Supplementation of Dihomo-γ-Linolenic Acid for Pollen-Induced Allergic Symptoms in Healthy Subjects: A Randomized, Double-Blinded, Placebo-Controlled Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Ethics
2.3. Participants
2.4. Randomising and Blinding
2.5. Treatment
2.6. Primary Outcomes
2.6.1. CSARS
2.6.2. JRQLQ
2.7. Secondary Outcomes
2.8. Pollen Count
2.9. Dietary Assement
2.10. Safety Evaluation
2.11. Sample Size
2.12. Statistical Analysis
3. Results
3.1. Participants
3.2. Pollen Counts
3.3. Primary Endpoint
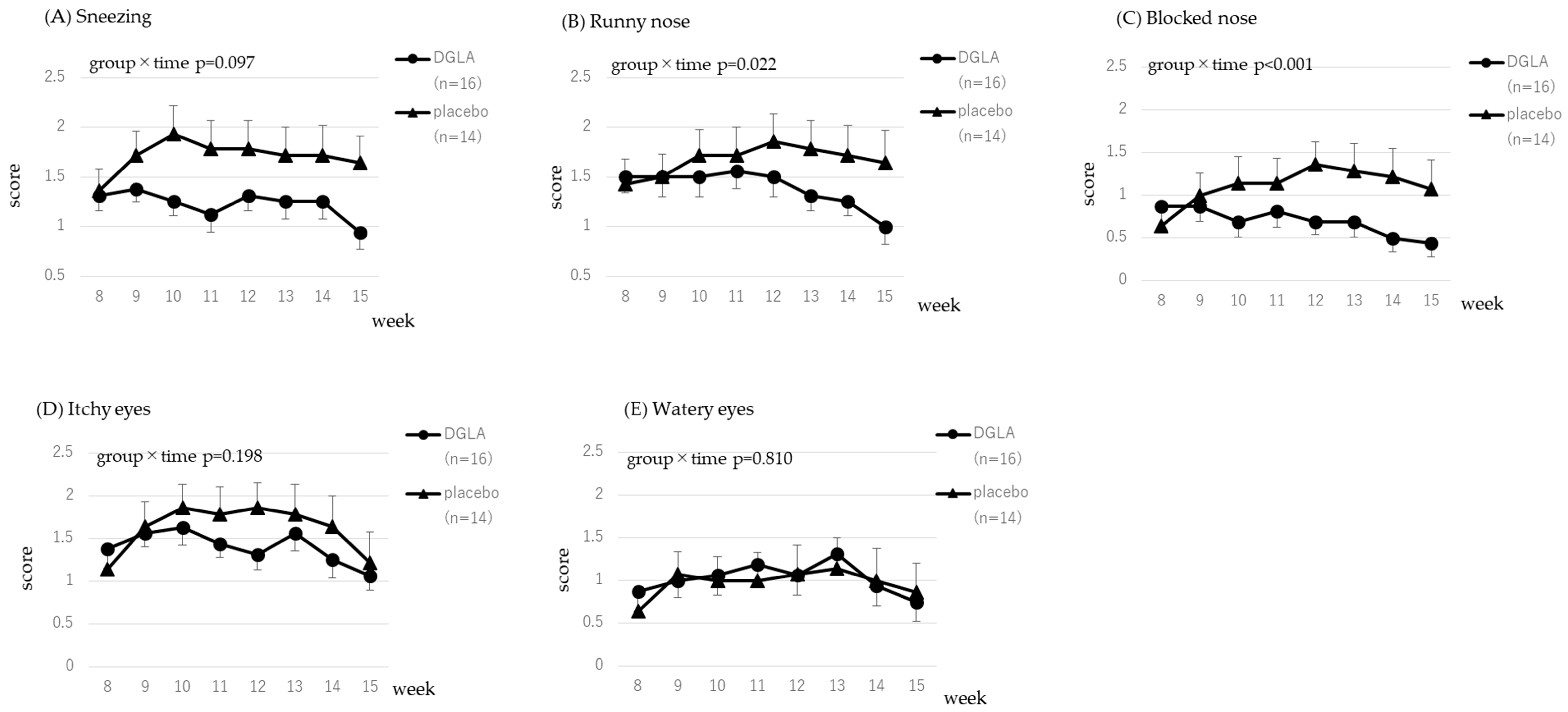
3.3.1. Cedar Pollen Associated Symptoms
3.3.2. Cypress Pollen Associated Symptoms
3.4. Secondary Endpoint
3.5. Adverse Events
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ruan, K.H.; Cervantes, V.; So, S.P. Engineering of a novel hybrid enzyme: An anti-inflammatory drug target with triple catalytic activities directly converting arachidonic acid into the inflammatory prostaglandin E2. Protein Eng. Des. Sel. 2009, 22, 733–740. [Google Scholar] [CrossRef] [Green Version]
- Kapoor, R.; Huang, Y.S. Gamma linolenic acid: An antiinflammatory omega-6 fatty acid. Curr. Pharm. Biotechnol. 2006, 7, 531–534. [Google Scholar] [CrossRef] [Green Version]
- Innes, J.K.; Calder, P.C. Omega-6 fatty acids and inflammation. Prostaglandins Leukot. Essent. Fat. Acids 2018, 132, 41–48. [Google Scholar] [CrossRef] [Green Version]
- Simopoulos, A.P. The importance of the omega-6/omega-3 fatty acid ratio in cardiovascular disease and other chronic diseases. Exp. Biol. Med. 2008, 233, 674–688. [Google Scholar] [CrossRef]
- Dommels, Y.E.; Haring, M.M.; Keestra, N.G.; Alink, G.M.; van Bladeren, P.J.; van Ommen, B. The role of cyclooxygenase in n-6 and n-3 polyunsaturated fatty acid mediated effects on cell proliferation, PGE(2) synthesis and cytotoxicity in human colorectal carcinoma cell lines. Carcinogenesis 2003, 24, 385–392. [Google Scholar] [CrossRef] [Green Version]
- Tabolacci, C.; Lentini, A.; Provenzano, B.; Gismondi, A.; Rossi, S.; Beninati, S. Similar antineoplastic effects of nimesulide, a selective COX-2 inhibitor, and prostaglandin E1 on B16-F10 murine melanoma cells. Melanoma Res. 2010, 20, 273–279. [Google Scholar] [CrossRef]
- Levin, G.; Duffin, K.L.; Obukowicz, M.G.; Hummert, S.L.; Fujiwara, H.; Needleman, P.; Raz, A. Differential metabolism of dihomo-gamma-linolenic acid and arachidonic acid by cyclo-oxygenase-1 and cyclo-oxygenase-2: Implications for cellular synthesis of prostaglandin E1 and prostaglandin E2. Biochem. J. 2002, 365, 489–496. [Google Scholar] [CrossRef] [Green Version]
- Fan, Y.Y.; Chapkin, R.S. Importance of dietary gamma-linolenic acid in human health and nutrition. J. Nutr. 1998, 128, 1411–1414. [Google Scholar] [CrossRef] [Green Version]
- Li, J.H.; Yang, P.; Li, A.L.; Wang, Y.; Ke, Y.N.; Li, X.L. Cardioprotective effect of liposomal prostaglandin E1 on a porcine model of myocardial infarction reperfusion no-reflow. J. Zhejiang Univ. Sci. B 2011, 12, 638–643. [Google Scholar] [CrossRef] [Green Version]
- Gupta, V.; Davis, M.; Hope-Weeks, L.J.; Ahsan, F. PLGA microparticles encapsulating prostaglandin E1-hydroxypropyl-β-cyclodextrin (PGE1-HPβCD) complex for the treatment of pulmonary arterial hypertension (PAH). Pharm. Res. 2011, 28, 1733–1749. [Google Scholar] [CrossRef]
- Williams, C.D.; Whitley, B.M.; Hoyo, C.; Grant, D.J.; Iraggi, J.D.; Newman, K.A.; Gerber, L.; Taylor, L.A.; McKeever, M.G.; Freedland, S.J. A high ratio of dietary n-6/n-3 polyunsaturated fatty acids is associated with increased risk of prostate cancer. Nutr. Res. 2011, 31, 1–8. [Google Scholar] [CrossRef]
- Dooper, M.M.; van Riel, B.; Graus, Y.M.; M’Rabet, L. Dihomo-gamma-linolenic acid inhibits tumour necrosis factor-alpha production by human leucocytes independently of cyclooxygenase activity. Immunology 2003, 110, 348–357. [Google Scholar] [CrossRef]
- Watanabe, N.; Masubuchi, D.; Itoh, M.; Teradu, S.; Yazawa, H.; Uemura, H. Oral administration of whole dihomo-γ-linolenic acid-producing Saccharomyces cerevisiae suppresses cutaneous inflammatory responses induced by croton oil application in mice. Appl. Microbiol. Biotechnol. 2014, 98, 8697–8706. [Google Scholar] [CrossRef]
- Wang, X.; Lin, H.; Gu, Y. Multiple roles of dihomo-γ-linolenic acid against proliferation diseases. Lipids Health Dis. 2012, 11, 25. [Google Scholar] [CrossRef] [Green Version]
- Mustonen, A.M.; Nieminen, P. Dihomo-γ-Linolenic Acid (20:3n-6)-Metabolism, Derivatives, and Potential Significance in Chronic Inflammation. Int. J. Mol. Sci. 2023, 24, 2116. [Google Scholar] [CrossRef]
- Sergeant, S.; Rahbar, E.; Chilton, F.H. Gamma-linolenic acid, Dihommo-gamma linolenic, Eicosanoids and Inflammatory Processes. Eur. J. Pharmacol. 2016, 785, 77–86. [Google Scholar] [CrossRef] [Green Version]
- Jamal, G.A. Pathogenesis of diabetic neuropathy: The role of the n-6 essential fatty acids and their eicosanoid derivatives. Diabet. Med. 1990, 7, 574–579. [Google Scholar] [CrossRef]
- Zurier, R.B.; Rossetti, R.G.; Jacobson, E.W.; DeMarco, D.M.; Liu, N.Y.; Temming, J.E.; White, B.M.; Laposata, M. gamma-Linolenic acid treatment of rheumatoid arthritis. A randomized, placebo-controlled trial. Arthritis Rheum. 1996, 39, 1808–1817. [Google Scholar] [CrossRef]
- van Gool, C.J.; Thijs, C.; Henquet, C.J.; van Houwelingen, A.C.; Dagnelie, P.C.; Schrander, J.; Menheere, P.P.; van den Brandt, P.A. Gamma-linolenic acid supplementation for prophylaxis of atopic dermatitis--a randomized controlled trial in infants at high familial risk. Am. J. Clin. Nutr. 2003, 77, 943–951. [Google Scholar] [CrossRef] [Green Version]
- Kanda, N.; Hoashi, T.; Saeki, H. Nutrition and Atopic Dermatitis. J. Nippon. Med. Sch. 2021, 88, 171–177. [Google Scholar] [CrossRef]
- Takai, S.; Jin, D.; Kawashima, H.; Kimura, M.; Shiraishi-Tateishi, A.; Tanaka, T.; Kakutani, S.; Tanaka, K.; Kiso, Y.; Miyazaki, M. Anti-atherosclerotic effects of dihomo-gamma-linolenic acid in ApoE-deficient mice. J. Atheroscler. Thromb. 2009, 16, 480–489. [Google Scholar] [CrossRef] [Green Version]
- Ouchi, S.; Miyazaki, T.; Shimada, K.; Sugita, Y.; Shimizu, M.; Murata, A.; Kato, T.; Aikawa, T.; Suda, S.; Shiozawa, T.; et al. Decreased circulating dihomo-gamma-linolenic acid levels are associated with total mortality in patients with acute cardiovascular disease and acute decompensated heart failure. Lipids Health Dis. 2017, 16, 150. [Google Scholar] [CrossRef] [Green Version]
- Nilsen, D.W.T.; Myhre, P.L.; Kalstad, A.; Schmidt, E.B.; Arnesen, H.; Seljeflot, I. Serum Levels of Dihomo-Gamma (γ)-Linolenic Acid (DGLA) Are Inversely Associated with Linoleic Acid and Total Death in Elderly Patients with a Recent Myocardial Infarction. Nutrients 2021, 13, 3475. [Google Scholar] [CrossRef]
- Kawashima, H.; Akimoto, K.; Higashiyama, K.; Fujikawa, S.; Shimizu, S. Industrial production of dihomo-γ-linolenic acid by a Δ5 desaturase-defective mutant of Mortierella alpina 1S-4 Fungus. J. Am. Oil Chem. Soc. 2000, 77, 1135. [Google Scholar] [CrossRef]
- Kawashima, H.; Tateishi, N.; Shiraishi, A.; Teraoka, N.; Tanaka, T.; Tanaka, A.; Matsuda, H.; Kiso, Y. Oral administration of dihomo-gamma-linolenic acid prevents development of atopic dermatitis in NC/Nga mice. Lipids 2008, 43, 37–43. [Google Scholar] [CrossRef]
- Amagai, Y.; Oida, K.; Matsuda, A.; Jung, K.; Kakutani, S.; Tanaka, T.; Matsuda, K.; Jang, H.; Ahn, G.; Xia, Y.; et al. Dihomo-γ-linolenic acid prevents the development of atopic dermatitis through prostaglandin D1 production in NC/Tnd mice. J. Dermatol. Sci. 2015, 79, 30–37. [Google Scholar] [CrossRef]
- Kakutani, S.; Kawashima, H.; Tanaka, T.; Shiraishi-Tateishi, A.; Kiso, Y. Uptake of dihomo-gamma-linolenic acid by murine macrophages increases series-1 prostaglandin release following lipopolysaccharide treatment. Prostaglandins Leukot. Essent. Fat. Acids 2010, 83, 23–29. [Google Scholar] [CrossRef]
- Flower, R.J.; Kingston, W.P. Proceedings: Prostaglandin D1 inhibits the increase in vascular permeability in rat skin produced by prostaglandin E1, E2 and D2. Br. J. Pharmacol. 1975, 55, 239–240. [Google Scholar] [CrossRef]
- Iversen, L.; Fogh, K.; Kragballe, K. Effect of dihomogammalinolenic acid and its 15-lipoxygenase metabolite on eicosanoid metabolism by human mononuclear leukocytes in vitro: Selective inhibition of the 5-lipoxygenase pathway. Arch. Dermatol. Res. 1992, 284, 222–226. [Google Scholar] [CrossRef]
- Santoli, D.; Zurier, R.B. Prostaglandin E precursor fatty acids inhibit human IL-2 production by a prostaglandin E-independent mechanism. J. Immunol. 1989, 143, 1303–1309. [Google Scholar] [CrossRef]
- Zurier, R.B.; Rossetti, R.G.; Seiler, C.M.; Laposata, M. Human peripheral blood T lymphocyte proliferation after activation of the T cell receptor: Effects of unsaturated fatty acids. Prostaglandins Leukot. Essent. Fat. Acids 1999, 60, 371–375. [Google Scholar] [CrossRef]
- Okubo, K.; Kurono, Y.; Fujieda, S.; Ogino, S.; Uchio, E.; Odajima, H.; Takenaka, H.; Baba, K. Japanese guideline for allergic rhinitis. Allergol. Int. 2011, 60, 171–189. [Google Scholar] [CrossRef] [Green Version]
- Larché, M.; Akdis, C.A.; Valenta, R. Immunological mechanisms of allergen-specific immunotherapy. Nat. Rev. Immunol. 2006, 6, 761–771. [Google Scholar] [CrossRef]
- Brożek, J.L.; Bousquet, J.; Agache, I.; Agarwal, A.; Bachert, C.; Bosnic-Anticevich, S.; Brignardello-Petersen, R.; Canonica, G.W.; Casale, T.; Chavannes, N.H.; et al. Allergic Rhinitis and its Impact on Asthma (ARIA) guidelines-2016 revision. J. Allergy Clin. Immunol. 2017, 140, 950–958. [Google Scholar] [CrossRef] [Green Version]
- Thyssen, J.P.; Vestergaard, C.; Deleuran, M.; de Bruin-Weller, M.S.; Bieber, T.; Taieb, A.; Seneschal, J.; Cork, M.J.; Paul, C.; Flohr, C.; et al. European Task Force on Atopic Dermatitis (ETFAD): Treatment targets and treatable traits in atopic dermatitis. J. Eur. Acad. Dermatol. Venereol. 2020, 34, e839–e842. [Google Scholar] [CrossRef]
- Kernoff, P.B.; Willis, A.L.; Stone, K.J.; Davies, J.A.; McNicol, G.P. Antithrombotic potential of dihomo-gamma-linolenic acid in man. Br. Med. J. 1977, 2, 1441–1444. [Google Scholar] [CrossRef] [Green Version]
- Teraoka, N.; Kawashima, H.; Shiraishi-Tateishi, A.; Tanaka, T.; Nakamura, J.; Kakutani, S.; Kiso, Y. Oral supplementation with dihomo-gamma-linolenic acid-enriched oil altered serum fatty acids in healthy men. Biosci. Biotechnol. Biochem. 2009, 73, 1453–1455. [Google Scholar] [CrossRef]
- Tanaka, T.; Kakutani, S.; Horikawa, C.; Kawashima, H.; Kiso, Y. Oral supplementation with dihomo-γ-linolenic acid (DGLA)-enriched oil increases serum DGLA content in healthy adults. Lipids 2012, 47, 643–646. [Google Scholar] [CrossRef]
- NAME Committees. Practical Guideline for the Management of Allergic Rhinitis in Japan, 2016 ed.; NAME Committees: Washington, DC, USA, 2016. (In Japanese)
- NAME Committees. Practical Guideline for the Management of Allergic Rhinitis in Japan, 2020 ed.; NAME Committees: Washington, DC, USA, 2020. (In Japanese)
- Okuda, M.; Ohkubo, K.; Goto, M.; Okamoto, H.; Konno, A.; Baba, K.; Ogino, S.; Enomoto, M.; Imai, T.; So, N.; et al. Comparative study of two Japanese rhinoconjunctivitis quality-of-life questionnaires. Acta Otolaryngol. 2005, 125, 736–744. [Google Scholar] [CrossRef]
- Okuda, M.; Okubo, K.; Gotoh, M.; Okamoto, Y.; Imano, A.; Baba, K.; Ogino, T.; Ishikawa, K.; Takenata, Y.; So, N.; et al. Standard questionnaire for QOL of Japanese patients with allergic rhinitis. Arerugi 2003, 52 (Suppl. S1), 21–56. [Google Scholar]
- Brown, A.J.; Pang, E.; Roberts, D.C. Persistent changes in the fatty acid composition of erythrocyte membranes after moderate intake of n-3 polyunsaturated fatty acids: Study design implications. Am. J. Clin. Nutr. 1991, 54, 668–673. [Google Scholar] [CrossRef] [PubMed]
- Miyajima, T.; Tsujino, T.; Saito, K.; Yokoyama, M. Effects of eicosapentaenoic acid on blood pressure, cell membrane fatty acids, and intracellular sodium concentration in essential hypertension. Hypertens. Res. 2001, 24, 537–542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suzuki, N.B.A.; Kakinuma, T.; Sano, Y.; Tanaka, M.; Ouchi, S.; Watanabe, T.; Yamamoto, K. A novel dietary questionnaire: The Calorie and Nutrition Diary (CAND). New Food Indust. 2019, 61, 721–732. [Google Scholar]
- Takano, H.; Osakabe, N.; Sanbongi, C.; Yanagisawa, R.; Inoue, K.; Yasuda, A.; Natsume, M.; Baba, S.; Ichiishi, E.; Yoshikawa, T. Extract of Perilla frutescens enriched for rosmarinic acid, a polyphenolic phytochemical, inhibits seasonal allergic rhinoconjunctivitis in humans. Exp. Biol. Med. 2004, 229, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, T. Lipid mediators in health and disease: Enzymes and receptors as therapeutic targets for the regulation of immunity and inflammation. Annu. Rev. Pharmacol. Toxicol. 2009, 49, 123–150. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, N.; Teradu, S.; Ohtani, M.; Uemura, H. Oral administration of whole dihomo-γ-linolenic acid-producing yeast suppresses allergic contact dermatitis in mice. Biosci. Biotechnol. Biochem. 2020, 84, 208–215. [Google Scholar] [CrossRef]
- Yamaoka, K.A.; Dugas, B.; Paul-Eugene, N.; Mencia-Huerta, J.M.; Braquet, P.; Kolb, J.P. Leukotriene B4 enhances IL-4-induced IgE production from normal human lymphocytes. Cell Immunol. 1994, 156, 124–134. [Google Scholar] [CrossRef]
- Sakurai, E.; Gunji, E.; Iizuka, Y.; Hikichi, N.; Maeyama, K.; Watanabe, T. In vivo microdialysis measurement of histamine in rat blood effects of compound 48/80 and histamine receptor antagonists. J. Pharmacol. Toxicol. Methods 1993, 29, 105–109. [Google Scholar] [CrossRef]





| Variable | DGLA Group (n = 18) | Placebo Group (n = 15) | p Value |
|---|---|---|---|
| n (Men/Women) | 7/11 | 4/11 | 0.712 |
| Age (years) | 48.1 ± 11.6 | 49.7 ± 13.0 | 0.698 |
| Weight (kg) | 59.3 ± 14.5 | 61.3 ± 24.5 | 0.772 |
| Height (cm) | 163.6 ± 9.8 | 160.3 ± 7.1 | 0.290 |
| BMI (kg/m2) | 21.9 ± 3.4 | 23.4 ± 7.2 | 0.438 |
| Variable | Baseline | Week 15 | ||||
|---|---|---|---|---|---|---|
| DGLA Group (n = 18) | Placebo Group (n = 15) | p Value | DGLA Group (n = 18) | Placebo Group (n = 15) | p Value | |
| DGLA (µg/mL) | 42.9 ± 20.9 | 44.9 ± 18.5 | 0.776 | 62.1 ± 19.5 *$$$ | 49.3 ± 15.7 | 0.011 |
| AA (µg/mL) | 211.8 ± 64.4 | 201.1 ± 46.7 | 0.593 | 228.3 ± 59.6 | 200.1 ± 30.7 | 0.106 |
| EPA (µg/mL) | 47.1 ± 28.6 | 47.8 ± 36.8 | 0.948 | 40.0 ± 24.3 | 59.0 ± 56.8 | 0.118 |
| DHA (µg/mL) | 104.8 ± 38.1 | 106.0 ± 37.3 | 0.930 | 107.7 ± 52.5 | 115.1 ± 34.1 | 0.500 |
| Variable | Time | DGLA Group (n = 18) | Placebo Group (n = 15) | p Value |
|---|---|---|---|---|
| Energy (kcal) | Baseline | 2345.9 ± 837.2 | 2309.2 ± 433.0 | 0.872 |
| Week 12 | 2725.1 ± 1201.0 | 2568.8 ± 727.9 | 0.649 | |
| Week 15 | 2773.7 ± 1643.3 | 2778.0 ± 760.7 | 0.992 | |
| Protein (g) | Baseline | 95.3 ± 46.9 | 90.7 ± 17.6 | 0.702 |
| Week 12 | 108.3 ± 49.3 | 101.2 ± 31.8 | 0.624 | |
| Week 15 | 105.4 ± 48.6 | 110.5 ± 32.1 | 0.722 | |
| Fat (g) | Baseline | 85.5 ± 43.2 | 78.9 ± 19.5 | 0.564 |
| Week 12 | 95.6 ± 49.0 | 89.6 ± 29.4 | 0.667 | |
| Week 15 | 96.7 ± 49.7 | 101.1 ± 34.1 | 0.768 | |
| Carbonate (g) | Baseline | 284.5 ± 82.9 | 294.6 ± 63.5 | 0.696 |
| Week 12 | 342.8 ± 169.1 | 326.6 ± 89.1 | 0.727 | |
| Week 15 | 358.2 ± 269.1 | 341.3 ± 96.6 | 0.806 | |
| Omega-6 fatty acid (g) | Baseline | 14.4 ± 9.0 | 14.0 ± 3.8 | 0.875 |
| Week 12 | 16.3 ± 8.7 | 15.3 ± 6.7 | 0.724 | |
| Week 15 | 16.8 ± 7.2 | 18.3 ± 7.4 | 0.562 | |
| DGLA (mg) | Baseline | 55.6 ± 29.7 | 47.9 ± 13.3 | 0.333 |
| Week 12 | 60.9 ± 27.9 | 56.7 ± 22.8 | 0.636 | |
| Week 15 | 59.7 ± 29.8 | 61.1 ± 20.4 | 0.874 |
| Variable | Baseline | Week 12 | ||||
|---|---|---|---|---|---|---|
| DGLA Group (n = 18) | Placebo Group (n = 15) | p Value | DGLA Group (n = 18) | Placebo Group (n = 15) | p Value | |
| Cedar-specific IgE (UA/mL) | 10.8 ± 15.9 | 14.3 ± 19.5 | 0.579 | 14.6 ± 20.2 $$$ | 16.1 ± 24.1 $$$ | 0.850 |
| Cypress-specific IgE (UA/mL) | 1.3 ± 1.6 | 2.8 ± 3.2 | 0.097 | 1.8 ± 2.5 $$$ | 3.2 ± 3.9 $$$ | 0.235 |
| House dust-specific IgE (UA/mL) | 2.7 ± 4.1 | 8.6 ± 25.5 | 0.343 | 2.7 ± 4.2 $$$ | 8.1 ± 24.3 $$$ | 0.345 |
| Mite-specific IgE (UA/mL) | 2.9 ± 4.3 | 8.5 ± 25.5 | 0.361 | 3.4 ± 5.3 $$$ | 8.8 ± 25.4 $$$ | 0.385 |
| Nonspecific IgE (IU/mL) | 158.5 ± 183.3 | 140.1 ± 214.6 | 0.792 | 176.6 ± 188.6 | 146.3 ± 235.5 | 0.583 |
| Histamine (ng/mL) | 1.1 ± 0.9 | 0.8 ± 0.8 | 0.347 | 1.4 ± 1.2 | 1.4 ± 1.2 | 0.876 |
| IL-6 (pg/mL) | 1.3 ± 0.7 | 1.4 ± 1.3 | 0.639 | 1.5 ± 0.9 $$$ | 1.3 ± 0.8 $$$ | 0.373 |
| Variable | Baseline | Week 12 | ||||
|---|---|---|---|---|---|---|
| DGLA Group (n = 18) | Placebo Group (n = 15) | p Value | DGLA Group (n = 18) | Placebo Group (n = 15) | p Value | |
| Nasal eosinophil (−) | 15 | 12 | 1.000 | 13 | 11 | 1.000 |
| Nasal eosinophil (±) | 0 | 0 | N.A. | 0 | 0 | N.A. |
| Nasal eosinophil (+) | 2 | 3 | 0.639 | 2 | 1 | 1.000 |
| Nasal eosinophil (2+) | 0 | 0 | N.A. | 3 | 3 | 1.000 |
| Nasal eosinophil (3+) | 1 | 0 | 1.000 | 0 | 0 | N.A. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yokoi, K.; Yanagimoto, K.; Hayamizu, K. Supplementation of Dihomo-γ-Linolenic Acid for Pollen-Induced Allergic Symptoms in Healthy Subjects: A Randomized, Double-Blinded, Placebo-Controlled Trial. Nutrients 2023, 15, 3465. https://doi.org/10.3390/nu15153465
Yokoi K, Yanagimoto K, Hayamizu K. Supplementation of Dihomo-γ-Linolenic Acid for Pollen-Induced Allergic Symptoms in Healthy Subjects: A Randomized, Double-Blinded, Placebo-Controlled Trial. Nutrients. 2023; 15(15):3465. https://doi.org/10.3390/nu15153465
Chicago/Turabian StyleYokoi, Kaori, Kenichi Yanagimoto, and Kohsuke Hayamizu. 2023. "Supplementation of Dihomo-γ-Linolenic Acid for Pollen-Induced Allergic Symptoms in Healthy Subjects: A Randomized, Double-Blinded, Placebo-Controlled Trial" Nutrients 15, no. 15: 3465. https://doi.org/10.3390/nu15153465
APA StyleYokoi, K., Yanagimoto, K., & Hayamizu, K. (2023). Supplementation of Dihomo-γ-Linolenic Acid for Pollen-Induced Allergic Symptoms in Healthy Subjects: A Randomized, Double-Blinded, Placebo-Controlled Trial. Nutrients, 15(15), 3465. https://doi.org/10.3390/nu15153465




