Concepts of Lactate Metabolic Clearance Rate and Lactate Clamp for Metabolic Inquiry: A Mini-Review
Abstract
1. Introduction
1.1. Background on Lactate Clearance
1.2. The Clamp Technique
2. Previous Studies Utilizing a Lactate Infusion or Clamp
2.1. Lactate Infusion or Bolus Studies
2.2. Lactate Clamp Studies
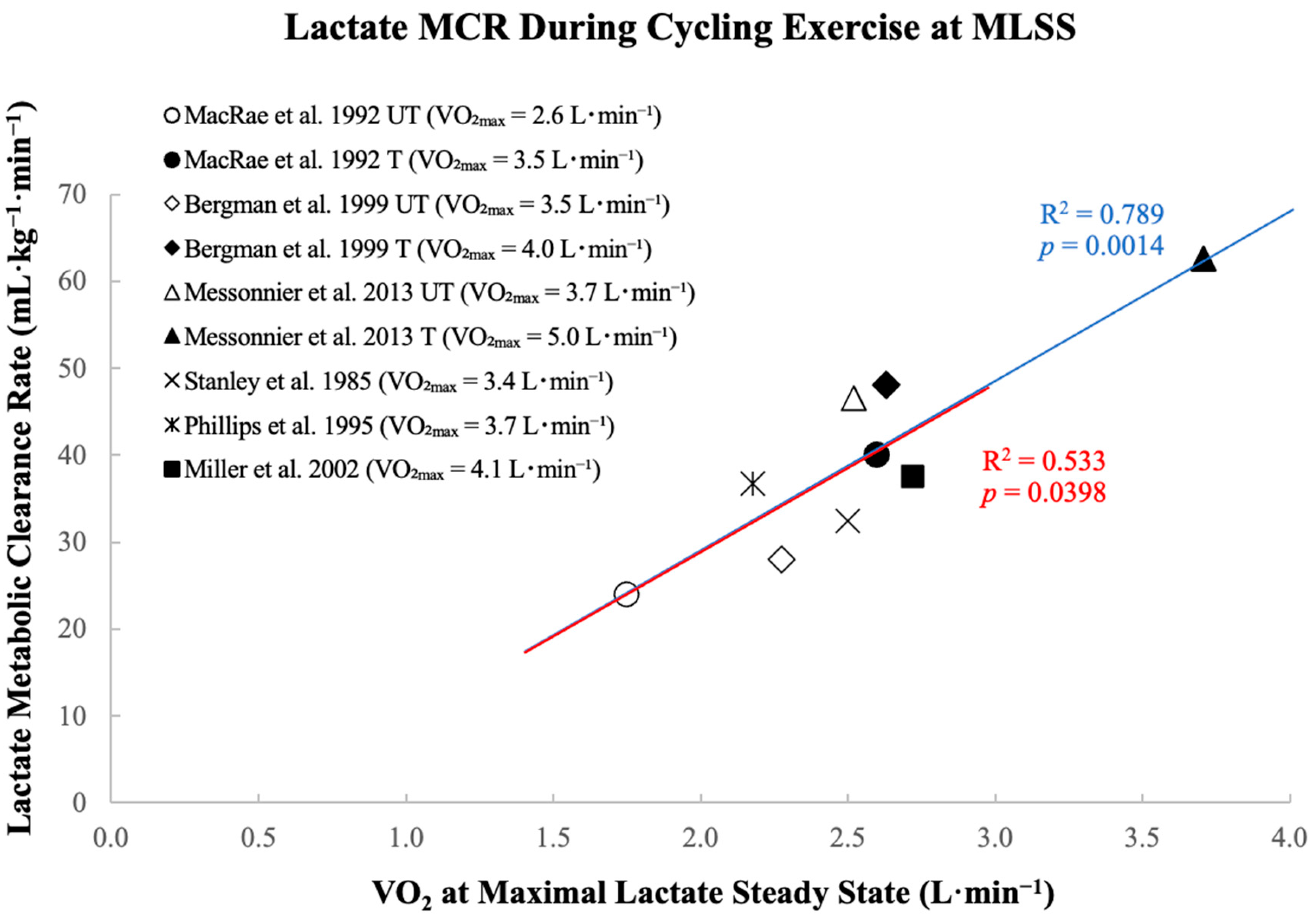
3. Metabolic Clearance Rate Limits Exercise Intensity at Maximal Lactate Steady State
4. Methodological Considerations with the Lactate Clamp
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gladden, L.B. Lactate Metabolism: A New Paradigm for the Third Millennium. J. Physiol. 2004, 558, 5–30. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, B.S.; Rogatzki, M.J.; Goodwin, M.L.; Kane, D.A.; Rightmire, Z.; Gladden, L.B. Lactate Metabolism: Historical Context, Prior Misinterpretations, and Current Understanding. Eur. J. Appl. Physiol. 2018, 118, 691–728. [Google Scholar] [CrossRef] [PubMed]
- Brooks, G.A. Lactate as a Fulcrum of Metabolism. Redox Biol. 2020, 35, 101454. [Google Scholar] [CrossRef] [PubMed]
- Hui, S.; Ghergurovich, J.M.; Morscher, R.J.; Jang, C.; Teng, X.; Lu, W.; Esparza, L.A.; Reya, T.; Zhan, L.; Guo, J.Y.; et al. Glucose Feeds the TCA Cycle via Circulating Lactate. Nature 2017, 551, 115–118. [Google Scholar] [CrossRef]
- Rogatzki, M.J.; Ferguson, B.S.; Goodwin, M.L.; Gladden, L.B. Lactate Is Always the End Product of Glycolysis. Front. Neurosci. 2015, 9, 22. [Google Scholar] [CrossRef]
- Bergman, B.C.; Wolfel, E.E.; Butterfield, G.E.; Lopaschuk, G.D.; Casazza, G.A.; Horning, M.A.; Brooks, G.A. Active Muscle and Whole Body Lactate Kinetics after Endurance Training in Men. J. Appl. Physiol. 1999, 87, 1684–1696. [Google Scholar] [CrossRef]
- Emhoff, C.A.W.; Messonnier, L.A.; Horning, M.A.; Fattor, J.A.; Carlson, T.J.; Brooks, G.A. Direct and Indirect Lactate Oxidation in Trained and Untrained Men. J. Appl. Physiol. 2013, 115, 829–838. [Google Scholar] [CrossRef]
- Emhoff, C.A.W.; Messonnier, L.A.; Horning, M.A.; Fattor, J.A.; Carlson, T.J.; Brooks, G.A. Gluconeogenesis and Hepatic Glycogenolysis during Exercise at the Lactate Threshold. J. Appl. Physiol. 2013, 114, 297–306. [Google Scholar] [CrossRef]
- Juel, C. Lactate-Proton Cotransport in Skeletal Muscle. Physiol. Rev. 1997, 77, 321–358. [Google Scholar] [CrossRef]
- Brooks, G.A.; Dubouchaud, H.; Brown, M.; Sicurello, J.P.; Butz, C.E. Role of Mitochondrial Lactate Dehydrogenase and Lactate Oxidation in the Intracellular Lactate Shuttle. Proc. Natl. Acad. Sci. USA 1999, 96, 1129–1134. [Google Scholar] [CrossRef]
- Brooks, G.A. The Lactate Shuttle during Exercise and Recovery. Med. Sci. Sports Exerc. 1986, 18, 360–368. [Google Scholar] [CrossRef]
- Brooks, G.A.; Arevalo, J.A.; Osmond, A.D.; Leija, R.G.; Curl, C.C.; Tovar, A.P. Lactate in Contemporary Biology: A Phoenix Risen. J. Physiol. 2022, 600, 1229–1251. [Google Scholar] [CrossRef]
- Liu, C.; Wu, J.; Zhu, J.; Kuei, C.; Yu, J.; Shelton, J.; Sutton, S.W.; Li, X.; Yun, S.J.; Mirzadegan, T.; et al. Lactate Inhibits Lipolysis in Fat Cells through Activation of an Orphan G-Protein-Coupled Receptor, GPR81. J. Biol. Chem. 2009, 284, 2811–2822. [Google Scholar] [CrossRef]
- Ahmed, K.; Tunaru, S.; Tang, C.; Müller, M.; Gille, A.; Sassmann, A.; Hanson, J.; Offermanns, S. An Autocrine Lactate Loop Mediates Insulin-Dependent Inhibition of Lipolysis through GPR81. Cell Metab. 2010, 11, 311–319. [Google Scholar] [CrossRef]
- Goodwin, M.L.; Harris, J.E.; Hernández, A.; Gladden, L.B. Blood Lactate Measurements and Analysis during Exercise: A Guide for Clinicians. J. Diabetes Sci. Technol. 2007, 1, 558–569. [Google Scholar] [CrossRef]
- Messonnier, L.A.; Emhoff, C.A.W.; Fattor, J.A.; Horning, M.A.; Carlson, T.J.; Brooks, G.A. Lactate Kinetics at the Lactate Threshold in Trained and Untrained Men. J. Appl. Physiol. 2013, 114, 1593–1602. [Google Scholar] [CrossRef]
- MacRae, H.S.; Dennis, S.C.; Bosch, A.N.; Noakes, T.D. Effects of Training on Lactate Production and Removal during Progressive Exercise in Humans. J. Appl. Physiol. 1992, 72, 1649–1656. [Google Scholar] [CrossRef] [PubMed]
- MacRae, H.H.; Noakes, T.D.; Dennis, S.C. Effects of Endurance Training on Lactate Removal by Oxidation and Gluconeogenesis during Exercise. Pflüg. Arch. Eur. J. Physiol. 1995, 430, 964–970. [Google Scholar] [CrossRef]
- Fattor, J.A.; Miller, B.F.; Jacobs, K.A.; Brooks, G.A. Catecholamine Response Is Attenuated during Moderate-Intensity Exercise in Response to the “Lactate Clamp”. Am. J. Physiol. Endocrinol. Metab. 2005, 288, E143–E147. [Google Scholar] [CrossRef]
- Chang, A.J.; Ortega, F.E.; Riegler, J.; Madison, D.V.; Krasnow, M.A. Oxygen Regulation of Breathing through an Olfactory Receptor Activated by Lactate. Nature 2015, 527, 240–244. [Google Scholar] [CrossRef]
- Torres-Torrelo, H.; Ortega-Sáenz, P.; Gao, L.; López-Barneo, J. Lactate Sensing Mechanisms in Arterial Chemoreceptor Cells. Nat. Commun. 2021, 12, 4166. [Google Scholar] [CrossRef] [PubMed]
- Messonnier, L.; Freund, H.; Féasson, L.; Prieur, F.; Castells, J.; Denis, C.; Linossier, M.T.; Geyssant, A.; Lacour, J.R. Blood Lactate Exchange and Removal Abilities after Relative High-Intensity Exercise: Effects of Training in Normoxia and Hypoxia. Eur. J. Appl. Physiol. 2001, 84, 403–412. [Google Scholar] [CrossRef] [PubMed]
- Dubouchaud, H.; Butterfield, G.E.; Wolfel, E.E.; Bergman, B.C.; Brooks, G.A. Endurance Training, Expression, and Physiology of LDH, MCT1, and MCT4 in Human Skeletal Muscle. Am. J. Physiol. Endocrinol. Metab. 2000, 278, E571–E579. [Google Scholar] [CrossRef]
- Quistorff, B.; Secher, N.H.; Van Lieshout, J.J. Lactate Fuels the Human Brain during Exercise. FASEB J. 2008, 22, 3443–3449. [Google Scholar] [CrossRef] [PubMed]
- Islam, H.; Townsend, L.K.; McKie, G.L.; Medeiros, P.J.; Gurd, B.J.; Hazell, T.J. Potential Involvement of Lactate and Interleukin-6 in the Appetite-Regulatory Hormonal Response to an Acute Exercise Bout. J. Appl. Physiol. 2017, 123, 614–623. [Google Scholar] [CrossRef] [PubMed]
- Brooks, G.A.; Osmond, A.D.; Arevalo, J.A.; Duong, J.J.; Curl, C.C.; Moreno-Santillan, D.D.; Leija, R.G. Lactate as a Myokine and Exerkine: Drivers and Signals of Physiology and Metabolism. J. Appl. Physiol. 2023, 134, 529–548. [Google Scholar] [CrossRef]
- Stainsby, W.; Welch, H. Lactate Metabolism of Contracting Dog Skeletal Muscle in Situ. Am. J. Physiol.-Leg. Content 1966, 211, 177–183. [Google Scholar] [CrossRef]
- Johnson, M.L.; Emhoff, C.-A.W.; Horning, M.A.; Brooks, G.A. Transpulmonary Lactate Shuttle. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2012, 302, R143–R149. [Google Scholar] [CrossRef]
- Brooks, G.A. The Science and Translation of Lactate Shuttle Theory. Cell Metab. 2018, 27, 757–785. [Google Scholar] [CrossRef]
- Hashimoto, T.; Hussien, R.; Brooks, G.A. Colocalization of MCT1, CD147, and LDH in Mitochondrial Inner Membrane of L6 Muscle Cells: Evidence of a Mitochondrial Lactate Oxidation Complex. Am. J. Physiol. Endocrinol. Metab. 2006, 290, E1237–E1244. [Google Scholar] [CrossRef]
- Goodpaster, B.H.; Sparks, L.M. Metabolic Flexibility in Health and Disease. Cell Metab. 2017, 25, 1027–1036. [Google Scholar] [CrossRef]
- San-Millán, I.; Brooks, G.A. Assessment of Metabolic Flexibility by Means of Measuring Blood Lactate, Fat, and Carbohydrate Oxidation Responses to Exercise in Professional Endurance Athletes and Less-Fit Individuals. Sports Med. 2018, 48, 467–479. [Google Scholar] [CrossRef]
- Prior, S.J.; Ryan, A.S.; Stevenson, T.G.; Goldberg, A.P. Metabolic Inflexibility during Submaximal Aerobic Exercise Is Associated with Glucose Intolerance in Obese Older Adults: Metabolic Inflexibility during Exercise in IGT. Obesity 2014, 22, 451–457. [Google Scholar] [CrossRef]
- Lowell, B.B.; Shulman, G.I. Mitochondrial Dysfunction and Type 2 Diabetes. Science 2005, 307, 384–387. [Google Scholar] [CrossRef]
- Jacobs, R.A.; Lundby, C. Mitochondria Express Enhanced Quality as Well as Quantity in Association with Aerobic Fitness across Recreationally Active Individuals up to Elite Athletes. J. Appl. Physiol. 2013, 114, 344–350. [Google Scholar] [CrossRef]
- Reaven, G.M.; Hollenbeck, C.; Jeng, C.-Y.; Wu, M.S.; Chen, Y.-D.I. Measurement of Plasma Glucose, Free Fatty Acid, Lactate, and Insulin for 24 h in Patients with NIDDM. Diabetes 1988, 37, 1020–1024. [Google Scholar] [CrossRef]
- Krentz, A.J.; Singh, B.M.; Nattrass, M. Impaired Glucose Tolerance Is Characterized by Multiple Abnormalities in the Regulation of Intermediary Metabolism. Diabet. Med. 1991, 8, 848–854. [Google Scholar] [CrossRef]
- Tzoulaki, I.; Castagné, R.; Boulangé, C.L.; Karaman, I.; Chekmeneva, E.; Evangelou, E.; Ebbels, T.M.D.; Kaluarachchi, M.R.; Chadeau-Hyam, M.; Mosen, D.; et al. Serum Metabolic Signatures of Coronary and Carotid Atherosclerosis and Subsequent Cardiovascular Disease. Eur. Heart J. 2019, 40, 2883–2896. [Google Scholar] [CrossRef]
- Huang, Z.; Klaric, L.; Krasauskaite, J.; McLachlan, S.; Strachan, M.W.J.; Wilson, J.F.; Price, J.F. Serum Metabolomic Profiles Associated with Subclinical and Clinical Cardiovascular Phenotypes in People with Type 2 Diabetes. Cardiovasc. Diabetol. 2022, 21, 62. [Google Scholar] [CrossRef]
- Wu, Y.; Dong, Y.; Atefi, M.; Liu, Y.; Elshimali, Y.; Vadgama, J.V. Lactate, a Neglected Factor for Diabetes and Cancer Interaction. Mediat. Inflamm. 2016, 2016, 6456018. [Google Scholar] [CrossRef]
- Radziuk, J.; Lickley, H.L.A. The Metabolic Clearance of Glucose: Measurement and Meaning. Diabetologia 1985, 28, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Steele, R.; Wall, J.S.; De Bodo, R.C.; Altszuler, N. Measurement of Size and Turnover Rate of Body Glucose Pool by the Isotope Dilution Method. Am. J. Physiol. 1956, 187, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Stanley, W.C.; Gertz, E.W.; Wisneski, J.A.; Morris, D.L.; Neese, R.A.; Brooks, G.A. Systemic Lactate Kinetics during Graded Exercise in Man. Am. J. Physiol. 1985, 249 Pt 1, E595–E602. [Google Scholar] [CrossRef] [PubMed]
- Miller, B.F.; Fattor, J.A.; Jacobs, K.A.; Horning, M.A.; Navazio, F.; Lindinger, M.I.; Brooks, G.A. Lactate and Glucose Interactions during Rest and Exercise in Men: Effect of Exogenous Lactate Infusion. J. Physiol. 2002, 544 Pt 3, 963–975. [Google Scholar] [CrossRef]
- Norwich, K.H.; Fluker, G.; Anthony, J.; Popescu, I.; Pagurek, B.; Hetenyi, G. The Development of a Glucose Clamp. Metabolism 1975, 24, 1221–1230. [Google Scholar] [CrossRef]
- Andersen, D.K.; Elahi, D.; Brown, J.C.; Tobin, J.D.; Andres, R. Oral Glucose Augmentation of Insulin Secretion. J. Clin. Investig. 1978, 62, 152–161. [Google Scholar] [CrossRef]
- DeFronzo, R.A.; Tobin, J.D.; Andres, R. Glucose Clamp Technique: A Method for Quantifying Insulin Secretion and Resistance. Am. J. Physiol. Endocrinol. Metab. Gastrointest. Physiol. 1979, 237, E214. [Google Scholar] [CrossRef]
- Elahi, D. In Praise of the Hyperglycemic Clamp: A Method for Assessment of β-Cell Sensitivity and Insulin Resistance. Diabetes Care 1996, 19, 278–286. [Google Scholar] [CrossRef]
- Reinauer, H.; Gries, F.A.; Hübinger, A.; Knode, O.; Severing, K.; Susanto, F. Determination of Glucose Turnover and Glucose Oxidation Rates in Man with Stahle Isotope Tracers. Clin. Chem. Lab. Med. 1990, 28, 505–512. [Google Scholar] [CrossRef]
- Kim, J.K. Hyperinsulinemic–Euglycemic Clamp to Assess Insulin Sensitivity In Vivo. In Type 2 Diabetes; Stocker, C., Ed.; Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2009; Volume 560, pp. 221–238. [Google Scholar] [CrossRef]
- Kolterman, O.G.; Insel, J.; Saekow, M.; Olefsky, J.M. Mechanisms of Insulin Resistance in Human Obesity: Evidence for Receptor and Postreceptor Defects. J. Clin. Investig. 1980, 65, 1272–1284. [Google Scholar] [CrossRef]
- Lang, V.; Bornet, F.R.; Vaugelade, P.; Van Ypersele De Strihou, M.; Luo, J.; Pacher, N.; Rossi, F.; La Droitte, P.; Duée, P.-H.; Slama, G. Euglycemic Hyperinsulinemic Clamp to Assess Posthepatic Glucose Appearance after Carbohydrate Loading. 2. Evaluation of Corn and Mung Bean Starches in Healthy Men. Am. J. Clin. Nutr. 1999, 69, 1183–1188. [Google Scholar] [CrossRef] [PubMed]
- Otowa-Suematsu, N.; Sakaguchi, K.; Kaneko, A.; Ito, J.; Morita, Y.; Miura, H.; Yamada, T.; So, A.; Komada, H.; Okada, Y.; et al. Relation of Cardiac Function to Insulin Resistance as Evaluated by Hyperinsulinemic-euglycemic Clamp Analysis in Individuals with Type 2 Diabetes. J. Diabetes Investig. 2021, 12, 2197–2202. [Google Scholar] [CrossRef] [PubMed]
- Ryan, B.J.; Schleh, M.W.; Ahn, C.; Ludzki, A.C.; Gillen, J.B.; Varshney, P.; Van Pelt, D.W.; Pitchford, L.M.; Chenevert, T.L.; Gioscia-Ryan, R.A.; et al. Moderate-Intensity Exercise and High-Intensity Interval Training Affect Insulin Sensitivity Similarly in Obese Adults. J. Clin. Endocrinol. Metab. 2020, 105, e2941–e2959. [Google Scholar] [CrossRef] [PubMed]
- Pinto, M.M.; Dubouchaud, H.; Jouve, C.; Rigaudière, J.-P.; Patrac, V.; Bouvier, D.; Hininger-Favier, I.; Walrand, S.; Demaison, L. A Chronic Low-Dose Magnesium L-Lactate Administration Has a Beneficial Effect on the Myocardium and the Skeletal Muscles. J. Physiol. Biochem. 2022, 78, 501–516. [Google Scholar] [CrossRef] [PubMed]
- Schiffer, T.; Schulte, S.; Sperlich, B.; Achtzehn, S.; Fricke, H.; Strüder, H.K. Lactate Infusion at Rest Increases BDNF Blood Concentration in Humans. Neurosci. Lett. 2011, 488, 234–237. [Google Scholar] [CrossRef]
- Zhang, S.; Hulver, M.W.; McMillan, R.P.; Cline, M.A.; Gilbert, E.R. The Pivotal Role of Pyruvate Dehydrogenase Kinases in Metabolic Flexibility. Nutr. Metab. 2014, 11, 10. [Google Scholar] [CrossRef]
- Chien, H.-C.; Constantin, D.; Greenhaff, P.L.; Constantin-Teodosiu, D. PPARα, δ and FOXO1 Gene Silencing Overturns Palmitate-Induced Inhibition of Pyruvate Oxidation Differentially in C2C12 Myotubes. Biology 2021, 10, 1098. [Google Scholar] [CrossRef]
- Gold, M.; Miller, H.I.; Issekutz, B.; Spitzer, J.J. Effect of Exercise and Lactic Acid Infusion on Individual Free Fatty Acids of Plasma. Am. J. Physiol. Leg. Content 1963, 205, 902–904. [Google Scholar] [CrossRef]
- Freminet, A.; Bursaux, E.; Poyart, C. Effect of Elevated Lactataemia on the Rates of Lactate Turnover and Oxidation in Rats. Pflug. Arch. 1974, 346, 75–86. [Google Scholar] [CrossRef]
- Gao, J.; Islam, M.A.; Brennan, C.M.; Dunning, B.E.; Foley, J.E. Lactate Clamp: A Method to Measure Lactate Utilization in Vivo. Am. J. Physiol. 1998, 38, E729–E733. [Google Scholar] [CrossRef]
- Boyd, A.E.; Giamber, S.R.; Mager, M.; Lebovitz, H.E. Lactate Inhibition of Lipolysis in Exercising Man. Metabolism 1974, 23, 531–542. [Google Scholar] [CrossRef]
- Ryan, W.J.; Sutton, J.R.; Toews, C.J.; Jones, N.L. Metabolism of Infused L(+)-Lactate during Exercise. Clin. Sci. 1979, 56, 139–146. [Google Scholar] [CrossRef]
- Chioléro, R.; Mavrocordatos, P.; Burnier, P.; Cayeux, M.C.; Schindler, C.; Jéquier, E.; Tappy, L. Effects of Infused Sodium Acetate, Sodium Lactate, and Sodium Beta-Hydroxybutyrate on Energy Expenditure and Substrate Oxidation Rates in Lean Humans. Am. J. Clin. Nutr. 1993, 58, 608–613. [Google Scholar] [CrossRef]
- Searle, G.L.; Feingold, K.R.; Hsu, F.S.; Clark, O.H.; Gertz, E.W.; Stanley, W.C. Inhibition of Endogenous Lactate Turnover with Lactate Infusion in Humans. Metabolism 1989, 38, 1120–1123. [Google Scholar] [CrossRef]
- Brooks, G.A. Anaerobic Threshold: Review of the Concept and Directions for Future Research. Med. Sci. Sports Exerc. 1985, 17, 22–34. [Google Scholar] [CrossRef]
- Tanaka, K. Lactate-Related Factors as a Critical Determinant of Endurance. Ann. Physiol. Anthropol. Seiri Jinruigaku Kenkyūkai Kaishi 1990, 9, 191–202. [Google Scholar] [CrossRef]
- Heuberger, J.A.A.C.; Gal, P.; Stuurman, F.E.; De Muinck Keizer, W.A.S.; Miranda, Y.M.; Cohen, A.F. Repeatability and Predictive Value of Lactate Threshold Concepts in Endurance Sports. PLoS ONE 2018, 13, e0206846. [Google Scholar] [CrossRef]
- Miller, B.F.; Fattor, J.A.; Jacobs, K.A.; Horning, M.A.; Suh, S.-H.; Navazio, F.; Brooks, G.A. Metabolic and Cardiorespiratory Responses to “the Lactate Clamp”. Am. J. Physiol. Endocrinol. Metab. 2002, 283, E889–E898. [Google Scholar] [CrossRef]
- Consoli, A.; Nurjhan, N.; Reilly, J.J.; Bier, D.M.; Gerich, J.E. Contribution of Liver and Skeletal Muscle to Alanine and Lactate Metabolism in Humans. Am. J. Physiol. 1990, 259 Pt 1, E677–E684. [Google Scholar] [CrossRef]
- Phillips, S.M.; Green, H.J.; Tarnopolsky, M.A.; Grant, S.M. Increased Clearance of Lactate after Short-Term Training in Men. J. Appl. Physiol. 1995, 79, 1862–1869. [Google Scholar] [CrossRef]
- Brooks, G.A.; Osmond, A.D.; Leija, R.G.; Curl, C.C.; Arevalo, J.A.; Duong, J.J.; Horning, M.A. The Blood Lactate/Pyruvate Equilibrium Affair. Am. J. Physiol.-Endocrinol. Metab. 2022, 322, E34–E43. [Google Scholar] [CrossRef] [PubMed]
- Miller, B.F.; Lindinger, M.I.; Fattor, J.A.; Jacobs, K.A.; LeBlanc, P.J.; Duong, M.; Heigenhauser, G.J.F.; Brooks, G.A. Hematological and Acid-Base Changes in Men during Prolonged Exercise with and without Sodium-Lactate Infusion. J. Appl. Physiol. 2005, 98, 856–865. [Google Scholar] [CrossRef] [PubMed]
- Kyun, S.; Yoo, C.; Hashimoto, T.; Tomi, H.; Teramoto, N.; Kim, J.; Lim, K. Effects of Exogenous Lactate Administration on Fat Metabolism and Glycogen Synthesis Factors in Rats. Phys. Act. Nutr. 2020, 24, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Jang, I.; Kim, J.; Kyun, S.; Hwang, D.; Lim, K. Acute Administration of Exogenous Lactate Increases Carbohydrate Metabolism during Exercise in Mice. Metabolites 2021, 11, 553. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Emhoff, C.-A.W.; Messonnier, L.A. Concepts of Lactate Metabolic Clearance Rate and Lactate Clamp for Metabolic Inquiry: A Mini-Review. Nutrients 2023, 15, 3213. https://doi.org/10.3390/nu15143213
Emhoff C-AW, Messonnier LA. Concepts of Lactate Metabolic Clearance Rate and Lactate Clamp for Metabolic Inquiry: A Mini-Review. Nutrients. 2023; 15(14):3213. https://doi.org/10.3390/nu15143213
Chicago/Turabian StyleEmhoff, Chi-An W., and Laurent A. Messonnier. 2023. "Concepts of Lactate Metabolic Clearance Rate and Lactate Clamp for Metabolic Inquiry: A Mini-Review" Nutrients 15, no. 14: 3213. https://doi.org/10.3390/nu15143213
APA StyleEmhoff, C.-A. W., & Messonnier, L. A. (2023). Concepts of Lactate Metabolic Clearance Rate and Lactate Clamp for Metabolic Inquiry: A Mini-Review. Nutrients, 15(14), 3213. https://doi.org/10.3390/nu15143213





