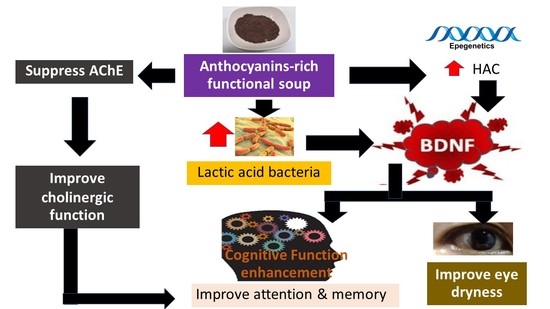
A Randomized, Double-Blind, Placebo-Controlled Study of an Anthocyanin-Rich Functional Ingredient on Cognitive Function and Eye Dryness in Late Adulthood Volunteers: Roles of Epigenetic and Gut Microbiome Modulations
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of the Functional Soup
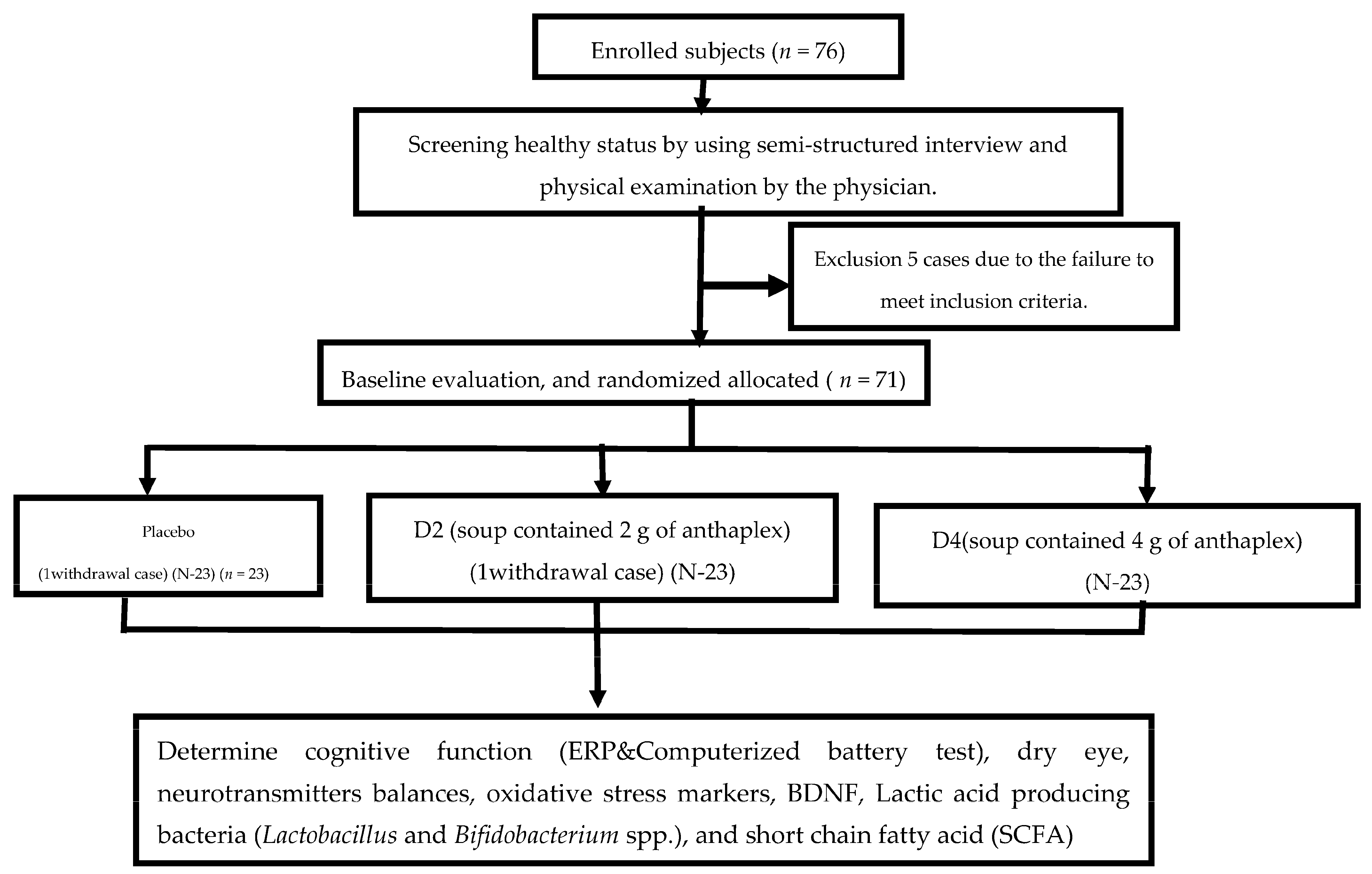
2.2. Study Design and Population
2.2.1. Sample Size
2.2.2. Participants
2.2.3. Study Design
2.3. Outcome Measurement
2.3.1. Cognitive Function Assessment
2.3.2. Working Memory Assessment
2.3.3. Biochemical Assays
Oxidative Stress Marker Assessment
Assessments of the Neurotransmitter Disturbances
Assessment of Brain-Derived Neurotrophic Factor (BDNF), Histone Acetylase, Histone Deacetylase, and DNA Methyltransferase
2.4. Assessment of Eye Dryness
2.5. Determine of Enumeration of Lactobacillus spp. and Bifidobacterium spp.
2.6. Short-Chain Fatty Acid (SCFA) Extraction and Determination
2.7. Statistical Analysis
3. Results
3.1. Demographic Data of Subjects
3.2. Changes in Cognition
3.3. Changes of Eye Dryness Severity
3.4. Changes of Neurotransmitters and Brain-Derived Neurotrophic Factor (BDNF)
3.5. Changes in pH, Amount of Lactic Acid Producing Bacteria, Lactobacillus spp., and Bifidobacterium spp. in Feces
3.6. Changes of Short-Chain Fatty Acid
3.7. Changes in Epigenetic Mechanism via Histone Acetyltransferase (HAT), Histone Deacetylase (HDAC), and DNA Methyltransferase (DNMT)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hilliam, M. Functional food how big is the market? World Food Ingred. 2000, 12, 50–52. [Google Scholar]
- Ali, A.; Rahut, D.B. Healthy foods as proxy for functional foods: Consumers’ awareness, perception, and demand for natural functional foods in Pakistan. Int. J. Food Sci. 2019, 2019, 6390650. [Google Scholar] [CrossRef] [PubMed]
- Nisar, B.; Sultan, A.; Rubab, S.L. Comparison of medicinally important natural products versus synthetic drugs-a short commentary. Nat. Prod. Chem. Res. 2017, 6, 2. [Google Scholar] [CrossRef]
- Rasoanaivo, P.; Wright, C.W.; Willcox, M.L.; Gilbert, B. Whole plant extracts versus single compounds for the treatment of malaria: Synergy and positive interactions. Malar. J. 2011, 10 (Suppl. 1), S4. [Google Scholar] [CrossRef] [Green Version]
- Ali, H.; Ali, D.; Almutairi, B.O.; Kumar, G.; Karga, G.A.; Masi, C.; Sundramurthy, V.P. Synergistic effect of conventional medicinal herbs against different pharmacological activity. BioMed Res. Int. 2022, 2022, 7337261. [Google Scholar] [CrossRef]
- Kirisattayakul, W.; Wattanathorn, J.; Iamsaard, S.; Jittiwat, J.; Suriharn, B.; Lertrat, K. Neuroprotective and memory-enhancing effect of the combined extract of purple waxy corn cob and pandan in ovariectomized rats. Oxid. Med. Cell. Longev. 2017, 2017, 5187102. [Google Scholar] [CrossRef] [Green Version]
- Wattanathorn, J.; Kirisattayakul, W.; Suriharn, B.; Lertrat, K. Functional drink containing the extracts of purple corn cob and pandan leaves, the novel cognitive enhancer, increases spatial memory and hippocampal neuron density through the improvement of extracellular signal regulated protein kinase expression, cholinergic function, and oxidative status in ovariectomized rats. Rejuvenation Res. 2018, 21, 431–441. [Google Scholar]
- Kaewkaen, P.; Tong-Un, T.; Wattanathorn, J.; Muchimapura, S.; Kaewrueng, W.; Wongcharoenwanakit, S. Mulberry fruit extract protects against memory impairment and hippocampal damage in animal model of vascular dementia. Evid. Based Complement. Altern. Med. 2012, 2012, 263520. [Google Scholar] [CrossRef] [Green Version]
- Shin, S.K.; Yoo, J.M.; Li, F.Y.; Baek, S.Y.; Kim, M.R. Mulberry fruit improves memory in scopolamine-treated mice: Role of cholinergic function, antioxidant system, and TrkB/Akt signaling. Nutr. Neurosci. 2021, 24, 940–950. [Google Scholar] [CrossRef]
- Thukham-Mee, W.; Wattanathorn, J.; Paholpak, P.; Ransikachi, P.; Piyavhatkul, N. The Positive modulation effect of a 6-week consumption of an anthocyanin-rich mulberry milk on working memory, cholinergic, and monoaminergic functions in healthy working-age adults. Oxid. Med. Cell. Longev. 2021, 2021, 5520059. [Google Scholar] [CrossRef]
- Thukham-Mee, W.; Wattanathorn, J.; Kirisattayakul, W.; Wannanon, P. Effect of single administration of mulberry milk on the cognitive function of 6–12-year-old children: Results from a randomized, placebo-controlled, crossover study. Oxid. Med. Cell. Longev. 2020, 2020, 6123759. [Google Scholar] [CrossRef] [PubMed]
- Vauzour, D.; Rendeiro, C.; D’Amato, A.; Waffo-Téguo, P.; Richard, T.; Mérillon, J.M.; Pontifex, M.G.; Connell, E.; Müller, M.; Butler, L.T.; et al. Anthocyanins promote learning through modulation of synaptic plasticity related proteins in an animal model of ageing. Antioxidants 2021, 10, 1235. [Google Scholar] [CrossRef] [PubMed]
- Kawvised, S.; Wattanathorn, J.; Thukham-Mee, W. Neuroprotective and cognitive-enhancing effects of microencapsulation of mulberry fruit extract in animal model of menopausal women with Metabolic syndrome. Oxid. Med. Cell. Longev. 2017, 2017, 2962316. [Google Scholar] [CrossRef] [PubMed]
- Nomi, Y.; Iwasaki-Kurashige, K.; Matsumoto, H. Therapeutic effects of anthocyanins for vision and eye health. Molecules 2019, 24, 3311. [Google Scholar] [CrossRef] [Green Version]
- Kizawa, Y.; Sekikawa, T.; Kageyama, M.; Tomobe, H.; Kobashi, R.; Yamada, T. Effects of anthocyanin, astaxanthin, and lutein on eye functions: A randomized, double-blind, placebo-controlled study. J. Clin. Biochem. Nutr. 2021, 69, 77–90. [Google Scholar] [CrossRef] [PubMed]
- Thiraphatthanavong, P.; Wattanathorn, J.; Muchimapura, S.; Wipawee, T.M.; Wannanon, P.; Terdthai, T.U.; Suriharn, B.; Lertrat, K. Preventive effect of Zea mays L. (purple waxy corn) on experimental diabetic cataract. BioMed Res. Int. 2014, 2014, 507435. [Google Scholar] [CrossRef] [Green Version]
- Shi, H.; Ge, X.; Ma, X.; Zheng, M.; Cui, X.; Pan, W.; Zheng, P.; Yang, X.; Zhang, P.; Hu, M.; et al. A fiber-deprived diet causes cognitive impairment and hippocampal microglia-mediated synaptic loss through the gut microbiota and metabolites. Microbiome 2021, 9, 223. [Google Scholar] [CrossRef]
- Prokopidis, K.; Giannos, P.; Ispoglou, T.; Witard, O.C.; Isanejad, M. Dietary fiber intake is associated with cognitive function in older adults: Data from the National Health and Nutrition Examination Survey. Am. J. Med. 2022, 135, e257–e262. [Google Scholar] [CrossRef]
- Yamagishi, K.; Maruyama, K.; Ikeda, A.; Nagao, M.; Noda, H.; Umesawa, M.; Hayama-Terada, M.; Muraki, I.; Okada, C.; Tanaka, M.; et al. Dietary fiber intake and risk of incident disabling dementia: The Circulatory Risk in Communities Study. Nutr. Neurosci. 2022, 6, 148–155. [Google Scholar] [CrossRef]
- Ganesan, S.; Raman, R.; Kulothungan, V.; Sharma, T. Influence of dietary-fibre intake on diabetes and diabetic retinopathy: Sankara Nethralaya-Diabetic Retinopathy Epidemiology and Molecular Genetic Study (report 26). Clin. Exp. Ophthalmol. 2012, 40, 288–294. [Google Scholar] [CrossRef]
- Zhang, G.; Sun, X.; Yuan, T.; Guo, C.; Zhou, Z.; Wang, L.; Dou, G. Certain detary nutrients Reduce the risk of eye affliction/retinopathy in individuals with diabetes: National Health and Nutrition Examination Survey, 2003–2018. Int. J. Environ. Res. Public Health 2022, 19, 12173. [Google Scholar] [CrossRef] [PubMed]
- Hair, R.; Sakaki, J.R.; Chun, O.K. Anthocyanins, microbiome and health benefits in aging. Molecules 2021, 26, 537. [Google Scholar] [CrossRef] [PubMed]
- Marques, C.; Fernandes, I.; Meireles, M.; Faria, A.; Spencer, J.P.E.; Mateus, N.; Calhau, C. Gut microbiota modulation accounts for the neuroprotective properties of anthocyanins. Sci. Rep. 2018, 8, 11341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kan, J.; Wu, F.; Wang, F.; Zheng, J.; Cheng, J.; Li, Y.; Yang, Y.; Du, J. Phytonutrients: Sources, bioavailability, interaction with gut microbiota, and their impacts on human health. Front. Nutr. 2022, 9, 960309. [Google Scholar] [CrossRef]
- Berding, K.; Carbia, C.; Cryan, J.F. Going with the grain: Fiber, cognition, and the microbiota-gut-brain-axis. Exp. Biol. Med. 2021, 246, 796–811. [Google Scholar] [CrossRef]
- La Torre, D.; Verbeke, K.; Dalile, B. Dietary fibre and the gut–brain axis: Microbiota-dependent and independent mechanisms of action. Gut Microbiome 2021, 2, E3. [Google Scholar] [CrossRef]
- Floyd, J.L.; Grant, M.B. The Gut-Eye Axis: Lessons learned from murine models. Ophthalmol. Ther. 2020, 9, 499–513. [Google Scholar] [CrossRef]
- Napolitano, P.; Filippelli, M.; Davinelli, S.; Bartollino, S.; dell’Omo, R.; Costagliola, C. Influence of gut microbiota on eye diseases: An overview. Ann. Med. 2021, 53, 750–761. [Google Scholar] [CrossRef]
- Choi, S.-J.; Jeon, H.; Lee, C.U.; Yoon, S.H.; Bae, S.K.; Chin, Y.-W.; Yoon, K.D. Isolation and development of quantification method for cyanidin-3-glucoside and cyanidin-3-rutinoside in mulberry fruit by high-performance countercurrent chromatography and high-performance liquid chromatography. Nat. Prod. Sci. 2015, 21, 20–24. [Google Scholar]
- Chuntakaruk, H.; Kongtawelert, P.; Pothacharoen, P. Chondroprotective effects of purple corn anthocyanins on advanced glycation end products induction through suppression of NF-κB and MAPK signaling. Sci. Rep. 2021, 11, 1895. [Google Scholar] [CrossRef]
- Pascual-Teresa, S.d.; Santos-Buelga, C.; Rivas-Gonzalo, J.C. LC–MS analysis of anthocyanins from purple corn cob. J. Sci. Food Agric. 2002, 82, 1003–1006. [Google Scholar] [CrossRef]
- Lao, F.; Giusti, M.M. Quantification of Purple Corn (Zea mays L.) Anthocyanins Using Spectrophotometric and HPLC Approaches: Method Comparison and Correlation. Food Anal. Methods 2016, 9, 1367–1380. [Google Scholar] [CrossRef]
- Hou, Z.; Qin, P.; Zhang, Y.; Cui, S.; Ren, G. Identification of anthocyanins isolated from black rice (Oryza sativa L.) and their degradation kinetics. Food Res. Int. 2013, 50, 691–696. [Google Scholar] [CrossRef]
- Wattanathorn, J.; Somboonporn, W.; Thukham-Mee, W.; Sungkamnee, S. Memory-Enhancing Effect of 8-Week Consumption of the Quercetin-Enriched Culinary Herbs-Derived Functional Ingredients: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Foods 2022, 11, 2678. [Google Scholar] [CrossRef] [PubMed]
- Berchio, C.; Micali, N. Cognitive assessment using ERP in child and adolescent psychiatry: Difficulties and opportunities. Psychiatry Res. Neuroimaging 2022, 319, 111424. [Google Scholar] [CrossRef] [PubMed]
- Lijffijt, M.; Lane, S.D.; Meier, S.L.; Boutros, N.N.; Burroughs, S.; Steinberg, J.L.; Moeller, F.G.; Swann, A.C. P50, N100, and P200 sensory gating: Relationships with behavioral inhibition, attention, and working memory. Psychophysiology 2009, 46, 1059–1068. [Google Scholar] [CrossRef] [Green Version]
- Pavarini, S.C.I.; Brigola, A.G.; Luchesi, B.M.; Souza, É.N.; Rossetti, E.S.; Fraga, F.J.; Guarisco, L.P.C.; Terassi, M.; Oliveira, N.A.; Hortense, P.; et al. On the use of the P300 as a tool for cognitive processing assessment in healthy aging: A review. Dement. Neuropsychol. 2018, 12, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Choi, K.H.; Kwon, O.S.; Cho, S.J.; Lee, S.; Kang, S.Y.; Ryu, Y.H. Change in the P300 index—A pilot randomized controlled trial of low-frequency electrical stimulation of acupuncture points in middle-aged men and women. BMC Complement. Altern. Med. 2017, 17, 246. [Google Scholar] [CrossRef] [Green Version]
- Wattanathorn, J.; Ohnon, W.; Thukhammee, W.; Muchmapura, S.; Wannanon, P.; Tong-Un, T. Cerebroprotective effect against cerebral ischemia of the combined extract of Oryza sativa and Anethum graveolens in metabolic syndrome Rats. Oxid. Med. Cell. Longev. 2019, 2019, 9658267. [Google Scholar] [CrossRef] [Green Version]
- Srichomphu, P.; Wattanathorn, J.; Thukham-Mee, W.; Muchimapura, S. Anxiety, insomnia, and memory impairment in metabolic syndrome rats are alleviated by the novel functional ingredients from Anacardium occidentale. Antioxidants 2022, 11, 2203. [Google Scholar] [CrossRef]
- Chung, C.C.; Pai, H.H.; Lung, C.; Jia-Hung, C.; Li-Nien, C.; Chien, T.H. Plasma Exosomal brain-derived neurotrophic Ffactor correlated with the postural instability and gait disturbance-related motor symptoms in patients with Parkinson’s Disease. Diagnostics 2020, 10, 684. [Google Scholar] [CrossRef]
- Gilbert, E. The eye signs of vitamin A deficiency. Community Eye Health J. 2013, 26, 66–67. [Google Scholar]
- Messer, E.M. The Pathophysiology, Diagnosis, and Treatment of Dry Eye Disease. Dtsch. Arztebl. Int. 2015, 112, 71–82. [Google Scholar]
- Buck, J.D.; Cleverdon, R.C. The spread plate as a method for the enumeration of marine bacteria 1, 2. Limnol. Oceanogr. 1960, 5, 78–80. [Google Scholar] [CrossRef]
- Ribeiro, W.R.; Vinolo, M.A.R.; Calixto, L.A.; Ferreira, C.M. Use of Gas Chromatography to Quantify Short Chain Fatty Acids in the Serum, Colonic Luminal Content and Feces of mice. Bio-Protocol 2018, 8, e3089. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.H.; Lin, Y. Resistant starch prepared from high-amylose maize starch with citric acid hydrolysis and its simulated fermentation in vitro. Eur. Food Res. Technol. 2009, 228, 1015–1021. [Google Scholar] [CrossRef]
- Bakusic, J.; Ghosh, M.; Polli, A.; Bekaert, B.; Schaufeli, W.; Claes, S.; Godderis, L. Epigenetic perspective on the role of brain-derived neurotrophic factor in burnout. Transl. Psychiatry 2020, 1, 354. [Google Scholar] [CrossRef]
- Bredy, T.W.; Wu, H.; Crego, C.; Zellhoefer, J.; Sun, Y.E.; Barad, M. Histone modifications around individual BDNF gene promoters in prefrontal cortex are associated with extinction of conditioned fear. Learn. Mem. 2007, 14, 268–276. [Google Scholar] [CrossRef] [Green Version]
- Kundakovic, M.; Gudsnuk, K.; Herbstman, J.B.; Tang, D.; Perera, F.P.; Champagne, F.A. DNA methylation of BDNF as a biomarker of early-life adversity. Proc. Natl. Acad. Sci. USA 2015, 112, 6807–6813. [Google Scholar] [CrossRef]
- Thornton, A.R.; Harmer, M.; Lavoie, B.A. Selective attention increases the temporal precision of the auditory N100 event-related potential. Hear. Res. 2007, 230, 73–79. [Google Scholar] [CrossRef]
- Naveh-Benjamin, M.; Guez, J.; Sorek, S. The effects of divided attention on encoding processes in memory: Mapping the locus of interference. Can. J. Exp. Psychol. 2007, 61, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Sano, M.; Rosen, W.; Stern, Y.; Rosen, J.; Mayeux, R. Simple reaction time as a measure of global attention in Alzheimer’s disease. J. Int. Neuropsychol. Soc. 1995, 1, 56–61. [Google Scholar] [CrossRef]
- Saenghong, N.; Wattanathorn, J.; Muchimapura, S.; Tongun, T.; Piyavhatkul, N.; Banchonglikitkul, C.; Kajsongkram, T. Zingiber officinale improves cognitive function of the middle-aged healthy women. Evid. Based Complement. Altern. Med. 2012, 2012, 383062. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peth-Nui, T.; Wattanathorn, J.; Muchimapura, S.; Tong-Un, T.; Piyavhatkul, N.; Rangseekajee, P.; Ingkaninan, K.; Vittaya-Areekul, S. Effects of 12-week Bacopa monnieri consumption on attention, cognitive processing, working memory, and functions of both cholinergic and monoaminergic systems in healthy elderly volunteers. Evid. Based Complement. Altern. Med. 2012, 2012, 606424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miranda, M.; Morici, J.F.; Zanoni, M.B.; Bekinschtein, P. Brain-derived neurotrophic factor: A key molecule for memory in the healthy and the pathological brain. Front. Cell. Neurosci. 2019, 13, 363. [Google Scholar] [CrossRef]
- Mahmoudvand, H.; Sheibani, V.; Keshavarz, H.; Shojaee, S.; Esmaeelpour, K.; Ziaali, N. Acetylcholinesterase inhibitor improves learning and memory impairment induced by Toxoplasma gondii Infection. Iran J. Parasitol. 2016, 11, 177–185. [Google Scholar]
- Colović, M.B.; Krstić, D.Z.; Lazarević-Pašti, T.D.; Bondžić, A.M.; Vasić, V.M. Acetylcholinesterase inhibitors: Pharmacology and toxicology. Curr. Neuropharmacol. 2013, 11, 315–335. [Google Scholar] [CrossRef] [Green Version]
- Sano, K.; Kawashima, M.; Imada, T.; Suzuki, T.; Nakamura, S.; Mimura, M.; Tanaka, K.F.; Tsubota, K. Enriched environment alleviates stress-induced dry-eye through the BDNF axis. Sci. Rep. 2019, 9, 3422. [Google Scholar] [CrossRef] [Green Version]
- Javadi, M.A.; Feizi, S. Dry eye syndrome. J. Ophthalmic Vis. Res. 2011, 6, 192–198. [Google Scholar]
- Kobashi, H.; Kamiya, K.; Sambe, T.; Nakagawa, R. Factors influencing subjective symptoms in dry eye disease. Int. J. Ophthalmol. 2018, 11, 1926–1931. [Google Scholar]
- Seen, S.; Tong, L. Dry eye disease and oxidative stress. Acta Ophthalmol. 2018, 96, e412–e420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- The definition and classification of dry eye disease: Report of the Definition and Classification Subcommittee of the International Dry Eye WorkShop (2007). Ocul. Surf. 2007, 5, 75–92. [CrossRef]
- Moon, J.; Yoon, C.H.; Choi, S.H.; Kim, M.K. Can gut microbiota affect dry eye syndrome? Int. J. Mol. Sci. 2020, 21, 8443. [Google Scholar] [CrossRef] [PubMed]
- Yun, S.W.; Son, Y.H.; Lee, D.Y.; Shin, Y.J.; Han, M.J.; Kim, D.H. Lactobacillus plantarum and Bifidobacterium bifidum alleviate dry eye in mice with exorbital lacrimal gland excision by modulating gut inflammation and microbiota. Food Funct. 2021, 12, 2489–2497. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, E.; Barrett, E.; Grenham, S.; Fitzgerald, P.; Stanton, C.; Ross, R.P.; Quigley, E.M.; Cryan, J.F.; Dinan, T.G. BDNF expression in the hippocampus of maternally separated rats: Does Bifidobacterium breve 6330 alter BDNF levels? Benef. Microbes 2011, 2, 199–207. [Google Scholar] [CrossRef]
- Kim, C.S.; Cha, L.; Sim, M.; Jung, S.; Chun, W.Y.; Baik, H.W.; Shin, D.M. Probiotic supplementation improves cognitive function and mood with changes in gut microbiota in community-dwelling older adults: A randomized, double-blind, placebo-controlled, multicenter trial. J. Gerontol. A Biol. Sci. Med. Sci. 2021, 76, 32–40. [Google Scholar] [CrossRef]
- Zhan, W.; Liao, X.; Xie, R.J.; Tian, T.; Yu, L.; Liu, X.; Liu, J.; Li, P.; Han, B.; Yang, T.; et al. The effects of blueberry anthocyanins on histone acetylation in rat liver fibrosis. Oncotarget 2017, 8, 96761–96773. [Google Scholar] [CrossRef] [Green Version]
- Kuo, H.D.; Wu, R.; Li, S.; Yang, A.Y.; Kong, A.N. Anthocyanin delphinidin prevents neoplastic transformation of mouse skin JB6 P+ Cells: Epigenetic re-activation of Nrf2-ARE pathway. AAPS J. 2019, 21, 83. [Google Scholar] [CrossRef]
- Boto-Ordóñez, M.; Urpi-Sarda, M.; Queipo-Ortuño, M.I.; Tulipani, S.; Tinahones, F.J.; Andres-Lacueva, C. High levels of Bifidobacteria are associated with increased levels of anthocyanin microbial metabolites: A randomized clinical trial. Food Funct. 2014, 5, 1932–1938. [Google Scholar] [CrossRef] [Green Version]
- Schroeder, B.O.; Birchenough, G.M.H.; Ståhlman, M.; Arike, L.; Johansson, M.E.V.; Hansson, G.C.; Bäckhed, F. Bifidobacteria or fiber protects against diet-induced microbiota-mediated colonic mucus deterioration. Cell Host Microbe 2018, 23, 27–40.e7. [Google Scholar] [CrossRef] [Green Version]
- Cernes, R.; Zimlichman, R.; Shargorodsky, M. Arterial elasticity in cardiovascular disease: Focus on hypertension, metabolic syndrome and diabetes. Adv. Cardiol. 2008, 45, 65–81. [Google Scholar] [PubMed]
- Jennings, A.; Welch, A.A.; Fairweather-Tait, S.J.; Kay, C.; Minihane, A.M.; Chowienczyk, P.; Jiang, B.; Cecelja, M.; Spector, T.; Macgregor, A.; et al. Higher anthocyanin intake is associated with lower arterial stiffness and central blood pressure in women. Am. J. Clin. Nutr. 2012, 96, 781–788. [Google Scholar] [CrossRef] [PubMed] [Green Version]
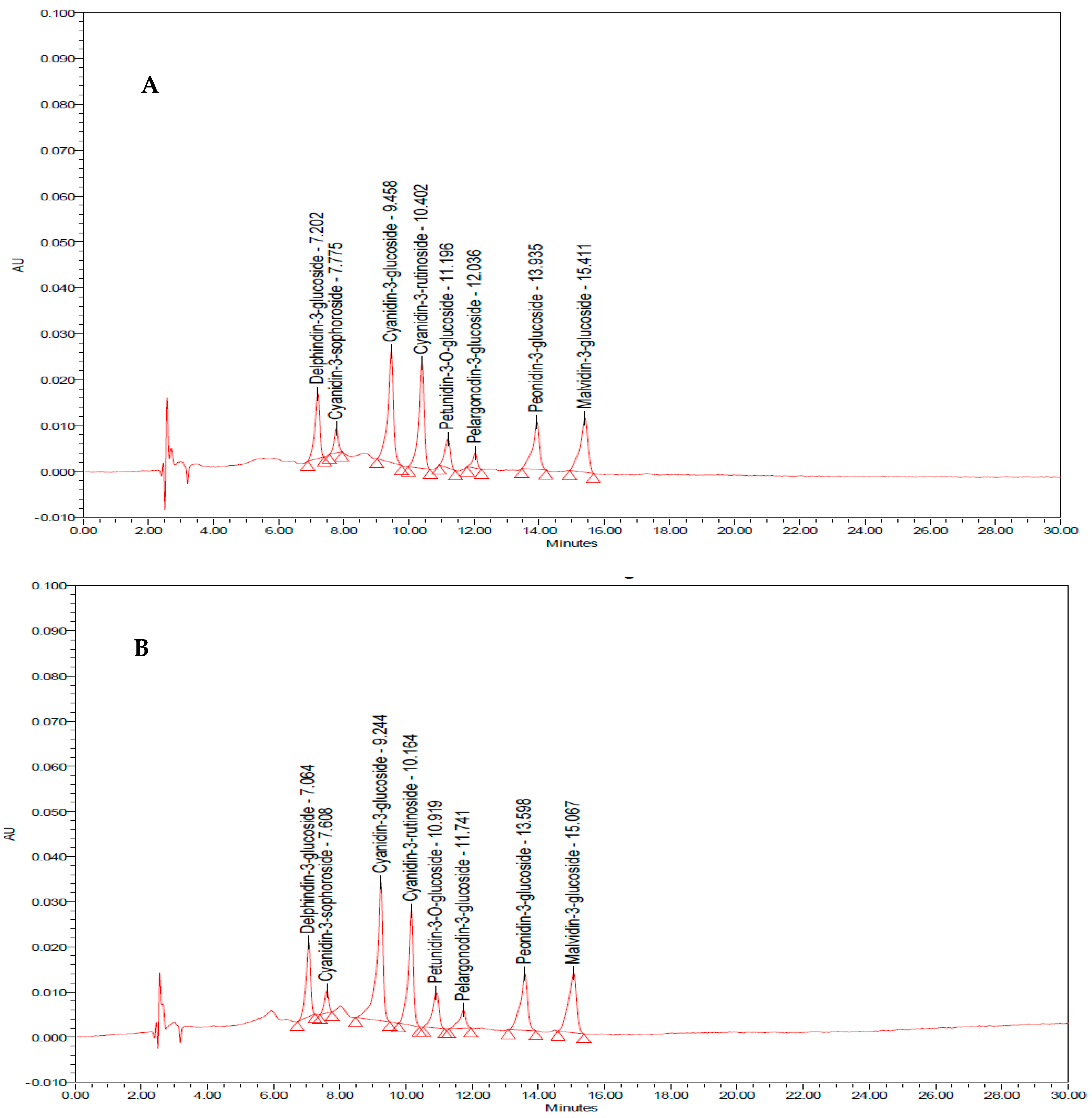
| Substance | Concentration (mg/g Sample) | |
|---|---|---|
| Soup Containing “Anthaplex” 2 g per Serving | Soup Containing “Anthaplex” 4 g per Serving | |
| Delphinidin-3-glucoside | 0.010 ± 0.002 | 0.011 ± 0.001 |
| Cyanidin-3-sophoroside | 0.0130 ± 0.001 | 0.016 ± 0.003 |
| Cyanidin-3-glucoside | 0.016 ± 0.004 | 0.021 ± 0.003 |
| Cyanidin-3-rutinoside | 0.016 ± 0.004 | 0.015 ± 0.001 |
| Petunidin-3-O-beta-D-glucoside | 0.013 ± 0.004 | 0.017 ± 0.004 |
| Pelargondin-3-glucoside | 0.014 ± 0.007 | 0.021 ± 0.003 |
| Peonidin-3-O-glucoside | 0.018 ± 0.002 | 0.022 ± 0.001 |
| Malvidin-3-glucoside | 0.015 ± 0.002 | 0.015 ± 0.001 |
| Dry Eye Severity Level | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| Discomfort, severity, frequency | Mild and/or episodic; occurs under environmental stress | Moderate episodic or chronic stress or no stress | Severe frequent or constant without stress | Severe and/or disabling and constant |
| Visual symptoms | None or episodic mild stress | Annoying and/or activity-limiting episodic | Annoying and/or constant-limiting activity | Constant and/or possibly disabling |
| Conjunctival injection | None to mild | None to mild | +/− | +/+++ |
| Corneal/tear signs | None to mild | Mild debris, ↓ miniscus | Filamentary keratitis, mucus clumping, ↓ tear debris | Filamentary keratitis, mucus clumping, ↓ tear debris, ulceration |
| Lid, meibomian glands | Meibomian gland dysfunction variably present | Meibomian gland dysfunction variably present | Frequent | Trichiasis, keratinization, symblepharon |
| Parameters | Baseline | ||
|---|---|---|---|
| Placebo | D2 g per Day | D4 g per Day | |
| Age (year) | 51.00 ± 0.85 | 50.30 ± 0.90 (p = 0.732) | 51.13 ± 0.82 (p = 0.929) |
| Gender (Male/Female) | 1/22 | 0/23 | 1/22 |
| Body Temperature (°C) | 36.25 ± 0.12 | 36.30 ± 0.08 (p = 0.748) | 36.36 ± 0.08 (p = 0.738) |
| Heart Rate (beats/min) | 72.39 ± 1.34 | 73.47 ± 1.95 (p = 0.361) | 72.91 ± 1.36 (p = 0.668) |
| Respiratory Rate (breaths/min) | 16.82 ± 0.20 | 16.73 ± 0.20 (p = 0.765) | 16.47 ± 0.20 (p = 0.233) |
| Systolic Blood Pressure (mmHg) | 119.05 ± 2.06 | 117.82 ± 1.64 (p = 0.613) | 114.53 ± 1.39 (p = 0.078) |
| Diastolic Blood Pressure (mmHg) | 78.56 ± 1.89 | 74.21 ± 1.66 (p = 0.091) | 73.78 ± 1.81 (p = 0.064) |
| Body Weight (kg) | 58.15 ± 1.76 | 59.17 ± 1.70 (p = 0.655) | 58.48 ± 1.34 (p = 0.885) |
| Body Height (cm) | 155.39 ± 0.99 | 155.39 ± 1.00 (p = 1.000) | 155.47 ± 1.58 (p = 0.960) |
| Body Mass Index (BMI) (kg/m2) | 24.02 ± 0.61 | 24.47 ± 0.61 (p = 0.618) | 24.30 ± 0.67 (p = 0.757) |
| Parameters | 8-Week | ||
|---|---|---|---|
| Placebo | D2 g per Day | D4 g per Day | |
| Age (year) | 51.00 ± 0.85 | 50.30 ± 0.90 (p = 0.732) | 51.13 ± 0.82 (p = 0.929) |
| Gender (Male/Female) | 1/22 | 0/23 | 1/22 |
| Body Temperature (°C) | 36.43 ± 0.06 | 36.35 ± 0.07 (p = 0.547) | 36.45 ± 0.06 (p = 0.982) |
| Heart Rate (beats/min) | 74.30 ± 1.65 | 70.95 ± 1.66 (p = 0.158) | 76.69 ± 1.64 (p = 0.311) |
| Respiratory Rate (breaths/min) | 16.43 ± 0.13 | 16.30 ± 0.13 (p = 0.428) | 16.13 ± 0.14 (p = 0.102) |
| Systolic Blood Pressure (mmHg) | 116.76 ± 1.76 | 113.91 ± 2.30 (p = 0.357) | 107.82 ± 2.34 ** (p = 0.005) |
| Diastolic Blood Pressure (mmHg) | 76.13 ± 1.91 | 71.47 ± 1.89 (p = 0.115) | 70.21 ± 2.34 * (p = 0.046) |
| Body Weight (kg) | 58.53 ± 1.78 | 58.99 ± 1.70 (p = 0.838) | 58.57 ± 1.27 (p = 0.983) |
| Body Height (cm) | 155.39 ± 0.99 | 155.39 ± 1.00 (p = 1.000) | 155.47 ± 1.58 (p = 0.960) |
| Body Mass Index (BMI) (kg/m2) | 24.17 ± 0.61 | 24.40 ± 0.61 (p = 0.800) | 24.35 ± 0.65 (p = 0.845) |
| Location | Wave | Treatment Group | Baseline | 8-Week |
|---|---|---|---|---|
| Fz | N100 Latency (ms) | Placebo | 106.40 ± 2.47 | 106.00 ± 2.08 |
| D 2 g per day | 109.00 ± 2.37 (p = 0.404) | 98.86 ± 2.22 * (p = 0.016) | ||
| D 4 g per day | 106.39 ± 2.35 (p = 0.539) | 103.86 ± 1.79 (p = 0.459) | ||
| N100 Amplitude (μV) | Placebo | 7.84 ± 0.93 | 7.36 ± 0.85 | |
| D 2 g per day | 7.97 ± 1.04 (p = 0.922) | 10.16 ± 0.72 * (p = 0.022) | ||
| D 4 g per day | 5.71 ± 0.69 (p = 0.097) | 10.47 ± 0.93 * (p = 0.011) | ||
| P300 Latency (ms) | Placebo | 345.00 ± 4.07 | 344.04 ± 3.68 | |
| D 2 g per day | 341.54 ± 3.70 (p = 0.485) | 343.78 ± 3.22 (p = 0.955) | ||
| D 4 g per day | 350.08 ± 2.45 (p = 0.300) | 344.91 ± 2.91 (p = 0.852) | ||
| P300 Amplitude (μV) | Placebo | 22.04 ± 1.75 | 23.83 ± 1.66 | |
| D 2 g per day | 20.99 ± 2.16 (p = 0.702) | 22.28 ± 1.57 (p = 0.472) | ||
| D 4 g per day | 21.00 ± 1.78 (p = 0.702) | 23.70 ± 1.23 (p = 0.953) | ||
| Cz | N100 Latency (ms) | Placebo | 106.36 ± 2.53 | 102.47 ± 2.94 |
| D 2 g per day | 110.77 ± 2.51 (p = 0.207) | 104.34 ± 2.12 (p = 0.585) | ||
| D 4 g per day | 106.60 ± 2.23 (p = 0.943) | 104.73 ± 2.04 (p = 0.509) | ||
| N100 Amplitude (μV) | Placebo | 7.84 ± 1.11 | 6.04 ± 0.91 | |
| D 2 g per day | 7.46 ± 0.88 (p = 0.897) | 9.55 ± 0.99 ** (p = 0.001) | ||
| D 4 g per day | 6.10 ± 0.75 (p = 0.233) | 8.25 ± 0.73 * (p = 0.014) | ||
| P300 Latency (ms) | Placebo | 341.60 ± 3.48 | 344.85 ± 3.40 | |
| D 2 g per day | 346.36 ± 3.52 (p = 0.331) | 341.39 ± 3.83 (p = 0.486) | ||
| D 4 g per day | 349.71 ± 3.29 (p = 0.104) | 347.77 ± 3.15 (p = 0.562) | ||
| P300 Amplitude (μV) | Placebo | 19.57 ± 1.98 | 21.25 ± 1.66 | |
| D 2 g per day | 19.95 ± 1.94 (p = 0.875) | 20.14 ± 1.90 (p = 0.652) | ||
| D 4 g per day | 19.55 ± 1.63 (p = 0.774) | 22.83 ± 1.53 (p = 0.510) |
| Cognitive Domains | Test Items | Treatment Group | Baseline | 8-Week |
|---|---|---|---|---|
| Word Recognition | Time (ms) | Placebo | 1497.31 ± 72.09 | 1300.95 ± 59.80 |
| D2 g per day | 1331.22 ± 64.44 (p = 0.110) | 1186.96 ± 49.87 (p = 0.132) | ||
| D4 g per day | 1453.52 ± 83.43 (p = 0.677) | 1300.04 ± 47.14 (p = 0.555) | ||
| %Accuracy | Placebo | 83.96 ± 2.29 | 86.37 ± 2.33 | |
| D2 g per day | 84.63 ± 1.59 (p = 0.962) | 87.24 ± 1.89 (p = 1.000) | ||
| D4 g per day | 83.01 ± 2.04 (p = 0.656) | 85.30 ± 1.43 (p = 0.329) | ||
| Picture Recognition | Time (ms) | Placebo | 1436.86 ± 50.65 | 1322.56 ± 48.01 |
| D2 g per day | 1359.25 ± 68.57 (p = 0.204) | 1291.03 ± 58.21 (p = 0.386) | ||
| D4 g per day | 1489.69 ± 67.97 (p = 0.496) | 1308.66 ± 43.16 (p = 0.393) | ||
| %Accuracy | Placebo | 88.47 ± 1.84 | 85.68 ± 2.20 | |
| D2 g per day | 86.52 ± 1.84 (p = 0.501) | 87.17 ± 1.36 (p = 0.920) | ||
| D4 g per day | 87.39 ± 1.82 (p = 0.727) | 86.52 ± 1.42 (p = 0.893) | ||
| Simple Reaction | Time (ms) | Placebo | 760.12 ± 32.36 | 727.65 ± 28.96 |
| D2 g per day | 703.79 ± 22.26 (p = 0.135) | 635.73 ± 18.94 * (p = 0.010) | ||
| D4 g per day | 730.33 ± 21.89 (p = 0.410) | 695.93 ± 21.16 (p = 0.455) | ||
| Digit Vigilance | Time (ms) | Placebo | 665.50 ± 14.05 | 655.04 ± 21.67 |
| D2 g per day | 672.60 ± 9.72 (p = 0.648) | 665.14 ± 9.79 (p = 0.538) | ||
| D4 g per day | 682.82 ± 8.46 (p = 0.267) | 685.99 ± 7.69 (p = 0.468) | ||
| %Accuracy | Placebo | 91.24 ± 1.31 | 91.47 ± 1.44 | |
| D2 g per day | 92.75 ± 1.10 (p = 0.460) | 93.97 ± 0.97 (p = 0.348) | ||
| D4 g per day | 90.91 ± 1.04 (p = 0.620) | 93.75 ± 0.89 (p = 0.528) | ||
| Choice Reaction Time | Time (ms) | Placebo | 884.09 ± 26.89 | 886.87 ± 32.03 |
| D2 g per day | 862.69 ± 25.46 (p = 0.474) | 864.63 ± 33.38 (p = 0.603) | ||
| D4 g per day | 885.38 ± 24.22 (p = 0.981) | 869.68 ± 23.71(p = 0.688) | ||
| %Accuracy | Placebo | 97.21 ± 0.71 | 98.17 ± 0.46 | |
| D2 g per day | 97.65 ± 0.44 (p = 0.909) | 97.50 ± 0.51 (p = 0.298) | ||
| D4 g per day | 97.69 ± 0.57 (p = 0.789) | 97.43 ± 0.66 (p = 0.454) | ||
| Spatial Memory | Time (ms) | Placebo | 1503.98 ± 68.44 | 1439.15 ± 77.30 |
| D2 g per day | 1450.06 ± 60.10 (p = 0.633) | 1452.06 ± 58.35 (p = 0.606) | ||
| D4 g per day | 1548.42 ± 70.67 (p = 0.683) | 1433.48 ± 52.32 (p = 0.668) | ||
| %Accuracy | Placebo | 90.17 ± 2.11 | 85.43 ± 3.23 | |
| D2 g per day | 90.33 ± 2.46 (p = 0.617) | 86.78 ± 2.70 (p = 0.903) | ||
| D4 g per day | 91.63 ± 1.69 (p = 0.765) | 85.78 ± 2.87 (p = 0.722) | ||
| Numeric Working Memory | Time (ms) | Placebo | 1200.45 ± 46.17 | 1131.49 ± 42.36 |
| D2 g per day | 1179.57 ± 58.96 (p = 0.462) | 1171.98 ± 48.10 (p = 0.470) | ||
| D4 g per day | 1207.86 ± 33.96 (p = 0.684) | 1166.01 ± 23.54 (p = 0.538) | ||
| %Accuracy | Placebo | 93.04 ± 2.41 | 89.55 ± 2.82 | |
| D2 g per day | 93.91 ± 1.71 (p = 0.477) | 96.07 ± 1.15 * (p = 0.015) | ||
| D4 g per day | 92.17 ± 2.07 (p = 0.311) | 94.92 ± 0.87 * (p = 0.046) |
| Treatment | Severity Score at Baseline | Severity Score after an 8 Week-Consumption Period |
|---|---|---|
| Placebo | 4.47 ± 0.14 | 4.42 ± 0.11 |
| D2 g per day | 4.72 ± 0.17 | 4.22 ± 0.11 # |
| D4 g per day | 4.47 ± 0.11 | 4.23 ± 0.10 |
| Parameters | Times | Placebo | D2 g per Day | D4 g per Day |
|---|---|---|---|---|
| AChE activity (nmol/mg protein) | Baseline | 6.43 ± 0.50 | 5.54 ± 0.40 (p = 0.249) | 5.97 ± 0.47 (p = 0.511) |
| 8-week | 6.35 ± 0.34 | 5.33 ± 0.27 * (p = 0.016) | 6.07 ± 0.23 (p = 0.525) | |
| MAO activity (umol/mg protein) | Baseline | 4.03 ± 0.34 | 3.66 ± 0.23 (p = 0.849) | 3.44 ± 0.23 (p = 0.166) |
| 8-week | 2.90 ± 0.16 | 2.89 ± 0.16 (p = 0.988) | 2.73 ± 0.09 (p = 0.782) | |
| MAO-A activity (umol/mg protein) | Baseline | 1.85 ± 0.36 | 1.48 ± 0.22 (p = 0.988) | 1.26 ± 0.22 (p = 0.328) |
| 8-week | 1.27 ± 0.20 | 1.15 ± 0.17 (p = 0.620) | 0.98 ± 0.12 (p = 0.465) | |
| MAO-B activity (umol/mg protein) | Baseline | 1.22 ± 0.19 | 0.99 ± 0.11 (p = 0.919) | 0.93 ± 0.12 (p = 0.474) |
| 8-week | 0.89 ± 0.10 | 0.82 ± 0.10 (p = 0.605) | 0.82 ± 0.08 (p = 0.612) | |
| GABA-T (nmol/mg protein) | Baseline | 0.22 ± 0.02 | 0.19 ± 0.01 (p = 0.988) | 0.18 ± 0.01 (p = 0.165) |
| 8-week | 0.18 ± 0.01 | 0.18 ± 0.01 (p = 0.838) | 0.16 ± 0.00 (p = 0.726) | |
| BDNF (pg/mL) | Baseline | 113.23 ± 3.82 | 118.74 ± 4.36 (p = 0.411) | 113.07 ± 5.48 (p = 0.979) |
| 8-week | 117.21 ± 4.18 | 146.20 ± 12.57 * (p = 0.033) | 143 ± 9.25 * (p = 0.043) |
| Parameters | Treatment Duration | Placebo | D2 g per Day | D4 g per Day |
|---|---|---|---|---|
| pH | Baseline | 7.43 ± 0.07 | 7.19 ± 0.07 | 7.35 ± 0.08 |
| 8-week | 6.83 ± 0.12 | 6.74 ± 0.08 * (p = 0.02) | 6.70 ± 0.07 * (p = 0.026) | |
| Amount of lactic acid producing bacteria (Log of CFU/mL) | Baseline | 8.13 ± 0.23 | 7.88 ± 0.17 (p = 0.734) | 8.00 ± 0.17 (p = 0.865) |
| 8-week | 7.76 ± 0.17 | 7.56 ± 0.15 (p = 0.147) | 7.55 ± 0.14 (p = 0.482) | |
| Amount of Lactobacillus spp. (Log of CFU/mL) | Baseline | 6.64 ± 0.29 | 6.79 ± 0.08 (p = 0.918) | 6.71 ± 0.24 (p = 0.564) |
| 8-week | 6.64 ± 0.30 | 6.72 ± 0.18 (p = 0.825) | 6.62 ± 0.32 (p = 0.363) | |
| Amount of Bifidobacterium spp. (Log of CFU/mL) | Baseline | 6.84 ± 0.56 | 6.56 ± 0.05 (p = 0.934) | 6.68 ± 0.25 (p = 0.998) |
| 8-week | 6.61 ± 0.04 | 7.76 ± 0.43 ** (p = 0.006) | 6.69 ± 0.20 (p = 0.417) |
| Parameters | Time Window of Treatment | Placebo | D2 g/Day | D4 g/Day |
|---|---|---|---|---|
| Acetate (mM) | Baseline | 4.70 ± 0.88 | 6.76 ± 1.37 (p = 0.351) | 6.74 ± 1.49 (p = 0.313) |
| 8-week | 6.79 ± 1.01 | 7.55 ± 1.13 (p = 0.598) | 8.57± 0.83 (p = 0.210) | |
| Propionate (mM) | Baseline | 2.27 ± 0.52 | 3.64 ± 0.83 (p = 0.235) | 3.70 ± 0.70 (p = 0.181) |
| 8-week | 3.09 ± 0.44 | 3.64± 0.67 (p = 0.487) | 4.85 ±0.53 * (p = 0.032) | |
| Butyrate (mM) | Baseline | 1.64 ± 0.30 | 3.10 ± 0.93 (p = 0.206) | 3.36 ± 0.61 (p = 0.835) |
| 8-week | 2.72 ± 0.52 | 3.49 ± 0.58 (p = 0.311) | 3.33 ± 0.43 (p = 0.405) |
| Parameters | Times | Placebo | D2 g/Day | D4 g/Day |
|---|---|---|---|---|
| Histone Acetyltransferase (HAT) | Baseline | 1.03 ± 0.048 | 1.09 ± 0.77 (p = 0.415) | 1.03 ± 0.039 (p = 0.967) |
| 8-week | 0.96 ± 0.042 | 1.06± 0.072 (p = 0.192) | 1.06 ± 0.036 (p = 0.922) | |
| Histone Deacetylase (HDAC) | Baseline | 7.41 ± 0.72 | 6.16 ± 0.51 (p = 0.171) | 7.27 ± 0.62 (p = 0.876) |
| 8-week | 7.48 ± 0.59 | 5.77± 0.48 * (p = 0.040) | 6.14 ± 0.57 (p = 0.104) | |
| DNA Methyltransferase (DNMT) | Baseline | 0.35 ± 0.05 | 0.35 ± 0.05 (p = 0.952) | 0.32 ± 0.05 (p = 0.745) |
| 8-week | 0.29 ± 0.04 | 0.32± 0.05 (p = 0.734) | 0.26± 0.04 (p = 0.732) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wattanathorn, J.; Tong-un, T.; Thukham-mee, W.; Paholpak, P.; Rangseekhajee, P. A Randomized, Double-Blind, Placebo-Controlled Study of an Anthocyanin-Rich Functional Ingredient on Cognitive Function and Eye Dryness in Late Adulthood Volunteers: Roles of Epigenetic and Gut Microbiome Modulations. Nutrients 2023, 15, 3499. https://doi.org/10.3390/nu15163499
Wattanathorn J, Tong-un T, Thukham-mee W, Paholpak P, Rangseekhajee P. A Randomized, Double-Blind, Placebo-Controlled Study of an Anthocyanin-Rich Functional Ingredient on Cognitive Function and Eye Dryness in Late Adulthood Volunteers: Roles of Epigenetic and Gut Microbiome Modulations. Nutrients. 2023; 15(16):3499. https://doi.org/10.3390/nu15163499
Chicago/Turabian StyleWattanathorn, Jintanaporn, Terdthai Tong-un, Wipawee Thukham-mee, Pongsatorn Paholpak, and Poonsri Rangseekhajee. 2023. "A Randomized, Double-Blind, Placebo-Controlled Study of an Anthocyanin-Rich Functional Ingredient on Cognitive Function and Eye Dryness in Late Adulthood Volunteers: Roles of Epigenetic and Gut Microbiome Modulations" Nutrients 15, no. 16: 3499. https://doi.org/10.3390/nu15163499
APA StyleWattanathorn, J., Tong-un, T., Thukham-mee, W., Paholpak, P., & Rangseekhajee, P. (2023). A Randomized, Double-Blind, Placebo-Controlled Study of an Anthocyanin-Rich Functional Ingredient on Cognitive Function and Eye Dryness in Late Adulthood Volunteers: Roles of Epigenetic and Gut Microbiome Modulations. Nutrients, 15(16), 3499. https://doi.org/10.3390/nu15163499