Association between Serum Selenium Levels and Lipids among People with and without Diabetes
Abstract
:1. Introduction
2. Methods
2.1. Study Design and Participants
2.2. Definition of Diabetes
2.3. Measurement of Serum Selenium Levels
2.4. Measurement of Serum Lipids Levels
2.5. Assessment of Other Covariates
2.6. Statistical Analysis
3. Results
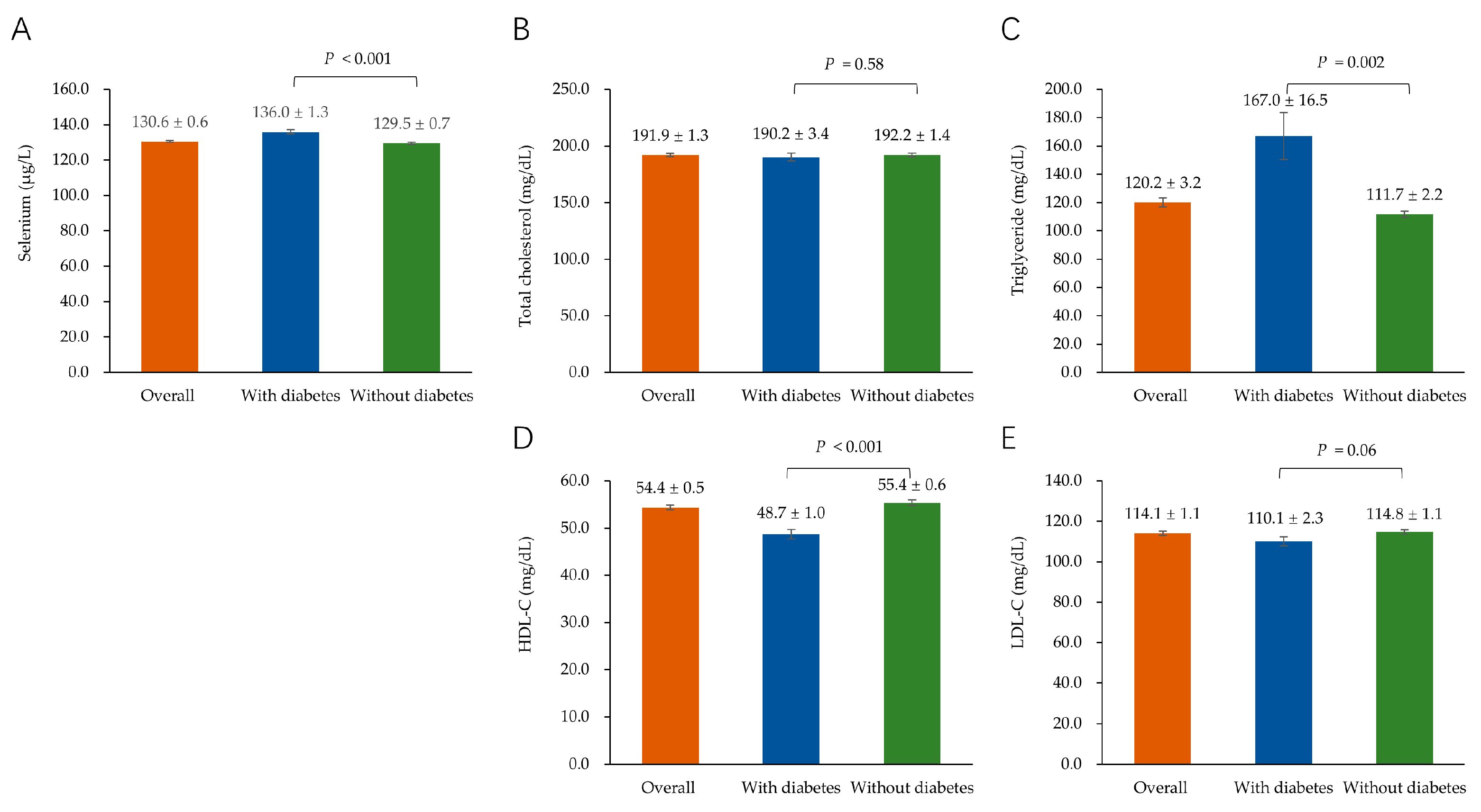
3.1. Characteristics of the Study Participants
3.2. Association of Serum Selenium with TC
3.3. Association of Serum Selenium with TG
3.4. Association of Serum Selenium with HDL-C
3.5. Association of Serum Selenium with LDL-C
3.6. Interaction between Serum Selenium and Diabetic Status for Lipids
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Grundy, S.M.; Cleeman, J.I.; Merz, C.N.; Brewer, H.B., Jr.; Clark, L.T.; Hunninghake, D.B.; Pasternak, R.C.; Smith, S.C., Jr.; Stone, N.J.; Coordinating Committee of the National Cholesterol Education Program. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III Guidelines. J. Am. Coll. Cardiol. 2004, 44, 720–732. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zoroddu, M.A.; Aaseth, J.; Crisponi, G.; Medici, S.; Peana, M.; Nurchi, V.M. The essential metals for humans: A brief overview. J. Inorg. Biochem. 2019, 195, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Barragan, R.; Sanchez-Gonzalez, C.; Aranda, P.; Sorli, J.V.; Asensio, E.M.; Portoles, O.; Ortega-Azorin, C.; Villamil, L.V.; Coltell, O.; Llopis, J.; et al. Single and combined associations of plasma and urine essential trace elements (Zn, Cu, Se, and Mn) with cardiovascular risk factors in a mediterranean population. Antioxidants 2022, 11, 1991. [Google Scholar] [CrossRef] [PubMed]
- Brown, K.M.; Arthur, J.R. Selenium, selenoproteins and human health: A review. Public Health Nutr. 2001, 4, 593–599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhuang, Y.; Li, L.; Feng, L.; Wang, S.; Su, H.; Liu, H.; Liu, H.; Wu, Y. Mitochondrion-targeted selenium nanoparticles enhance reactive oxygen species-mediated cell death. Nanoscale 2020, 12, 1389–1396. [Google Scholar] [CrossRef]
- Peng, K.Y.; Watt, M.J.; Rensen, S.; Greve, J.W.; Huynh, K.; Jayawardana, K.S.; Meikle, P.J.; Meex, R.C.R. Mitochondrial dysfunction-related lipid changes occur in nonalcoholic fatty liver disease progression. J. Lipid Res. 2018, 59, 1977–1986. [Google Scholar] [CrossRef] [Green Version]
- Biczo, G.; Vegh, E.T.; Shalbueva, N.; Mareninova, O.A.; Elperin, J.; Lotshaw, E.; Gretler, S.; Lugea, A.; Malla, S.R.; Dawson, D.; et al. Mitochondrial dysfunction, through impaired autophagy, leads to endoplasmic reticulum stress, deregulated lipid metabolism, and pancreatitis in animal models. Gastroenterology 2018, 154, 689–703. [Google Scholar] [CrossRef] [Green Version]
- Guo, X.; Yang, Q.; Zhang, W.; Chen, Y.; Ren, J.; Gao, A. Associations of blood levels of trace elements and heavy metals with metabolic syndrome in Chinese male adults with microRNA as mediators involved. Environ. Pollut. 2019, 248, 66–73. [Google Scholar] [CrossRef]
- Zinkhan, E.K.; Yu, B.; Schlegel, A. Prenatal exposure to a maternal high fat diet increases hepatic cholesterol accumulation in intrauterine growth restricted rats in part through microRNA-122 inhibition of Cyp7a1. Front. Physiol. 2018, 9, 645. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Z.; Barcus, M.; Kim, J.; Lum, K.L.; Mills, C.; Lei, X.G. High dietary selenium intake alters lipid metabolism and protein synthesis in liver and muscle of pigs. J. Nutr. 2016, 146, 1625–1633. [Google Scholar] [CrossRef] [Green Version]
- Ju, W.; Ji, M.; Li, X.; Li, Z.; Wu, G.; Fu, X.; Yang, X.; Gao, X. Relationship between higher serum selenium level and adverse blood lipid profile. Clin. Nutr. 2018, 37, 1512–1517. [Google Scholar] [CrossRef] [PubMed]
- Bukkens, S.G.; de Vos, N.; Kok, F.J.; Schouten, E.G.; de Bruijn, A.M.; Hofman, A. Selenium status and cardiovascular risk factors in healthy Dutch subjects. J. Am. Coll. Nutr. 1990, 9, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Krauss, R.M. Lipids and lipoproteins in patients with type 2 diabetes. Diabetes Care 2004, 27, 1496–1504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mueller, A.S.; Mueller, K.; Wolf, N.M.; Pallauf, J. Selenium and diabetes: An enigma? Free Radic. Res. 2009, 43, 1029–1059. [Google Scholar] [CrossRef]
- American Diabetes Association. 2. Classification and diagnosis of diabetes: Standards of medical care in diabetes-2019. Diabetes Care 2019, 42, S13–S28. [Google Scholar] [CrossRef] [Green Version]
- U.S. Department of Health and Human Services. Physical Activity Guidelines for Americans. Available online: https://health.gov/paguidelines/guidelines/chapter5.aspx (accessed on 19 October 2021).
- Reedy, J.; Lerman, J.L.; Krebs-Smith, S.M.; Kirkpatrick, S.I.; Pannucci, T.E.; Wilson, M.M.; Subar, A.F.; Kahle, L.L.; Tooze, J.A. Evaluation of the Healthy Eating Index-2015. J. Acad. Nutr. Diet. 2018, 118, 1622–1633. [Google Scholar] [CrossRef]
- Christensen, K.; Werner, M.; Malecki, K. Serum selenium and lipid levels: Associations observed in the National Health and Nutrition Examination Survey (NHANES) 2011–2012. Environ. Res. 2015, 140, 76–84. [Google Scholar] [CrossRef]
- Bleys, J.; Navas-Acien, A.; Stranges, S.; Menke, A.; Miller, E.R., 3rd; Guallar, E. Serum selenium and serum lipids in US adults. Am. J. Clin. Nutr. 2008, 88, 416–423. [Google Scholar] [CrossRef] [Green Version]
- Stranges, S.; Laclaustra, M.; Ji, C.; Cappuccio, F.P.; Navas-Acien, A.; Ordovas, J.M.; Rayman, M.; Guallar, E. Higher selenium status is associated with adverse blood lipid profile in British adults. J. Nutr. 2010, 140, 81–87. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.Q.; Shen, G.; Lo, K.; Huang, J.Y.; Liu, L.; Chen, C.L.; Yu, Y.L.; Sun, S.; Zhang, B.; Feng, Y.Q. Association of circulating selenium concentration with dyslipidemia: Results from the NHANES. J. Trace Elem. Med. Biol. 2020, 58, 126438. [Google Scholar] [CrossRef]
- Chen, C.; Jin, Y.; Unverzagt, F.W.; Cheng, Y.; Hake, A.M.; Liang, C.; Ma, F.; Su, L.; Liu, J.; Bian, J.; et al. The association between selenium and lipid levels: A longitudinal study in rural elderly Chinese. Arch. Gerontol. Geriatr. 2015, 60, 147–152. [Google Scholar] [CrossRef] [Green Version]
- Combs, G.F., Jr. Biomarkers of selenium status. Nutrients 2015, 7, 2209–2236. [Google Scholar] [CrossRef] [Green Version]
- Faghihi, T.; Radfar, M.; Barmal, M.; Amini, P.; Qorbani, M.; Abdollahi, M.; Larijani, B. A randomized, placebo-controlled trial of selenium supplementation in patients with type 2 diabetes: Effects on glucose homeostasis, oxidative stress, and lipid profile. Am. J. Ther. 2014, 21, 491–495. [Google Scholar] [CrossRef]
- Asemi, Z.; Jamilian, M.; Mesdaghinia, E.; Esmaillzadeh, A. Effects of selenium supplementation on glucose homeostasis, inflammation, and oxidative stress in gestational diabetes: Randomized, double-blind, placebo-controlled trial. Nutrition 2015, 31, 1235–1242. [Google Scholar] [CrossRef]
- Bahmani, F.; Kia, M.; Soleimani, A.; Asemi, Z.; Esmaillzadeh, A. Effect of selenium supplementation on glycemic control and lipid profiles in patients with diabetic nephropathy. Biol. Trace Elem. Res. 2016, 172, 282–289. [Google Scholar] [CrossRef]
- Valentine, W.M.; Abel, T.W.; Hill, K.E.; Austin, L.M.; Burk, R.F. Neurodegeneration in mice resulting from loss of functional selenoprotein P or its receptor apolipoprotein E receptor 2. J. Neuropathol. Exp. Neurol. 2008, 67, 68–77. [Google Scholar] [CrossRef] [Green Version]
- Olson, G.E.; Winfrey, V.P.; Hill, K.E.; Burk, R.F. Megalin mediates selenoprotein P uptake by kidney proximal tubule epithelial cells. J. Biol. Chem. 2008, 283, 6854–6860. [Google Scholar] [CrossRef] [Green Version]
- Sengupta, A.; Carlson, B.A.; Hoffmann, V.J.; Gladyshev, V.N.; Hatfield, D.L. Loss of housekeeping selenoprotein expression in mouse liver modulates lipoprotein metabolism. Biochem. Biophys. Res. Commun. 2008, 365, 446–452. [Google Scholar] [CrossRef] [Green Version]
- Wang, N.; Tan, H.Y.; Li, S.; Xu, Y.; Guo, W.; Feng, Y. Supplementation of micronutrient selenium in metabolic diseases: Its role as an antioxidant. Oxid. Med. Cell. Longev. 2017, 2017, 7478523. [Google Scholar] [CrossRef]
- Navarro-Alarcon, M.; López-G de la Serrana, H.; Perez-Valero, V.; Lopez-Martinez, C. Serum and urine selenium concentrations as indicators of body status in patients with diabetes mellitus. Sci. Total Environ. 1999, 228, 79–85. [Google Scholar] [CrossRef]
- Kljai, K.; Runje, R. Selenium and glycogen levels in diabetic patients. Biol. Trace Elem. Res. 2001, 83, 223–229. [Google Scholar] [CrossRef] [PubMed]
| Variables | Overall Participants | Participants with Diabetes | Participants without Diabetes | p Value |
|---|---|---|---|---|
| Number | 4132 | 782 | 3350 | |
| Age, y | 47.0 ± 0.5 | 59.1 ± 0.8 | 44.8 ± 0.5 | <0.001 |
| BMI, kg/m2 | 35.8 ± 2.5 | 44.5 ± 7.9 | 34.2 ± 2.5 | 0.13 |
| Sex | 0.10 | |||
| Male | 2069 (51.0) | 438 (56.5) | 1631 (50.0) | |
| Female | 2063 (49.0) | 344 (43.5) | 1719 (50.0) | |
| Race/ethnicity | 0.54 | |||
| Mexican American | 567 (8.6) | 142 (8.6) | 425 (8.6) | |
| Non-Hispanic White | 1653 (66.3) | 243 (66.5) | 1410 (66.2) | |
| Non-Hispanic Black | 829 (10.6) | 187 (12.2) | 642 (10.3) | |
| Others | 1083 (14.6) | 210 (12.8) | 873 (14.9) | |
| Marital status | <0.001 | |||
| Married | 2434 (63.2) | 471 (65.8) | 1963 (62.7) | |
| Single | 885 (19.8) | 85 (8.8) | 800 (21.8) | |
| Others | 811 (17.0) | 225 (25.4) | 586 (15.5) | |
| Family income–poverty ratio | 0.57 | |||
| ≤1.0 | 863 (15.7) | 183 (17.4) | 680 (15.4) | |
| 1.0 ≤ 3.0 | 1537 (37.6) | 305 (38.8) | 1232 (37.3) | |
| >3.0 | 1383 (46.7) | 204 (43.8) | 1179 (47.3) | |
| Education level | <0.001 | |||
| Less than high school | 892 (16.1) | 266 (20.4) | 626 (15.3) | |
| High school or equivalent | 888 (20.6) | 182 (24.9) | 706 (19.8) | |
| College or above | 2350 (63.3) | 332 (54.7) | 2018 (64.9) | |
| Physical activity | <0.001 | |||
| Inactive | 1022 (21.0) | 294 (32.5) | 728 (18.9) | |
| Moderate | 556 (13.9) | 111 (16.8) | 445 (13.4) | |
| Vigorous | 2535 (65.0) | 369 (50.7) | 2166 (67.7) | |
| Smoking status | <0.001 | |||
| Non-smoker | 2377 (56.1) | 394 (48.9) | 1983 (57.5) | |
| Former smoker | 949 (24.3) | 255 (35.3) | 694 (22.3) | |
| Current smoker | 804 (19.5) | 132 (15.8) | 672 (20.2) | |
| Drinking status | 0.023 | |||
| Non-drinker | 562 (10.9) | 135 (13.0) | 427 (10.5) | |
| Former drinker | 597 (14.1) | 179 (18.1) | 418 (13.4) | |
| Current drinker | 2602 (75.0) | 400 (68.9) | 2202 (76.2) | |
| HEI-2015 score | 54.0 ± 0.4 | 54.1 ± 0.8 | 54.0 ± 0.4 | 0.91 |
| Selenium (μg/L) | Per SD Increase | ||||||
|---|---|---|---|---|---|---|---|
| Tertile 1 | Tertile 2 | Tertile 3 | |||||
| Reference | β (95% CI) | p Value | β (95% CI) | p Value | β (95% CI) | p Value | |
| Overall participants | <121.30 | 121.30–135.40 | >135.40 | ||||
| Crude | 0.00 | 8.48 (2.60, 14.36) | 0.006 | 14.08 (7.83, 20.33) | <0.001 | 6.18 (4.05, 8.31) | <0.001 |
| Model 1 | 0.00 | 7.50 (1.72, 13.29) | 0.012 | 13.18 (6.72, 19.65) | <0.001 | 5.97 (3.82, 8.12) | <0.001 |
| Model 2 | 0.00 | 6.65 (0.33, 12.97) | 0.040 | 12.75 (6.02, 19.47) | <0.001 | 5.54 (3.34, 7.74) | <0.001 |
| Participants with diabetes | <126.00 | 126.00–141.20 | >141.20 | ||||
| Crude | 0.00 | −10.48 (−25.24, 4.29) | 0.16 | −9.97 (−27.04, 7.10) | 0.25 | −2.38 (−8.71, 3.94) | 0.45 |
| Model 1 | 0.00 | −6.67 (−20.47, 7.12) | 0.34 | −5.85 (−22.90, 11.21) | 0.49 | −0.83 (−7.03, 5.37) | 0.79 |
| Model 2 | 0.00 | −6.16 (−20.45, 8.14) | 0.39 | −4.41 (−22.79, 13.97) | 0.63 | −0.76 (−7.42, 5.90) | 0.82 |
| Participants without diabetes | <120.50 | 120.50–133.90 | >133.90 | ||||
| Crude | 0.00 | 8.38 (2.04, 14.72) | 0.011 | 19.27 (12.65, 25.90) | <0.001 | 7.69 (5.35, 10.03) | <0.001 |
| Model 1 | 0.00 | 7.27 (1.10, 13.45) | 0.022 | 18.03 (11.53, 24.53) | <0.001 | 7.33 (5.11, 9.56) | <0.001 |
| Model 2 | 0.00 | 6.41 (−0.26, 13.08) | 0.06 | 17.88 (10.89, 24.87) | <0.001 | 6.89 (4.51, 9.26) | <0.001 |
| Selenium (μg/L) | Per SD Increase | ||||||
|---|---|---|---|---|---|---|---|
| Tertile 1 | Tertile 2 | Tertile 3 | |||||
| Reference | β (95% CI) | p Value | β (95% CI) | p Value | β (95% CI) | p Value | |
| Overall participants | <121.30 | 121.30–135.40 | >135.40 | ||||
| Crude | 0.00 | 19.02 (3.15, 34.88) | 0.020 | 29.75 (16.58, 42.92) | <0.001 | 12.54 (6.62, 18.46) | <0.001 |
| Model 1 | 0.00 | 13.73 (−1.43, 28.90) | 0.07 | 22.50 (8.68, 36.33) | 0.002 | 9.38 (3.46, 15.30) | 0.003 |
| Model 2 | 0.00 | 9.17 (−5.15, 23.50) | 0.20 | 23.31 (8.22, 38.40) | 0.003 | 8.85 (2.68, 15.01) | 0.006 |
| Participants with diabetes | <126.00 | 126.00–141.20 | >141.20 | ||||
| Crude | 0.00 | 4.68 (−64.29, 73.65) | 0.89 | 33.09 (−4.16, 70.34) | 0.08 | 20.63 (0.09, 41.17) | 0.049 |
| Model 1 | 0.00 | 8.12 (−61.70, 77.93) | 0.82 | 25.47 (−10.95, 61.90) | 0.17 | 19.08 (−1.14, 39.29) | 0.06 |
| Model 2 | 0.00 | −21.27 (−50.93, 8.39) | 0.16 | 38.90 (−6.77, 84.57) | 0.09 | 21.85 (−3.41, 47.10) | 0.09 |
| Participants without diabetes | <120.50 | 120.50–133.90 | >133.90 | ||||
| Crude | 0.00 | 10.39 (−0.32, 21.11) | 0.06 | 26.00 (11.77, 40.22) | <0.001 | 8.19 (3.43, 12.96) | 0.001 |
| Model 1 | 0.00 | 5.24 (−5.45, 15.94) | 0.33 | 20.39 (6.34, 34.44) | 0.005 | 5.63 (0.79, 10.47) | 0.024 |
| Model 2 | 0.00 | 4.04 (−8.23, 16.32) | 0.51 | 22.39 (6.92, 37.86) | 0.006 | 5.57 (0.44, 10.70) | 0.034 |
| Selenium (μg/L) | Per SD Increase | ||||||
|---|---|---|---|---|---|---|---|
| Tertile 1 | Tertile 2 | Tertile 3 | |||||
| Reference | β (95% CI) | p Value | β (95% CI) | p Value | β (95% CI) | p Value | |
| Overall participants | <121.30 | 121.30–135.40 | >135.40 | ||||
| Crude | 0.00 | −2.07 (−4.45, 0.30) | 0.09 | −1.31 (−3.60, 0.99) | 0.26 | −0.46 (−1.39, 0.47) | 0.32 |
| Model 1 | 0.00 | −1.27 (−3.68, 1.14) | 0.30 | −0.07 (−2.60, 2.45) | 0.95 | 0.19 (−0.76, 1.14) | 0.69 |
| Model 2 | 0.00 | −1.32 (−3.83, 1.19) | 0.30 | −0.64 (−3.48, 2.20) | 0.65 | −0.03 (−1.05, 0.99) | 0.95 |
| Participants with diabetes | <126.00 | 126.00–141.20 | >141.20 | ||||
| Crude | 0.00 | 3.66 (−1.28, 8.60) | 0.14 | −1.76 (−5.97, 2.44) | 0.40 | −0.21 (−2.10, 1.67) | 0.82 |
| Model 1 | 0.00 | 3.93 (−0.45, 8.30) | 0.08 | 0.59 (−3.35, 4.53) | 0.76 | 0.50 (−1.11, 2.10) | 0.53 |
| Model 2 | 0.00 | 3.02 (−1.82, 7.86) | 0.22 | −0.50 (−4.95, 3.95) | 0.82 | 0.44 (−1.41, 2.28) | 0.64 |
| Participants without diabetes | <120.50 | 120.50–133.90 | >133.90 | ||||
| Crude | 0.00 | −2.36 (−4.67, −0.04) | 0.046 | −0.47 (−3.01, 2.06) | 0.71 | −0.15 (−1.13, 0.84) | 0.76 |
| Model 1 | 0.00 | −1.29 (−3.47, 0.90) | 0.24 | 0.60 (−2.01, 3.21) | 0.65 | 0.44 (−0.52, 1.41) | 0.36 |
| Model 2 | 0.00 | −0.95 (−3.31, 1.41) | 0.42 | −0.16 (−3.14, 2.82) | 0.91 | 0.10 (−0.94, 1.14) | 0.85 |
| Selenium (μg/L) | Per SD Increase | ||||||
|---|---|---|---|---|---|---|---|
| Tertile 1 | Tertile 2 | Tertile 3 | |||||
| Reference | β (95% CI) | p Value | β (95% CI) | p Value | β (95% CI) | p Value | |
| Overall participants | <121.30 | 121.30–135.40 | >135.40 | ||||
| Crude | 0.00 | 7.66 (2.66, 12.65) | 0.003 | 9.63 (4.73, 14.53) | <0.001 | 4.22 (2.13, 6.30) | <0.001 |
| Model 1 | 0.00 | 6.81 (1.74, 11.89) | 0.010 | 8.78 (3.50, 14.06) | 0.002 | 3.91 (1.77, 6.06) | <0.001 |
| Model 2 | 0.00 | 6.09 (0.79, 11.40) | 0.025 | 8.87 (3.29, 14.46) | 0.003 | 3.80 (1.52, 6.09) | 0.002 |
| Participants with diabetes | <126.00 | 126.00–141.20 | >141.20 | ||||
| Crude | 0.00 | −9.51 (−22.03, 3.01) | 0.13 | −14.07 (−27.42, −0.71) | 0.039 | −6.00 (−10.80, −1.19) | 0.016 |
| Model 1 | 0.00 | −6.86 (−19.18, 5.46) | 0.27 | −11.55 (−25.68, 2.57) | 0.11 | −4.90 (−9.93, 0.13) | 0.06 |
| Model 2 | 0.00 | −3.86 (−16.11, 8.38) | 0.53 | −9.45 (−23.11, 4.21) | 0.17 | −4.51 (−9.27, 0.25) | 0.06 |
| Participants without diabetes | <120.50 | 120.50–133.90 | >133.90 | ||||
| Crude | 0.00 | 8.29 (3.16, 13.42) | 0.002 | 14.61 (9.51, 19.71) | <0.001 | 6.11 (3.83, 8.39) | <0.001 |
| Model 1 | 0.00 | 7.08 (1.92, 12.24) | 0.008 | 13.29 (8.03, 18.55) | <0.001 | 5.64 (3.39, 7.89) | <0.001 |
| Model 2 | 0.00 | 5.94 (0.33, 11.55) | 0.038 | 13.43 (7.68, 19.18) | <0.001 | 5.51 (3.02, 8.00) | <0.001 |
| TC | TG | HDL-C | LDL-C | |
|---|---|---|---|---|
| Crude | 0.002 | 0.30 | 0.97 | <0.001 |
| Model 1 | 0.003 | 0.30 | 0.80 | <0.001 |
| Model 2 | 0.007 | 0.39 | 0.64 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nie, Q.; Wang, C.; Zhou, L. Association between Serum Selenium Levels and Lipids among People with and without Diabetes. Nutrients 2023, 15, 3190. https://doi.org/10.3390/nu15143190
Nie Q, Wang C, Zhou L. Association between Serum Selenium Levels and Lipids among People with and without Diabetes. Nutrients. 2023; 15(14):3190. https://doi.org/10.3390/nu15143190
Chicago/Turabian StyleNie, Qi, Changsi Wang, and Li Zhou. 2023. "Association between Serum Selenium Levels and Lipids among People with and without Diabetes" Nutrients 15, no. 14: 3190. https://doi.org/10.3390/nu15143190




