Low-Bacterial Diet in Cancer Patients: A Systematic Review
Abstract
:1. Introduction
Objective of this Systematic Review
2. Materials and Methods
2.1. Search Strategy
2.2. Registration of the Systematic Review Protocol
2.3. Research Question and Criteria
2.4. Evaluation of the Risk of Bias and Methodological Quality of Studies
2.5. Data Extraction
2.6. Data Synthesis
2.7. Acronyms
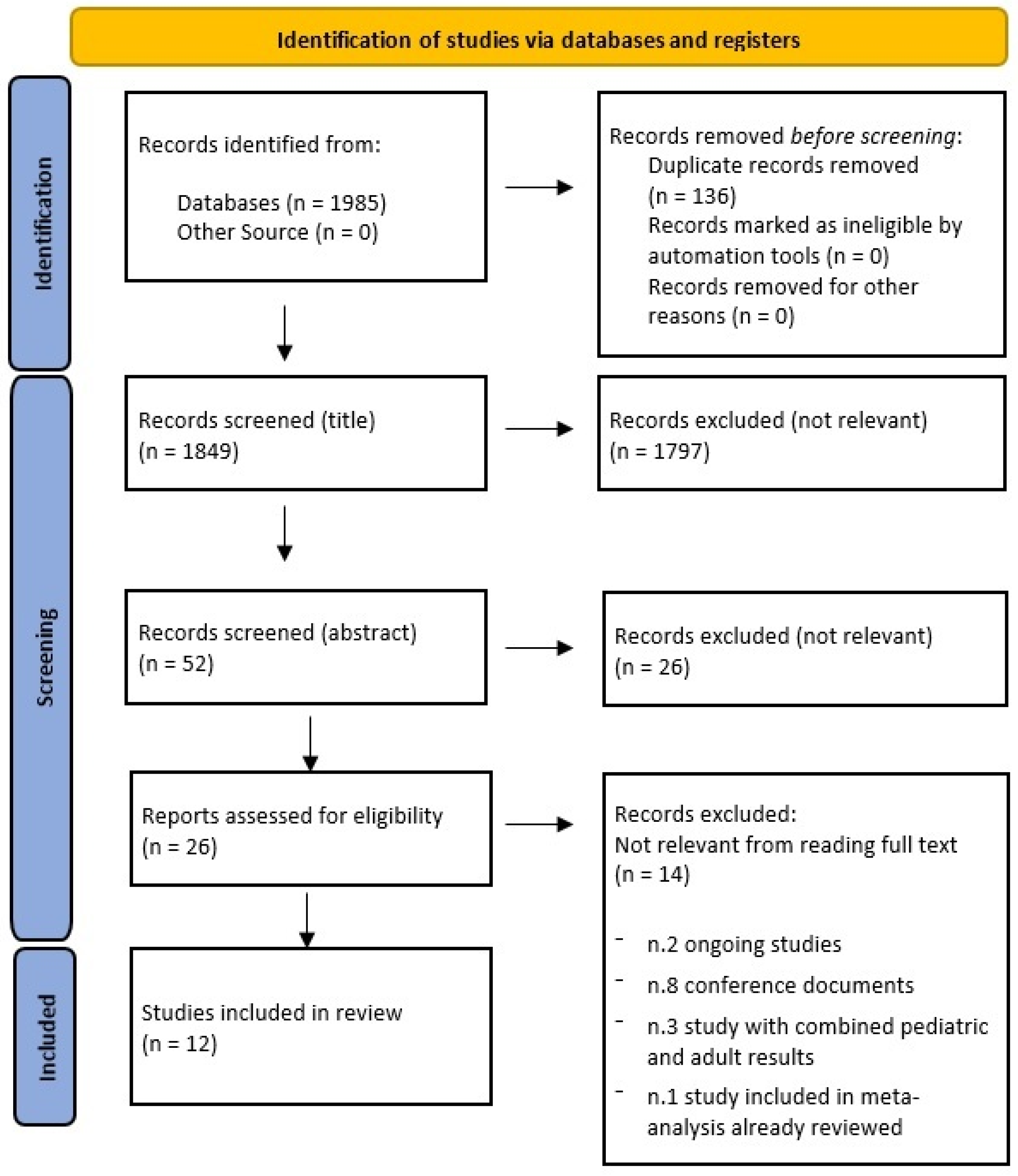
3. Results
3.1. General Characteristics of the Included Studies
3.2. Application of the Low-Bacterial Diet
3.3. Duration of the Low-Bacterial Diet
3.4. Infection Rates
3.5. Mortality Rates
3.6. Quality of Life
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Peric, Z.; Botti, S.; Stringer, J.; Krawczyk, J.; van der Werf, S.; van Biezen, A.; Aljurf, M.; Murray, J.; Liptrott, S.; Greenfield, D.M.; et al. Variability of Nutritional Practices in Peritransplant Period After Allogeneic Hematopoietic Stem Cell Transplantation: A Survey by the Complications and Quality of Life Working Party of the EBMT. Bone Marrow Transpl. 2018, 53, 1030–1037. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trifilio, S.; Helenowski, I.; Giel, M.; Gobel, B.; Pi, J.; Greenberg, D.; Mehta, J. Questioning the Role of a Neutropenic Diet Following Hematopoetic Stem Cell Transplantation. Biol. Blood Marrow Transpl. 2012, 18, 1385–1390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McQuade, R.M.; Stojanovska, V.; Abalo, R.; Bornstein, J.C.; Nurgali, K. Chemotherapy-Induced Constipation and Diarrhea: Pathophysiology, Current and Emerging Treatments. Front. Pharmacol. 2016, 7, 414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akbarali, H.I.; Muchhala, K.H.; Jessup, D.K.; Cheatham, S. Chemotherapy Induced Gastrointestinal Toxicities. In Advances in Cancer Research; Elsevier: Amsterdam, The Netherlands, 2022. [Google Scholar] [CrossRef]
- Ma, Y.; Lu, X.; Liu, H. Neutropenic Diet Cannot Reduce the Risk of Infection and Mortality in Oncology Patients with Neutropenia. Front. Oncol. 2022, 12, 836371. [Google Scholar] [CrossRef]
- Álvarez, Y.E.; Bataller, R.D.L.P.; Altozano, J.P.; Martínez, S.R.; Álvarez, A.S.; Cordellat, A.B.; Vázquez, E.B.; Jaime, J.C.; Escobar, I.G.; Zambrano, C.B. SEOM Clinical Guidelines for Anaemia Treatment in Cancer Patients (2020). Clin. Transl. Oncol. 2021, 23, 931–939. [Google Scholar] [CrossRef]
- Sonbol, M.B.; Firwana, B.; Diab, M.; Zarzour, A.; Witzig, T.E. The Effect of a Neutropenic Diet on Infection and Mortality Rates in Cancer Patients: A Meta-Analysis. Nutr. Cancer 2015, 67, 1232–1240. [Google Scholar] [CrossRef]
- Elad, S.; Yarom, N.; Zadik, Y.; Kuten-Shorrer, M.; Sonis, S.T. The Broadening Scope of Oral Mucositis and Oral Ulcerative Mucosal Toxicities of Anticancer Therapies. CA A Cancer J. Clin. 2021, 72, 57–77. [Google Scholar] [CrossRef]
- Tietsche de Moraes Hungria, V.; Chiattone, C.; Pavlovsky, M.; Abenoza, L.M.; Agreda, G.P.; Armenta, J.; Arrais, C.; Avendaño Flores, O.; Barroso, F.; Basquiera, A.L.; et al. Epidemiology of Hematologic Malignancies in Real-World Settings: Findings From the Hemato-Oncology Latin America Observational Registry Study. J. Glob. Oncol. 2019, 5, 1–19. [Google Scholar] [CrossRef]
- Bajic, J.E.; Johnston, I.N.; Howarth, G.S.; Hutchinson, M.R. From the Bottom-Up: Chemotherapy and Gut-Brain Axis Dysregulation. Front. Behav. Neurosci. 2018, 12, 104. [Google Scholar] [CrossRef] [Green Version]
- Moslemi, D.; Nokhandani, A.M.; Otaghsaraei, M.T.; Moghadamnia, Y.; Kazemi, S.; Moghadamnia, A.A. Management of Chemo/Radiation-Induced Oral Mucositis in Patients With Head and Neck Cancer: A Review of the Current Literature. Radiother. Oncol. 2016, 120, 13–20. [Google Scholar] [CrossRef]
- Heng, M.S.; Barbon Gauro, J.; Yaxley, A.; Thomas, J. Does a Neutropenic Diet Reduce Adverse Outcomes in Patients Undergoing Chemotherapy? Eur. J. Cancer Care 2019, 29, e13155. [Google Scholar] [CrossRef]
- Van Dalen, E.C.; Mank, A.; Leclercq, E.; Mulder, R.L.; Davies, M.; Kersten, M.J.; van de Wetering, M.D. Low Bacterial Diet Versus Control Diet to Prevent Infection in Cancer Patients Treated with Chemotherapy Causing Episodes of Neutropenia. Cochrane Database Syst. Rev. 2016, 2019, CD006247. [Google Scholar] [CrossRef] [PubMed]
- Preisler, H.D.; Goldstein, I.M.; Henderson, E.S. Gastrointestinal “sterilization” in the treatment of patients with acute leukemia. Cancer 1970, 26, 1076–1081. [Google Scholar] [CrossRef] [PubMed]
- Ball, S.; Brown, T.J.; Das, A.; Khera, R.; Khanna, S.; Gupta, A. Effect of Neutropenic Diet on Infection Rates in Cancer Patients With Neutropenia. Am. J. Clin. Oncol. 2019, 42, 270–274. [Google Scholar] [CrossRef] [PubMed]
- Jeon, O.K.; Lee, Y.H.; Kim, M.H. Knowledge of and Compliance with Neutropenic Diet in Patients With Hematologic Malignancy Undergoing Chemotherapy. Asian Oncol. Nurs. 2018, 18, 75–85. [Google Scholar] [CrossRef]
- Stuhler, R. Diets for Patients Undergoing Haematopoietic Stem Cell Transplant—A Review. LymphoSign J. 2020, 7, 115–121. [Google Scholar] [CrossRef]
- Jakob, C.E.M.; Classen, A.Y.; Stecher, M.; Engert, A.; Freund, M.; Hamprecht, A.; Jazmati, N.; Wisplinghoff, H.; Hallek, M.; Cornely, O.A.; et al. Association Between the Dietary Regimen and Infection-Related Complications in Neutropenic High-Risk Patients With Cancer. Eur. J. Cancer 2021, 155, 281–290. [Google Scholar] [CrossRef]
- Carr, S.E.; Halliday, V. Investigating the Use of the Neutropenic Diet: A Survey of UK Dietitians. J. Hum. Nutr. Diet. 2014, 28, 510–515. [Google Scholar] [CrossRef]
- August, D.A.; Huhmann, M.B. A.S.P.E.N. clinical guidelines: Nutrition support therapy during adult anticancer treatment and in hematopoietic cell transplantation. J. Parenter. Enter. Nutr. 2009, 33, 472–500. [Google Scholar] [CrossRef]
- Muscaritoli, M.; Arends, J.; Bachmann, P.; Baracos, V.; Barthelemy, N.; Bertz, H.; Bozzetti, F.; Hütterer, E.; Isenring, E.; Kaasa, S.; et al. ESPEN Practical Guideline: Clinical Nutrition in Cancer. Clin. Nutr. 2021, 40, 2898–2913. [Google Scholar] [CrossRef]
- Taplitz, R.A.; Kennedy, E.B.; Bow, E.J.; Crews, J.; Gleason, C.; Hawley, D.K.; Langston, A.A.; Nastoupil, L.J.; Rajotte, M.; Rolston, K.V.; et al. Antimicrobial prophylaxis for adult patients with cancer-related immunosuppression: ASCO and IDSA clinical practice guideline update. J. Clin. Oncol. 2018, 36, 3043–3054. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. Food Safety for Older Adults and People with Cancer, Diabetes, HIV/AIDS, Organ Transplants and Autoimmune Diseases. Available online: https://www.fda.gov/food/people-risk-foodborne-illness/food-safety-er-adults-and-people-cancer-diabetes-hivaids-organ-transplants-and-autoimmune (accessed on 12 December 2022).
- Fang, Y.; Liu, M.; Zhang, W.; Xie, C.; Liu, Z. Nutrition Support Practices of Hematopoietic Stem Cell Transplantation Centers in Mainland China. Curr. Med. Sci. 2020, 40, 691–698. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Richardson, W.S.; Wilson, M.C.; Nishikawa, J.; Hayward, R.S. The Well-Built Clinical Question: A Key to Evidence-Based Decisions. ACP J. Club 1995, 123, A12. [Google Scholar] [CrossRef]
- Aromataris, E.; Fernandez, R.; Godfrey, C.M.; Holly, C.; Khalil, H.; Tungpunkom, P. Summarizing Systematic Reviews. Int. J. Evidence-Based Healthc. 2015, 13, 132–140. [Google Scholar] [CrossRef] [Green Version]
- Moola, S.; Munn, Z.; Tufanaru, C.; Aromataris, E.; Sears, K.; Sfetc, R.; Currie, M.; Lisy, K.; Qureshi, R.; Mattis, P.; et al. Chapter 7: Systematic Reviews of Etiology and Risk. In JBI Manual for Evidence Synthesis; JBI: Adelaide, Australia, 2020. [Google Scholar] [CrossRef]
- Toenges, R.; Greinix, H.; Lawitschka, A.; Halter, J.; Baumgartner, A.; Simon, A.; Arends, J.; Jäger, P.; Middeke, M.; Hilgendorf, I.; et al. Current Practice in Nutrition After Allogeneic Hematopoietic Stem Cell Transplantation—Results From a Survey Among Hematopoietic Stem Cell Transplant Centers. Clin. Nutr. 2021, 40, 1571–1577. [Google Scholar] [CrossRef] [PubMed]
- Brown, T.J.; Mukhija, D.; Premnath, N.; Venkatraman, A.; Jiv Singh Nagpal, S.; Gupta, A. Dissemination of Information on Neutropenic Diet by Top US Cancer Centers: In-Line With the Evidence? Nutr. Cancer 2019, 71, 1272–1275. [Google Scholar] [CrossRef]
- Gupta, A.; Brown, T.J.; Singh, S.; Sen, A.; Agrawal, D.; Li, H.C.; Moriates, C.; Johnson, D.H.; Sadeghi, N. Applying the ‘COST’ (Culture, Oversight, Systems Change, and Training) Framework to De-Adopt the Neutropenic Diet. Am. J. Med. 2019, 132, 42–47. [Google Scholar] [CrossRef]
- Mancin, S.; Bertone, A.; Cattani, D.; Morenghi, E.; Passoni, L.; Donizetti, D.; Sökeland, F.; Azzolini, E.; Mazzoleni, B. Malnutrition risk as a negative prognostic factor in COVID-19 patients. Clin. Nutr. ESPEN 2021, 45, 369–373. [Google Scholar] [CrossRef]
- Mancin, S.; Sguanci, M.; Cattani, D.; Soekeland, F.; Axiak, G.; Mazzoleni, B.; De Marinis, M.G.; Piredda, M. Nutritional knowledge of nursing students: A systematic literature review. Nurse Educ. Today 2023, 126, 105826. [Google Scholar] [CrossRef]
| Study | Design | Sample (n) | Study Group (ND) | Control Group (SD) |
|---|---|---|---|---|
| Ma et al. [5] | Meta-analysis | LBD: 113 SD: 106 | 67.3% | 62.3% |
| Toenges et al. [29] | Cross-sectional | LBD: 28 | 82.1% | // |
| Jakob et al. [18] | Case–control | LBD: 1043 SD: 1043 | 0.2% | 0.35% |
| Ball et al. [15] | Meta-analysis | LBD: 113 SD: 106 | 67.3% | 62.3% |
| Heng et al. [12] | Cross-sectional | LBD: 79 SD: 75 | 58.2% | 42.7% |
| Sonbol et al. [7] | Meta-analysis | LBD: 88 SD: 85 | 8% | 20% |
| Study | Design | Sample (n) | Study Group (ND) | Control Group (SD) |
|---|---|---|---|---|
| Ma et al. [5] | Meta-analysis | LBD: 113 SD: 106 | 43.6% | 38.7% |
| Jakob et al. [18] | Case–control | LBD: 1043 SD: 1043 | 3.3% | 5% |
| Sonbol et al. [7] | Meta-analysis | LBD: 88 SD: 85 | 43.6%% | 38.7% |
| Study | Design | Sample (n) | Study Group (ND) | Control Group (SD) |
|---|---|---|---|---|
| Jakob et al. [18] | Case–control | LBD: 1043 SD: 1043 | Diarrhea: 51.1% Nausea: 74.6% Weight loss: 64.8% | Diarrhea: 40.2% Nausea: 63.3% Weight loss: 60.2% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matteucci, S.; De Pasquale, G.; Pastore, M.; Morenghi, E.; Pipitone, V.; Soekeland, F.; Caccialanza, R.; Mazzoleni, B.; Mancin, S. Low-Bacterial Diet in Cancer Patients: A Systematic Review. Nutrients 2023, 15, 3171. https://doi.org/10.3390/nu15143171
Matteucci S, De Pasquale G, Pastore M, Morenghi E, Pipitone V, Soekeland F, Caccialanza R, Mazzoleni B, Mancin S. Low-Bacterial Diet in Cancer Patients: A Systematic Review. Nutrients. 2023; 15(14):3171. https://doi.org/10.3390/nu15143171
Chicago/Turabian StyleMatteucci, Sofia, Giulia De Pasquale, Manuela Pastore, Emanuela Morenghi, Veronica Pipitone, Fanny Soekeland, Riccardo Caccialanza, Beatrice Mazzoleni, and Stefano Mancin. 2023. "Low-Bacterial Diet in Cancer Patients: A Systematic Review" Nutrients 15, no. 14: 3171. https://doi.org/10.3390/nu15143171
APA StyleMatteucci, S., De Pasquale, G., Pastore, M., Morenghi, E., Pipitone, V., Soekeland, F., Caccialanza, R., Mazzoleni, B., & Mancin, S. (2023). Low-Bacterial Diet in Cancer Patients: A Systematic Review. Nutrients, 15(14), 3171. https://doi.org/10.3390/nu15143171







