Oat Beta-Glucan Alone and in Combination with Hydrochlorothiazide Lowers High Blood Pressure in Male but Not Female Spontaneously Hypertensive Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Ethics
2.2. Animal Model of Hypertension and Study Treatment Regimen
2.3. Transthoracic Echocardiography
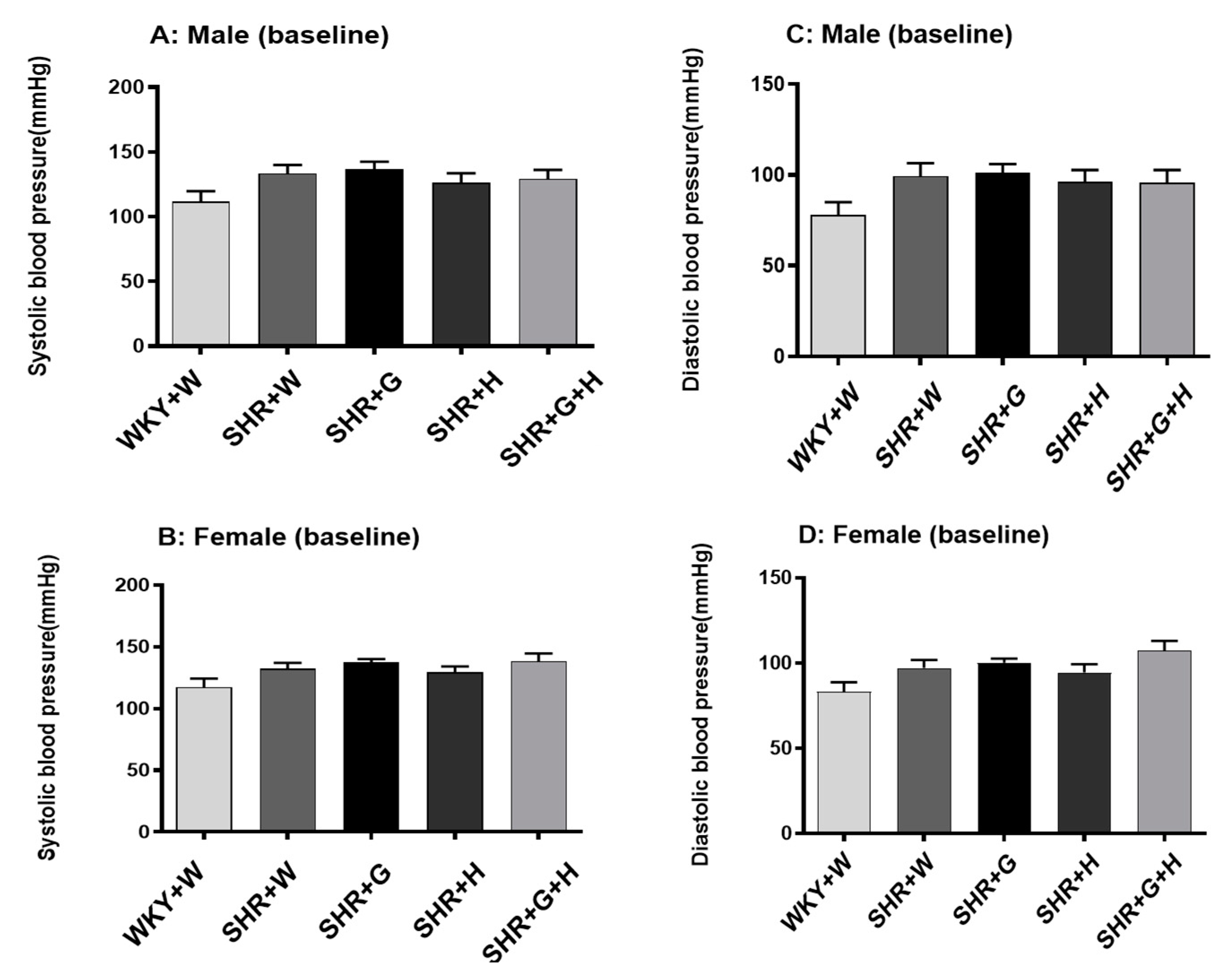
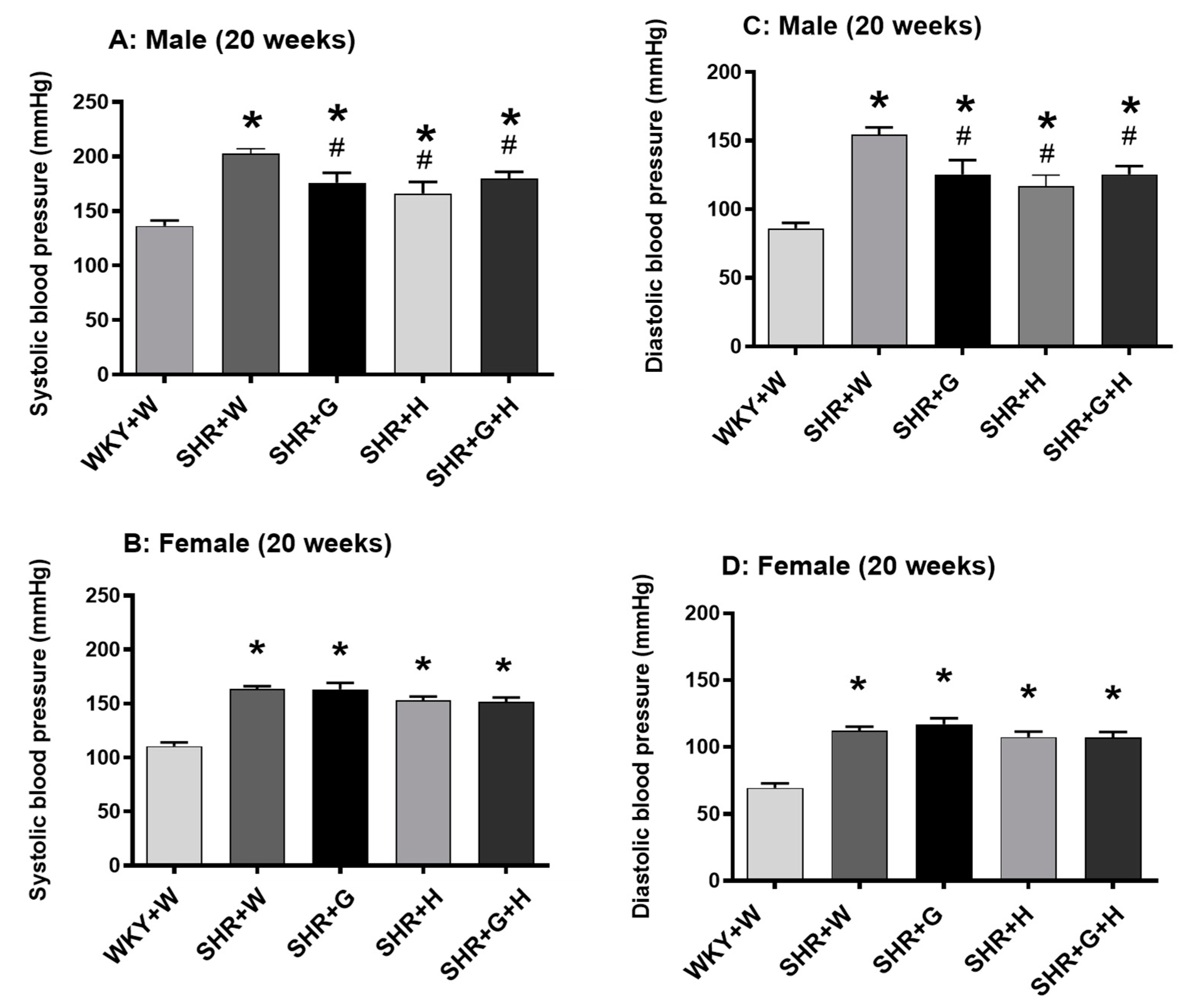
2.4. BP Measurement
2.5. Serum Biochemical Assays
2.6. Statistical Analysis
3. Results
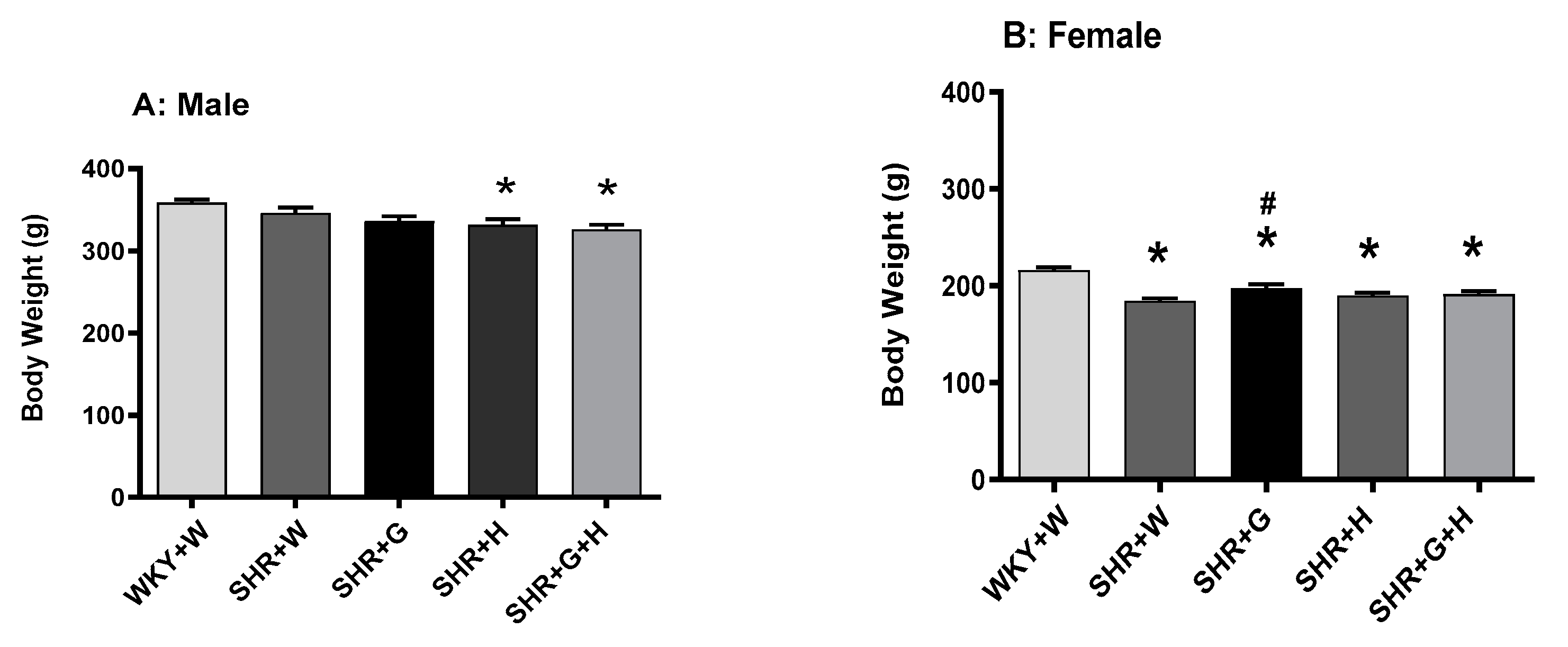
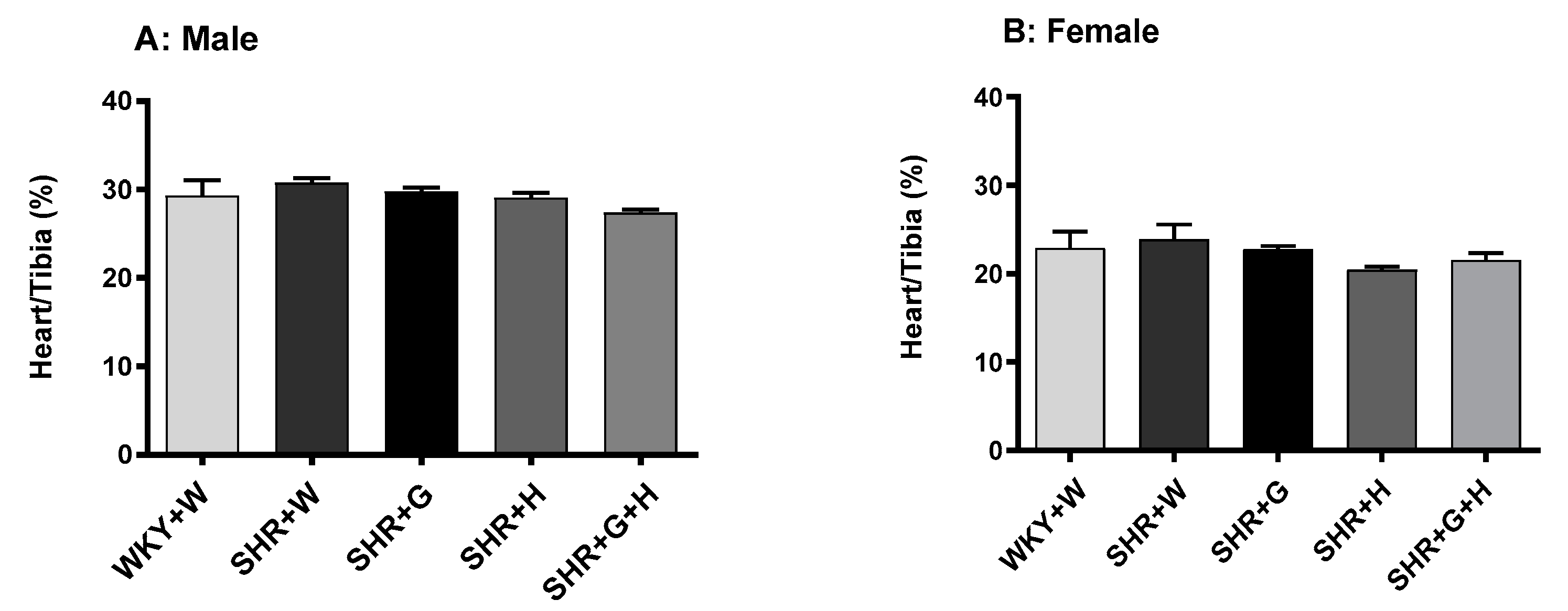
3.1. Body Weight and Heart/Tibia Length in SHRs and WKY
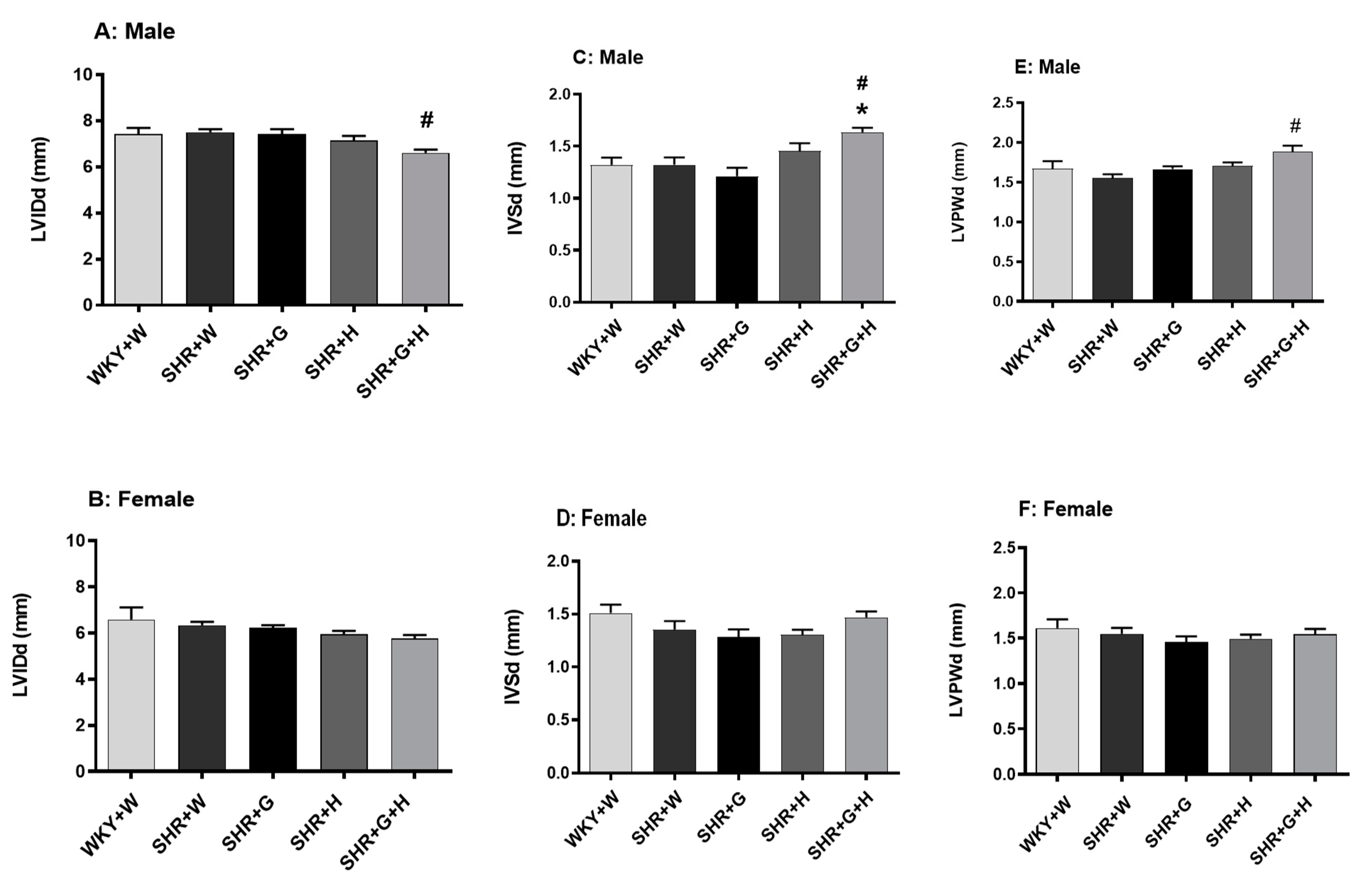
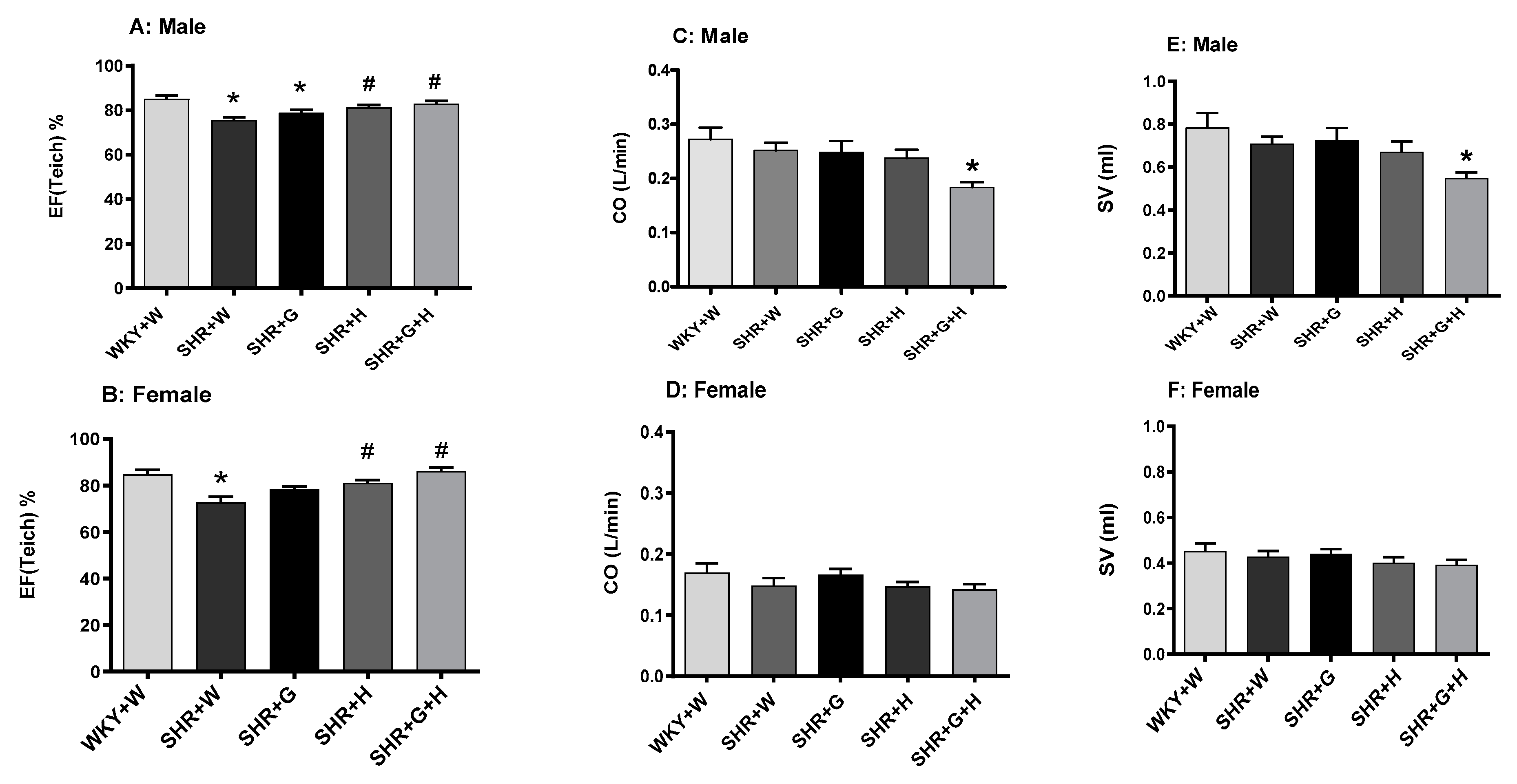
3.2. Heart Structure and Function in WKY Rats and SHRs
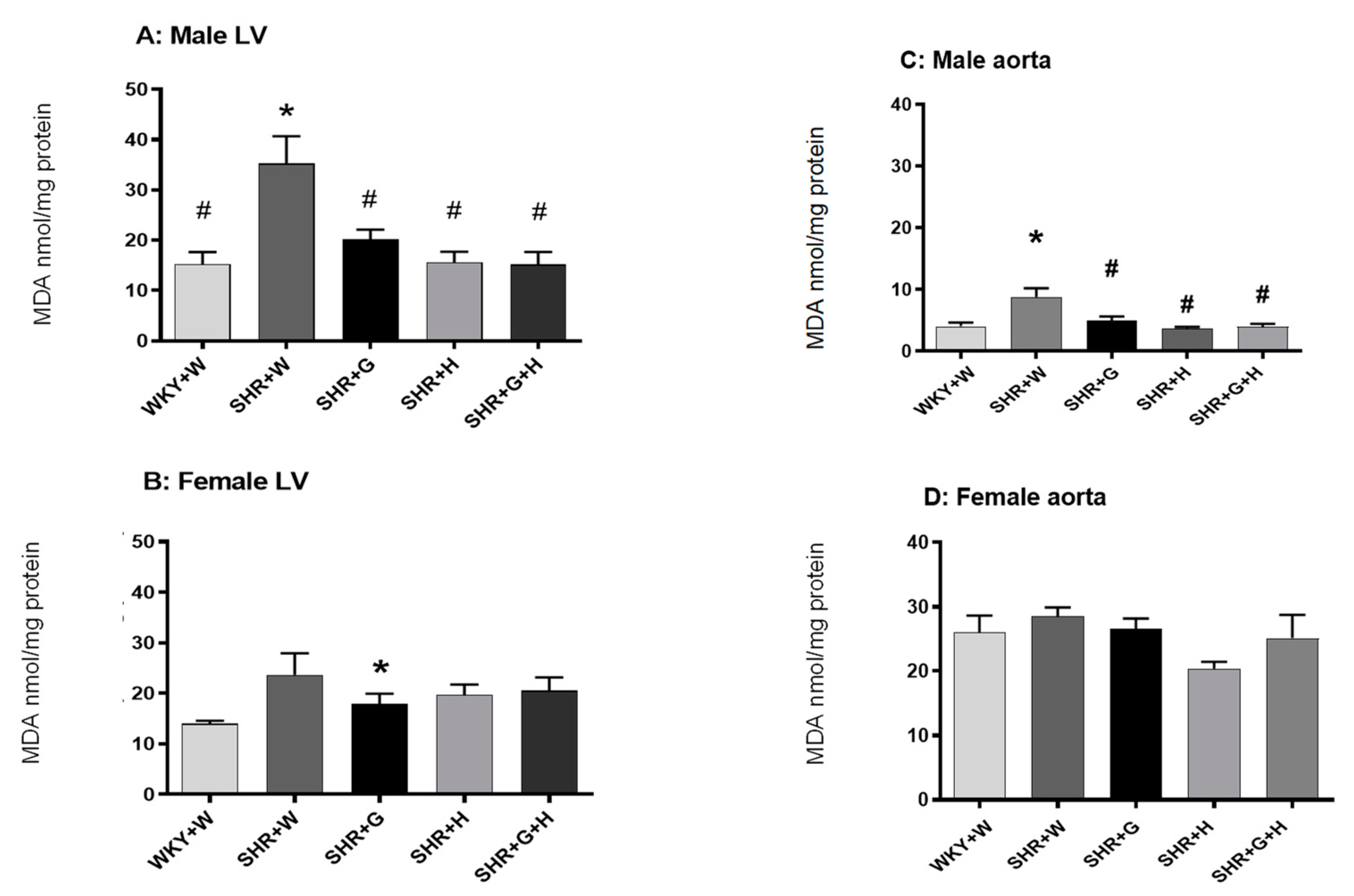
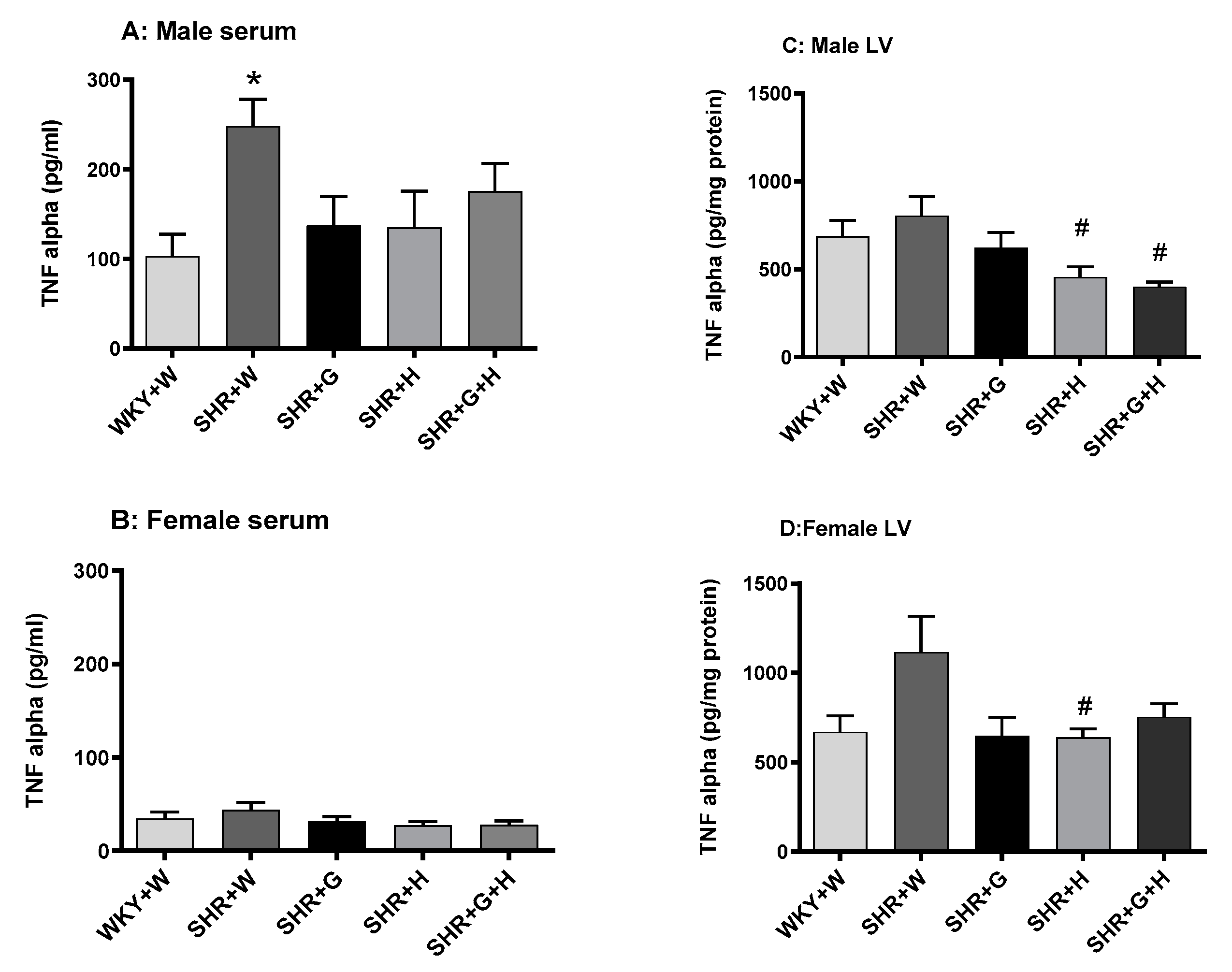
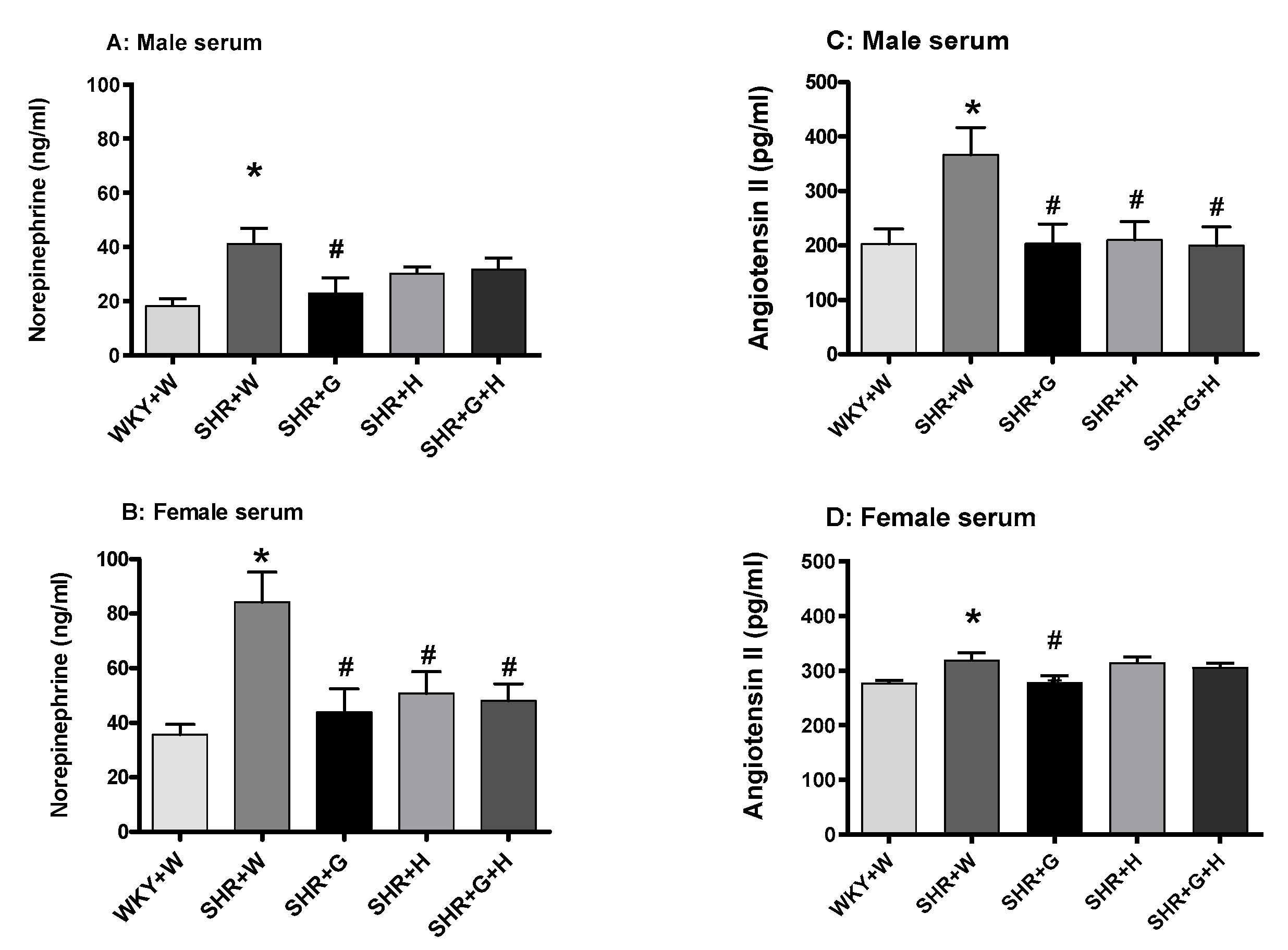
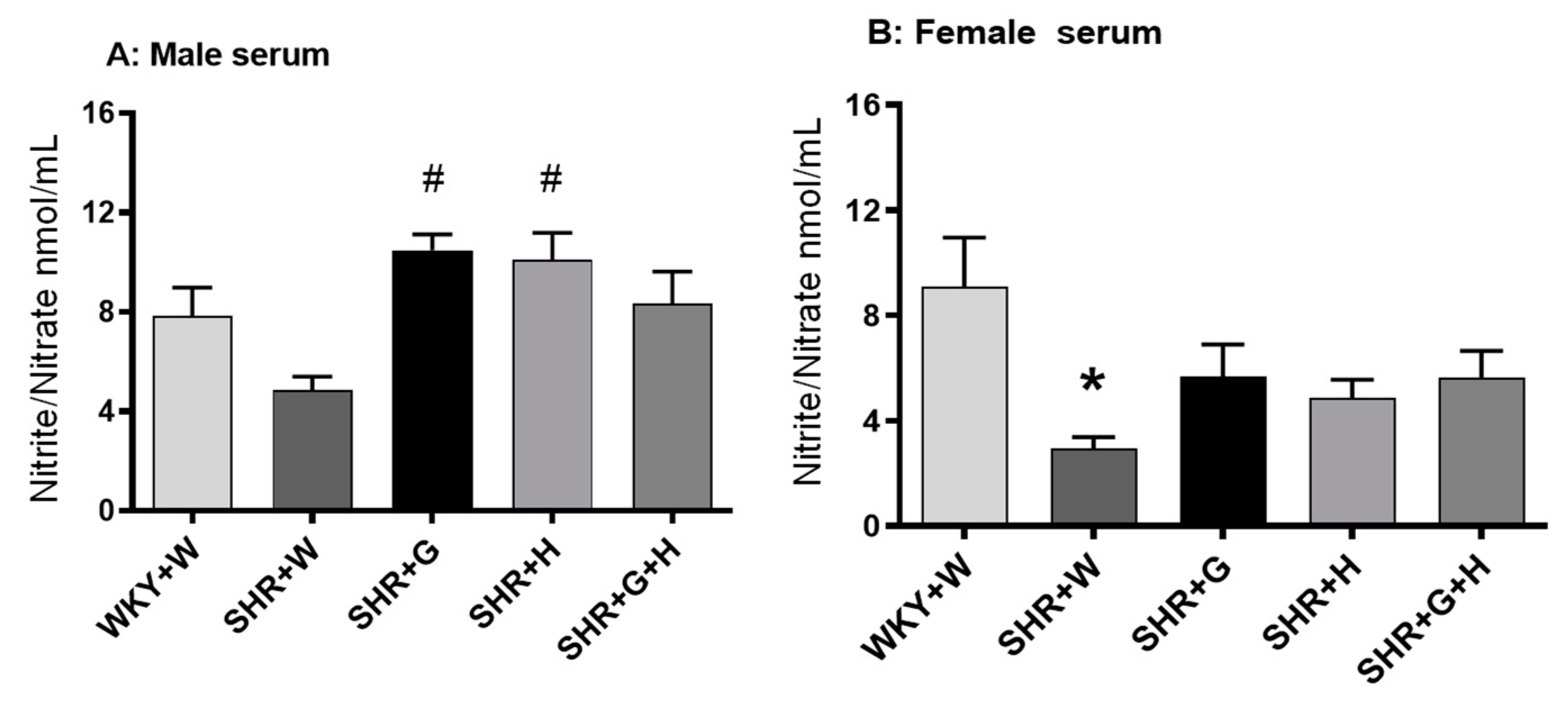
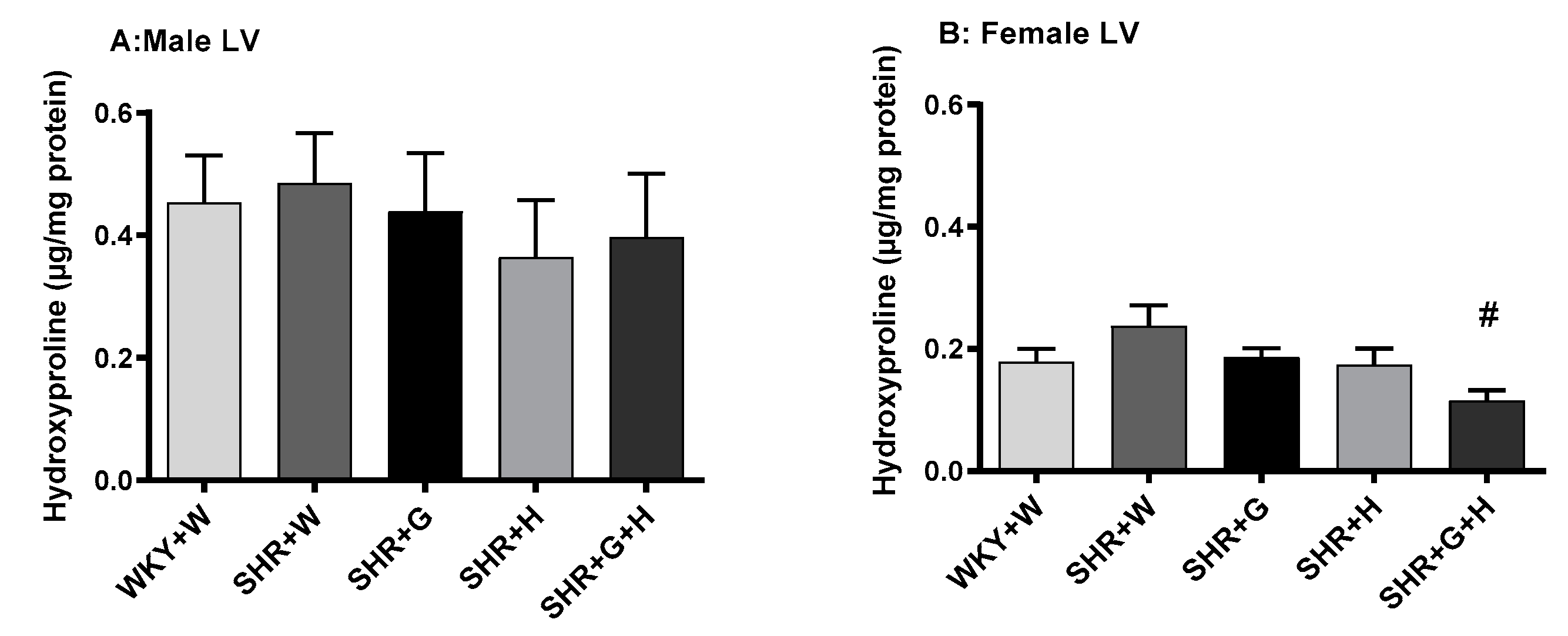
3.3. Biochemical Parameters in WKY Rats and SHRs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in blood pressure from 1975 to 2015: A pooled analysis of 1479 population-based measurement studies with 19.1 million participants. Lancet 2017, 389, 37–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salem, H.; Hasan, D.M.; Eameash, A.; El-Mageed, H.A.; Hasan, S.; Ali, R. Worldwide prevalence of hypertension: A pooled meta-analysis of 1670 studies in 71 countries with 29.5 million participants. J. Am. Coll. Cardiol. 2018, 71 (Suppl. 11), A1819. [Google Scholar] [CrossRef]
- Oparil, S.; Acelajado, M.C.; Bakris, G.L.; Berlowitz, D.R.; Cifkova, R.; Dominiczak, A.F.; Grassi, G.; Jordan, J.; Poulter, N.R.; Rodgers, A.; et al. Hypertension. Nat. Rev. Dis. Primers 2018, 4, 18014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ekstrom, M.; Hellman, A.; Hasselstrom, J.; Hage, C.; Kahan, T.; Ugander, M.; Wallen, H.; Persson, H.; Linde, C. The transition from hypertension to hypertensive heart disease and heart failure: The PREFERS Hypertension study. ESC Heart Fail. 2020, 7, 737–746. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.F. Sexual dimorphism of hypertension. Curr. Opin. Nephrol. Hypertens. 1996, 5, 181–185. [Google Scholar] [CrossRef]
- Lattimer, J.M.; Haub, M.D. Effects of dietary fiber and its components on metabolic health. Nutrients 2010, 2, 1266–1289. [Google Scholar] [CrossRef] [Green Version]
- Brown, L.; Rosner, B.; Willett, W.W.; Sacks, F.M. Cholesterol-lowering effects of dietary fiber: A meta-analysis. Am. J. Clin. Nutr. 1999, 69, 30–42. [Google Scholar] [CrossRef] [Green Version]
- Whitehead, A.; Beck, E.J.; Tosh, S.; Wolever, T.M. Cholesterol-lowering effects of oat beta-glucan: A meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2014, 100, 1413–1421. [Google Scholar] [CrossRef] [Green Version]
- Ho, H.V.; Sievenpiper, J.L.; Zurbau, A.; Blanco Mejia, S.; Jovanovski, E.; Au-Yeung, F.; Jenkins, A.L.; Vuksan, V. A systematic review and meta-analysis of randomized controlled trials of the effect of barley beta-glucan on LDL-C, non-HDL-C and apoB for cardiovascular disease risk reduction(i–iv). Eur. J. Clin. Nutr. 2016, 70, 1239–1245. [Google Scholar] [CrossRef]
- Maki, K.C.; Galant, R.; Samuel, P.; Tesser, J.; Witchger, M.S.; Ribaya-Mercado, J.D.; Blumberg, J.B.; Geohas, J. Effects of consuming foods containing oat beta-glucan on blood pressure, carbohydrate metabolism and biomarkers of oxidative stress in men and women with elevated blood pressure. Eur. J. Clin. Nutr. 2007, 61, 786–795. [Google Scholar] [CrossRef] [Green Version]
- Marques, F.Z.; Nelson, E.; Chu, P.Y.; Horlock, D.; Fiedler, A.; Ziemann, M.; Tan, J.K.; Kuruppu, S.; Rajapakse, N.W.; El-Osta, A.; et al. High-Fiber Diet and Acetate Supplementation Change the Gut Microbiota and Prevent the Development of Hypertension and Heart Failure in Hypertensive Mice. Circulation 2017, 135, 964–977. [Google Scholar] [CrossRef]
- Bing, O.H.; Conrad, C.H.; Boluyt, M.O.; Robinson, K.G.; Brooks, W.W. Studies of prevention, treatment and mechanisms of heart failure in the aging spontaneously hypertensive rat. Heart Fail. Rev. 2002, 7, 71–88. [Google Scholar] [CrossRef]
- Doggrell, S.A.; Brown, L. Rat models of hypertension, cardiac hypertrophy and failure. Cardiovasc. Res. 1998, 39, 89–105. [Google Scholar] [CrossRef]
- Juric, D.; Wojciechowski, P.; Das, D.K.; Netticadan, T. Prevention of concentric hypertrophy and diastolic impairment in aortic-banded rats treated with resveratrol. Am. J. Physiol. Heart Circ. Physiol. 2007, 292, H2138–H2143. [Google Scholar] [CrossRef] [Green Version]
- Aloud, B.M.; Raj, P.; McCallum, J.; Kirby, C.; Louis, X.L.; Jahan, F.; Yu, L.; Hiebert, B.; Duhamel, T.A.; Wigle, J.T.; et al. Cyanidin 3-O-glucoside prevents the development of maladaptive cardiac hypertrophy and diastolic heart dysfunction in 20-week-old spontaneously hypertensive rats. Food Funct. 2018, 9, 3466–3480. [Google Scholar] [CrossRef]
- Zheng, X.; Qiu, Y.; Zhong, W.; Baxter, S.; Su, M.; Li, Q.; Xie, G.; Ore, B.M.; Qiao, S.; Spencer, M.D.; et al. A targeted metabolomic protocol for short-chain fatty acids and branched-chain amino acids. Metabolomics 2013, 9, 818–827. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, S.; Hu, R.; Leete, J.; Layton, A.T. Understanding sex differences in long-term blood pressure regulation: Insights from experimental studies and computational modeling. Am. J. Physiol. Heart Circ. Physiol. 2019, 316, H1113–H1123. [Google Scholar] [CrossRef]
- Olivera, S.; Graham, D. Sex differences in preclinical models of hypertension. J. Hum. Hypertens. 2022. [Google Scholar] [CrossRef]
- Ouchi, Y.; Share, L.; Crofton, J.T.; Iitake, K.; Brooks, D.P. Sex difference in the development of deoxycorticosterone-salt hypertension in the rat. Hypertension 1987, 9, 172–177. [Google Scholar] [CrossRef] [Green Version]
- Wiinberg, N.; Hoegholm, A.; Christensen, H.R.; Bang, L.E.; Mikkelsen, K.L.; Nielsen, P.E.; Svendsen, T.L.; Kampmann, J.P.; Madsen, N.H.; Bentzon, M.W. 24-h ambulatory blood pressure in 352 normal Danish subjects, related to age and gender. Am. J. Hypertens. 1995, 8 Pt 1, 978–986. [Google Scholar] [CrossRef]
- Samargandy, S.; Matthews, K.A.; Brooks, M.M.; Barinas-Mitchell, E.; Magnani, J.W.; Thurston, R.C.; El Khoudary, S.R. Trajectories of Blood Pressure in Midlife Women: Does Menopause Matter? Circ. Res. 2022, 130, 312–322. [Google Scholar] [CrossRef] [PubMed]
- Gu, Q.; Burt, V.L.; Paulose-Ram, R.; Dillon, C.F. Gender differences in hypertension treatment, drug utilization patterns, and blood pressure control among US adults with hypertension: Data from the National Health and Nutrition Examination Survey 1999–2004. Am. J. Hypertens. 2008, 21, 789–798. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lagi, A.; Cencetti, S. Hypertensive emergencies: A new clinical approach. Clin. Hypertens. 2015, 21, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva-Antonialli, M.M.; Tostes, R.C.; Fernandes, L.; Fior-Chadi, D.R.; Akamine, E.H.; Carvalho, M.H.; Fortes, Z.B.; Nigro, D. A lower ratio of AT1/AT2 receptors of angiotensin II is found in female than in male spontaneously hypertensive rats. Cardiovasc. Res. 2004, 62, 587–593. [Google Scholar] [CrossRef] [PubMed]
- Wright, J.T., Jr.; Whelton, P.K.; Johnson, K.C.; Snyder, J.K.; Reboussin, D.M.; Cushman, W.C.; Williamson, J.D.; Pajewski, N.M.; Cheung, A.K.; Lewis, C.E.; et al. SPRINT Revisited: Updated Results and Implications. Hypertension 2021, 78, 1701–1710. [Google Scholar] [CrossRef]
- Kim, E.S.H.; Menon, V. Status of Women in Cardiovascular Clinical Trials. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 279–283. [Google Scholar] [CrossRef]
- Tamargo, J.; Rosano, G.; Walther, T.; Duarte, J.; Niessner, A.; Kaski, J.C.; Ceconi, C.; Drexel, H.; Kjeldsen, K.; Savarese, G.; et al. Gender differences in the effects of cardiovascular drugs. Eur. Heart J. Cardiovasc. Pharmacother. 2017, 3, 163–182. [Google Scholar] [CrossRef] [Green Version]
- Musini, V.M.; Nazer, M.; Bassett, K.; Wright, J.M. Blood pressure-lowering efficacy of monotherapy with thiazide diuretics for primary hypertension. Cochrane Database Syst. Rev. 2014, CD003824. [Google Scholar] [CrossRef] [Green Version]
- Gerdts, E.; Sudano, I.; Brouwers, S.; Borghi, C.; Bruno, R.M.; Ceconi, C.; Cornelissen, V.; Dievart, F.; Ferrini, M.; Kahan, T.; et al. Sex differences in arterial hypertension. Eur. Heart J. 2022, 43, 4777–4788. [Google Scholar] [CrossRef]
- Bazzano, L.A.; Green, T.; Harrison, T.N.; Reynolds, K. Dietary approaches to prevent hypertension. Curr. Hypertens. Rep. 2013, 15, 694–702. [Google Scholar] [CrossRef] [Green Version]
- Llanaj, E.; Dejanovic, G.M.; Valido, E.; Bano, A.; Gamba, M.; Kastrati, L.; Minder, B.; Stojic, S.; Voortman, T.; Marques-Vidal, P.; et al. Effect of oat supplementation interventions on cardiovascular disease risk markers: A systematic review and meta-analysis of randomized controlled trials. Eur. J. Nutr. 2022, 61, 1749–1778. [Google Scholar] [CrossRef]
- Liatis, S.; Tsapogas, P.; Chala, E.; Dimosthenopoulos, C.; Kyriakopoulos, K.; Kapantais, E.; Katsilambros, N. The consumption of bread enriched with betaglucan reduces LDL-cholesterol and improves insulin resistance in patients with type 2 diabetes. Diabetes Metab. 2009, 35, 115–120. [Google Scholar] [CrossRef]
- He, J.; Streiffer, R.H.; Muntner, P.; Krousel-Wood, M.A.; Whelton, P.K. Effect of dietary fiber intake on blood pressure: A randomized, double-blind, placebo-controlled trial. J. Hypertens. 2004, 22, 73–80. [Google Scholar] [CrossRef]
- Thandapilly, S.J.; Wojciechowski, P.; Behbahani, J.; Louis, X.L.; Yu, L.; Juric, D.; Kopilas, M.A.; Anderson, H.D.; Netticadan, T. Resveratrol prevents the development of pathological cardiac hypertrophy and contractile dysfunction in the SHR without lowering blood pressure. Am. J. Hypertens. 2010, 23, 192–196. [Google Scholar] [CrossRef] [Green Version]
- Aziz, F.; Tk, L.A.; Enweluzo, C.; Dutta, S.; Zaeem, M. Diastolic heart failure: A concise review. J. Clin. Med. Res. 2013, 5, 327–334. [Google Scholar] [CrossRef] [Green Version]
- Mureddu, G.F.; de Simone, G.; Greco, R.; Rosato, G.F.; Contaldo, F. Left ventricular filling in arterial hypertension. Influence of obesity and hemodynamic and structural confounders. Hypertension 1997, 29, 544–550. [Google Scholar] [CrossRef]
- de Simone, G.; Greco, R.; Mureddu, G.; Romano, C.; Guida, R.; Celentano, A.; Contaldo, F. Relation of left ventricular diastolic properties to systolic function in arterial hypertension. Circulation 2000, 101, 152–157. [Google Scholar] [CrossRef]
- Mattioli, A.V.; Zennaro, M.; Bonatti, S.; Bonetti, L.; Mattioli, G. Regression of left ventricular hypertrophy and improvement of diastolic function in hypertensive patients treated with telmisartan. Int. J. Cardiol. 2004, 97, 383–388. [Google Scholar] [CrossRef]
- Wachtell, K.; Bella, J.N.; Rokkedal, J.; Palmieri, V.; Papademetriou, V.; Dahlof, B.; Aalto, T.; Gerdts, E.; Devereux, R.B. Change in diastolic left ventricular filling after one year of antihypertensive treatment: The Losartan Intervention For Endpoint Reduction in Hypertension (LIFE) Study. Circulation 2002, 105, 1071–1076. [Google Scholar] [CrossRef] [Green Version]
- Muller-Brunotte, R.; Kahan, T.; Malmqvist, K.; Ring, M.; Edner, M. Tissue velocity echocardiography shows early improvement in diastolic function with irbesartan and atenolol therapy in patients with hypertensive left ventricular hypertrophy. Results form the Swedish Irbesartan Left Ventricular Hypertrophy Investigation vs Atenolol (SILVHIA). Am. J. Hypertens. 2006, 19, 927–936. [Google Scholar]
- Ito, H.; Ishii, K.; Kihara, H.; Kasayuki, N.; Nakamura, F.; Shimada, K.; Fukuda, S.; Iwakura, K.; Yoshikawa, J. Adding thiazide to a renin-angiotensin blocker improves left ventricular relaxation and improves heart failure in patients with hypertension. Hypertens. Res. 2012, 35, 93–99. [Google Scholar] [CrossRef] [Green Version]
- Sullivan, J.C.; Sasser, J.M.; Pollock, J.S. Sexual dimorphism in oxidant status in spontaneously hypertensive rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 292, R764–R768. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khullar, M.; Relan, V.; Sehrawat, B.S. Antioxidant activities and oxidative stress byproducts in human hypertension. Hypertension 2004, 43, e7–e8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raj, P.; Ames, N.; Joseph Thandapilly, S.; Yu, L.; Netticadan, T. The effects of oat ingredients on blood pressure in spontaneously hypertensive rats. J. Food Biochem. 2020, 44, e13402. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, D.; Haque, M.; Sriramula, S.; Mariappan, N.; Pariaut, R.; Francis, J. Role of proinflammatory cytokines and redox homeostasis in exercise-induced delayed progression of hypertension in spontaneously hypertensive rats. Hypertension 2009, 54, 1393–1400. [Google Scholar] [CrossRef] [Green Version]
- Adler, S.; Huang, H. Oxidant stress in kidneys of spontaneously hypertensive rats involves both oxidase overexpression and loss of extracellular superoxide dismutase. Am. J. Physiol. Renal Physiol. 2004, 287, F907–F913. [Google Scholar] [CrossRef] [Green Version]
- Chan, S.H.; Tai, M.H.; Li, C.Y.; Chan, J.Y. Reduction in molecular synthesis or enzyme activity of superoxide dismutases and catalase contributes to oxidative stress and neurogenic hypertension in spontaneously hypertensive rats. Free Radic. Biol. Med. 2006, 40, 2028–2039. [Google Scholar] [CrossRef]
- Sanz-Rosa, D.; Oubiña, M.P.; Cediel, E.; Heras, N.d.l.; Vegazo, O.; Jiménez, J.; Lahera, V.; Cachofeiro, V. Effect of AT1 receptor antagonism on vascular and circulating inflammatory mediators in SHR: Role of NF-κB/IκB system. Am. J. Physiol.-Heart Circ. Physiol. 2005, 288, H111–H115. [Google Scholar] [CrossRef] [Green Version]
- Patrick, D.M.; Van Beusecum, J.P.; Kirabo, A. The role of inflammation in hypertension: Novel concepts. Curr. Opin. Physiol. 2021, 19, 92–98. [Google Scholar] [CrossRef]
- Forstermann, U.; Xia, N.; Li, H. Roles of Vascular Oxidative Stress and Nitric Oxide in the Pathogenesis of Atherosclerosis. Circ. Res. 2017, 120, 713–735. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raj, P.; Sayfee, K.; Yu, L.; Sabra, A.; Wijekoon, C.; Malunga, L.; Thandapilly, S.J.; Netticadan, T. Oat Beta-Glucan Alone and in Combination with Hydrochlorothiazide Lowers High Blood Pressure in Male but Not Female Spontaneously Hypertensive Rats. Nutrients 2023, 15, 3180. https://doi.org/10.3390/nu15143180
Raj P, Sayfee K, Yu L, Sabra A, Wijekoon C, Malunga L, Thandapilly SJ, Netticadan T. Oat Beta-Glucan Alone and in Combination with Hydrochlorothiazide Lowers High Blood Pressure in Male but Not Female Spontaneously Hypertensive Rats. Nutrients. 2023; 15(14):3180. https://doi.org/10.3390/nu15143180
Chicago/Turabian StyleRaj, Pema, Karen Sayfee, Liping Yu, Ali Sabra, Champa Wijekoon, Lovemore Malunga, Sijo Joseph Thandapilly, and Thomas Netticadan. 2023. "Oat Beta-Glucan Alone and in Combination with Hydrochlorothiazide Lowers High Blood Pressure in Male but Not Female Spontaneously Hypertensive Rats" Nutrients 15, no. 14: 3180. https://doi.org/10.3390/nu15143180




