Diet and Glycemic Index in Children with Type 1 Diabetes
Abstract
:1. Introduction
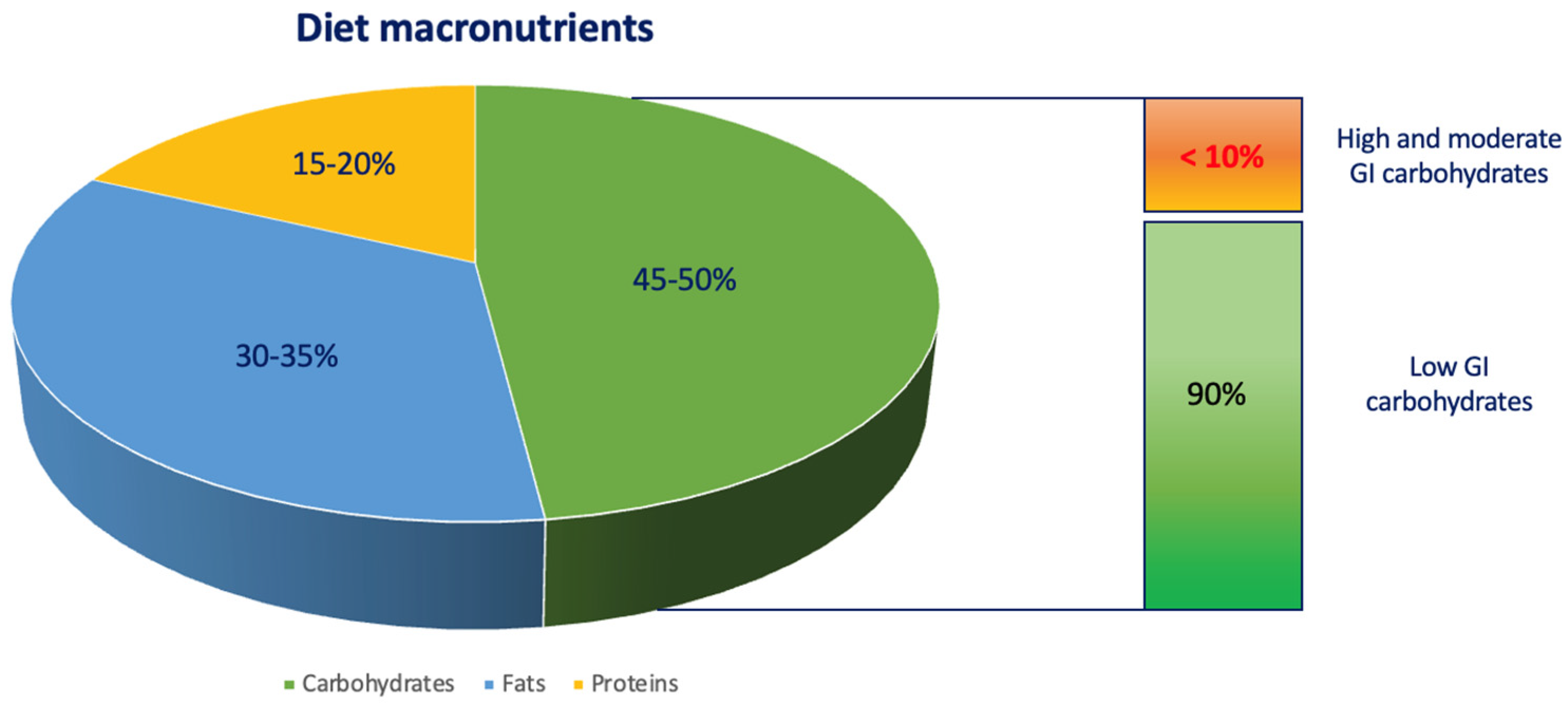
2. Diet in Children with T1D
2.1. Carbohydrate Structure and Classification
2.2. GI and Glucose Blood Levels
3. Diet and Growth in Children with T1D
4. Studies Focusing on Diets Characterized by Different Quantities and Qualities of Carbohydrates in Children with T1D
4.1. Studies Analyzing the Effects of Carbohydrate-Restricted Diets
4.2. Studies Analyzing the Effects of Low-GI-Index Diets
| Population | Type of Diet | Method | Results | Limitations | Ref. |
|---|---|---|---|---|---|
| Children and adults with T1D | Very-low-carbohydrates diet (VLCD) | Evaluation of HbA1c, insulin requirements and adverse events with VLCD diet through an online survey | Good glycemic control of T1D with low rates of adverse events | Potential risk of DKA, dyslipidemia, inappropriate caloric intake, adverse effects on growth and pubertal development and patient’s poor diet adherence | [69,70] |
| Children with T1D and CGM | Low-carbohydrates diet | Impact of low-carbohydrate diet on glycemic control | Higher time in range (TIR) and better values of HbA1c | Patient’s poor diet adherence | [71] |
| Children with T1D and CGM | Low-GI foods | Effects of low-GI foods compared to the usual diet | Lower postprandial glycemic values and less glycemic variability | Not known long-term metabolic effects and poor children adherence | [78] |
| Children with T1D | Low-GI diet | Effects of low-GI foods compared to ISPAD-recommended optimized mixed diet (OMD) | No significant differences about HbA1c levels; fat intake increased in the low-GI diet group and energy and fat intake reduced in OMD group | Patient’s poor diet adherence | [79] |
| Young people with T1D, CGM and following the basal–bolus insulin regimen | Low-GI diet | Comparison between low- and high-GI diets | Lower glycemic values, daily glycemic fluctuations, no increased risk of severe hypoglycemia | - | [83] |
| Children with T1D | Flexible low-GI diet | Evaluation of long-term effects (12 months) on metabolic control and quality of life of a flexible low-GI diet versus a traditional carbohydrate-counting diet | Significant improvement in HbA1c values and reduction in hyperglycemic episodes; no differences in insulin requirements and hypoglycemic episodes | Restricted food choice options and increased consumption of fatty foods | [85,86] |
| Children with T1D, not well controlled | High- and low-GI breakfast | Effect of a low- and high-GI breakfast on postprandial glycemia | Postprandial glycemia resulted most influenced by adequate doses of preprandial insulin administered rather than the GI of foods | - | [87] |
| Children with T1D and CGM on basal–bolus insulin regimen | High- and low-GI breakfast | Effect of a meal with the same amounts of macronutrients and different GIs | Fewer glycemic fluctuations and postprandial glycemic peaks in low-GI meal group | - | [88] |
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lovic, D.; Piperidou, A.; Zografou, I.; Grassos, H.; Pittaras, A.; Manolis, A. The Growing Epidemic of Diabetes Mellitus. Curr. Vasc. Pharmacol. 2020, 18, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Abela, A.G.; Fava, S. Why is the Incidence of Type 1 Diabetes Increasing? Curr. Diabetes Rev. 2021, 17, 22–34. [Google Scholar] [CrossRef]
- Zimmet, P.; Alberti, K.G.; Magliano, D.J.; Bennett, P.H. Diabetes mellitus statistics on prevalence and mortality: Facts and fallacies. Nat. Rev. Endocrinol. 2016, 12, 616–622. [Google Scholar] [CrossRef]
- Xia, Y.; Xie, Z.; Huang, G.; Zhou, Z. Incidence and trend of type 1 diabetes and the underlying environmental determinants. Diabetes Metab. Res. Rev. 2019, 35, e3075. [Google Scholar] [CrossRef] [Green Version]
- Ward, Z.J.; Yeh, J.M.; Reddy, C.L.; Gomber, A.; Ross, C.; Rittiphairoj, T.; Manne-Goehler, J.; Abdalla, A.T.; Abdullah, M.A.; Ahmed, A.; et al. Estimating the total incidence of type 1 diabetes in children and adolescents aged 0–19 years from 1990 to 2050: A global simulation-based analysis. Lancet Diabetes Endocrinol. 2022, 10, 848–858. [Google Scholar] [CrossRef]
- American Diabetes Association. Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 2014, 37, S81–S90. [Google Scholar] [CrossRef] [Green Version]
- Beck, R.W.; Bergenstal, R.M.; Laffel, L.M.; Pickup, J.C. Advances in technology for management of type 1 diabetes. Lancet 2019, 394, 1265–1273. [Google Scholar] [CrossRef] [PubMed]
- Sims, E.K.; Carr, A.L.J.; Oram, R.A.; DiMeglio, L.A.; Evans-Molina, C. 100 years of insulin: Celebrating the past, present and future of diabetes therapy. Nat. Med. 2021, 27, 1154–1164. [Google Scholar] [CrossRef] [PubMed]
- Mańkiewicz-Żurawska, I.; Jarosz-Chobot, P. Nutrition of children and adolescents with type 1 diabetes in the recommendations of the Mediterranean diet. Pediatr. Endocrinol. Diabetes Metab. 2019, 25, 74–80. [Google Scholar] [CrossRef]
- Node, K.; Inoue, T. Postprandial hyperglycemia as an etiological factor in vascular failure. Cardiovasc. Diabetol. 2009, 8, 23. [Google Scholar] [CrossRef] [Green Version]
- Leiter, L.A.; Ceriello, A.; Davidson, J.A.; Hanefeld, M.; Monnier, L.; Owens, D.R.; Tajima, N.; Tuomilehto, J.; Group, I.G. Postprandial glucose regulation: New data andnew implications. Clin. Ther. 2005, 27, S42–S56. [Google Scholar] [CrossRef] [PubMed]
- Cavalot, F.; Petrelli, A.; Traversa, M.; Bonomo, K.; Fiora, E.; Conti, M.; Anfossi, G.; Costa, G.; Trovati, M. Postprandial Blood Glucose Is a Stronger Predictor of Cardiovascular Events Than Fasting Blood Glucose in Type 2 Diabetes Mellitus, Particularly in Women: Lessons from the San Luigi Gonzaga Diabetes Study. J. Clin. Endocrinol. Metab. 2006, 91, 813–819. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ceriello, A. Postprandial Glucose Regulation and Diabetic Complications. Arch. Intern. Med. 2004, 164, 2090. [Google Scholar] [CrossRef]
- Smart, C.E.; Annan, F.; Higgins, L.A.; Jelleryd, E.; Lopez, M.; Acerini, C.L. ISPAD Clinical Practice Consensus Guidelines 2018: Nutritional management in children and adolescents with diabetes. Pediatr. Diabetes 2018, 19, 136–154. [Google Scholar] [CrossRef]
- Stenvers, D.J.; Schouten, L.J.; Jurgens, J.; Endert, E.; Kalsbeek, A.; Fliers, E.; Bisschop, P.H. Breakfast replacement with a low-glycaemic response liquid formula in patients with type 2 diabetes: A randomised clinical trial. Br. J. Nutr. 2014, 112, 504–512. [Google Scholar] [CrossRef] [Green Version]
- Lobos, D.R.; Vicuña, I.A.; Novik, V.; Vega, C.A. Effect of high and low glycemic index breakfast on postprandial metabolic parameters and satiety in subjects with type 2 diabetes mellitus under intensive insulin therapy: Controlled clinical trial. Clin. Nutr. ESPEN 2017, 20, 12–16. [Google Scholar] [CrossRef]
- Chang, C.R.; Francois, M.E.; Little, J.P. Restricting carbohydrates at breakfast is sufficient to reduce 24-hour exposure to postprandial hyperglycemia and improve glycemic variability. Am. J. Clin. Nutr. 2019, 109, 1302–1309. [Google Scholar] [CrossRef]
- de Carvalho, C.M.; de Paula, T.P.; Viana, L.V.; Machado, V.M.; de Almeida, J.C.; Azevedo, M.J. Plasma glucose and insulin responses after consumption of breakfasts with different sources of soluble fiber in type 2 diabetes patients: A randomized crossover clinical trial. Am. J. Clin. Nutr. 2017, 106, 1238–1245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martínez-González, M.Á.; De la Fuente-Arrillaga, C.; Nunez-Cordoba, J.M.; Basterra-Gortari, F.J.; Beunza, J.J.; Vazquez, Z.; Benito, S.; Tortosa, A.; Bes-Rastrollo, M. Adherence to Mediterranean diet and risk of developing diabetes: Prospective cohort study. BMJ 2008, 336, 1348–1351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bell, K.J.; Smart, C.E.; Steil, G.M.; Brand-Miller, J.C.; King, B.; Wolpert, H.A. Impact of Fat, Protein, and Glycemic Index on Postprandial Glucose Control in Type 1 Diabetes: Implications for Intensive Diabetes Management in the Continuous Glucose Monitoring Era. Diabetes Care 2015, 38, 1008–1015. [Google Scholar] [CrossRef] [Green Version]
- Vlachos, D.; Malisova, S.; Lindberg, F.A.; Karaniki, G. Glycemic Index (GI) or Glycemic Load (GL) and Dietary Interventions for Optimizing Postprandial Hyperglycemia in Patients with T2 Diabetes: A Review. Nutrients 2020, 12, 1561. [Google Scholar] [CrossRef]
- Mazzocchi, A.; Leone, L.; Agostoni, C.; Pali-Schöll, I. The Secrets of the Mediterranean Diet. Does [Only] Olive Oil Matter? Nutrients 2019, 11, 2941. [Google Scholar] [CrossRef] [Green Version]
- Smart, C.E.; Evans, M.; O’connell, S.M.; McElduff, P.; Lopez, P.E.; Jones, T.W.; Davis, E.A.; King, B.R. Both Dietary Protein and Fat Increase Postprandial Glucose Excursions in Children with Type 1 Diabetes, and the Effect Is Additive. Diabetes Care 2013, 36, 3897–3902. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neu, A.; Behret, F.; Braun, R.; Herrlich, S.; Liebrich, F.; Loesch-Binder, M.; Schneider, A.; Schweizer, R. Higher glucose concentrations following protein- and fat-rich meals—The Tuebingen Grill Study: A pilot study in adolescents with type 1 diabetes. Pediatr. Diabetes 2015, 16, 587–591. [Google Scholar] [CrossRef]
- Abdou, M.; Hafez, M.H.; Anwar, G.M.; Fahmy, W.A.; Abd Alfattah, N.M.; Salem, R.I.; Arafa, N. Effect of high protein and fat diet on postprandial blood glucose levels in children and adolescents with type 1 diabetes in Cairo, Egypt. Diabetes Metab. Syndr. Clin. Res. Rev. 2021, 15, 7–12. [Google Scholar] [CrossRef]
- Sharon, N. Carbohydrates. Sci. Am. 1980, 243, 90–116. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Castillo, C.P.; Hudson, G.J.; Englyst, H.N.; Dewey, P.; James, W.P.T. The importance of dietary carbohydrates. Arch. Latinoam. Nutr. 2002, 52, 321–335. [Google Scholar] [PubMed]
- Cummings, J.H.; Stephen, A.M. Carbohydrate terminology and classification. Eur. J. Clin. Nutr. 2007, 61, S5–S18. [Google Scholar] [CrossRef] [Green Version]
- Kiely, L.J.; Hickey, R.M. Characterization and Analysis of Food-Sourced Carbohydrates; Humana: New York, NY, USA, 2022; pp. 67–95. [Google Scholar]
- Qi, X.; Tester, R.F. Fructose, galactose and glucose—In health and disease. Clin. Nutr. ESPEN 2019, 33, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Varki, A. Biological roles of oligosaccharides: All of the theories are correct. Glycobiology 1993, 3, 97–130. [Google Scholar] [CrossRef] [PubMed]
- Englyst, H.N.; Cummings, J.H. Non-Starch Polysaccharides (Dietary Fiber) and Resistant Starch; Springer: Boston, MA, USA, 1990; pp. 205–225. [Google Scholar]
- Lattimer, J.M.; Haub, M.D. Effects of Dietary Fiber and Its Components on Metabolic Health. Nutrients 2010, 2, 1266–1289. [Google Scholar] [CrossRef] [Green Version]
- Delbianco, M.; Bharate, P.; Varela-Aramburu, S.; Seeberger, P.H. Carbohydrates in Supramolecular Chemistry. Chem. Rev. 2016, 116, 1693–1752. [Google Scholar] [CrossRef] [PubMed]
- Hall, M.B.; Mertens, D.R. A 100-Year Review: Carbohydrates—Characterization, digestion, and utilization. J. Dairy Sci. 2017, 100, 10078–10093. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hojsak, I. 1.3.4 Digestible and Non-Digestible Carbohydrates; Karger Publishers: Basel, Switzerland, 2022; pp. 60–64. [Google Scholar]
- Brand-Miller, J.; Buyken, A.E. The Relationship between Glycemic Index and Health. Nutrients 2020, 12, 536. [Google Scholar] [CrossRef] [Green Version]
- Semenza, G. Intestinal Digestion and Absorption of Sugars. Biochem. Soc. Trans. 1975, 3, 221–223. [Google Scholar] [CrossRef] [PubMed]
- Frost, G.; Dornhorst, A. The relevance of the glycaemic index to our understanding of dietary carbohydrates. Diabet. Med. 2000, 17, 336–345. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, D.M. Growth in patients with type 1 diabetes. Curr. Opin. Endocrinol. Diabetes Obes. 2016, 24, 67. [Google Scholar] [CrossRef]
- El–Jamal, S.; Elfane, H.; Chamlal, H.; Barakat, I.; Daif, H.; Mziwira, M.; Fassouane, A.; Belahsen, R. Assessment of diet quality in children and adolescents with type 1 diabetes. Rocz Panstw Zakl Hig. 2022, 73, 413–422. [Google Scholar]
- Kaur, B.; Ranawana, V.; Henry, J. The Glycemic Index of Rice and Rice Products: A Review, and Table of GI Values. Crit. Rev. Food Sci. Nutr. 2016, 56, 215–236. [Google Scholar] [CrossRef] [Green Version]
- Reynolds, A.; Mann, J.; Cummings, J.; Winter, N.; Mete, E.; Te Morenga, L. Carbohydrate quality and human health: A series of systematic reviews and meta-analyses. Lancet 2019, 393, 434–445. [Google Scholar] [CrossRef] [Green Version]
- Jenkins, D.J.; Wolever, T.M.; Taylor, R.H.; Barker, H.; Fielden, H.; Baldwin, J.M.; Bowling, A.C.; Newman, H.C.; Jenkins, A.L.; Goff, D.V. Glycemic index of foods: A physiological basis for carbohydrate exchange. Am. J. Clin. Nutr. 1981, 34, 362–366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salmerón, J. Dietary Fiber, Glycemic Load, and Risk of Non—Insulin-dependent Diabetes Mellitus in Women. JAMA J. Am. Med. Assoc. 1997, 277, 472. [Google Scholar] [CrossRef] [PubMed]
- Vega-López, S.; Venn, B.; Slavin, J. Relevance of the Glycemic Index and Glycemic Load for Body Weight, Diabetes, and Cardiovascular Disease. Nutrients 2018, 10, 1361. [Google Scholar] [CrossRef] [Green Version]
- Sheard, N.F.; Clark, N.G.; Brand-Miller, J.C.; Franz, M.J.; Pi-Sunyer, F.X.; Mayer-Davis, E.; Kulkarni, K.; Geil, P. Dietary Carbohydrate (Amount and Type) in the Prevention and Management of Diabetes. Diabetes Care 2004, 27, 2266–2271. [Google Scholar] [CrossRef] [Green Version]
- Ludwig, D.S.; Hu, F.B.; Tappy, L.; Brand-Miller, J. Dietary carbohydrates: Role of quality and quantity in chronic disease. BMJ 2018, 361, k2340. [Google Scholar] [CrossRef] [Green Version]
- Atkinson, F.S.; Brand-Miller, J.C.; Foster-Powell, K.; Buyken, A.E.; Goletzke, J. International tables of glycemic index and glycemic load values 2021: A systematic review. Am. J. Clin. Nutr. 2021, 114, 1625–1632. [Google Scholar] [CrossRef] [PubMed]
- Foster-Powell, K.; Holt, S.H.; Brand-Miller, J.C. International table of glycemic index and glycemic load values: 2002. Am. J. Clin. Nutr. 2002, 76, 5–56. [Google Scholar] [CrossRef] [Green Version]
- Wolever, T.M. Effect of macronutrients on the glycemic index. Am. J. Clin. Nutr. 2017, 106, 704–705. [Google Scholar] [CrossRef] [Green Version]
- Henry, C.J.; Kaur, B.; Quek, R.Y.C. Chrononutrition in the management of diabetes. Nutr. Diabetes 2020, 10, 6. [Google Scholar] [CrossRef] [Green Version]
- Srichaikul, K.; Jenkins, D.J.A. The Glycemic Index, Rate of Digestion of Carbohydrate Foods, and Their Potential Link with Cardiovascular Disease. J. Nutr. 2022, 152, 920–921. [Google Scholar] [CrossRef]
- Zafar, M.I.; Mills, K.E.; Zheng, J.; Regmi, A.; Hu, S.Q.; Gou, L.; Chen, L.L. Low-glycemic index diets as an intervention for diabetes: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2019, 110, 891–902. [Google Scholar] [CrossRef] [PubMed]
- Kappeler, L.; Clemessy, M.; Saget, S.; Decourtye, L.; Le Bouc, Y. Regulation of growth: Epigenetic mechanisms? Ann. Endocrinol. 2017, 78, 92–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santi, E.; Tascini, G.; Toni, G.; Berioli, M.G.; Esposito, S. Linear Growth in Children and Adolescents with Type 1 Diabetes Mellitus. Int. J. Environ. Res. Public Health 2019, 16, 3677. [Google Scholar] [CrossRef] [Green Version]
- Chiarelli, F.; Giannini, C.; Mohn, A. Growth, growth factors and diabetes. Eur. J. Endocrinol. 2004, 151, U109–U117. [Google Scholar] [CrossRef] [Green Version]
- Seckold, R.; Fisher, E.; de Bock, M.; King, B.R.; Smart, C.E. The ups and downs of low-carbohydrate diets in the management of Type 1 diabetes: A review of clinical outcomes. Diabet. Med. 2019, 36, 326–334. [Google Scholar] [CrossRef]
- Koren, D. Growth and development in type 1 diabetes. Curr. Opin. Endocrinol. Diabetes Obes. 2022, 29, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Akil, A.A.-S.; Yassin, E.; Al-Maraghi, A.; Aliyev, E.; Al-Malki, K.; Fakhro, K.A. Diagnosis and treatment of type 1 diabetes at the dawn of the personalized medicine era. J. Transl. Med. 2021, 19, 137. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Tian, J.; Tang, C.; Fang, X.; Miao, R.; Wu, H.; Wang, X.; Tong, X. The Influence of Different Types of Diabetes on Vascular Complications. J. Diabetes Res. 2022, 2022, 3448618. [Google Scholar] [CrossRef] [PubMed]
- Domingueti, C.P.; Dusse, L.M.S.; Carvalho, M.d.G.; de Sousa, L.P.; Gomes, K.B.; Fernandes, A.P. Diabetes mellitus: The linkage between oxidative stress, inflammation, hypercoagulability and vascular complications. J. Diabetes Complicat. 2016, 30, 738–745. [Google Scholar] [CrossRef]
- Sperling, M.A.; Laffel, L.M. Current Management of Glycemia in Children with Type 1 Diabetes Mellitus. N. Engl. J. Med. 2022, 386, 1155–1164. [Google Scholar] [CrossRef]
- Lizama Fuentes, F.; Ormeño Rojas, S.; Mourguiart Liberona, F.; Fuentes Cammell, J.; López-Alegría, F. Impact on the quality of life of adolescents with diabetes mellitus type 1. Rev. Chil. Pediatr. 2020, 91, 968–981. [Google Scholar] [CrossRef]
- Xu, Y.; Bergenstal, R.M.; Dunn, T.C.; Ram, Y.; Ajjan, R.A. Interindividual variability in average glucose-glycated haemoglobin relationship in type 1 diabetes and implications for clinical practice. Diabetes Obes. Metab. 2022, 24, 1779–1787. [Google Scholar] [CrossRef]
- Marks, B.E.; Wolfsdorf, J.I. Monitoring of paediatric type 1 diabetes. Curr. Opin. Pediatr. 2022, 34, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Ahola, A.J.; Forsblom, C.; Harjutsalo, V.; Groop, P.-H. Dietary carbohydrate intake and cardio-metabolic risk factors in type 1 diabetes. Diabetes Res. Clin. Pract. 2019, 155, 107818. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lamichhane, A.P.; Crandell, J.L.; Jaacks, L.M.; Couch, S.C.; Lawrence, J.M.; Mayer-Davis, E.J. Longitudinal associations of nutritional factors with glycated hemoglobin in youth with type 1 diabetes: The SEARCH Nutrition Ancillary Study. Am. J. Clin. Nutr. 2015, 101, 1278–1285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garonzi, C.; Forsander, G.; Maffeis, C. Impact of Fat Intake on Blood Glucose Control and Cardiovascular Risk Factors in Children and Adolescents with Type 1 Diabetes. Nutrients 2021, 13, 2625. [Google Scholar] [CrossRef]
- Lennerz, B.S.; Barton, A.; Bernstein, R.K.; Dikeman, R.D.; Diulus, C.; Hallberg, S.; Rhodes, E.T.; Ebbeling, C.B.; Westman, E.C.; Yancy, W.S.; et al. Management of Type 1 Diabetes with a Very Low-Carbohydrate Diet. Pediatrics 2018, 141, e20173349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lejk, A.; Chrzanowski, J.; Cieślak, A.; Fendler, W.; Myśliwiec, M. Effect of Nutritional Habits on the Glycemic Response to Different Carbohydrate Diet in Children with Type 1 Diabetes Mellitus. Nutrients 2021, 13, 3815. [Google Scholar] [CrossRef]
- Cherubini, V.; Marino, M.; Marigliano, M.; Maffeis, C.; Zanfardino, A.; Rabbone, I.; Giorda, S.; Schiaffini, R.; Lorubbio, A.; Rollato, S.; et al. Rethinking Carbohydrate Intake and Time in Range in Children and Adolescents with Type 1 Diabetes. Nutrients 2021, 13, 3869. [Google Scholar] [CrossRef]
- de Souza Bosco Paiva, C.; Lima, M.H.M. Introducing a very low carbohydrate diet for a child with type 1 diabetes. Br. J. Nurs. 2019, 28, 1015–1019. [Google Scholar] [CrossRef]
- Neuman, V.; Plachy, L.; Pruhova, S.; Kolouskova, S.; Petruzelkova, L.; Obermannova, B.; Vyzralkova, J.; Konecna, P.; Vosahlo, J.; Romanova, M.; et al. Low-Carbohydrate Diet among Children with Type 1 Diabetes: A Multi-Center Study. Nutrients 2021, 13, 3903. [Google Scholar] [CrossRef] [PubMed]
- Seidelmann, S.B.; Claggett, B.; Cheng, S.; Henglin, M.; Shah, A.; Steffen, L.M.; Folsom, A.R.; Rimm, E.B.; Willett, W.C.; Solomon, S.D. Dietary carbohydrate intake and mortality: A prospective cohort study and meta-analysis. Lancet Public Health 2018, 3, e419–e428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Güleryüz, C.; Eker, E.; Şakar, M.; Genç, F.N.; Elmaoğulları, S.; Çetinkaya, S.; Erdeve, S. Unfavorbale Effects of Low-Carbonhydrate Diet in a Pediatric Patient with Type 1 Diabetes Mellitus. J. Clin. Res. Pediatr. Endocrinol. 2022. [Google Scholar] [CrossRef]
- Hancock, M.; Burns, K.; Gan, S.K.; Chew, G.T. Low-carbohydrate diets in type 1 diabetes: Balancing benefits and risks. Curr. Opin. Endocrinol. Diabetes Obes. 2023, 30, 113–122. [Google Scholar] [CrossRef]
- Rovner, A.J.; Nansel, T.R.; Gellar, L. The Effect of a Low-Glycemic Diet vs a Standard Diet on Blood Glucose Levels and Macronutrient Intake in Children with Type 1 Diabetes. J. Am. Diet Assoc. 2009, 109, 303–307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marquard, J.; Stahl, A.; Lerch, C.; Wolters, M.; Grotzke-Leweling, M.; Mayatepek, E.; Meissner, T. A prospective clinical pilot-trial comparing the effect of an optimized mixed diet versus a flexible low-glycemic index diet on nutrient intake and HbA1c levels in children with type 1 diabetes. J. Pediatr. Endocrinol. Metab. 2011, 24, 441–447. [Google Scholar] [CrossRef]
- Bantle, J.P.; Wylie-Rosett, J.; Albright, A.L.; Apovian, C.M.; Clark, N.G.; Franz, M.J.; Hoogwerf, B.J.; Lichtenstein, A.H.; Mayer-Davis, E.; Mooradian, A.D.; et al. Nutrition Recommendations and Interventions for Diabetes. Diabetes Care 2008, 31, S61–S78. [Google Scholar]
- American Diabetes Association. Lifestyle Management: Standards of Medical Care in Diabetes—2019. Diabetes Care 2019, 42, S46–S60. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Xia, W.; Zhao, Z.; Zhang, H. Effects comparison between low glycemic index diets and high glycemic index diets on HbA1c and fructosamine for patients with diabetes: A systematic review and meta-analysis. Prim. Care Diabetes 2015, 9, 362–369. [Google Scholar] [CrossRef]
- Nansel, T.R.; Gellar, L.; McGill, A. Effect of Varying Glycemic Index Meals on Blood Glucose Control Assessed with Continuous Glucose Monitoring in Youth with Type 1 Diabetes on Basal-Bolus Insulin Regimens. Diabetes Care 2008, 31, 695–697. [Google Scholar] [CrossRef] [Green Version]
- Nansel, T.R.; Lipsky, L.M.; Liu, A. Greater diet quality is associated with more optimal glycemic control in a longitudinal study of youth with type 1 diabetes. Am. J. Clin. Nutr. 2016, 104, 81–87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gilbertson, H.R.; Brand-Miller, J.C.; Thorburn, A.W.; Evans, S.; Chondros, P.; Werther, G.A. The Effect of Flexible Low Glycemic Index Dietary Advice versus Measured Carbohydrate Exchange Diets on Glycemic Control in Children with Type 1 Diabetes. Diabetes Care 2001, 24, 1137–1143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gilbertson, H.R.; Thorburn, A.W.; Brand-Miller, J.C.; Chondros, P.; Werther, G.A. Effect of low-glycemic-index dietary advice on dietary quality and food choice in children with type 1 diabetes. Am. J. Clin. Nutr. 2003, 77, 83–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weyman-Daum, M.; Fort, P.; Recker, B.; Lanes, R.; Lifshitz, F. Glycemic response in children with insulin-dependent diabetes mellitus after high- or low-glycemic-index breakfast. Am. J. Clin. Nutr. 1987, 46, 798–803. [Google Scholar] [CrossRef] [PubMed]
- Ryan, R.L.; King, B.R.; Anderson, D.G.; Attia, J.R.; Collins, C.E.; Smart, C.E. Influence of and Optimal Insulin Therapy for a Low–Glycemic Index Meal in Children with Type 1 Diabetes Receiving Intensive Insulin Therapy. Diabetes Care 2008, 31, 1485–1490. [Google Scholar] [CrossRef] [Green Version]

| Food Category | Mean GI |
|---|---|
| Bakery products | 58 |
| Beverages | 50 |
| Breads | 64 |
| Breakfast cereals | 61 |
| Rice | 67 |
| Cookies | 49 |
| Crackers | 55 |
| Dairy products | 35 |
| Fruits | 51 |
| Legumes | 34 |
| Nuts | 22 |
| Pasta | 52 |
| Savonary snacks | 60 |
| Snack bars | 44 |
| Soups | 49 |
| Potatoes | 71 |
| Other vegetables | 66 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quarta, A.; Guarino, M.; Tripodi, R.; Giannini, C.; Chiarelli, F.; Blasetti, A. Diet and Glycemic Index in Children with Type 1 Diabetes. Nutrients 2023, 15, 3507. https://doi.org/10.3390/nu15163507
Quarta A, Guarino M, Tripodi R, Giannini C, Chiarelli F, Blasetti A. Diet and Glycemic Index in Children with Type 1 Diabetes. Nutrients. 2023; 15(16):3507. https://doi.org/10.3390/nu15163507
Chicago/Turabian StyleQuarta, Alessia, Miriana Guarino, Roberta Tripodi, Cosimo Giannini, Francesco Chiarelli, and Annalisa Blasetti. 2023. "Diet and Glycemic Index in Children with Type 1 Diabetes" Nutrients 15, no. 16: 3507. https://doi.org/10.3390/nu15163507
APA StyleQuarta, A., Guarino, M., Tripodi, R., Giannini, C., Chiarelli, F., & Blasetti, A. (2023). Diet and Glycemic Index in Children with Type 1 Diabetes. Nutrients, 15(16), 3507. https://doi.org/10.3390/nu15163507







