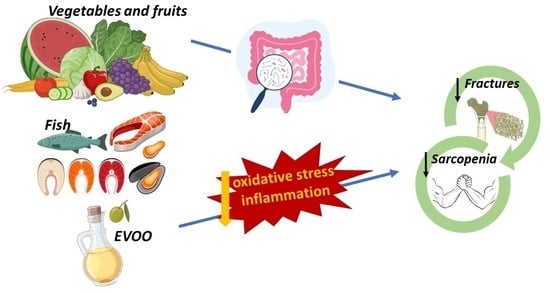
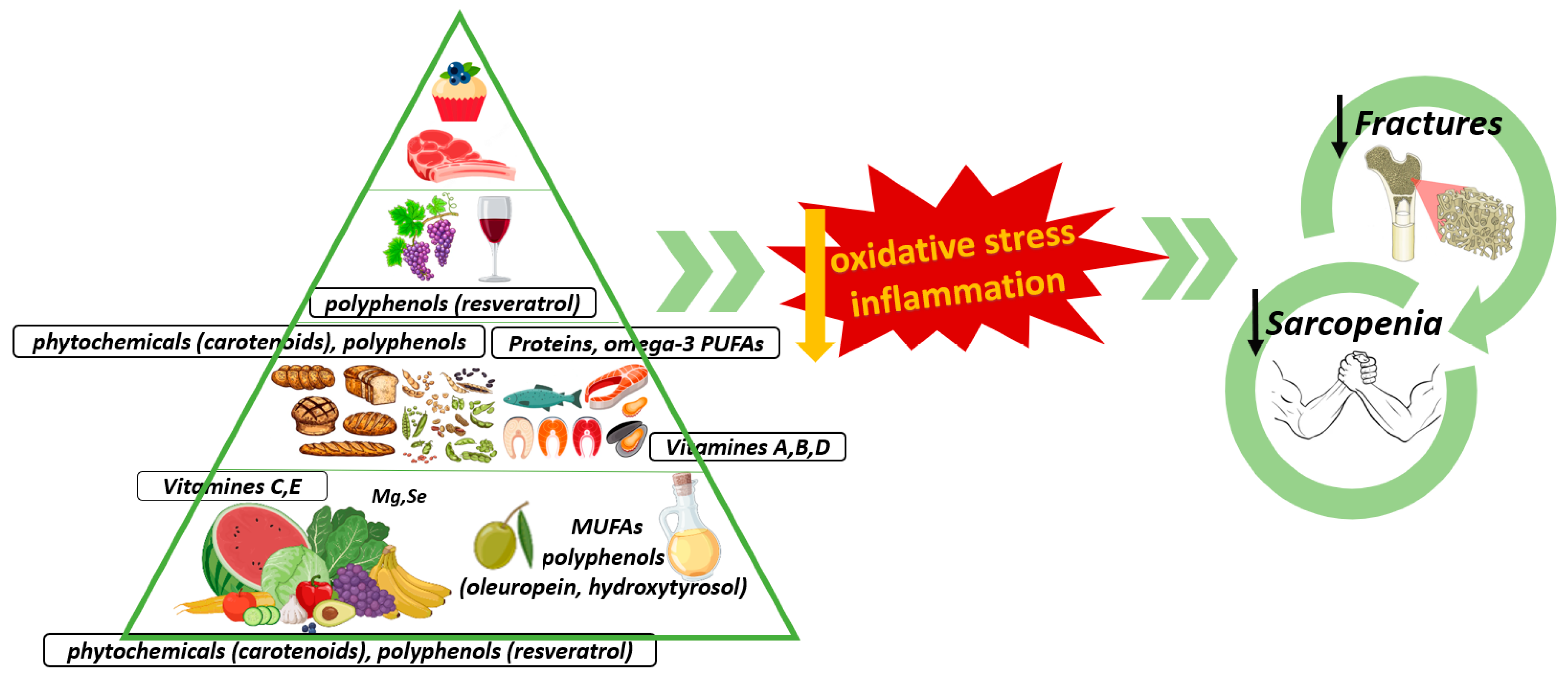
The Influence of the Mediterranean Dietary Pattern on Osteoporosis and Sarcopenia
Abstract
:1. Introduction
2. Materials and Methods
3. The Mediterranean Diet
4. Relationships between Osteoporosis and Sarcopenia
5. Interplay between Osteoporosis and the Mediterranean Diet
5.1. Bone Mineral Status and Bone Biomarkers
5.2. Osteoporotic Fractures
6. Interplay between Sarcopenia and the Mediterranean Diet
6.1. Strength, Muscle Mass and Function
6.2. Frailty and Falls
7. The Microbiome: A Moderator between Diet, Bone and Muscle Health
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Barrea, L.; Muscogiuri, G.; Di Somma, C.; Tramontano, G.; De Luca, V.; Illario, M.; Colao, A.; Savastano, S. Association between Mediterranean diet and hand grip strength in older adult women. Clin. Nutr. 2019, 38, 721–729. [Google Scholar] [CrossRef] [PubMed]
- Haring, B.; Crandall, C.J.; Wu, C.; LeBlanc, E.S.; Shikany, J.M.; Carbone, L.; Orchard, T.; Thomas, F.; Wactawaski-Wende, J.; Li, W.; et al. Dietary Patterns and Fractures in Postmenopausal Women: Results From the Women’s Health Initiative. JAMA Int. Med. 2016, 176, 645. [Google Scholar] [CrossRef] [PubMed]
- Malmir, H.; Saneei, P.; Larijani, B.; Esmaillzadeh, A. Adherence to Mediterranean diet in relation to bone mineral density and risk of fracture: A systematic review and meta-analysis of observational studies. Eur. J. Nutr. 2018, 57, 2147–2160. [Google Scholar] [CrossRef]
- Baum, J.; Wolfe, R. The Link between Dietary Protein Intake, Skeletal Muscle Function and Health in Older Adults. Healthcare 2015, 3, 529–543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heaney, R.P. Dairy and Bone Health. J. Am. Coll. Nutr. 2009, 28 (Suppl. S1), 82S–90S. [Google Scholar] [CrossRef]
- Cervo, M.M.C.; Scott, D.; Seibel, M.J.; Cumming, R.G.; Naganathan, V.; Blyth, F.M.; Le Couteur, D.G.; Handelsman, D.J.; Ribeiro, R.V.; Waite, L.M.; et al. Adherence to Mediterranean Diet and Its Associations with Circulating Cytokines, Musculoskeletal Health and Incident Falls in Community-Dwelling Older Men: The Concord Health and Ageing in Men Project. Clin. Nutr. 2021, 40, 5753–5763. [Google Scholar] [CrossRef]
- García-Gavilán, J.F.; Bulló, M.; Canudas, S.; Martínez-González, M.A.; Estruch, R.; Giardina, S.; Fitó, M.; Corella, D.; Ros, E.; Salas-Salvadó, J. Extra virgin olive oil consumption reduces the risk of osteoporotic fractures in the PREDIMED trial. Clin Nutr. 2018, 37, 329–335. [Google Scholar] [CrossRef] [Green Version]
- Welch, A.A.; Kelaiditi, E.; Jennings, A.; Steves, C.J.; Spector, T.D.; MacGregor, A. Dietary Magnesium Is Positively Associated With Skeletal Muscle Power and Indices of Muscle Mass and May Attenuate the Association Between Circulating C-Reactive Protein and Muscle Mass in Women: Effects of dietary magnesium on skeletal muscle. J. Bone Miner Res. 2016, 31, 317–325. [Google Scholar] [CrossRef] [Green Version]
- Warensjö Lemming, E.; Byberg, L. Is a Healthy Diet Also Suitable for the Prevention of Fragility Fractures? Nutrients 2020, 12, 2642. [Google Scholar] [CrossRef]
- Perna, S.; Avanzato, I.; Nichetti, M.; D’Antona, G.; Negro, M.; Rondanelli, M. Association between Dietary Patterns of Meat and Fish Consumption with Bone Mineral Density or Fracture Risk: A Systematic Literature. Nutrients 2017, 9, 1029. [Google Scholar] [CrossRef]
- Nih Consensus Development Panel on Osteoporosis Prevention, Diagnosis, and Therapy. Osteoporosis Prevention, Diagnosis, and Therapy. JAMA J. Am. Med. Assoc. 2001, 285, 785–795. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnell, O.; Hertzman, P. What Evidence Is There for the Prevention and Screening of Osteoporosis? World Health Organization; Regional Office for Europe; 2006. Available online: http://www.euro.who.int/document/e88668.pdf (accessed on 28 April 2023).
- Johnel, O.; Gullberg, B.; Allander, E.; Kanis, J.A.; the MEDOS Study Group. The apparent incidence of hip fracture in Europe: A study of national register sources. Osteoporos. Int. Noviembre 1992, 2, 298–302. [Google Scholar] [CrossRef] [PubMed]
- Kanis, J.A. The incidence of hip fracture in Europe. Osteoporos. Int. 1993, 3, 10–15. [Google Scholar] [CrossRef]
- Rosés, C.; Cuevas-Sierra, A.; Quintana, S.; Riezu-Boj, J.I.; Martínez, J.A.; Milagro, F.I.; Barceló, A. Gut Microbiota Bacterial Species Associated with Mediterranean Diet-Related Food Groups in a Northern Spanish Population. Nutrients 2021, 13, 636. [Google Scholar] [CrossRef] [PubMed]
- Cronin, O.; Lanham-New, S.; Corfe, B.; Gregson, C.L.; Darling, A.L.; Ahmadi, K.R.; Gibson, P.S.; Tobias, J.H.; Ward, K.A.; Traka, M.H.; et al. Role of the Microbiome in Regulating Bone Metabolism and Susceptibility to Osteoporosis. Calcif. Tissue Int. 2022, 110, 273–284. [Google Scholar] [CrossRef] [PubMed]
- Lefevre, C.; Bindels, L. Role of the Gut Microbiome in Skeletal Muscle Physiology and Pathophysiology. Curr. Osteoporos. Rep. 2022, 20, 422–432. [Google Scholar] [CrossRef]
- Feart, C.; Lorrain, S.; Ginder Coupez, V.; Samieri, C.; Letenneur, L.; Paineau, D.; Barberger-Gateau, P. Adherence to a Mediterranean diet and risk of fractures in French older persons. Osteoporos. Int. 2013, 24, 3031–3041. [Google Scholar] [CrossRef] [Green Version]
- Fougère, B.; Mazzuco, S.; Spagnolo, P.; Guyonnet, S.; Vellas, B.; Cesari, M.; Gallucci, M. Association between the Mediterranean-style dietary pattern score and physical performance: Results from TRELONG study. J. Nutr. Health Aging 2016, 20, 415–419. [Google Scholar] [CrossRef]
- Talegawkar, S.A.; Bandinelli, S.; Bandeen-Roche, K.; Chen, P.; Milaneschi, Y.; Tanaka, T.; Semba, R.D.; Guralnik, J.M.; Ferrucci, L. A Higher Adherence to a Mediterranean-Style Diet Is Inversely Associated with the Development of Frailty in Community-Dwelling Elderly Men and Women. J. Nutr. 2012, 142, 2161–2166. [Google Scholar] [CrossRef] [Green Version]
- Martini, D. Health Benefits of Mediterranean Diet. Nutrients 2019, 11, 1802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andreo-López, M.C.; Contreras-Bolívar, V.; Muñoz-Torres, M.; García-Fontana, B.; García-Fontana, C. Influence of the Mediterranean Diet on Healthy Aging. Int. J. Mol. Sci. 2023, 24, 4491. [Google Scholar] [CrossRef] [PubMed]
- Bach-Faig, A.; Berry, E.M.; Lairon, D.; Reguant, J.; Trichopoulou, A.; Dernini, S.; Medina, F.X.; Battino, M.; Belahsen, R.; Miranda, G.; et al. Mediterranean diet pyramid today. Science and cultural updates. Public Health Nutr. 2011, 14, 2274–2284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Román, G.C.; Jackson, R.E.; Gadhia, R.; Román, A.N.; Reis, J. Mediterranean diet: The role of long-chain ω-3 fatty acids in fish; polyphenols in fruits, vegetables, cereals, coffee, tea, cacao and wine; probiotics and vitamins in prevention of stroke, age-related cognitive decline, and Alzheimer disease. Rev. Neurol. 2019, 175, 724–741. [Google Scholar] [CrossRef]
- Fung, T.; Rexrode, K.; Mantzoros, C.; Manson, J.; Willett, W.; Hu, F. Mediterranean diet and incidence and mortality of coronary heart disease and stroke in women. FASEB J. 2009, 119, 1093–1100. [Google Scholar]
- Di Renzo, L.; Gualtieri, P.; De Lorenzo, A. Diet, Nutrition and Chronic Degenerative Diseases. Nutrients 2021, 13, 1372. [Google Scholar] [CrossRef]
- Zhou, S.; Qian, B.; Wang, L.; Zhang, C.; Hogan, M.V.; Li, H. Altered bone-regulating myokine expression in skeletal muscle Of Duchenne muscular dystrophy mouse models: Altered Bone-Regulating Myokines in DMD. Muscle Nerve 2018, 58, 573–582. [Google Scholar] [CrossRef]
- Brotto, M.; Bonewald, L. Bone and muscle: Interactions beyond mechanical. Bone 2015, 80, 109–114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gomes, M.M.; Silva, M.M.R.D.; Araújo, I.M.D.; Paula, F.J.A.D. Bone, fat, and muscle interactions in health and disease. Arch. Endocrinol. Metab. 2022, 66, 611–620. [Google Scholar] [CrossRef]
- Kaplan, S.J.; Pham, T.N.; Arbabi, S.; Gross, J.A.; Damodarasamy, M.; Bentov, I.; Taitsman, L.A.; Mitchell, S.H.; Reed, M.J. Association of Radiologic Indicators of Frailty With 1-Year Mortality in Older Trauma Patients: Opportunistic Screening for Sarcopenia and Osteopenia. JAMA Surg. 2017, 152, e164604. [Google Scholar] [CrossRef]
- Nielsen, B.R.; Abdulla, J.; Andersen, H.E.; Schwarz, P.; Suetta, C. Sarcopenia and osteoporosis in older people: A systematic review and meta-analysis. Eur. Geriatr. Med. 2018, 9, 419–434. [Google Scholar] [CrossRef]
- Tarantino, U.; Greggi, C.; Visconti, V.V.; Cariati, I.; Tallarico, M.; Fauceglia, M.; Iundusi, R.; Albanese, M.; Chiaramonte, C.; Gasbarra, E. T-Score and Handgrip Strength Association for the Diagnosis of Osteosarcopenia: A Systematic Review and Meta-Analysis. J. Clin. Med. 2021, 10, 2597. [Google Scholar] [CrossRef] [PubMed]
- Shetty, S.; John, B.; Mohan, S.; Paul, T.V. Vertebral fracture assessment by dual-energy X-ray absorptiometry along with bone mineral density in the evaluation of postmenopausal osteoporosis. Arch. Osteoporos. 2020, 15, 25. [Google Scholar] [CrossRef]
- McCloskey, E.V.; Odén, A.; Harvey, N.C.; Leslie, W.D.; Hans, D.; Johansson, H.; Kanis, J.A. Adjusting Fracture Probability by Trabecular Bone Score. Calcif. Tissue Int. 2015, 96, 500–509. [Google Scholar] [CrossRef] [PubMed]
- Harvey, N.C.; Glüer, C.C.; Binkley, N.; McCloskey, E.V.; Brandi, M.L.; Cooper, C.; Kendler, D.; Lamy, O.; Laslop, A.; Camargos, B.M.; et al. Trabecular bone score (TBS) as a new complementary approach for osteoporosis evaluation in clinical practice. Bone 2015, 78, 216–224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palomo, T.; Muszkat, P.; Weiler, F.G.; Dreyer, P.; Brandão, C.M.A.; Silva, B.C. Update on trabecular bone score. Arch. Endocrinol. Metab. 2022, 66, 694–706. [Google Scholar] [CrossRef]
- Pérez-Rey, J.; Roncero-Martín, R.; Rico-Martín, S.; Rey-Sánchez, P.; Pedrera-Zamorano, J.; Pedrera-Canal, M.; López-Espuela, F.; Lavado García, J.M. Adherence to a Mediterranean Diet and Bone Mineral Density in Spanish Premenopausal Women. Nutrients 2019, 11, 555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hernlund, E.; Svedbom, A.; Ivergård, M.; Compston, J.; Cooper, C.; Stenmark, J.; McCloskey, E.V.; Jönsson, B.; Kanis, J.A. Osteoporosis in the European Union: Medical management, epidemiology and economic burden: A report prepared in collaboration with the International Osteoporosis Foundation (IOF) and the European Federation of Pharmaceutical Industry Associations (EFPIA). Arch. Osteoporos. 2013, 8, 136. [Google Scholar] [CrossRef] [Green Version]
- Papadopoulou, S. Sarcopenia: A Contemporary Health Problem among Older Adult Populations. Nutrients 2020, 12, 1293. [Google Scholar] [CrossRef]
- Azagra, R.; López-Expósito, F.; Martin-Sánchez, J.C.; Aguyé, A.; Moreno, N.; Cooper, C.; Díez-Pérez, A.; Dennison, E.M. Changing trends in the epidemiology of hip fracture in Spain. Osteoporos. Int. 2014, 25, 1267–1274. [Google Scholar] [CrossRef] [Green Version]
- Weltgesundheitsorganisation (Ed.) Prevention and management of osteoporosis: Report of a WHO scientific group. In Proceedings of the WHO Scientific Group Meeting on Prevention and Management of Osteoporosis, Geneva, Switzerland, 7–10 April 2003; WHO Technical Report Series; World Health Organization: Geneva, Switzerland, 2003; 192p. [Google Scholar]
- Kim, S.W.; Lee, H.A.; Cho, E.H. Low Handgrip Strength is Associated with Low Bone Mineral Density and Fragility Fractures in Postmenopausal Healthy Korean Women. J. Korean Med. Sci. 2012, 27, 744. [Google Scholar] [CrossRef] [Green Version]
- Byberg, L.; Bellavia, A.; Larsson, S.C.; Orsini, N.; Wolk, A.; Michaëlsson, K. Mediterranean Diet and Hip Fracture in Swedish Men and Women: Mediterranean Diet And Hip Fracture in Swedish Men and Women. J. Bone Miner. Res. 2016, 31, 2098–2105. [Google Scholar] [CrossRef] [Green Version]
- Nikkhah, A.; Ejtahed, H.S.; Ettehad Marvasti, F.; Taghavi, M.; Pakmehr, A.; Hajipour, F.; Larijani, B. The critical role of gut microbiota dysbiosis in skeletal muscle wasting: A systematic review. J. Appl. Microbiol. 2023, 134, lxac014. [Google Scholar] [CrossRef] [PubMed]
- Rizzoli, R.; Biver, E.; Brennan-Speranza, T.C. Nutritional intake and bone health. Lancet Diabetes Endocrinol. 2021, 9, 606–621. [Google Scholar] [CrossRef] [PubMed]
- Gaffney-Stomberg, E. The Impact of Trace Minerals on Bone Metabolism. Biol. Trace Elem. Res. 2019, 188, 26–34. [Google Scholar] [CrossRef]
- Lin, P.H.; Ginty, F.; Appel, L.J.; Aickin, M.; Bohannon, A.; Garnero, P.; Barclay, D.; Svetkey, L.P. The DASH Diet and Sodium Reduction Improve Markers of Bone Turnover and Calcium Metabolism in Adults. J. Nutr. 2003, 133, 3130–3136. [Google Scholar] [CrossRef] [Green Version]
- Movassagh, E.Z.; Vatanparast, H. Current Evidence on the Association of Dietary Patterns and Bone Health: A Scoping Review. Adv. Nutr. 2017, 8, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.; Wang, N.; Gao, Y.; Zhou, Z.; Liu, W.; Pan, C.; Yin, P.; Yu, X.; Tang, M. β-Carotene suppresses osteoclastogenesis and bone resorption by suppressing NF-κB signaling pathway. Life Sci. 2017, 174, 15–20. [Google Scholar] [CrossRef]
- Park, S.J.; Joo, S.E.; Min, H.; Park, J.K.; Kim, Y.; Kim, S.S.; Ahn, Y. Dietary Patterns and Osteoporosis Risk in Postmenopausal Korean Women. Osong. Public Health Res. Perspect. 2012, 3, 199–205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benetou, V.; Orfanos, P.; Pettersson-Kymmer, U.; Bergström, U.; Svensson, O.; Johansson, I.; Berrino, F.; Tumino, R.; Borch, K.B.; Lund, E. Mediterranean diet and incidence of hip fractures in a European cohort. Osteoporos. Int. 2013, 24, 1587–1598. [Google Scholar] [CrossRef]
- Rivas Velasco, A. Adherencia a la Dieta Mediterránea y Salud Ósea. Nutr. Hosp. 2014, 29, 989–996. [Google Scholar]
- Jennings, A.; Cashman, K.D.; Gillings, R.; Cassidy, A.; Tang, J.; Fraser, W.; Dowling, K.G.; Hull, G.L.J.; Berendsen, A.A.M.; de Groot, L.C.P.G.M.; et al. A Mediterranean-like dietary pattern with vitamin D3 (10 µg/d) supplements reduced the rate of bone loss in older Europeans with osteoporosis at baseline: Results of a 1-y randomized controlled trial. Am. J. Clin. Nutr. 2018, 108, 633–640. [Google Scholar] [CrossRef] [Green Version]
- Noori, M.; Jayedi, A.; Khan, T.A.; Moradi, S.; Shab-Bidar, S. Mediterranean dietary pattern and bone mineral density: A systematic review and dose-response meta-analysis of observational studies. Eur. J. Clin. Nutr. 2022, 76, 1657–1664. [Google Scholar] [CrossRef]
- Chen, G.D.; Dong, X.W.; Zhu, Y.Y.; Tian, H.Y.; He, J.; Chen, Y.M. Adherence to the Mediterranean diet is associated with a higher BMD in middle-aged and elderly Chinese. Sci. Rep. 2016, 6, 25662. [Google Scholar] [CrossRef] [Green Version]
- Zeng, F.F.; Xue, W.Q.; Cao, W.T.; Wu, B.H.; Xie, H.L.; Fan, F.; Zhu, H.L.; Chen, Y.M. Diet-quality scores and risk of hip fractures in elderly urban Chinese in Guangdong, China: A case–control study. Osteoporos. Int. 2014, 25, 2131–2141. [Google Scholar] [CrossRef]
- Kontogianni, M.D.; Melistas, L.; Yannakoulia, M.; Malagaris, I.; Panagiotakos, D.B.; Yiannakouris, N. Association between dietary patterns and indices of bone mass in a sample of Mediterranean women. Nutrition 2009, 25, 165–171. [Google Scholar] [CrossRef]
- Marini, H.; Bitto, A.; Altavilla, D.; Burnett, B.P.; Polito, F.; Di Stefano, V.; Minutoli, L.; Atteritano, M.; Levy, R.M.; D’Anna, R.; et al. Breast Safety and Efficacy of Genistein Aglycone for Postmenopausal Bone Loss: A Follow-Up Study. J. Clin. Endocrinol. Metab. 2008, 93, 4787–4796. [Google Scholar] [CrossRef] [Green Version]
- Zeng, L.F.; Luo, M.H.; Liang, G.H.; Yang, W.Y.; Xiao, X.; Wei, X.; Yu, J.; Guo, D.; Chen, H.Y.; Pan, J.K.; et al. Can Dietary Intake of Vitamin C-Oriented Foods Reduce the Risk of Osteoporosis, Fracture, and BMD Loss? Systematic Review With Meta-Analyses of Recent Studies. Front. Endocrinol. 2020, 10, 844. [Google Scholar] [CrossRef] [Green Version]
- Qu, Z.; Yang, F.; Yan, Y.; Hong, J.; Wang, W.; Li, S.; Jiang, G.; Yan, S. Relationship between Serum Nutritional Factors and Bone Mineral Density: A Mendelian Randomization Study. J. Clin. Endocrinol. Metab. 2021, 106, e2434–e2443. [Google Scholar] [CrossRef]
- Roncero-Martín, R.; Aliaga Vera, I.; Moreno-Corral, L.; Moran, J.; Lavado-Garcia, J.; Pedrera-Zamorano, J.; Pedrera-Canal, M. Olive Oil Consumption and Bone Microarchitecture in Spanish Women. Nutrients 2018, 10, 968. [Google Scholar] [CrossRef] [Green Version]
- Puel, C.; Coxam, V.; Davicco, M.J. Régime méditerranéen et ostéoporose. Méd. Sci. 2007, 23, 756–760. [Google Scholar] [CrossRef] [PubMed]
- Puel, C.; Mardon, J.; Agalias, A.; Davicco, M.J.; Lebecque, P.; Mazur, A.; Horcajada, M.N.; Skaltsounis, A.L.; Coxam, V. Major Phenolic Compounds in Olive Oil Modulate Bone Loss in an Ovariectomy/Inflammation Experimental Model. J. Agric. Food Chem. 2008, 56, 9417–9422. [Google Scholar] [CrossRef]
- Chin, K.Y.; Ima-Nirwana, S. Olives and Bone: A Green Osteoporosis Prevention Option. Int. J. Environ. Res. Public Health 2016, 13, 755. [Google Scholar] [CrossRef]
- García-Martínez, O.; De Luna-Bertos, E.; Ramos-Torrecillas, J.; Ruiz, C.; Milia, E.; Lorenzo, M.L.; Jimenez, B.; Sánchez-Ortiz, A.; Rivas, A. Phenolic Compounds in Extra Virgin Olive Oil Stimulate Human Osteoblastic Cell Proliferation. PLoS ONE 2016, 11, e0150045. [Google Scholar] [CrossRef] [Green Version]
- Hagiwara, K.; Goto, T.; Araki, M.; Miyazaki, H.; Hagiwara, H. Olive polyphenol hydroxytyrosol prevents bone loss. Eur. J. Pharmacol. 2011, 662, 78–84. [Google Scholar] [CrossRef]
- Santiago-Mora, R.; Casado-Díaz, A.; De Castro, M.D.; Quesada-Gómez, J.M. Oleuropein enhances osteoblastogenesis and inhibits adipogenesis: The effect on differentiation in stem cells derived from bone marrow. Osteoporos. Int. 2011, 22, 675–684. [Google Scholar] [CrossRef]
- Fernández-Real, J.M.; Bulló, M.; Moreno-Navarrete, J.M.; Ricart, W.; Ros, E.; Estruch, R.; Salas-Salvadó, J. A Mediterranean Diet Enriched with Olive Oil Is Associated with Higher Serum Total Osteocalcin Levels in Elderly Men at High Cardiovascular Risk. J. Clin. Endocrinol. Metab. 2012, 97, 3792–3798. [Google Scholar] [CrossRef]
- Tousen, Y.; Ichimaru, R.; Kondo, T.; Inada, M.; Miyaura, C.; Ishimi, Y. The Combination of Soy Isoflavones and Resveratrol Preserve Bone Mineral Density in Hindlimb-Unloaded Mice. Nutrients 2020, 12, 2043. [Google Scholar] [CrossRef]
- Marrone, J.A.; Maddalozzo, G.F.; Branscum, A.J.; Hardin, K.; Cialdella-Kam, L.; Philbrick, K.A.; Breggia, A.C.; Rosen, C.J.; Turner, R.T.; Iwaniec, U.T. Moderate alcohol intake lowers biochemical markers of bone turnover in postmenopausal women. Menopause 2012, 19, 974–979. [Google Scholar] [CrossRef] [Green Version]
- Yin, J.; Winzenberg, T.; Quinn, S.; Giles, G.; Jones, G. Beverage-specific alcohol intake and bone loss in older men and women: A longitudinal study. Eur. J. Clin. Nutr. 2011, 65, 526–532. [Google Scholar] [CrossRef]
- Díaz-Castro, J.; Kajarabille, N.; Pulido-Moran, M.; Moreno-Fernandez, J.; Lopez-Frias, M.; Ochoa, J. Influence of Omega-3 Fatty Acids on Bone Turnover. In Omega-3 Fatty Acids; Springer: Cham, Switzerland, 2016; pp. 285–291. [Google Scholar]
- Orchard, T.S.; Ing, S.W.; Lu, B.; Belury, M.A.; Johnson, K.; Wactawski-Wende, J.; Jackson, R.D. The association of red blood cell n-3 and n-6 fatty acids with bone mineral density and hip fracture risk in the women’s health initiative. J. Bone Miner. Res. 2013, 28, 505–515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pérez-López, F.R.; Chedraui, P.; Haya, J.; Cuadros, J.L. Effects of the Mediterranean diet on longevity and age-related morbid conditions. Maturitas 2009, 64, 67–79. [Google Scholar] [CrossRef] [PubMed]
- Rivas, A.; Romero, A.; Mariscal-Arcas, M.; Monteagudo, C.; Feriche, B.; Lorenzo, M.L.; Olea, F. Mediterranean diet and bone mineral density in two age groups of women. Int. J. Food Sci. Nutr. 2013, 64, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Dhanwal, D.K.; Dennison, E.M.; Harvey, N.C.; Cooper, C. Epidemiology of hip fracture: Worldwide geographic variation. Indian J. Orthop. 2011, 45, 15–22. [Google Scholar] [CrossRef]
- Tamura, Y.; Omura, T.; Toyoshima, K.; Araki, A. Nutrition Management in Older Adults with Diabetes: A Review on the Importance of Shifting Prevention Strategies from Metabolic Syndrome to Frailty. Nutrients 2020, 12, 3367. [Google Scholar] [CrossRef]
- Tuttle, C.S.L.; Thang, L.A.N.; Maier, A.B. Markers of inflammation and their association with muscle strength and mass: A systematic review and meta-analysis. Ageing Res. Rev. 2020, 64, 101185. [Google Scholar] [CrossRef]
- Cauley, J.A.; Danielson, M.E.; Boudreau, R.M.; Forrest, K.Y.Z.; Zmuda, J.M.; Pahor, M.; Tylavsky, F.A.; Cummings, S.R.; Harris, T.B.; Newman, A.B.; et al. Inflammatory Markers and Incident Fracture Risk in Older Men and Women: The Health Aging and Body Composition Study. J. Bone Miner. Res. 2007, 22, 1088–1095. [Google Scholar] [CrossRef]
- Mitchell, A.; Fall, T.; Melhus, H.; Wolk, A.; Michaëlsson, K.; Byberg, L. Is the effect of Mediterranean diet on hip fracture mediated through type 2 diabetes mellitus and body mass index? Int. J. Epidemiol. 2021, 50, 234–244. [Google Scholar] [CrossRef]
- Palomeras-Vilches, A.; Viñals-Mayolas, E.; Bou-Mias, C.; Jordà-Castro, M.; Agüero-Martínez, M.; Busquets-Barceló, M.; Pujol-Busquets, G.; Carrion, C.; Bosque-Prous, M.; Serra-Majem, L.; et al. Adherence to the Mediterranean Diet and Bone Fracture Risk in Middle-Aged Women: A Case Control Study. Nutrients 2019, 11, 2508. [Google Scholar] [CrossRef] [Green Version]
- Gimeno, E.; Castellote, A.I. The effects of harvest and extraction methods on the antioxidant content (phenolics, a-tocopherol, and b-carotene) in virgin olive oil. Food Chem. 2002, 78, 207–211. [Google Scholar] [CrossRef]
- Sadeghi, O.; Djafarian, K.; Ghorabi, S.; Khodadost, M.; Nasiri, M.; Shab-Bidar, S. Dietary intake of fish, n-3 polyunsaturated fatty acids and risk of hip fracture: A systematic review and meta-analysis on observational studies. Crit. Rev. Food Sci. Nutr. 2019, 59, 1320–1333. [Google Scholar] [CrossRef] [PubMed]
- Malmir, H.; Shab-Bidar, S.; Djafarian, K. Vitamin C intake in relation to bone mineral density and risk of hip fracture and osteoporosis: A systematic review and meta-analysis of observational studies. Br. J. Nutr. 2018, 119, 847–858. [Google Scholar] [CrossRef]
- Byberg, L.; Bellavia, A.; Orsini, N.; Wolk, A.; Michaëlsson, K. Fruit and Vegetable Intake and Risk of Hip Fracture: A Cohort Study of Swedish Men and Women: Fruit and Vegetable Intake and Risk of Hip Fracture. J. Bone Miner. Res. 2015, 30, 976–984. [Google Scholar] [CrossRef] [Green Version]
- Michaëlsson, K.; Wolk, A.; Lemming, E.W.; Melhus, H.; Byberg, L. Intake of Milk or Fermented Milk Combined With Fruit and Vegetable Consumption in Relation to Hip Fracture Rates: A Cohort Study of Swedish Women: Hip fracture rates and milk/fermented milk + fruit/vegetable consumption. J. Bone Miner. Res. 2018, 33, 449–457. [Google Scholar] [CrossRef] [Green Version]
- García-Gavilán, J.F.; Bulló, M.; Camacho-Barcia, L.; Rosique-Esteban, N.; Hernández-Alonso, P.; Basora, J.; Martínez-González, M.A.; Estruchm, R.; Fitó, M.; Salas-Salvadó, J. Higher dietary glycemic index and glycemic load values increase the risk of osteoporotic fracture in the PREvención con DIeta MEDiterránea (PREDIMED)-Reus trial. Am. J. Clin. Nutr. 2018, 107, 1035–1042. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DiGirolamo, D.J.; Kiel, D.P.; Esser, K.A. Bone and Skeletal Muscle: Neighbors With Close Ties: Bone and skeletal muscle: Neighbors with close ties. J. Bone Miner. Res. 2013, 28, 1509–1518. [Google Scholar] [CrossRef] [Green Version]
- Welch, A.A. Nutritional influences on age-related skeletal muscle loss. Proc. Nutr. Soc. 2014, 73, 16–33. [Google Scholar] [CrossRef]
- Pan, L.; Xie, W.; Fu, X.; Lu, W.; Jin, H.; Lai, J.; Zhang, A.; Yu, Y.; Li, Y.; Xiao, W. Inflammation and sarcopenia: A focus on circulating inflammatory cytokines. Exp. Gerontol. 2021, 154, 111544. [Google Scholar] [CrossRef]
- Mendes, J.; Afonso, C.; Borges, N.; Santos, A.; Moreira, P.; Padrão, P.; Negrão, R.; Amaral, T.F. Adherence to a Mediterranean Dietary Pattern and Functional Parameters: A Cross-Sectional Study in an Older Population. J. Nutr. Health Aging 2020, 24, 138–146. [Google Scholar] [CrossRef]
- Lauretani, F.; Semba, R.D.; Bandinelli, S.; Giacomini, V.; Corsi, A.M.; Ferrucci, L. Low Plasma Carotenoids and Skeletal Muscle Strength Decline over Six Years. J. Gerontol. A Biol. Sci. Med. Sci. 2008, 63, 376–383. [Google Scholar] [CrossRef] [Green Version]
- Semba, R.D.; Lauretani, F.; Ferrucci, L. Carotenoids as protection against sarcopenia in older adults. Arch. Biochem. Biophys. 2007, 458, 141–145. [Google Scholar] [CrossRef] [Green Version]
- Sim, M.; Blekkenhorst, L.C.; Lewis, J.R.; Bondonno, C.P.; Devine, A.; Zhu, K.; Woodman, R.J.; Prince, R.L.; Hodgson, J.M. Vegetable and fruit intake and injurious falls risk in older women: A prospective cohort study. Br. J. Nutr. 2018, 120, 925–934. [Google Scholar] [CrossRef] [PubMed]
- Tak, Y.; Lee, J.; Yi, Y.; Kim, Y.; Lee, S.; Cho, B.; Cho, Y.H. Association of Handgrip Strength with Dietary Intake in the Korean Population: Findings Based on the Seventh Korea National Health and Nutrition Examination Survey (KNHANES VII-1), 2016. Nutrients 2018, 10, 1180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gallo, L.H.; Rodrigues, E.V.; Filho, J.M.; Harris-Love, M.O.; Gomes, A.R.S. Effects of virtual dance exercise on skeletal muscle architecture and function of community dwelling older women. J. Musculoskelet. Neuronal Interact. 2019, 19, 50–61. [Google Scholar]
- Isanejad, M.; Sirola, J.; Mursu, J.; Rikkonen, T.; Kröger, H.; Tuppurainen, M.; Erkkilä, A.T. Association of the Baltic Sea and Mediterranean diets with indices of sarcopenia in elderly women, OSPTRE-FPS study. Eur. J. Nutr. 2018, 57, 1435–1448. [Google Scholar] [CrossRef]
- Kelaiditi, E.; Jennings, A.; Steves, C.J.; Skinner, J.; Cassidy, A.; MacGregor, A.J.; Welch, A.A. Measurements of skeletal muscle mass and power are positively related to a Mediterranean dietary pattern in women. Osteoporos. Int. 2016, 27, 3251–3260. [Google Scholar] [CrossRef] [Green Version]
- Mangano, K.M.; Sahni, S.; Kiel, D.P.; Tucker, K.L.; Dufour, A.B.; Hannan, M.T. Dietary protein is associated with musculoskeletal health independently of dietary pattern: The Framingham Third Generation Study. Am. J. Clin. Nutr. 2017, 105, 714–722. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.; Yang, H.; Song, S.; Liu, J.; Wang, Z.; Wang, J. Bioactive Components in Whole Grains for the Regulation of Skeletal Muscle Function. Foods 2022, 11, 2752. [Google Scholar] [CrossRef]
- Liguori, I.; Russo, G.; Aran, L.; Bulli, G.; Curcio, F.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Sarcopenia: Assessment of disease burden and strategies to improve outcomes. Clin. Interv. Aging 2018, 13, 913–927. [Google Scholar] [CrossRef] [Green Version]
- Slavin, J.L.; Lloyd, B. Health Benefits of Fruits and Vegetables. Adv. Nutr. 2012, 3, 506–516. [Google Scholar] [CrossRef] [Green Version]
- Smith, G.I.; Atherton, P.; Reeds, D.N.; Mohammed, B.S.; Rankin, D.; Rennie, M.J.; Mittendorfer, B. Omega-3 polyunsaturated fatty acids augment the muscle protein anabolic response to hyperinsulinaemia–hyperaminoacidaemia in healthy young and middle-aged men and women. Clin. Sci. 2011, 121, 267–278. [Google Scholar] [CrossRef] [Green Version]
- Salucci, S.; Bartoletti-Stella, A.; Bavelloni, A.; Aramini, B.; Blalock, W.L.; Fabbri, F.; Vannini, I.; Sambri, V.; Stella, F.; Faenza, I. Extra Virgin Olive Oil (EVOO), a Mediterranean Diet Component, in the Management of Muscle Mass and Function Preservation. Nutrients 2022, 14, 3567. [Google Scholar] [CrossRef]
- Takayama, M.; Arai, Y.; Sasaki, S.; Hashimoto, M.; Shimizu, K.; Abe, Y.; Hirose, N. Association of marine-origin N-3 polyunsaturated fatty acids consumption and functional mobility in the community-dwelling oldest old. J. Nutr. Health Aging 2013, 17, 82–89. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Sayer, A.A. Sarcopenia. Lancet 2019, 393, 2636–2646. [Google Scholar] [CrossRef]
- O’Connell, M.L.; Coppinger, T.; McCarthy, A.L. The role of nutrition and physical activity in frailty: A review. Clin. Nutr. ESPEN 2020, 35, 1–11. [Google Scholar] [CrossRef]
- Rashidi Pour Fard, N.; Amirabdollahian, F.; Haghighatdoost, F. Dietary patterns and frailty: A systematic review and meta-analysis. Nutr. Rev. 2019, 77, 498–513. [Google Scholar] [CrossRef]
- Alaghehband, F.R.; Erkkilä, A.T.; Rikkonen, T.; Sirola, J.; Kröger, H.; Isanejad, M. Association of Baltic Sea and Mediterranean diets with frailty phenotype in older women, Kuopio OSTPRE-FPS study. Eur. J. Nutr. 2021, 60, 821–831. [Google Scholar] [CrossRef]
- Rahi, B.; Ajana, S.; Tabue-Teguo, M.; Dartigues, J.F.; Peres, K.; Feart, C. High adherence to a Mediterranean diet and lower risk of frailty among French older adults community-dwellers: Results from the Three-City-Bordeaux Study. Clin. Nutr. 2018, 37, 1293–1298. [Google Scholar] [CrossRef]
- García-Esquinas, E.; Rahi, B.; Peres, K.; Colpo, M.; Dartigues, J.F.; Bandinelli, S.; Feart, C.; Rodríguez-Artalejo, F. Consumption of fruit and vegetables and risk of frailty: A dose-response analysis of 3 prospective cohorts of community-dwelling older adults. Am. J. Clin. Nutr. 2016, 104, 132–142. [Google Scholar] [CrossRef] [Green Version]
- Kojima, G.; Avgerinou, C.; Iliffe, S.; Walters, K. Adherence to Mediterranean Diet Reduces Incident Frailty Risk: Systematic Review and Meta-Analysis. J. Am. Geriatr. Soc. 2018, 66, 783–788. [Google Scholar] [CrossRef] [Green Version]
- Soysal, P.; Isik, A.T.; Carvalho, A.F.; Fernandes, B.S.; Solmi, M.; Schofield, P.; Veronese, N.; Stubbs, B. Oxidative stress and frailty: A systematic review and synthesis of the best evidence. Maturitas 2017, 99, 66–72. [Google Scholar] [CrossRef] [Green Version]
- Hao, J.; Zhou, P.; Qiu, H. Association between Ultra-Processed Food Consumption and Frailty in American Elder People: Evidence from a Cross-Sectional Study. J. Nutr. Health Aging 2022, 26, 688–697. [Google Scholar] [CrossRef]
- Struijk, E.A.; Fung, T.T.; Sotos-Prieto, M.; Rodriguez-Artalejo, F.; Willett, W.C.; Hu, F.B.; Lopez-Garcia, E. Red meat consumption and risk of frailty in older women. J. Cachexia Sarcopenia Muscle 2022, 13, 210–219. [Google Scholar] [CrossRef]
- Jin, M.; Qian, Z.; Yin, J.; Xu, W.; Zhou, X. The role of intestinal microbiota in cardiovascular disease. J. Cell. Mol. Med. 2019, 23, 2343–2350. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Mantrana, I.; Selma-Royo, M.; Alcantara, C.; Collado, M.C. Shifts on Gut Microbiota Associated to Mediterranean Diet Adherence and Specific Dietary Intakes on General Adult Population. Front. Microbiol. 2018, 9, 890. [Google Scholar] [CrossRef] [Green Version]
- Li, J.Y.; Yu, M.; Pal, S.; Tyagi, A.M.; Dar, H.; Adams, J.; Weitzmann, M.N.; Jones, R.M.; Pacifici, R. Parathyroid hormone–dependent bone formation requires butyrate production by intestinal microbiota. J. Clin. Investig. 2020, 130, 1767–1781. [Google Scholar] [CrossRef] [Green Version]
- Thomas, R.L.; Jiang, L.; Adams, J.S.; Xu, Z.Z.; Shen, J.; Janssen, S.; Ackermann, G.; Vanderschueren, D.; Pauwels, S.; Knight, R.; et al. Vitamin D metabolites and the gut microbiome in older men. Nat. Commun. 2020, 11, 5997. [Google Scholar] [CrossRef]
- Takimoto, T.; Hatanaka, M.; Hoshino, T.; Takara, T.; Tanaka, K.; Shimizu, A.; Morita, H.; Nakamura, T. Effect of Bacillus subtilis C-3102 on bone mineral density in healthy postmenopausal Japanese women: A randomized, placebo-controlled, double-blind clinical trial. Biosci. Microbiota Food Health 2018, 37, 87–96. [Google Scholar] [CrossRef] [Green Version]
- Lambert, M.N.T.; Thybo, C.B.; Lykkeboe, S.; Rasmussen, L.M.; Frette, X.; Christensen, L.P.; Jeppesen, P.B. Combined bioavailable isoflavones and probiotics improve bone status and estrogen metabolism in postmenopausal osteopenic women: A randomized controlled trial. Am. J. Clin. Nutr. 2017, 106, 909–920. [Google Scholar] [CrossRef] [Green Version]
- Manickam, R.; Duszka, K.; Wahli, W. PPARs and Microbiota in Skeletal Muscle Health and Wasting. Int. J. Mol. Sci. 2020, 21, 8056. [Google Scholar]
- Hsu, C.F.; Huang, C.C.; Liu, T.T.; Yang, U.C.; Liu, C.W.; Huang, S.F.; Yang, Y.Y.; Huang, Y.H.; Hou, M.C.; Lin, H.C. Deletion of intestinal SIRT1 exacerbated muscle wasting in cirrhotic mice by decreasing the intestinal concentration of short-chain fatty acids and inflammation. J. Pharmacol. Sci. 2021, 147, 376–385. [Google Scholar] [CrossRef] [PubMed]
- Canfora, H.; Blaak, J. The Short-Chain Fatty Acid Acetate in Body Weight Control and Insulin Sensitivity. Nutrients 2019, 11, 1943. [Google Scholar]
- Ticinesi, A.; Mancabelli, L.; Tagliaferri, S.; Nouvenne, A.; Milani, C.; Del Rio, D.; Lauretani, F.; Maggio, M.G.; Ventura, M.; Meschi, T. The Gut-Muscle Axis in Older Subjects with Low Muscle Mass and Performance: A Proof of Concept Study Exploring Fecal Microbiota Composition and Function with Shotgun Metagenomics Sequencing. Int. J. Mol. Sci. 2020, 21, 8946. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, T.S.; Rampelli, S.; Jeffery, I.B.; Santoro, A.; Neto, M.; Capri, M.; Giampieri, E.; Jennings, A.; Candela, M.; Turroni, S.; et al. Mediterranean diet intervention alters the gut microbiome in older people reducing frailty and improving health status: The NU-AGE 1-year dietary intervention across five European countries. Gut 2020, 69, 1218–1228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Typical Foods of the Meddiet | BMD | Osteoporotic Fractures | Strength | Muscle Mass |
|---|---|---|---|---|
| EVOO | + | + | + | + |
| Vegetables and Fruits | + | + | + | + |
| Legumes | + | + | Unknown | Unknown |
| Grains | + | + | Unknown | + |
| Nuts | Unknown | Unknown | + | Unknown |
| Fish | + | + | + | + |
| Wine | + | + | + | Unknown |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andreo-López, M.C.; Contreras-Bolívar, V.; García-Fontana, B.; García-Fontana, C.; Muñoz-Torres, M. The Influence of the Mediterranean Dietary Pattern on Osteoporosis and Sarcopenia. Nutrients 2023, 15, 3224. https://doi.org/10.3390/nu15143224
Andreo-López MC, Contreras-Bolívar V, García-Fontana B, García-Fontana C, Muñoz-Torres M. The Influence of the Mediterranean Dietary Pattern on Osteoporosis and Sarcopenia. Nutrients. 2023; 15(14):3224. https://doi.org/10.3390/nu15143224
Chicago/Turabian StyleAndreo-López, María Carmen, Victoria Contreras-Bolívar, Beatriz García-Fontana, Cristina García-Fontana, and Manuel Muñoz-Torres. 2023. "The Influence of the Mediterranean Dietary Pattern on Osteoporosis and Sarcopenia" Nutrients 15, no. 14: 3224. https://doi.org/10.3390/nu15143224
APA StyleAndreo-López, M. C., Contreras-Bolívar, V., García-Fontana, B., García-Fontana, C., & Muñoz-Torres, M. (2023). The Influence of the Mediterranean Dietary Pattern on Osteoporosis and Sarcopenia. Nutrients, 15(14), 3224. https://doi.org/10.3390/nu15143224