Med. 2018, 20, 358–363. [Google Scholar] [CrossRef] [PubMed]
- Tournadre, A.; Vial, G.; Capel, F.; Soubrier, M.; Boirie, Y. Sarcopenia. Jt. Bone Spine 2019, 86, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Alabadi, B.; Civera, M.; De la Rosa, A.; Martinez-Hervas, S.; Gomez-Cabrera, M.C.; Real, J.T. Frailty Is Associated with Oxidative Stress in Older Patients with Type 2 Diabetes. Nutrients 2021, 13, 3983. [Google Scholar] [CrossRef]
- American Diabetes Association. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2023. Diabetes Care 2021, 44 (Suppl. S1), S15–S33. [Google Scholar] [CrossRef]
- Vellas, B.; Guigoz, Y.; Garry, P.J.; Nourhashemi, F.; Bennahum, D.; Lauque, S.; Albarede, J.L. The Mini Nutritional Assessment (MNA) and Its Use in Grading the Nutritional State of Elderly Patients. Nutrition 1999, 15, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Kyle, U.G.; Bosaeus, I.; De Lorenzo, A.D.; Deurenberg, P.; Elia, M.; Manuel Gómez, J.; Lilienthal Heitmann, B.; Kent-Smith, L.; Melchior, J.-C.; Pirlich, M.; et al. Bioelectrical Impedance Analysis—Part II: Utilization in Clinical Practice. Clin. Nutr. 2004, 23, 1430–1453. [Google Scholar] [CrossRef] [PubMed]
- Kyle, U.G.; Bosaeus, I.; De Lorenzo, A.D.; Deurenberg, P.; Elia, M.; Gómez, J.M.; Heitmann, B.L.; Kent-Smith, L.; Melchior, J.-C.; Pirlich, M.; et al. Bioelectrical Impedance Analysis—Part I: Review of Principles and Methods. Clin. Nutr. 2004, 23, 1226–1243. [Google Scholar] [CrossRef]
- Fess, E.; Moran, C. Clinical Assessment Recommendations; American Society of Hand Therapists: Chicago, IL, USA, 1981; pp. 6–8. Available online: https://www.asht.org/practice/clinical-assessment-recommendations (accessed on 7 June 2023).
- Trinder, P. Enzymatic Determination of Glucose in Blood Serum. Ann. Clin. Biochem. 1969, 6, 24–27. [Google Scholar] [CrossRef]
- Little, R.R. Glycated Hemoglobin Standardization—National Glycohemoglobin Standardization Program (NGSP) Perspective. Clin. Chem. Lab. Med. 2003, 20, 1191–1198. [Google Scholar] [CrossRef]
- Inglés, M.; Serra-Añó, P.; Gambini, J.; Abu-Sharif, F.; Dromant, M.; Garcia-Valles, R.; Pareja-Galeano, H.; Garcia-Lucerga, C.; Gomez-Cabrera, M.C. Active paraplegics are protected against exercise-induced oxidative damage through the induction of antioxidant enzymes. Spinal Cord 2016, 54, 830–837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maliszewska, K.; Adamska-Patruno, E.; Goscik, J.; Lipinska, D.; Citko, A.; Krahel, A.; Miniewska, K.; Fiedorczuk, J.; Moroz, M.; Gorska, M.; et al. The Role of Muscle Decline in Type 2 Diabetes Development: A 5-Year Prospective Observational Cohort Study. Nutrients 2019, 11, 834. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Son, J.W.; Lee, S.S.; Kim, S.R.; Yoo, S.J.; Cha, B.Y.; Son, H.Y.; Cho, N.H. Low muscle mass and risk of type 2 diabetes in middle-aged and older adults: Findings from the KoGES. Diabetologia 2017, 60, 865–872. [Google Scholar] [CrossRef] [Green Version]
- Kim, T.N.; Park, M.S.; Yang, S.J.; Yoo, H.J.; Kang, H.J.; Song, W.; Seo, J.A.; Kim, S.G.; Kim, N.H.; Baik, S.H.; et al. Prevalence and determinant factors of sarcopenia in patients with type 2 diabetes: The Korean Sarcopenic Obesity Study (KSOS). Diabetes Care 2010, 33, 1497–1499. [Google Scholar] [CrossRef] [Green Version]
- Kalyani, R.R.; Tra, Y.; Egan, J.M.; Ferrucci, L.; Brancati, F. Hyperglycemia is associated with relatively lower lean body mass in older adults. J. Nutr. Health Aging 2014, 18, 737–743. [Google Scholar] [CrossRef] [Green Version]
- Bell, K.E.; Paris, M.T.; Avrutin, E.; Mourtzakis, M. Altered features of body composition in older adults with type 2 diabetes and prediabetes compared with matched controls. J. Cachexia Sarcopenia Muscle 2022, 13, 1087–1099. [Google Scholar] [CrossRef]
- Lemos, T.; Gallagher, D. Current body composition measurement techniques. Curr. Opin. Endocrinol. Diabetes Obes. 2017, 24, 310–314. [Google Scholar] [CrossRef]
- Jensen, B.; Braun, W.; Geisler, C.; Both, M.; Klückmann, K.; Müller, M.J.; Bosy-Westphal, A. Limitations of Fat-Free Mass for the Assessment of Muscle Mass in Obesity. Obes. Facts 2019, 12, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Petzke, K.J.; Freudenberg, A.; Klaus, S. Beyond the role of dietary protein and amino acids in the prevention of diet-induced obesity. Int. J. Mol. Sci. 2014, 15, 1374–1391. [Google Scholar] [CrossRef] [Green Version]
- Westerterp-Plantenga, M.S.; Luscombe-Marsh, N.; Lejeune, M.P.G.M.; Diepvens, K.; Nieuwenhuizen, A.; Engelen, M.P.K.J.; Deutz, N.E.P.; Azzout-Marniche, D.; Tome, D.; Westerterp, K.R. Dietary protein, metabolism, and body-weight regulation: Dose-response effects. Int. J. Obes. 2006, 30, S16–S23. [Google Scholar] [CrossRef] [Green Version]
- Bauer, J.; Biolo, G.; Cederholm, T.; Cesari, M.; Cruz-Jentoft, A.J.; Morley, J.E.; Phillips, S.; Sieber, C.; Stehle, P.; Teta, D.; et al. Evidence-based recommendations for optimal dietary protein intake in older people: A position paper from the PROT-AGE Study Group. J. Am. Med. Dir. Assoc. 2013, 14, 542–559. [Google Scholar] [CrossRef]
- Amador-Licona, N.; Moreno-Vargas, E.V.; Martinez-Cordero, C.; Amador-Licona, N.; Moreno-Vargas, E.V.; Martinez-Cordero, C. Protein intake, serum lipids and muscle strenght in the elderly. Nutr. Hosp. 2018, 35, 65–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Merz, K.E.; Thurmond, D.C. Role of Skeletal Muscle in Insulin Resistance and Glucose Uptake. Compr. Physiol. 2020, 10, 785–809. [Google Scholar] [CrossRef]
- Ritov, V.B.; Menshikova, E.V.; Azuma, K.; Wood, R.; Toledo, F.G.; Goodpaster, B.H.; Ruderman, N.B.; Kelley, D.E. Deficiency of electron transport chain in human skeletal muscle mitochondria in type 2 diabetes mellitus and obesity. Am. J. Physiol. Endocrinol. Metab. 2010, 298, E49–E58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tabara, Y.; Ikezoe, T.; Yamanaka, M.; Setoh, K.; Segawa, H.; Kawaguchi, T.; Kosugi, S.; Nakayama, T.; Ichihashi, N.; Tsuboyama, T.; et al. Advanced Glycation End Product Accumulation Is Associated with Low Skeletal Muscle Mass, Weak Muscle Strength, and Reduced Bone Density: The Nagahama Study. J. Gerontol. A Biol. Sci. Med. Sci. 2019, 74, 1446–1453. [Google Scholar] [CrossRef] [PubMed]
- Gougeon, R. Insulin resistance of protein metabolism in type 2 diabetes and impact on dietary needs: A review. Can. J. Diabetes 2013, 37, 115–120. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Srikanthan, P.; Karlamangla, A.S. Relative muscle mass is inversely associated with insulin resistance and prediabetes. Findings from the third National Health and Nutrition Examination Survey. J. Clin. Endocrinol. Metab. 2011, 96, 2898–2903. [Google Scholar] [CrossRef] [Green Version]
- Atlantis, E.; Martin, S.A.; Haren, M.T.; Taylor, A.W.; Wittert, G.A.; Members of the Florey Adelaide Male Ageing Study. Inverse associations between muscle mass, strength, and the metabolic syndrome. Metabolism 2009, 58, 1013–1022. [Google Scholar] [CrossRef]
- Qiao, Y.S.; Chai, Y.H.; Gong, H.J.; Zhuldyz, Z.; Stehouwer, C.D.A.; Zhou, J.B.; Simó, R. The Association Between Diabetes Mellitus and Risk of Sarcopenia: Accumulated Evidences from Observational Studies. Front. Endocrinol. 2021, 12, 782391. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Mao, J.; Zhou, W. Prediabetes is associated with loss of appendicular skeletal muscle mass and sarcopenia. Front. Nutr. 2023, 10, 1109824. [Google Scholar] [CrossRef] [PubMed]
- Kaga, H.; Tamura, Y.; Someya, Y.; Naito, H.; Tabata, H.; Kakehi, S.; Yamasaki, N.; Sato, M.; Kadowaki, S.; Suzuki, R. Predia-betes is an independent risk factor for sarcopenia in older men, but not in older women: The Bunkyo Health Study. J. Cachexia Sarcopenia Muscle 2022, 13, 2835–2842. [Google Scholar] [CrossRef] [PubMed]
- Cleasby, M.E.; Jamieson, P.M.; Atherton, P.J. Insulin resistance and sarcopenia: Mechanistic links between common co-morbidities. J. Endocrinol. 2016, 229, R67–R81. [Google Scholar] [CrossRef] [PubMed]
- Wandrag, L.; Brett, S.J.; Frost, G.; Hickson, M. Impact of supplementation with amino acids or their metabolites on muscle wasting in patients with critical illness or other muscle wasting illness: A systematic review. J. Hum. Nutr. Diet. 2015, 28, 313–330. [Google Scholar] [CrossRef]
- Malafarina, V.; Uriz-Otano, F.; Iniesta, R.; Gil-Guerrero, L. Effectiveness of nutritional supplementation on muscle mass in treatment of sarcopenia in old age: A systematic review. J. Am. Med. Dir. Assoc. 2013, 14, 10–17. [Google Scholar] [CrossRef]
- Gielen, E.; Beckwée, D.; Delaere, A.; De Breucker, S.; Vandewoude, M.; Bautmans, I.; Sarcopenia Guidelines Development Group of the Belgian Society of Gerontology and Geriatrics (BSGG). Nutritional interventions to improve muscle mass, muscle strength, and physical performance in older people: An umbrella review of systematic reviews and meta-analyses. Nutr. Rev. 2021, 79, 121–147. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.D.; Tsauo, J.Y.; Wu, Y.T.; Cheng, C.P.; Chen, H.C.; Huang, Y.C.; Chen, H.C.; Liou, T.H. Effects of protein supplementation combined with resistance exercise on body composition and physical function in older adults: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2017, 106, 1078–1091. [Google Scholar] [CrossRef] [Green Version]
- Naseeb, M.A.; Volpe, S.L. Protein and exercise in the prevention of sarcopenia and aging. Nutr. Res. 2017, 40, 1–20. [Google Scholar] [CrossRef]
- Nyberg, S.T.; Batty, G.D.; Pentti, J.; Virtanen, M.; Alfredsson, L.; Fransson, E.I.; Goldberg, M.; Heikkilä, K.; Jokela, M.; Knutsson, A.; et al. Obesity and loss of disease-free years owing to major non-communicable diseases: A multicohort study. Lancet Public Health 2018, 3, e490–e497. [Google Scholar] [CrossRef] [Green Version]
- Westman, E.C.; Yancy, W.S. Using a low-carbohydrate diet to treat obesity and type 2 diabetes mellitus. Curr. Opin. Endocrinol. Diabetes Obes. 2020, 27, 255–260. [Google Scholar] [CrossRef]
- Kumar, S.; Behl, T.; Sachdeva, M.; Sehgal, A.; Kumari, S.; Kumar, A.; Kaur, G.; Yadav, H.N.; Bungau, S. Implicating the effect of ketogenic diet as a preventive measure to obesity and diabetes mellitus. Life Sci. 2021, 1, 118661. [Google Scholar] [CrossRef]
- Flaim, C.; Kob, M.; Di Pierro, A.M.; Herrmann, M.; Lucchin, L. Effects of a whey protein supplementation on oxidative stress, body composition and glucose metabolism among overweight people affected by diabetes mellitus or impaired fasting glucose: A pilot study. J. Nutr. Biochem. 2017, 50, 95–102. [Google Scholar] [CrossRef]
- Shahar, S.; Kamaruddin, N.S.; Badrasawi, M.; Sakian, N.I.M.; Abd Manaf, Z.; Yassin, Z.; Joseph, L. Effectiveness of exercise and protein supplementation intervention on body composition, functional fitness, and oxidative stress among elderly Malays with sarcopenia. Clin. Interv. Aging 2013, 8, 1365–1375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paoli, A.; Cerullo, G. Investigating the Link between Ketogenic Diet, NAFLD, Mitochondria, and Oxidative Stress: A Narrative Review. Antioxidants 2023, 12, 1065. [Google Scholar] [CrossRef] [PubMed]
- Rotariu, D.; Babes, E.E.; Tit, D.M.; Moisi, M.; Bustea, C.; Stoicescu, M.; Radu, A.F.; Vesa, C.M.; Behl, T.; Bungau, A.F. Oxidative stress—Complex pathological issues concerning the hallmark of cardiovascular and metabolic disorders. Biomed. Pharmacother. 2022, 152, 113238. [Google Scholar] [CrossRef] [PubMed]
- Soysal, P.; Isik, A.T.; Carvalho, A.F.; Fernandes, B.S.; Solmi, M.; Schofield, P.; Veronese, N.; Stubbs, B. Oxidative stress and frailty: A systematic review and synthesis of the best evidence. Maturitas 2017, 99, 66–72. [Google Scholar] [CrossRef] [Green Version]
- Inglés, M.; Gambini, J.; Carnicero, J.A.; García-García, F.J.; Rodríguez-Mañas, L.; Olaso-González, G.; Dromant, M.; Borrás, C.; Viña, J. Oxidative stress is related to frailty, not to age or sex, in a geriatric population: Lipid and protein oxidation as biomarkers of frailty. J. Am. Geriatr. Soc. 2014, 62, 1324–1328. [Google Scholar] [CrossRef]
- Asmat, U.; Abad, K.; Ismail, K. Diabetes mellitus and oxidative stress—A concise review. Saudi Pharm. J. 2016, 24, 547–553. [Google Scholar] [CrossRef] [Green Version]
- Valentim, M.A.; Brahmbhatt, A.N.; Tupling, A.R. Skeletal and cardiac muscle calcium transport regulation in health and disease. Biosci. Rep. 2022, 42, BSR20211997. [Google Scholar] [CrossRef]
- Derbré, F.; Gratas-Delamarche, A.; Gómez-Cabrera, M.C.; Viña, J. Inactivity-induced oxidative stress: A central role in age-related sarcopenia? Eur. J. Sport Sci. 2014, 14, S98–S108. [Google Scholar] [CrossRef]
- Li, H.; Manwani, B.; Leng, S.X. Frailty, inflammation, and immunity. Aging Dis. 2011, 2, 466–473. [Google Scholar] [PubMed]
- Hecker, M.; Wagner, A.H. Role of protein carbonylation in diabetes. J. Inherit. Metab. Dis. 2018, 41, 29–38. [Google Scholar] [CrossRef]
- Nehme, R.; Nahas, P.C.; de Oliveira, E.P. Association between serum uric acid and muscle strength in older adults with or without gout diagnosis: NHANES 2011–2014. Aging Clin. Exp. Res. 2023. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Li, N.; Jia, W.; Wang, N.; Liang, M.; Yang, X.; Du, G. Skeletal muscle atrophy: From mechanisms to treatments. Pharmacol. Res. 2021, 172, 105807. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Sayer, A.A. Sarcopenia. Lancet 2019, 393, 2636–2646. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, C.M.; Ingles, M.; Salvador-Pascual, A.; Cominetti, M.R.; Gomez-Cabrera, M.C.; Viña, J. Sarcopenia, frailty and their prevention by exercise. Free Radic. Biol. Med. 2019, 20, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Sanz-Cánovas, J.; López-Sampalo, A.; Cobos-Palacios, L.; Ricci, M.; Hernández-Negrín, H.; Mancebo-Sevilla, J.J.; Álvarez-Recio, E.; López-Carmona, M.D.; Pérez-Belmonte, L.M.; Gómez-Huelgas, R.; et al. Management of Type 2 Diabetes Mellitus in Elderly Patients with Frailty and/or Sarcopenia. Int. J. Environ. Res. Public Health 2022, 19, 8677. [Google Scholar] [CrossRef]
- Vesa, C.M.; Bungau, S.G. Novel Molecules in Diabetes Mellitus, Dyslipidemia and Cardiovascular Disease. Int. J. Mol. Sci. 2023, 24, 4029. [Google Scholar] [CrossRef]
- Pan, R.; Zhang, Y.; Wang, R.; Xu, Y.; Ji, H.; Zhao, Y. Effect of SGLT-2 inhibitors on body composition in patients with type 2 diabetes mellitus: A meta-analysis of randomized controlled trials. PLoS ONE 2022, 17, e0279889. [Google Scholar] [CrossRef]
- Ida, S.; Kaneko, R.; Imataka, K.; Okubo, K.; Shirakura, Y.; Azuma, K.; Fujiwara, R.; Murata, K. Effects of Antidiabetic Drugs on Muscle Mass in Type 2 Diabetes Mellitus. Curr. Diabetes Rev. 2021, 17, 293–303. [Google Scholar] [CrossRef]
- Ozeki, Y.; Masaki, T.; Kamata, A.; Miyamoto, S.; Yoshida, Y.; Okamoto, M.; Gotoh, K.; Shibata, H. The Effectiveness of GLP-1 Receptor Agonist Semaglutide on Body Composition in Elderly Obese Diabetic Patients: A Pilot Study. Medicines 2022, 9, 47. [Google Scholar] [CrossRef] [PubMed]
- Osaka, T.; Hamaguchi, M.; Fukui, M. Favorable Appendicular Skeletal Muscle Mass Changes in Older Patients with Type 2 Diabetes Receiving GLP-1 Receptor Agonist and Basal Insulin Co-Therapy. Clin. Med. Insights Endocrinol. Diabetes 2023, 16, 11795514231161885. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | Total (n = 100) |
|---|---|
| Age (years) | 70.3 ± 3.8 |
| Time of T2D evolution (years) | 17.8 ± 10.7 |
| Body mass index (kg/m2) | 30.8 ± 4.2 |
| Brachial circumference (cm) | 31.5 ± 3.4 |
| Calf circumference (cm) | 37.2 ± 3.1 |
| Fat mass index (kg/m2) | 8.9 ± 3.8 |
| Fat-free mass index (kg/m2) | 21.7 ± 2.6 |
| Skeletal muscle mass index (kg/m2) | 10.1 ± 1.9 |
| Appendicular skeletal muscle mass index (kg/m2) | 7.9 ± 1.0 |
| Maximum muscle strength (kg) | 28.6 ± 10.0 |
| Gait speed (m/s) | 0.8 ± 0.2 |
| Protein intake (%) | 20.0 ± 4.2 |
| MNA score | 26.5 ± 2.1 |
| Glucose (mg/dL) | 148.2 ± 45.7 |
| HbA1c (%) | 7.4 ± 1.1 |
| Total cholesterol (mg/dL) | 156.1 ± 30.2 |
| LDL cholesterol (mg/dL) | 93.0 ± 22.6 |
| HDL cholesterol (mg/dL) | 47.7 ± 12.8 |
| Triglycerides (mg/dL) | 134.5 ± 62.3 |
| Albumin (g/dL) | 4.2 ± 0.2 |
| C-reactive protein | 4.5 ± 8.9 |
| Malondialdehyde (µM) | 6.0 ± 5.8 |
| Protein carbonyls (U.A.) | 81.3 ± 20.9 |
| Characteristic | Women (n = 52) | Men (n = 48) |
|---|---|---|
| Age (years) | 70.6 ± 3.6 | 70.0 ± 4.0 |
| Time of T2D evolution (years) | 20.2 ± 11.2 | 15.3 ± 9.7 * |
| Body mass index (kg/m2) | 31.0 ± 4.4 | 30.5 ± 4.2 |
| Brachial circumference (cm) | 32.1 ± 3.7 | 30.9 ± 3.0 |
| Calf circumference (cm) | 36.7 ± 3.1 | 37.9 ± 3.1 * |
| Fat mass index (kg/m2) | 10.7 ± 3.8 | 7.0 ± 2.7 * |
| Fat-free mass index (kg/m2) | 20.1 ± 1.8 | 23.3 ± 2.3 * |
| Skeletal muscle mass index (kg/m2) | 8.6 ± 1.0 | 11.6 ± 1.4 * |
| Appendicular skeletal muscle mass index (kg/m2) | 7.3 ± 0.8 | 8.6 ± 0.9 * |
| Maximum muscle strength (kg) | 21.0 ± 4.4 | 36.9 ± 7.6 * |
| Gait speed (m/s) | 0.86 ± 0.2 | 0.71 ± 0.2 * |
| Protein intake (%) | 20.3 ± 4.3 | 19.8 ± 4.0 |
| MNA score | 25.8 ± 2.2 | 27.3 ± 1.7 * |
| Glucose (mg/dL) | 147.1 ± 47.7 | 149.3 ± 43.7 |
| HbA1c (%) | 7.3 ± 1.0 | 7.4 ± 1.1 |
| Total cholesterol (mg/dL) | 165.7 ± 29.9 | 145.4 ± 27.1 * |
| LDL cholesterol (mg/dL) | 96.4 ± 23.6 | 89.1 ± 21.1 |
| HDL cholesterol (mg/dL) | 51.6 ± 14.4 | 43.4 ± 9.2 * |
| Triglycerides (mg/dL) | 141.4 ± 57.4 | 126.8 ± 67.0 |
| Albumin (g/dL) | 4.2 ± 0.2 | 4.2 ± 0.3 |
| C-reactive protein | 4.2 ± 6.7 | 4.8 ± 10.9 |
| Malondialdehyde (µM) | 6.1 ± 5.6 | 5.8 ± 6.0 |
| Protein carbonyls (U.A.) | 84.5 ± 26.3 | 78.0 ± 12.9 |
| Characteristic | Total (n = 100) | Women (n = 52) | Men (n = 48) | |||
|---|---|---|---|---|---|---|
| ≤25th Percentile (n = 25) | >25th Percentile (n = 74) | ≤25th Percentile (n = 11) | >25th Percentile (n = 39) | ≤25th Percentile (n = 12) | >25th Percentile (n = 36) | |
| Time of T2D evolution (years) | 19.7 ± 12.2 | 17.2 ± 10.4 | 24.2 ± 13.4 | 18.9 ± 10.4 | 14.9 ± 8.8 | 15.4 ± 10.2 |
| Brachial circumference (cm) | 29.3 ± 2.8 * | 32.1 ± 3.3 | 30.1 ± 3.5 * | 32.7 ± 3.7 | 28.2 ± 1.2 * | 31.4 ± 2.7 |
| Calf circumference (cm) | 35.5 ± 2.2 * | 37.7 ± 3.2 | 36.4 ± 2.1 | 36.7 ± 3.4 | 34.6 ± 1.9 * | 38.7 ± 2.6 |
| Maximum muscle strength (kg) | 28.4 ± 11.2 | 28.8 ± 9.8 | 21.1 ± 5.4 | 21.0 ± 4.4 | 36.4 ± 10.6 | 37.0 ± 6.7 |
| Gait speed (m/s) | 0.80 ± 0.26 | 0.79 ± 0.18 | 0.83 ± 0.26 | 0.88 ± 0.19 | 0.78 ± 0.27 | 0.70 ± 0.09 |
| Protein intake (%) | 18.2 ± 4.1 * | 20.7 ± 4.0 | 18.3 ± 4.8 * | 21.0 ± 4.1 | 18.1 ± 3.5 | 20.4 ± 4.0 |
| MNA score | 26.2 ± 2.2 | 26.6 ± 2.1 | 25.7 ± 2.2 | 25.7 ± 2.2 | 26.8 ± 2.2 | 27.4 ± 1.5 |
| Glucose (mg/dL) | 160.6 ± 52.0 | 143.9 ± 43.1 | 178.3 ± 41.1 * | 136.8 ± 45.2 | 141.3 ± 57.4 | 151.3 ± 40.0 |
| HbA1c (%) | 7.6 ± 1.4 | 7.3 ± 0.9 | 7.8 ± 1.1 * | 7.2 ± 0.9 | 7.3 ± 1.6 | 7.4 ± 1.0 |
| Malondialdehyde (µM) | 7.1 ± 6.5 | 5.7 ± 5.6 | 8.0 ± 7.2 | 5.6 ± 5.1 | 6.2 ± 6.0 | 5.7 ± 6.1 |
| Protein carbonyls (U.A.) | 76.5 ± 15.1 | 84.3 ± 21.4 | 76.8 ± 18.6 | 88.7 ± 27.5 | 76.1 ± 11.6 | 79.9 ± 11.4 |
| Characteristic | Total (n = 100) | Women (n = 52) | Men (n = 48) | |||
|---|---|---|---|---|---|---|
| ≤25th Percentile (n = 25) | >25th Percentile (n = 74) | ≤25th Percentile (n = 11) | >25th Percentile (n = 39) | ≤25th Percentile (n = 12) | >25th Percentile (n = 36) | |
| Time of T2D evolution (years) | 17.0 ± 9.2 | 18.1 ± 11.3 | 18.4 ± 9.0 | 21.0 ± 11.9 | 15.5 ± 9.6 | 15.2 ± 9.9 |
| Brachial circumference (cm) | 30.7 ± 4.0 | 31.8 ± 3.2 | 32.0 ± 4.5 | 32.1 ± 3.5 | 29.0 ± 2.7 * | 31.4 ± 2.9 |
| Calf circumference (cm) | 35.8 ± 2.3 * | 37.7 ± 3.2 | 36.1 ± 2.2 | 36.8 ± 3.4 | 35.4 ± 2.6 * | 38.6 ± 2.8 |
| Maximum muscle strength (kg) | 26.0 ± 10.5 | 29.6 ± 9.8 | 19.1 ± 3.6 | 21.6 ± 4.5 | 33.5 ± 10.4 | 38.0 ± 6.1 |
| Gait speed (m/s) | 0.79 ± 0.20 | 0.79 ± 0.20 | 0.80 ± 0.16 | 0.89 ± 0.22 | 0.78 ± 0.25 | 0.69 ± 0.09 |
| Protein intake (%) | 19.0 ± 4.6 | 20.4 ± 4.0 | 19.9 ± 5.5 | 20.4 ± 4.0 | 18.1 ± 3.4 | 20.3 ± 4.0 |
| MNA score | 26.6 ± 2.5 | 26.5 ± 1.9 | 26.1 ± 2.9 | 25.7 ± 1.9 | 27.1 ± 2.1 | 27.4 ± 1.6 |
| Glucose (mg/dL) | 153.0 ± 50.1 | 147.2 ± 44.3 | 167.6 ± 49.6 | 141.3 ± 45.9 | 137.3 ± 47.5 | 153.3 ± 42.4 |
| HbA1c (%) | 7.5 ± 1.2 | 7.3 ± 1.0 | 7.9 ± 1.1 * | 7.2 ± 0.9 | 7.2 ± 1.3 | 7.5 ± 1.1 |
| Malondialdehyde (µM) | 9.0 ± 7.3 * | 4.9 ± 4.8 | 9.0 ± 7.1 * | 5.0 ± 4.7 | 9.1 ± 7.7 * | 4.7 ± 4.9 |
| Protein carbonyls (U.A.) | 78.4 ± 17.4 | 82.8 ± 21.9 | 77.0 ± 21.9 | 88.3 ± 27.3 | 79.9 ± 11.8 | 77.5 ± 13.4 |
| Characteristic | Total (n = 100) | Women (n = 52) | Men (n = 48) | |||
|---|---|---|---|---|---|---|
| ≤25th Percentile (n = 25) | >25th Percentile (n = 74) | ≤25th Percentile (n = 11) | >25th Percentile (n = 39) | ≤25th Percentile (n = 12) | >25th Percentile (n = 36) | |
| Time of T2D evolution (years) | 19.3 ± 11.7 | 17.2 ± 10.4 | 24.2 ± 13.1 | 18.8 ± 10.3 | 14.7 ± 8.4 | 15.5 ± 10.4 |
| Brachial circumference (cm) | 30.8 ± 4.5 | 31.8 ± 2.9 | 32.7 ± 5.3 | 31.9 ± 3.0 | 29.0 ± 2.5 * | 31.7 ± 2.8 |
| Calf circumference (cm) | 35.7 ± 2.1 * | 37.8 ± 3.3 | 36.1 ± 2.0 | 36.8 ± 3.4 | 35.3 ± 2.2 * | 39.0 ± 2.7 |
| Maximum muscle strength (kg) | 27.8 ± 10.6 | 29.0 ± 9.9 | 20.0 ± 5.0 | 21.3 ± 4.2 | 35.1 ± 9.2 | 37.7 ± 6.7 |
| Gait speed (m/s) | 0.81 ± 0.23 | 0.78 ± 0.18 | 0.88 ± 0.22 | 0.86 ± 0.21 | 0.74 ± 0.23 | 0.70 ± 0.10 |
| Protein intake (%) | 19.3 ± 4.6 | 20.3 ± 4.0 | 20.8 ± 5.6 | 20.1 ± 3.9 | 18.0 ± 3.1 * | 20.6 ± 4.1 |
| MNA score | 26.2 ± 2.3 | 26.6 ± 2.0 | 25.7 ± 2.4 | 25.8 ± 2.1 | 26.7 ± 2.0 | 27.5 ± 1.5 |
| Glucose (mg/dL) | 158.0 ± 51.2 | 144.8 ± 42.9 | 164.0 ± 46.1 | 142.0 ± 47.6 | 152.4 ± 56.6 | 147.9 ± 37.4 |
| HbA1c (%) | 7.5 ± 1.2 | 7.3 ± 1.0 | 7.8 ± 1.1 * | 7.2 ± 0.9 | 7.3 ± 1.3 | 7.5 ± 1.1 |
| Malondialdehyde (µM) | 7.8 ± 6.2 * | 5.2 ± 5.5 | 8.4 ± 6.6 * | 5.1 ± 5.0 | 7.1 ± 6.0 | 5.2 ± 6.0 |
| Protein carbonyls (U.A.) | 79.7 ± 18.6 | 82.5 ± 21.9 | 80.9 ± 24.0 | 87.0 ± 27.2 | 78.6 ± 12.3 | 77.9 ± 13.4 |
| A. Dependent Variable: Fat-Free Mass Index | B | SE | Wald | Significance | Exp(B) |
| (Constant) | −1.431 | 4.779 | 0.090 | 0.765 | 0.239 |
| Brachial circumference (cm) | 0.191 | 0.118 | 2.638 | 0.104 | 1.211 |
| Glucose (mg/dL) | −0.017 | 0.010 | 3.122 | 0.077 | 0.983 |
| HbA1c (%) | −0.097 | 0.428 | 0.051 | 0.821 | 0.908 |
| B. Dependent Variable: Skeletal Muscle Mass | B | SE | Wald | Significance | Exp(B) |
| (Constant) | 7.583 | 3.235 | 5.494 | 0.019 | 1964.920 |
| Malondialdehyde (µM) | −0.142 | 0.064 | 4.881 | 0.027 | 0.868 |
| Glucose (mg/dL) | −0.002 | 0.009 | 0.049 | 0.829 | 0.998 |
| HbA1c (%) | −0.703 | 0.466 | 2.278 | 0.131 | 0.495 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alabadi, B.; Civera, M.; De la Rosa, A.; Martinez-Hervas, S.; Gomez-Cabrera, M.C.; Real, J.T. Low Muscle Mass Is Associated with Poorer Glycemic Control and Higher Oxidative Stress in Older Patients with Type 2 Diabetes. Nutrients 2023, 15, 3167. https://doi.org/10.3390/nu15143167
Alabadi B, Civera M, De la Rosa A, Martinez-Hervas S, Gomez-Cabrera MC, Real JT. Low Muscle Mass Is Associated with Poorer Glycemic Control and Higher Oxidative Stress in Older Patients with Type 2 Diabetes. Nutrients. 2023; 15(14):3167. https://doi.org/10.3390/nu15143167
Chicago/Turabian StyleAlabadi, Blanca, Miguel Civera, Adrián De la Rosa, Sergio Martinez-Hervas, Mari Carmen Gomez-Cabrera, and José T. Real. 2023. "Low Muscle Mass Is Associated with Poorer Glycemic Control and Higher Oxidative Stress in Older Patients with Type 2 Diabetes" Nutrients 15, no. 14: 3167. https://doi.org/10.3390/nu15143167
APA StyleAlabadi, B., Civera, M., De la Rosa, A., Martinez-Hervas, S., Gomez-Cabrera, M. C., & Real, J. T. (2023). Low Muscle Mass Is Associated with Poorer Glycemic Control and Higher Oxidative Stress in Older Patients with Type 2 Diabetes. Nutrients, 15(14), 3167. https://doi.org/10.3390/nu15143167





