The Utilization of Serum Folate and Homocysteine Tests and the Prevalence of Folate Deficiency in Reproductive-Age Korean Women during the COVID-19 Pandemic
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Subjects
2.2. Definitions
2.3. Analytical Methods
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics of the Subjects
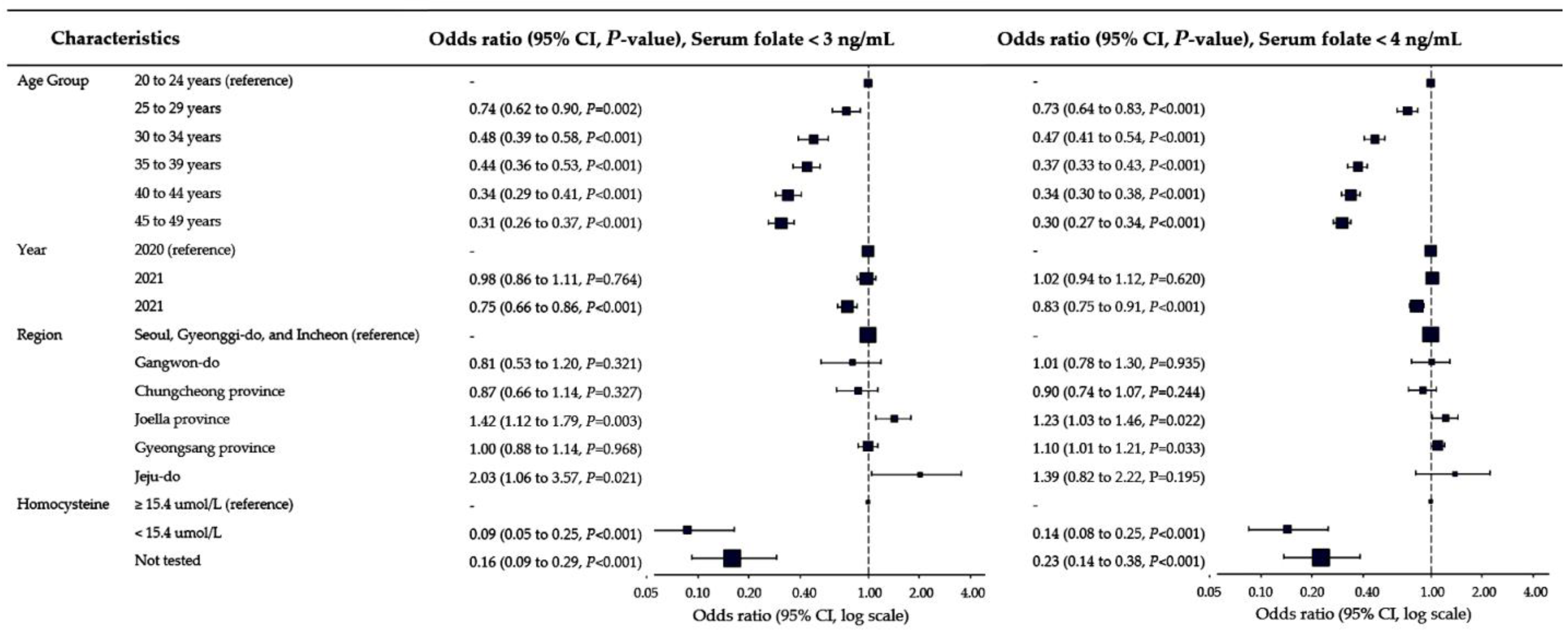
3.2. Prevalence of Folate Deficiency and Associated Factors
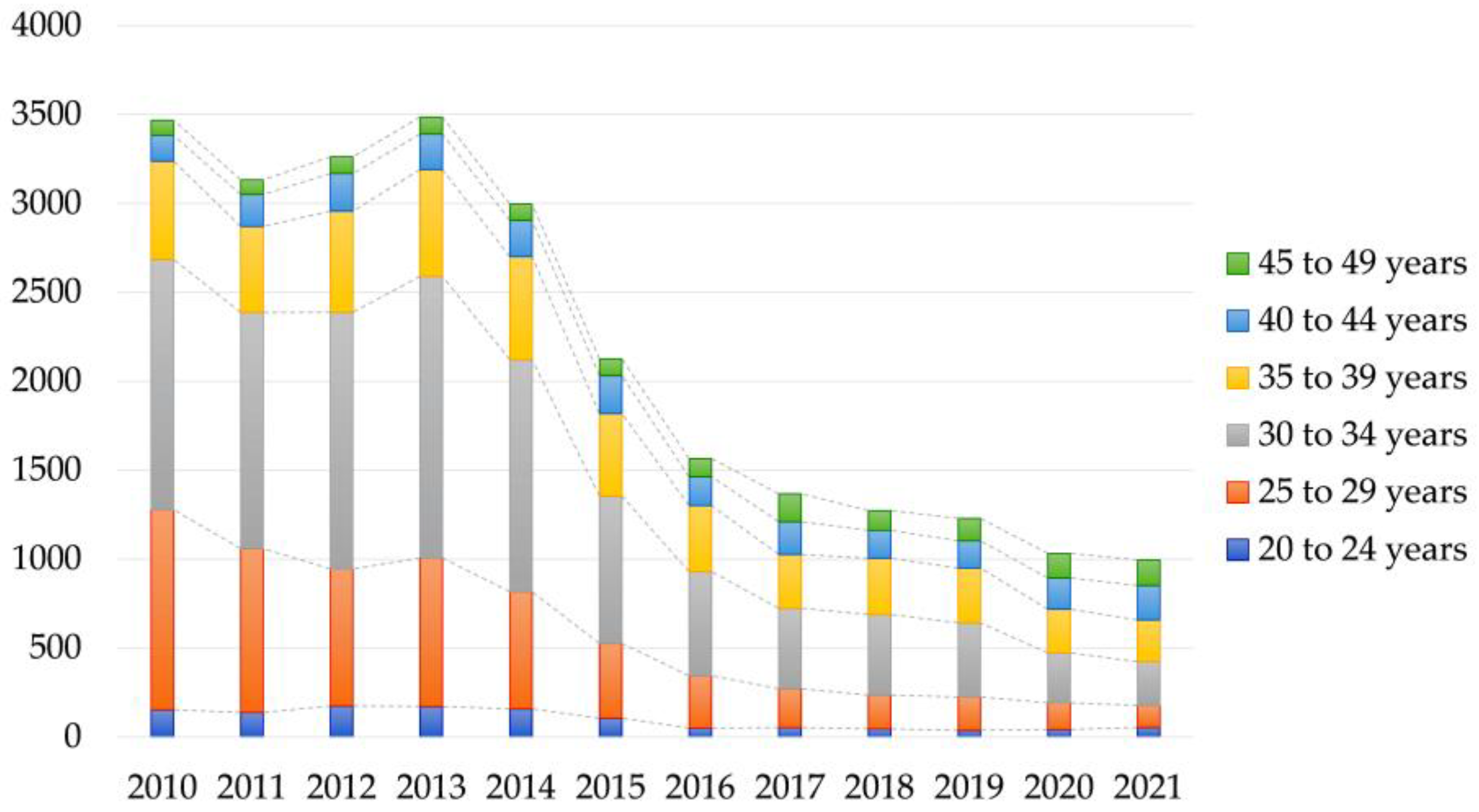
3.3. Number of Patients Managed with Folate Deficiency in the Public Database
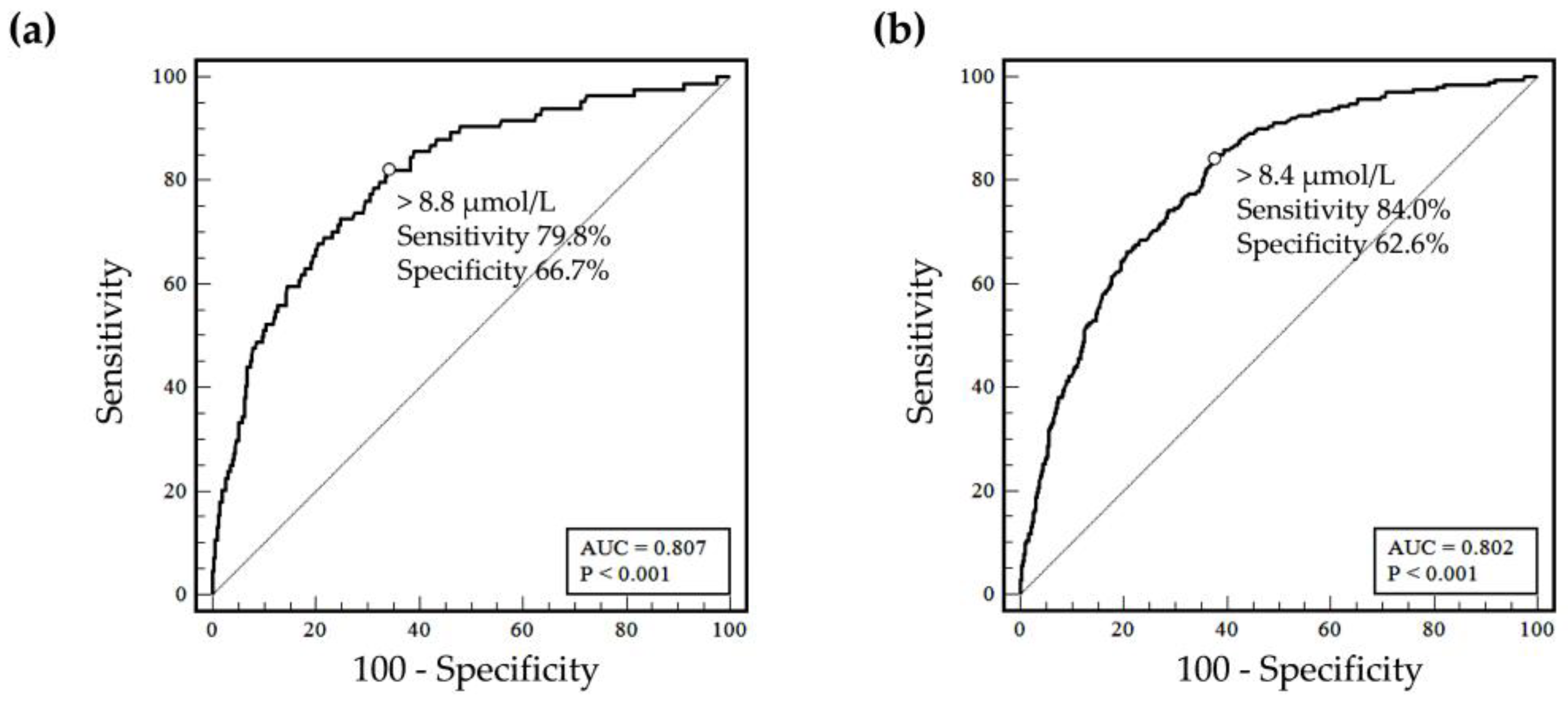
3.4. Homocysteine to Define Folate Deficiency
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kancherla, V.; Botto, L.D.; Rowe, L.A.; Shlobin, N.A.; Caceres, A.; Arynchyna-Smith, A.; Zimmerman, K.; Blount, J.; Kibruyisfaw, Z.; Ghotme, K.A.; et al. Preventing birth defects, saving lives, and promoting health equity: An urgent call to action for universal mandatory food fortification with folic acid. Lancet Glob. Health 2022, 10, e1053–e1057. [Google Scholar] [CrossRef]
- Atta, C.A.; Fiest, K.M.; Frolkis, A.D.; Jette, N.; Pringsheim, T.; St Germaine-Smith, C.; Rajapakse, T.; Kaplan, G.G.; Metcalfe, A. Global Birth Prevalence of Spina Bifida by Folic Acid Fortification Status: A Systematic Review and Meta-Analysis. Am. J. Public Health 2016, 106, e24–e34. [Google Scholar] [CrossRef]
- Blencowe, H.; Kancherla, V.; Moorthie, S.; Darlison, M.W.; Modell, B. Estimates of global and regional prevalence of neural tube defects for 2015: A systematic analysis. Ann. N. Y. Acad. Sci. 2018, 1414, 31–46. [Google Scholar] [CrossRef]
- Uta, M.; Neamtu, R.; Bernad, E.; Mocanu, A.G.; Gluhovschi, A.; Popescu, A.; Dahma, G.; Dumitru, C.; Stelea, L.; Citu, C.; et al. The Influence of Nutritional Supplementation for Iron Deficiency Anemia on Pregnancies Associated with SARS-CoV-2 Infection. Nutrients 2022, 14, 836. [Google Scholar] [CrossRef] [PubMed]
- Acosta-Elias, J.; Espinosa-Tanguma, R. The Folate Concentration and/or Folic Acid Metabolites in Plasma as Factor for COVID-19 Infection. Front. Pharmacol. 2020, 11, 1062. [Google Scholar] [CrossRef] [PubMed]
- Choi, R.; Choi, S.; Lim, Y.; Cho, Y.Y.; Kim, H.J.; Kim, S.W.; Chung, J.H.; Oh, S.Y.; Lee, S.Y. A Prospective Study on Serum Methylmalonic Acid and Homocysteine in Pregnant Women. Nutrients 2016, 8, 797. [Google Scholar] [CrossRef]
- Department of Nutrition for Health and Development (NHD); World Health Organization. Serum and Red Blood Cell Folate Concentrations for Assessing Folate Status in Populations. 2015. Available online: https://apps.who.int/iris/bitstream/handle/10665/162114/WHO_NMH_NHD_EPG_15.01.pdf?sequence=1 (accessed on 8 June 2023).
- World Health Organization. WHO Guideline: Optimal Serum and Red Blood Cell Folate Concentrations in Women of Reproductive Age for Prevention of Neural Tube Defects. 2015. Available online: https://apps.who.int/iris/bitstream/handle/10665/161988/9789241549042_eng.pdf (accessed on 8 June 2023).
- Stabler, S.P. Clinical practice. Vitamin B12 deficiency. N. Engl. J. Med. 2013, 368, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Choi, R.; Oh, Y.; Park, M.J.; Kim, S.; Kim, Y.; Lee, S.G.; Lee, E.H. Test utilization for the diagnosis of vitamin B12 and folate deficiency in local clinics in Korea. J. Clin. Lab. Anal. 2020, 34, e23441. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Micronutrients Database. Available online: https://platform.who.int/nutrition/micronutrients-database (accessed on 8 June 2023).
- Jenkins, D.J.A.; Spence, D.; Giovannucci, E.L.; Kim, Y.-I.; Josse, R.G.; Vieth, R.; Sahye-Pudaruth, S.; Paquette, M.; Patel, D.; Mejia, S.B.; et al. Supplemental Vitamins and Minerals for Cardiovascular Disease Prevention and Treatment: JACC Focus Seminar. J. Am. Coll. Cardiol. 2021, 77, 423–436. [Google Scholar] [CrossRef]
- O’Connor, E.A.; Evans, C.V.; Ivlev, I.; Rushkin, M.C.; Thomas, R.G.; Martin, A.; Lin, J.S. Vitamin and Mineral Supplements for the Primary Prevention of Cardiovascular Disease and CancerUpdated Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA 2022, 327, 2334–2347. [Google Scholar] [CrossRef]
- Herrmann, W.; Herrmann, M. The Controversial Role of HCY and Vitamin B Deficiency in Cardiovascular Diseases. Nutrients 2022, 14, 1412. [Google Scholar] [CrossRef]
- Spence, J.D.; Hankey, G.J. Problem in the Recent American Heart Association Guideline on Secondary Stroke Prevention: B Vitamins to Lower Homocysteine Do Prevent Stroke. Stroke 2022, 53, 2702–2708. [Google Scholar] [CrossRef]
- Aparicio-Ugarriza, R.; Palacios, G.; Alder, M.; González-Gross, M. A review of the cut-off points for the diagnosis of vitamin B12 deficiency in the general population. Clin. Chem. Lab. Med. 2015, 53, 1149–1159. [Google Scholar] [CrossRef] [PubMed]
- Cluitmans, J.C.A.; van den Ouweland, J.M.W. Reference values of a new serum folate assay traceable to the WHO International Standard. Clin. Chem. Lab. Med. 2019, 57, e176–e178. [Google Scholar] [CrossRef] [PubMed]
- Choi, R.; Oh, Y.; Park, M.J.; Lee, S.G.; Lee, E.H. Reference Interval for Korean Serum Folate Assay Traceable to the WHO International Standard. Clin. Lab. 2021, 67, 945–950. [Google Scholar] [CrossRef]
- Ferraro, S.; Panteghini, M. Folate and vitamin B12 assays after recalibration to the WHO International Standard 03/178: Making the interpretation as simple as possible, but not simpler. Clin. Chem. Lab. Med. 2019, 57, 1112–1114. [Google Scholar] [CrossRef]
- Braga, F.; Frusciante, E.; Ferraro, S.; Panteghini, M. Trueness evaluation and verification of inter-assay agreement of serum folate measuring systems. Clin. Chem. Lab. Med. 2020, 58, 1697–1705. [Google Scholar] [CrossRef] [PubMed]
- Rogers, L.M.; Cordero, A.M.; Pfeiffer, C.M.; Hausman, D.B.; Tsang, B.L.; De-Regil, L.M.; Rosenthal, J.; Razzaghi, H.; Wong, E.C.; Weakland, A.P.; et al. Global folate status in women of reproductive age: A systematic review with emphasis on methodological issues. Ann. N. Y. Acad. Sci. 2018, 1431, 35–57. [Google Scholar] [CrossRef]
- Choi, R.; Oh, Y.; Park, M.J.; Lee, S.G.; Lee, E.H. Prevalence of Folate Deficiency in Korean Women of Reproductive Age Using a Serum Folate Assay Traceable to the WHO International Standard. Clin. Lab. 2021, 67, 2157–2161. [Google Scholar] [CrossRef]
- Han, Y.-H.; Hyun, T. Folate: 2020 Dietary reference intakes and nutritional status of Koreans. J. Nutr. Health 2022, 55, 330–347. [Google Scholar] [CrossRef]
- United States Department of Agriculture. Dietary Guidelines for Americans 2020–2025. Available online: https://www.dietaryguidelines.gov/resources/2020-2025-dietary-guidelines-online-materials (accessed on 12 July 2023).
- Lamers, Y. Folate recommendations for pregnancy, lactation, and infancy. Ann. Nutr. Metab. 2011, 59, 32–37. [Google Scholar] [CrossRef]
- Public Health England. Government Dietary Recommendations. 2016. Available online: https://www.gov.uk/government/publications/the-eatwell-guide (accessed on 12 July 2023).
- British Nutrition Foundation. Nutrition Requirements. 2021. Available online: https://www.nutrition.org.uk/healthy-sustainable-diets/nutrient-requirements/ (accessed on 12 July 2023).
- Ministry of Health and Welfare, The Korean Nutrition Society. Dietary Reference Intakes for Koreans 2020. Sejong. 2020. Available online: https://www.kns.or.kr/fileroom/fileroom.asp?BoardID=Kdr (accessed on 12 July 2023).
- Ministry of Health and Welfare, The Korean Nutrition Society. Dietary Reference Intakes for Koreans: Application. 2022. Available online: http://www.mohw.go.kr/upload/viewer/skin/doc.html?fn=1643348559723_20220128144239.pdf&rs=/upload/viewer/result/202307/ (accessed on 12 July 2023).
- Murphy, R.; Marshall, K.; Zagorin, S.; Devarshi, P.P.; Mitmesser, S.H. Socioeconomic Inequalities Impact the Ability of Pregnant Women and Women of Childbearing Age to Consume Nutrients Needed for Neurodevelopment: An Analysis of NHANES 2007–2018. Nutrients 2022, 14, 3823. [Google Scholar] [CrossRef]
- Public Health England; Food Standards Agency. UK National Diet and Nutrition Survey. NDNS: Results from Years 9 to 11 (2016 to 2017 and 2018 to 2019). Available online: https://www.gov.uk/government/statistics/ndns-results-from-years-9-to-11-2016-to-2017-and-2018-to-2019 (accessed on 18 July 2023).
- Berger, M.M.; Shenkin, A.; Schweinlin, A.; Amrein, K.; Augsburger, M.; Biesalski, H.-K.; Bischoff, S.C.; Casaer, M.P.; Gundogan, K.; Lepp, H.-L.; et al. ESPEN micronutrient guideline. Clin. Nutr. 2022, 41, 1357–1424. [Google Scholar] [CrossRef]
- Choi, S.K. Women’s Dietary Problems over the Life Course. Available online: https://www.kihasa.re.kr/api/external/viewer/doc.html?fn=17238:1141979838254169727129.pdf&rs=/api/external/viewer/upload/kihasa2021/publish (accessed on 12 July 2023).
- Jin, T.; Park, E.Y.; Kim, B.; Oh, J.K. Non-linear association between serum folate concentrations and dyslipidemia: Korea National Health and Nutrition Examination Survey 2016–2018. Epidemiol. Health 2022, 44, e2022046. [Google Scholar] [CrossRef]
- Lee, M.R.; Jung, S.M. Serum Folate Related to Five Measurements of Obesity and High-Sensitivity C-Reactive Protein in Korean Adults. Nutrients 2022, 14, 3461. [Google Scholar] [CrossRef] [PubMed]
- Choi, R.; Park, W.; Chun, G.; Lee, S.G.; Lee, E.H. Utilization of Glucose-6-Phosphate Dehydrogenase Test and the Prevalence of Enzyme Deficiency in Korea. J. Clin. Med. 2023, 12, 3179. [Google Scholar] [CrossRef]
- Health Insurance Review & Assessment Service. HIRA Bigdata Open Portal. Statistics for Diseases. Available online: https://opendata.hira.or.kr/op/opc/olap4thDsInfoTab1.do#none (accessed on 12 July 2023).
- CLSI. Defining, Establishing, and Verifying Reference Intervals in the Clinical Laboratory; Approved Guideline—Third Edition; CLSI document EP28A3c; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2008. [Google Scholar]
- CLSI. Evaluation of Qualitative, Binary Output Examination Performance, 3rd ed.; CLSI guideline EP12; Clinical and Laboratory Standarads Institute: Wayne, PA, USA, 2023. [Google Scholar]
- Korea Disease Control and Prevention Agency. The Korea National Health and Nutrition Examination Survey. Available online: https://knhanes.kdca.go.kr/knhanes/eng/index.do (accessed on 8 June 2023).
- Kim, M.J.; Kim, J.; Hwang, E.J.; Song, Y.; Kim, H.; Hyun, T. Awareness, knowledge, and use of folic acid among non-pregnant Korean women of childbearing age. Nutr. Res. Pract. 2018, 12, 78–84. [Google Scholar] [CrossRef]
- Pfeiffer, C.M.; Sternberg, M.R.; Zhang, M.; Fazili, Z.; Storandt, R.J.; Crider, K.S.; Yamini, S.; Gahche, J.J.; Juan, W.; Wang, C.-Y.; et al. Folate status in the US population 20 y after the introduction of folic acid fortification. Am. J. Clin. Nutr. 2019, 110, 1088–1097. [Google Scholar] [CrossRef] [PubMed]
- Saravanan, P.; Sukumar, N.; Adaikalakoteswari, A.; Goljan, I.; Venkataraman, H.; Gopinath, A.; Bagias, C.; Yajnik, C.S.; Stallard, N.; Ghebremichael-Weldeselassi, Y.; et al. Association of maternal vitamin B12 and folate levels in early pregnancy with gestational diabetes: A prospective UK cohort study (PRiDE study). Diabetologia 2021, 64, 2170–2182. [Google Scholar] [CrossRef] [PubMed]
- FIGO Working Group on Best Practice in Maternal–Fetal Medicine. Best practice in maternal–fetal medicine. Int. J. Gynaecol. Obstet. 2015, 128, 80–82. [Google Scholar] [CrossRef]
- Statistics Korea. Korean Statistical Information Service. Available online: https://kosis.kr/eng/statisticsList/statisticsListIndex.do?menuId=M_01_01&vwcd=MT_ETITLE&parmTabId=M_01_01 (accessed on 3 May 2023).
- Choi, R.; Chun, G.; Park, M.J.; Lee, S.G.; Lee, E.H. Prevalence of Iron Deficiency Anemia Indicated for Intravenous Iron Treatment in the Korean Population. Nutrients 2023, 15, 614. [Google Scholar] [CrossRef] [PubMed]
- Choi, R.; Cho, S.E.; Lee, S.G.; Lee, E.H. Recent Information on Vitamin D Deficiency in an Adult Korean Population Visiting Local Clinics and Hospitals. Nutrients 2022, 14, 1978. [Google Scholar] [CrossRef] [PubMed]

| Characteristics | Data | |
|---|---|---|
| Age | Age, years (median, IQR) | 40.6 (33.1 to 45.6) |
| 20 to 24 years (n, %) | 2242 (8.1%) | |
| 25 to 29 years (n, %) | 2755 (9.9%) | |
| 30 to 34 years (n, %) | 3252 (11.7%) | |
| 35 to 39 years (n, %) | 4764 (17.2%) | |
| 40 to 44 years (n, %) | 7044 (25.4%) | |
| 45 to 49 years (n, %) | 7701 (27.7%) | |
| Tested year | Tested in 2020 (n, %) | 7573 (27.3%) |
| Tested in 2021 (n, %) | 10,261 (37.0%) | |
| Tested in 2022 (n, %) | 9924 (35.8%) | |
| Geographical region | Seoul, Incheon, and Gyeonggi-do (n, %) | 18,938 (68.2%) |
| Gangwon-do (n, %) | 577 (2.1%) | |
| Chungcheong province (n, %) | 1231 (4.4%) | |
| Jeolla province (n, %) | 1147 (4.1%) | |
| Gyeongsang province (n, %) | 5740 (20.7%) | |
| Jeju Island (n, %) | 125 (0.5%) | |
| Group | Group 1 (serum folate only; n, %) | 25,429 (91.6%) |
| Group 2 (serum folate and homocysteine; n, %) | 2329 (8.4%) | |
| Serum biomarker | Serum folate, ng/mL (median, IQR) | 8.10 (5.30 to 13.10) |
| Serum homocysteine, µmol/L (median, IQR) | 7.94 (6.65 to 9.49) | |
| Characteristics | Serum Folate (ng/mL) | Folate Deficiency (<3 ng/mL) | Folate Deficiency (<4 ng/mL) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | p-Value | n | % | p-Value | n | % | p-Value | ||
| Age group | 20 to 24 years | 7.40 | 5.58 | <0.0001 | 251 | 11.2 | <0.0001 | 558 | 24.9 | <0.0001 |
| 25 to 29 years | 8.71 | 6.69 | 233 | 8.5 | 531 | 19.3 | ||||
| 30 to 34 years | 10.81 | 8.25 | 179 | 5.5 | 429 | 13.2 | ||||
| 35 to 39 years | 11.60 | 8.50 | 248 | 5.2 | 525 | 11.0 | ||||
| 40 to 44 years | 11.02 | 8.10 | 290 | 4.1 | 712 | 10.1 | ||||
| 45 to 49 years | 10.95 | 7.53 | 293 | 3.8 | 707 | 9.2 | ||||
| Tested year | Tested in 2020 | 9.90 | 7.20 | <0.0001 | 448 | 5.9 | <0.0001 | 991 | 13.1 | <0.0001 |
| Tested in 2021 | 10.38 | 7.77 | 599 | 5.8 | 1373 | 13.4 | ||||
| Tested in 2022 | 11.07 | 8.25 | 447 | 4.5 | 1098 | 11.1 | ||||
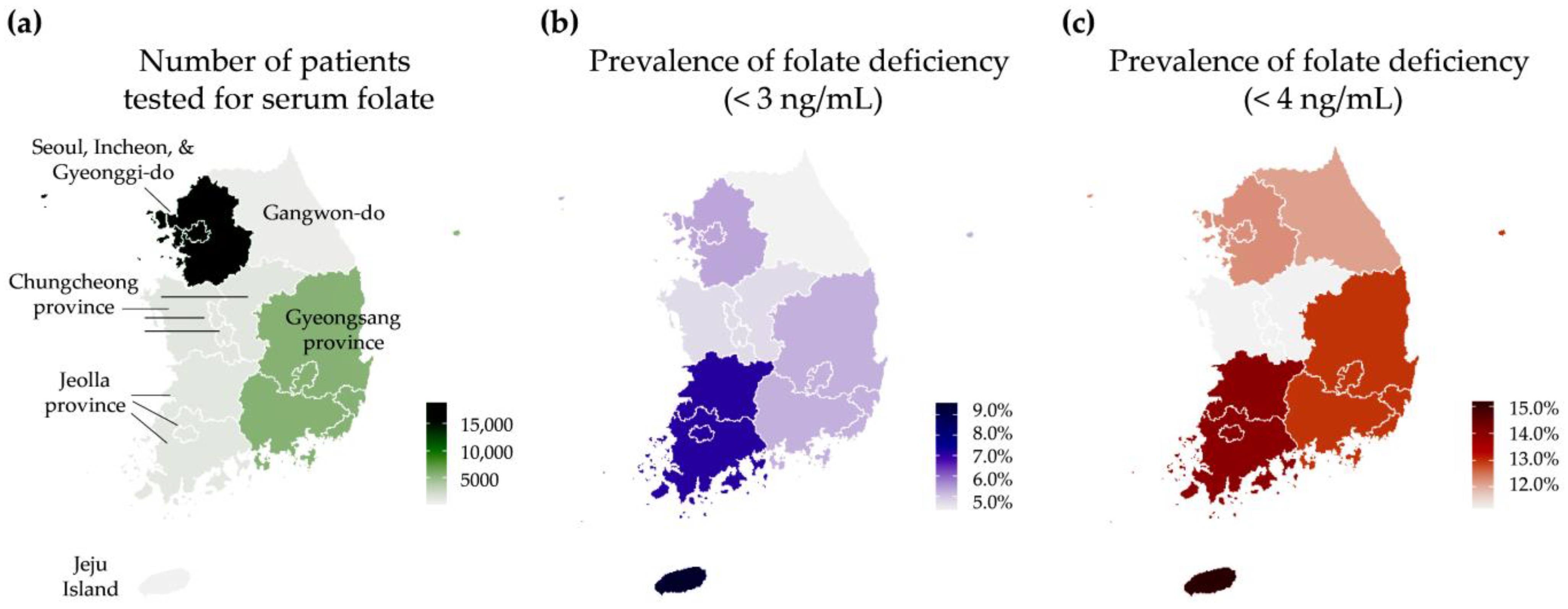
| Geographical region | Seoul, Incheon, and Gyeonggi-do | 10.78 | 8.05 | <0.0001 | 1016 | 5.4 | 0.0159 | 2328 | 12.3 | 0.2003 |
| Gangwon-do | 10.12 | 7.76 | 25 | 4.3 | 70 | 12.1 | ||||
| Chungcheong province | 9.64 | 6.62 | 58 | 4.7 | 138 | 11.2 | ||||
| Jeolla province | 9.62 | 6.76 | 82 | 7.1 | 161 | 14.0 | ||||
| Gyeongsang province | 9.96 | 7.38 | 301 | 5.2 | 746 | 13.0 | ||||
| Jeju island | 10.28 | 7.66 | 12 | 9.6 | 19 | 15.2 | ||||
| Group | Group 1 (serum folate only) | 10.36 | 7.73 | <0.0001 | 1410 | 5.5 | <0.0001 | 3249 | 12.8 | <0.0001 |
| Group 2 (serum folate and homocysteine) | 11.98 | 8.55 | 84 | 3.6 | 213 | 9.1 | ||||
| Homocysteine Cut-off | Folate Deficiency | Folate < 3 ng/mL | Folate < 4 ng/mL | ||
|---|---|---|---|---|---|
| Deficiency (n) | No Deficiency (n) | Deficiency (n) | No Deficiency (n) | ||
| Transferred from the manufacturer’s information, ≥15.4 µmol/L | Deficiency (n) | 17 | 47 | 25 | 39 |
| No deficiency (n) | 67 | 2198 | 188 | 2077 | |
| Overall agreement (%, 95% CI) | 95.1 (94.2 to 95.9) | 90.3 (89.0 to 91.4) | |||
| Positive agreement (%, 95% CI) | 26.6 (17.3 to 38.5) | 39.1 (28.1 to 51.3) | |||
| Negative agreement (%, 96% CI) | 97.0 (96.3 to 97.7) | 91.7 (90.5 to 92.8) | |||
| ROC analysis, >8.4 µmol/L | Deficiency (n) | 72 | 898 | 179 | 791 |
| No deficiency (n) | 12 | 1347 | 34 | 1325 | |
| Overall agreement (%, 95% CI) | 60.9 (58.9 to 92.9) | 64.6 (62.6 to 66.5) | |||
| Positive agreement (%, 95% CI) | 85.7 (79.7 to 91.6) | 84.0 (78.5 to 88.3) | |||
| Negative agreement (%, 96% CI) | 60.0 (58.0 to 62.0) | 62.6 (60.5 to 64.7) | |||
| ROC analysis, > 8.8 µmol/L | Deficiency (n) | 67 | 748 | 160 | 655 |
| No deficiency (n) | 17 | 1497 | 53 | 1461 | |
| Overall agreement (%, 95% CI) | 79.8 (70.0 to 87.0) | 75.1 (68.9 to 80.4) | |||
| Positive agreement (%, 95% CI) | 66.7 (64.7 to 68.6) | 69.0 (67.0 to 71.0) | |||
| Negative agreement (%, 96% CI) | 67.2 (65.2 to 69.0) | 69.6 (67.7 to 71.4) | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, R.; Park, W.; Chun, G.; Lee, S.G.; Lee, E.H. The Utilization of Serum Folate and Homocysteine Tests and the Prevalence of Folate Deficiency in Reproductive-Age Korean Women during the COVID-19 Pandemic. Nutrients 2023, 15, 3236. https://doi.org/10.3390/nu15143236
Choi R, Park W, Chun G, Lee SG, Lee EH. The Utilization of Serum Folate and Homocysteine Tests and the Prevalence of Folate Deficiency in Reproductive-Age Korean Women during the COVID-19 Pandemic. Nutrients. 2023; 15(14):3236. https://doi.org/10.3390/nu15143236
Chicago/Turabian StyleChoi, Rihwa, Wonseo Park, Gayoung Chun, Sang Gon Lee, and Eun Hee Lee. 2023. "The Utilization of Serum Folate and Homocysteine Tests and the Prevalence of Folate Deficiency in Reproductive-Age Korean Women during the COVID-19 Pandemic" Nutrients 15, no. 14: 3236. https://doi.org/10.3390/nu15143236
APA StyleChoi, R., Park, W., Chun, G., Lee, S. G., & Lee, E. H. (2023). The Utilization of Serum Folate and Homocysteine Tests and the Prevalence of Folate Deficiency in Reproductive-Age Korean Women during the COVID-19 Pandemic. Nutrients, 15(14), 3236. https://doi.org/10.3390/nu15143236






