Industrial Use of Phosphate Food Additives: A Mechanism Linking Ultra-Processed Food Intake to Cardiorenal Disease Risk?
Abstract
:1. Introduction
2. Ultra-Processed Food, Cardiorenal Disease, and Phosphate Additives
2.1. Inorganic Phosphate Additives
2.2. Multiple Approved Technical Functions Complicate Study of Phosphate Additives and Regulation of Use
2.3. Exposure Estimates for Inorganic Phosphate Additives
2.4. Is There a Potential Mechanism for Excess Inorganic Phosphate Additives to Promote Cardiorenal Disease Risk?
2.5. Evidence of Hormonal Disruption from Dietary Interventions Studies with Foods Containing Phosphate Additives
2.6. Role of Excess Phosphate Intake in the Endocrine Disruption of Phosphate Homeostasis Leading to Cardiorenal Disease and Mortality
3. Regulatory Action Needed to Ensure Safety of Industrial Inorganic Phosphate Food Additives in UPF
4. Future Considerations for At-Risk Populations to Minimize Phosphate Intake
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Capozzi, F.; Magkos, F.; Fava, F.; Milani, G.P.; Agostoni, C.; Astrup, A.; Saguy, I.S. A Multidisciplinary Perspective of Ultra-Processed Foods and Associated Food Processing Technologies: A View of the Sustainable Road Ahead. Nutrients 2021, 13, 3948. [Google Scholar] [CrossRef] [PubMed]
- Moradi, S.; Entezari, M.H.; Mohammadi, H.; Jayedi, A.; Lazaridi, A.V.; Kermani, M.A.H.; Miraghajani, M. Ultra-processed food consumption and adult obesity risk: A systematic review and dose-response meta-analysis. Crit. Rev. Food Sci. Nutr. 2023, 63, 249–260. [Google Scholar] [CrossRef] [PubMed]
- Neri, D.; Steele, E.M.; Khandpur, N.; Cediel, G.; Zapata, M.E.; Rauber, F.; Marrón-Ponce, J.A.; Machado, P.; da Costa Louzada, M.L.; Andrade, G.C.; et al. Ultraprocessed food consumption and dietary nutrient profiles associated with obesity: A multicountry study of children and adolescents. Obes. Rev. 2022, 23, e13387. [Google Scholar] [CrossRef] [PubMed]
- Juul, F.; Vaidean, G.; Lin, Y.; Deierlein, A.L.; Parekh, N. Ultra-Processed Foods and Incident Cardiovascular Disease in the Framingham Offspring Study. J. Am. Coll. Cardiol. 2021, 77, 1520–1531. [Google Scholar] [CrossRef]
- Du, S.; Kim, H.; Rebholz, C.M. Higher Ultra-Processed Food Consumption Is Associated with Increased Risk of Incident Coronary Artery Disease in the Atherosclerosis Risk in Communities Study. J. Nutr. 2021, 151, 3746–3754. [Google Scholar] [CrossRef]
- Zhang, Z.; Jackson, S.L.; Steele, E.M.; Gillespie, C.; Yang, Q. Relationship Between Ultraprocessed Food Intake and Cardiovascular Health Among U.S. Adolescents: Results From the National Health and Nutrition Examination Survey 2007–2018. J. Adolesc. Health 2022, 70, 249–257. [Google Scholar] [CrossRef]
- Du, S.; Kim, H.; Crews, D.C.; White, K.; Rebholz, C.M. Association Between Ultraprocessed Food Consumption and Risk of Incident CKD: A Prospective Cohort Study. Am. J. Kidney Dis. 2022, 80, 589–598.e581. [Google Scholar] [CrossRef]
- Chen, Z.; Khandpur, N.; Desjardins, C.; Wang, L.; Monteiro, C.A.; Rossato, S.L.; Fung, T.T.; Manson, J.E.; Willett, W.C.; Rimm, E.B.; et al. Ultra-Processed Food Consumption and Risk of Type 2 Diabetes: Three Large Prospective U.S. Cohort Studies. Diabetes Care 2023, 46, 1335–1344. [Google Scholar] [CrossRef]
- Canhada, S.L.; Vigo, Á.; Luft, V.C.; Levy, R.B.; Alvim Matos, S.M.; Del Carmen Molina, M.; Giatti, L.; Barreto, S.; Duncan, B.B.; Schmidt, M.I. Ultra-Processed Food Consumption and Increased Risk of Metabolic Syndrome in Adults: The ELSA-Brasil. Diabetes Care 2023, 46, 369–376. [Google Scholar] [CrossRef]
- Kliemann, N.; Rauber, F.; Bertazzi Levy, R.; Viallon, V.; Vamos, E.P.; Cordova, R.; Freisling, H.; Casagrande, C.; Nicolas, G.; Aune, D.; et al. Food processing and cancer risk in Europe: Results from the prospective EPIC cohort study. Lancet Planet Health 2023, 7, e219–e232. [Google Scholar] [CrossRef]
- Hang, D.; Wang, L.; Fang, Z.; Du, M.; Wang, K.; He, X.; Khandpur, N.; Rossato, S.L.; Wu, K.; Hu, Z.; et al. Ultra-processed food consumption and risk of colorectal cancer precursors: Results from 3 prospective cohorts. J. Natl. Cancer Inst. 2023, 115, 155–164. [Google Scholar] [CrossRef]
- Weinstein, G.; Vered, S.; Ivancovsky-Wajcman, D.; Ravona-Springer, R.; Heymann, A.; Zelber-Sagi, S.; Shahar, D.R.; Beeri, M.S. Consumption of Ultra-Processed Food and Cognitive Decline among Older Adults With Type-2 Diabetes. J. Gerontol. A Biol. Sci. Med. Sci. 2023, 78, 134–142. [Google Scholar] [CrossRef]
- Guglielmetti, M.; Grosso, G.; Ferraris, C.; Bergamaschi, R.; Tavazzi, E.; La Malfa, A.; Wahidah, H.A.; Tagliabue, A. Ultra-processed foods consumption is associated with multiple sclerosis severity. Front. Neurol. 2023, 14, 1086720. [Google Scholar] [CrossRef]
- Dehghan, M.; Mente, A.; Rangarajan, S.; Mohan, V.; Swaminathan, S.; Avezum, A.; Lear, S.A.; Rosengren, A.; Poirier, P.; Lanas, F.; et al. Ultra-processed foods and mortality: Analysis from the Prospective Urban and Rural Epidemiology study. Am. J. Clin. Nutr. 2023, 117, 55–63. [Google Scholar] [CrossRef]
- Yuan, L.; Hu, H.; Li, T.; Zhang, J.; Feng, Y.; Yang, X.; Li, Y.; Wu, Y.; Li, X.; Huang, H.; et al. Dose-response meta-analysis of ultra-processed food with the risk of cardiovascular events and all-cause mortality: Evidence from prospective cohort studies. Food Funct. 2023, 14, 2586–2596. [Google Scholar] [CrossRef]
- Tobias, D.K.; Hall, K.D. Eliminate or reformulate ultra-processed foods? Biological mechanisms matter. Cell Metab. 2021, 33, 2314–2315. [Google Scholar] [CrossRef]
- Juul, F.; Vaidean, G.; Parekh, N. Ultra-processed Foods and Cardiovascular Diseases: Potential Mechanisms of Action. Adv. Nutr. 2021, 12, 1673–1680. [Google Scholar] [CrossRef]
- Monteiro, C.A.; Cannon, G.; Moubarac, J.C.; Levy, R.B.; Louzada, M.L.C.; Jaime, P.C. The UN Decade of Nutrition, the NOVA food classification and the trouble with ultra-processing. Public Health Nutr. 2018, 21, 5–17. [Google Scholar] [CrossRef] [Green Version]
- Gibney, M.J.; Forde, C.G.; Mullally, D.; Gibney, E.R. Ultra-processed foods in human health: A critical appraisal. Am. J. Clin. Nutr. 2017, 106, 717–724. [Google Scholar] [CrossRef] [Green Version]
- Sneed, N.M.; Ukwuani, S.; Sommer, E.C.; Samuels, L.R.; Truesdale, K.P.; Matheson, D.; Noerper, T.E.; Barkin, S.L.; Heerman, W.J. Reliability and validity of assigning ultraprocessed food categories to 24-h dietary recall data. Am. J. Clin. Nutr. 2023, 117, 182–190. [Google Scholar] [CrossRef]
- Steele, E.M.; O’Connor, L.E.; Juul, F.; Khandpur, N.; Galastri Baraldi, L.; Monteiro, C.A.; Parekh, N.; Herrick, K.A. Identifying and Estimating Ultraprocessed Food Intake in the US NHANES According to the Nova Classification System of Food Processing. J. Nutr. 2023, 153, 225–241. [Google Scholar] [CrossRef] [PubMed]
- Zancheta Ricardo, C.; Duran, A.C.; Grilo, M.F.; Rebolledo, N.; Díaz-Torrente, X.; Reyes, M.; Corvalán, C. Impact of the use of food ingredients and additives on the estimation of ultra-processed foods and beverages. Front. Nutr. 2022, 9, 1046463. [Google Scholar] [CrossRef] [PubMed]
- Calvo, M.S.; Sherman, R.A.; Uribarri, J. Dietary Phosphate and the Forgotten Kidney Patient: A Critical Need for FDA Regulatory Action. Am. J. Kidney Dis. 2019, 73, 542–551. [Google Scholar] [CrossRef] [PubMed]
- Chazelas, E.; Druesne-Pecollo, N.; Esseddik, Y.; de Edelenyi, F.S.; Agaesse, C.; De Sa, A.; Lutchia, R.; Rebouillat, P.; Srour, B.; Debras, C.; et al. Exposure to food additive mixtures in 106,000 French adults from the NutriNet-Santé cohort. Sci. Rep. 2021, 11, 19680. [Google Scholar] [CrossRef] [PubMed]
- Food and Drug Administration. Overview of Food Ingredients, Additives and Colors. Substances Added to Food (Formerly EAFUS). Available online: https://www.fda.gov/food/food-ingredients-packaging (accessed on 6 August 2023).
- Dunford, E.K.; Miles, D.R.; Popkin, B. Food Additives in Ultra-Processed Packaged Foods: An Examination of US Household Grocery Store Purchases. J. Acad. Nutr. Diet. 2023, 123, 889–901. [Google Scholar] [CrossRef]
- Calvo, M.S.; Uribarri, J. Food Additive Use in Ultraprocessed Foods: Can Processing Use of Industrial Additives Contribute to Adverse Health Outcomes in Children? J. Acad. Nutr. Diet. 2023, 123, 861–864. [Google Scholar] [CrossRef]
- Calvo, M.S.; Moshfegh, A.J.; Tucker, K.L. Assessing the health impact of phosphorus in the food supply: Issues and considerations. Adv. Nutr. 2014, 5, 104–113. [Google Scholar] [CrossRef] [Green Version]
- Uribarri, J.; Calvo, M.S. Hidden sources of phosphorus in the typical American diet: Does it matter in nephrology? Semin. Dial. 2003, 16, 186–188. [Google Scholar] [CrossRef]
- Picard, K.; Griffiths, M.; Senior, P.A.; Mager, D.R.; Richard, C. Phosphorus Additives and Their Impact on Phosphorus Content in Foods-An Analysis of the USDAs Branded Foods Product Database. J. Ren. Nutr. 2023, 33, 443–449. [Google Scholar] [CrossRef]
- Juul, F.; Parekh, N.; Martinez-Steele, E.; Monteiro, C.A.; Chang, V.W. Ultra-processed food consumption among US adults from 2001 to 2018. Am. J. Clin. Nutr. 2022, 115, 211–221. [Google Scholar] [CrossRef]
- Baldridge, A.S.; Huffman, M.D.; Taylor, F.; Xavier, D.; Bright, B.; Van Horn, L.V.; Neal, B.; Dunford, E. The Healthfulness of the US Packaged Food and Beverage Supply: A Cross-Sectional Study. Nutrients 2019, 11, 1704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uribarri, J.; Calvo, M.S. Introduction to dietary phosphorus excess and health. Ann. N. Y. Acad. Sci. 2013, 1301, iii–iv. [Google Scholar] [CrossRef] [PubMed]
- Khor, B.H.; Sualeheen, A.; Sahathevan, S.; Chinna, K.; Gafor, A.H.A.; Bavanandan, S.; Goh, B.L.; Morad, Z.; Daud, Z.A.M.; Khosla, P.; et al. Association of dietary patterns with serum phosphorus in maintenance haemodialysis patients: A cross-sectional study. Sci. Rep. 2020, 10, 12278. [Google Scholar] [CrossRef] [PubMed]
- Su, G.; Saglimbene, V.; Wong, G.; Bernier-Jean, A.; Carrero, J.J.; Natale, P.; Ruospo, M.; Hegbrant, J.; Craig, J.C.; Strippoli, G.F.M. Dietary Phosphorus, Its Sources, and Mortality in Adults on Haemodialysis: The DIET-HD Study. Nutrients 2022, 14, 4064. [Google Scholar] [CrossRef] [PubMed]
- Dhingra, R.; Sullivan, L.M.; Fox, C.S.; Wang, T.J.; D’Agostino, R.B., Sr.; Gaziano, J.M.; Vasan, R.S. Relations of serum phosphorus and calcium levels to the incidence of cardiovascular disease in the community. Arch. Intern. Med. 2007, 167, 879–885. [Google Scholar] [CrossRef] [Green Version]
- Chang, A.R.; Lazo, M.; Appel, L.J.; Gutiérrez, O.M.; Grams, M.E. High dietary phosphorus intake is associated with all-cause mortality: Results from NHANES III. Am. J. Clin. Nutr. 2014, 99, 320–327. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto, K.T.; Robinson-Cohen, C.; de Oliveira, M.C.; Kostina, A.; Nettleton, J.A.; Ix, J.H.; Nguyen, H.; Eng, J.; Lima, J.A.; Siscovick, D.S.; et al. Dietary phosphorus is associated with greater left ventricular mass. Kidney Int. 2013, 83, 707–714. [Google Scholar] [CrossRef] [Green Version]
- Cai, Q.; Duan, M.J.; Dekker, L.H.; Carrero, J.J.; Avesani, C.M.; Bakker, S.J.L.; de Borst, M.H.; Navis, G.J. Ultraprocessed food consumption and kidney function decline in a population-based cohort in the Netherlands. Am. J. Clin. Nutr. 2022, 116, 263–273. [Google Scholar] [CrossRef]
- Bell, R.R.; Draper, H.H.; Tzeng, D.Y.; Shin, H.K.; Schmidt, G.R. Physiological responses of human adults to foods containing phosphate additives. J. Nutr. 1977, 107, 42–50. [Google Scholar] [CrossRef]
- Calvo, M.S.; Kumar, R.; Heath, H., 3rd. Elevated secretion and action of serum parathyroid hormone in young adults consuming high phosphorus, low calcium diets assembled from common foods. J. Clin. Endocrinol. Metab. 1988, 66, 823–829. [Google Scholar] [CrossRef]
- Calvo, M.S.; Kumar, R.; Heath, H. Persistently elevated parathyroid hormone secretion and action in young women after four weeks of ingesting high phosphorus, low calcium diets. J. Clin. Endocrinol. Metab. 1990, 70, 1334–1340. [Google Scholar] [CrossRef] [PubMed]
- Kemi, V.E.; Rita, H.J.; Kärkkäinen, M.U.; Viljakainen, H.T.; Laaksonen, M.M.; Outila, T.A.; Lamberg-Allardt, C.J. Habitual high phosphorus intakes and foods with phosphate additives negatively affect serum parathyroid hormone concentration: A cross-sectional study on healthy premenopausal women. Public Health Nutr. 2009, 12, 1885–1892. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sullivan, C.; Sayre, S.S.; Leon, J.B.; Machekano, R.; Love, T.E.; Porter, D.; Marbury, M.; Sehgal, A.R. Effect of food additives on hyperphosphatemia among patients with end-stage renal disease: A randomized controlled trial. JAMA 2009, 301, 629–635. [Google Scholar] [CrossRef] [PubMed]
- Itkonen, S.T.; Karp, H.J.; Kemi, V.E.; Kokkonen, E.M.; Saarnio, E.M.; Pekkinen, M.H.; Kärkkäinen, M.U.; Laitinen, E.K.; Turanlahti, M.I.; Lamberg-Allardt, C.J. Associations among total and food additive phosphorus intake and carotid intima-media thickness—A cross-sectional study in a middle-aged population in Southern Finland. Nutr. J. 2013, 12, 94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gutiérrez, O.M.; Luzuriaga-McPherson, A.; Lin, Y.; Gilbert, L.C.; Ha, S.W.; Beck, G.R., Jr. Impact of Phosphorus-Based Food Additives on Bone and Mineral Metabolism. J. Clin. Endocrinol. Metab. 2015, 100, 4264–4271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moore, L.W.; Nolte, J.V.; Gaber, A.O.; Suki, W.N. Association of dietary phosphate and serum phosphorus concentration by levels of kidney function. Am. J. Clin. Nutr. 2015, 102, 444–453. [Google Scholar] [CrossRef] [Green Version]
- Itkonen, S.T.; Rita, H.J.; Saarnio, E.M.; Kemi, V.E.; Karp, H.J.; Kärkkäinen, M.U.; Pekkinen, M.H.; Laitinen, E.K.; Risteli, J.; Koivula, M.K.; et al. Dietary phosphorus intake is negatively associated with bone formation among women and positively associated with some bone traits among men-a cross-sectional study in middle-aged Caucasians. Nutr. Res. 2017, 37, 58–66. [Google Scholar] [CrossRef] [Green Version]
- de Fornasari, M.L.; Dos Santos Sens, Y.A. Replacing Phosphorus-Containing Food Additives With Foods Without Additives Reduces Phosphatemia in End-Stage Renal Disease Patients: A Randomized Clinical Trial. J. Ren. Nutr. 2017, 27, 97–105. [Google Scholar] [CrossRef]
- Saito, Y.; Sakuma, M.; Narishima, Y.; Yoshida, T.; Kumagai, H.; Arai, H. Greater consumption of noodle is associated with higher serum phosphorus levels: A cross-sectional study on healthy participants. J. Clin. Biochem. Nutr. 2021, 68, 78–85. [Google Scholar] [CrossRef]
- Fulgoni, K.; Fulgoni, V.L., 3rd; Wallace, T.C. Association of Total, Added, and Natural Phosphorus Intakes with Biomarkers of Health Status and Mortality in Healthy Adults in the United States. Nutrients 2022, 14, 1738. [Google Scholar] [CrossRef]
- Duong, C.N.; Akinlawon, O.J.; Gung, J.; Noel, S.E.; Bigornia, S.; Flanagan, K.; Pourafshar, S.; Lin, P.H.; Davenport, C.A.; Pendergast, J.; et al. Bioavailability of phosphorus and kidney function in the Jackson Heart Study. Am. J. Clin. Nutr. 2022, 116, 541–550. [Google Scholar] [CrossRef]
- Moroșan, E.; Popovici, V.; Elian, V.; Dărăban, A.M.; Rusu, A.I.; Licu, M.; Mititelu, M.; Karampelas, O. The Impact of Medical Nutrition Intervention on the Management of Hyperphosphatemia in Hemodialysis Patients with Stage 5 Chronic Kidney Disease: A Case Series. Int. J. Environ. Res. Public Health 2023, 20, 5049. [Google Scholar] [CrossRef]
- Mazariegos-Ramos, E.; Guerrero-Romero, F.; Rodríguez-Morán, M.; Lazcano-Burciaga, G.; Paniagua, R.; Amato, D. Consumption of soft drinks with phosphoric acid as a risk factor for the development of hypocalcemia in children: A case-control study. J. Pediatr. 1995, 126, 940–942. [Google Scholar] [CrossRef]
- Fernando, G.R.; Martha, R.M.; Evangelina, R. Consumption of soft drinks with phosphoric acid as a risk factor for the development of hypocalcemia in postmenopausal women. J. Clin. Epidemiol. 1999, 52, 1007–1010. [Google Scholar] [CrossRef]
- Kristensen, M.; Jensen, M.; Kudsk, J.; Henriksen, M.; Mølgaard, C. Short-term effects on bone turnover of replacing milk with cola beverages: A 10-day interventional study in young men. Osteoporos. Int. 2005, 16, 1803–1808. [Google Scholar] [CrossRef]
- Tucker, K.L.; Morita, K.; Qiao, N.; Hannan, M.T.; Cupples, L.A.; Kiel, D.P. Colas, but not other carbonated beverages, are associated with low bone mineral density in older women: The Framingham Osteoporosis Study. Am. J. Clin. Nutr. 2006, 84, 936–942. [Google Scholar] [CrossRef] [Green Version]
- Guarnotta, V.; Riela, S.; Massaro, M.; Bonventre, S.; Inviati, A.; Ciresi, A.; Pizzolanti, G.; Benvenga, S.; Giordano, C. The Daily Consumption of Cola Can Determine Hypocalcemia: A Case Report of Postsurgical Hypoparathyroidism-Related Hypocalcemia Refractory to Supplemental Therapy with High Doses of Oral Calcium. Front. Endocrinol. 2017, 8, 7. [Google Scholar] [CrossRef] [Green Version]
- Gallagher, J.C. Sodas and colas are associated with an increase in fractures. Menopause 2019, 26, 1229–1231. [Google Scholar] [CrossRef]
- Kim, Y.A.; Yoo, J.H. Associations between cola consumption and bone mineral density in Korean adolescents and young adults: A cross-sectional study using data from the Korea National Health and Nutrition Examination Survey, 2008–2011. J. Nutr. Sci. 2020, 9, e56. [Google Scholar] [CrossRef]
- Volk, C.; Schmidt, B.; Brandsch, C.; Kurze, T.; Schlegelmilch, U.; Grosse, I.; Ulrich, C.; Girndt, M.; Stangl, G.I. Acute Effects of an Inorganic Phosphorus Additive on Mineral Metabolism and Cardiometabolic Risk Factors in Healthy Subjects. J. Clin. Endocrinol. Metab. 2022, 107, e852–e864. [Google Scholar] [CrossRef]
- Nishida, Y.; Taketani, Y.; Yamanaka-Okumura, H.; Imamura, F.; Taniguchi, A.; Sato, T.; Shuto, E.; Nashiki, K.; Arai, H.; Yamamoto, H.; et al. Acute effect of oral phosphate loading on serum fibroblast growth factor 23 levels in healthy men. Kidney Int. 2006, 70, 2141–2147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferrari, S.L.; Bonjour, J.P.; Rizzoli, R. Fibroblast growth factor-23 relationship to dietary phosphate and renal phosphate handling in healthy young men. J. Clin. Endocrinol. Metab. 2005, 90, 1519–1524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vervloet, M.G.; van Ittersum, F.J.; Büttler, R.M.; Heijboer, A.C.; Blankenstein, M.A.; ter Wee, P.M. Effects of dietary phosphate and calcium intake on fibroblast growth factor-23. Clin. J. Am. Soc. Nephrol. 2011, 6, 383–389. [Google Scholar] [CrossRef] [Green Version]
- Vervloet, M.G. Shedding Light on the Complex Regulation of FGF23. Metabolites 2022, 12, 401. [Google Scholar] [CrossRef] [PubMed]
- Donat-Vargas, C.; Guallar-Castillon, P.; Nyström, J.; Larsson, S.C.; Kippler, M.; Vahter, M.; Faxén-Irving, G.; Michaelsson, K.; Wolk, A.; Stenvinkel, P.; et al. Urinary phosphate is associated with cardiovascular disease incidence. J. Intern. Med. 2023. [Google Scholar] [CrossRef]
- Lang, F.; Leibrock, C.; Pandyra, A.A.; Stournaras, C.; Wagner, C.A.; Föller, M. Phosphate Homeostasis, Inflammation and the Regulation of FGF-23. Kidney Blood Press Res. 2018, 43, 1742–1748. [Google Scholar] [CrossRef]
- Kandarini, Y.; Mahadita, G.W.; Herawati, S.; Wibhuti, I.B.R.; Widiana, I.G.R.; Ayu, N.P. High C-Terminal Fibroblast Growth Factor-23, Intact Parathyroid Hormone, and Interleukin-6 as Determinants of Valvular Calcification in Regular Hemodialysis Patients. Int. J. Gen. Med. 2022, 15, 4227–4236. [Google Scholar] [CrossRef]
- Saxena, A.; Sachan, T.; Gupta, A.; Kapoor, V. Effect of Dietary Phosphorous Restriction on Fibroblast Growth 2 Factor-23 and sKlotho Levels in Patients with Stages 1–2 Chronic Kidney Disease. Nutrients 2022, 14, 3302. [Google Scholar] [CrossRef]
- Raikou, V.D. Serum phosphate and chronic kidney and cardiovascular disease: Phosphorus potential implications in general population. World J. Nephrol. 2021, 10, 76–87. [Google Scholar] [CrossRef]
- Chazelas, E.; Deschasaux, M.; Srour, B.; Kesse-Guyot, E.; Julia, C.; Alles, B.; Druesne-Pecollo, N.; Galan, P.; Hercberg, S.; Latino-Martel, P.; et al. Food additives: Distribution and co-occurrence in 126,000 food products of the French market. Sci. Rep. 2020, 10, 3980. [Google Scholar] [CrossRef] [Green Version]
- Institute of Medicine (US). Standing Committee on the Scientific Evaluation of Dietary Reference Intakes. In Phosphorus; National Academic Press: Washington, DC, USA, 1997. [Google Scholar]
- Brannon, P.M.; Weaver, C.M.; Anderson, C.A.; Donovan, S.M.; Murphy, S.P.; Yaktine, A.L. Scanning for new evidence to prioritize updates to the Dietary Reference Intakes: Case studies for thiamin and phosphorus. Am. J. Clin. Nutr. 2016, 104, 1366–1377. [Google Scholar] [CrossRef] [Green Version]
- Fulgoni, K.; Fulgoni, V.L., 3rd. Trends in Total, Added, and Natural Phosphorus Intake in Adult Americans, NHANES 1988–1994 to NHANES 2015–2016. Nutrients 2021, 13, 2249. [Google Scholar] [CrossRef]
- Martinez Steele, E.; Marrón Ponce, J.A.; Cediel, G.; Louzada, M.L.C.; Khandpur, N.; Machado, P.; Moubarac, J.C.; Rauber, F.; Corvalán, C.; Levy, R.B.; et al. Potential reductions in ultra-processed food consumption substantially improve population cardiometabolic-related dietary nutrient profiles in eight countries. Nutr. Metab. Cardiovasc. Dis. 2022, 32, 2739–2750. [Google Scholar] [CrossRef]
- Molina, R.E.; Bohrer, B.M.; Mejia, S.M.V. Phosphate alternatives for meat processing and challenges for the industry: A critical review. Food Res. Int. 2023, 166, 112624. [Google Scholar] [CrossRef] [PubMed]
- Mafra, D.; Borges, N.A.; Lindholm, B.; Shiels, P.G.; Evenepoel, P.; Stenvinkel, P. Food as medicine: Targeting the uraemic phenotype in chronic kidney disease. Nat. Rev. Nephrol. 2021, 17, 153–171. [Google Scholar] [CrossRef] [PubMed]
- Capozzi, F. Food Innovation in the Frame of Circular Economy by Designing Ultra-Processed Foods Optimized for Sustainable Nutrition. Front. Nutr. 2022, 9, 886220. [Google Scholar] [CrossRef] [PubMed]
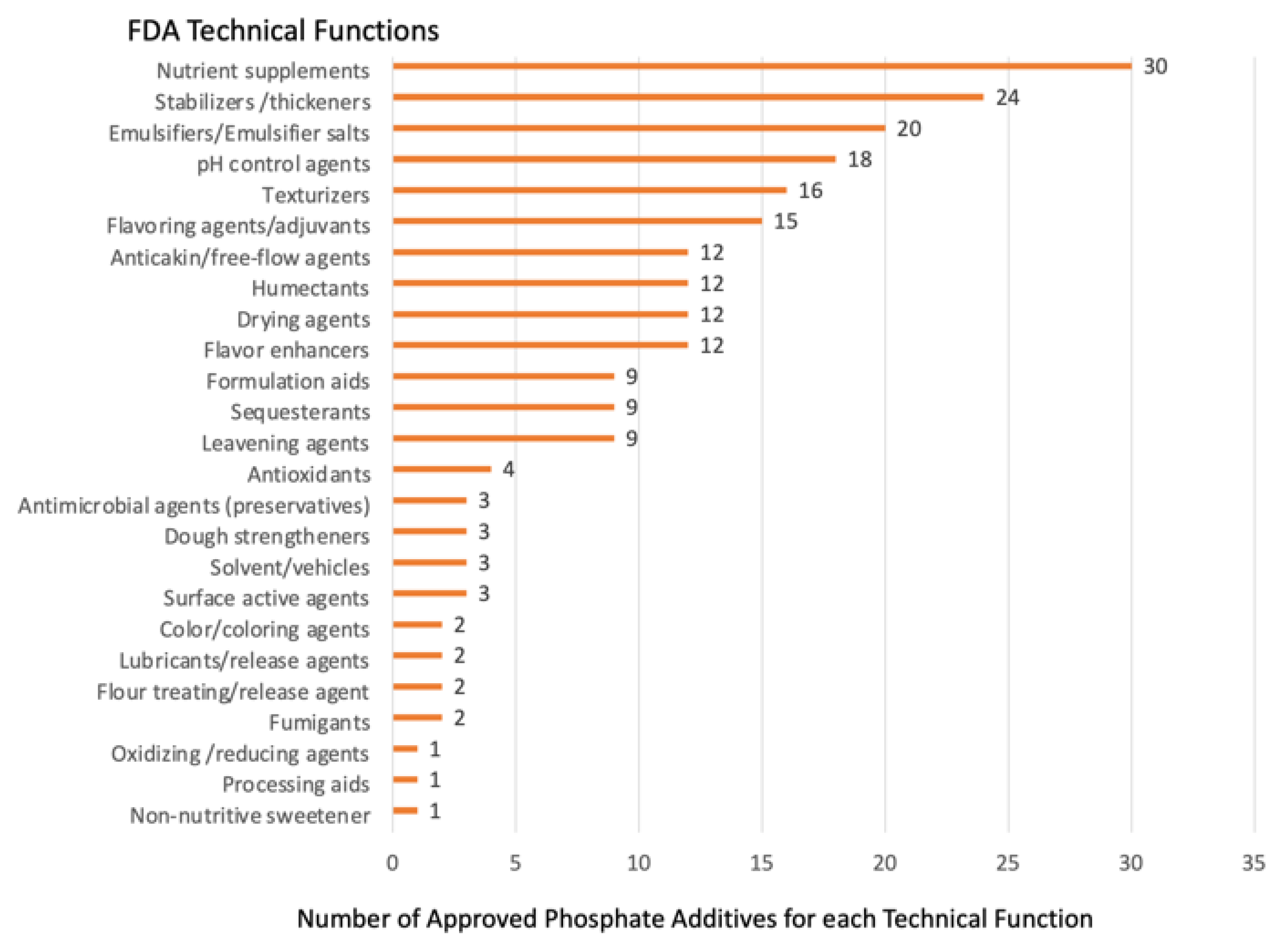

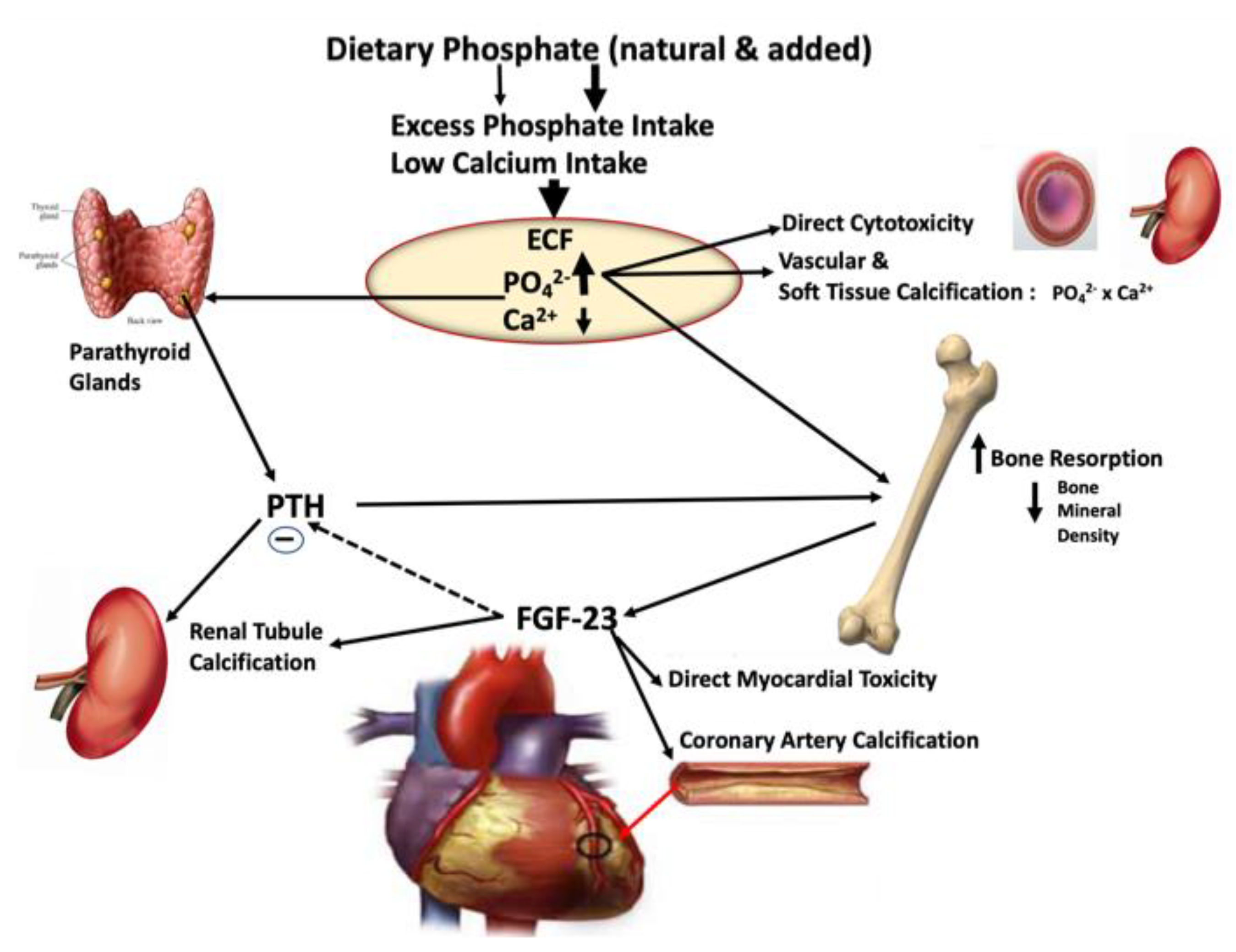

| Studies Using Market-Available Foods Containing Phosphate Additives [Reference] | Study Outcomes |
|---|---|
| Bell RR et al., 1977 [40] | Elevated parathyroid hormone biomarkers with high-phosphate additives in foods, >200 mg P/day, dietary Ca:P ratio = 0.35 |
| Calvo MS et al., 1988 [41] | Elevated parathyroid hormone after 2 weeks of grocery store foods with high-phosphate additives and low calcium content, dietary Ca:P ratio = 0.3 |
| Calvo MS et al., 1990 [42] | Parallel study design with test diet high in phosphate additives, low calcium content from purchased foods showing persistent elevation in parathyroid hormone but decrease in calcitriol after consuming for 4 weeks, Ca:P ratio = 0.3 |
| Kemi VE et al., 2009 [43] | A cross-sectional population study in healthy Finnish women showed higher parathyroid hormone concentrations in those consuming diets with Ca:P ratio = 0.56. |
| Sullivan C et al., 2009 [44] | Avoidance of foods with phosphate additives reduced serum phosphorus in dialysis patients. |
| Itkonen ST et al., 2013 [45] | Greater intakes of foods high in phosphate additives were associated with greater carotid intima thickness. |
| Gutiérrez OM et al., 2015 [46] | After a week of low phosphate additive diets (Ca:P = 0.77), a week on a diet high in phosphate additive (Ca:P = 0.47) increased fibroblast growth factor-23 and markers of bone metabolism. |
| Moore, LW, et al., 2015 [47] | Dairy and cereal grain products with phosphate food additives significantly increased serum phosphorus in early chronic kidney disease. |
| Itkonen, ST, et al., 2017 [48] | Phosphate intake in women, not in men, was negatively associated with bone formation markers. |
| De Fornasari ML et al., 2017 [49] | Phosphate additive–free food consumption reduced hyperphosphatemia and parathyroid hormone concentrations in dialysis patients. |
| Saito Y et al., 2021 [50] | Consumers of instant ramen noodles (high in phosphate additives) had higher serum phosphate. |
| Fulgoni K et al., 2022 [51] | Added phosphate intake was consistently inversely associated with HDL cholesterol in both men and women (NHANES data). |
| Duong CN et al., 2022 [52] | Phosphorus contents in foods were designated as natural or added, and intake was weighted by their bioavailability (used an algorithm based on literature). Added, but not natural, phosphorus was negatively linked to the estimated glomerular filtration rate in the Jackson Heart Study. |
| Moroșan E et al., 2023 [53] | Dialysis patients treated with personalized nutrition therapy substituting processed high phosphate-additive foods with low phosphate-additive foods and use of phosphate binders significantly reduced phosphatemia after 60 days of treatment. |
| Studies Examining Cola Intake [Reference] | Study Outcome |
|---|---|
| Mazariegos-Ramos E et al., 1995 [54] | Hypocalcemia in children was directly associated with the consumption of soft drinks containing phosphoric acid. |
| Fernando GR et al., 1999 [55] | Hypocalcemia in postmenopausal women was associated with the consumption of soft drinks processed with phosphoric acid. |
| Kristensen M et al., 2005 [56] | Increased serum phosphate, parathyroid hormone, bone turnover marker osteocalcin, and serum and urinary markers of bone resorption were among the acute effects of replacing milk with cola beverages for 10 days. |
| Tucker KL et al., 2006 [57] | Cola beverage (containing phosphoric acid) consumption was associated with low bone mineral density in older women. |
| Guarnotta V et al., 2019 [58] | Daily cola consumption was associated with hypocalcemia. |
| Gallagher JC 2019 [59] | Carbonated beverage and cola consumption in young Korean males was inversely associated with the whole body, whole femur, and femoral neck bone mineral density. |
| Kim YA and Yoo JH 2020 [60] | A cross-sectional population study of cola consumption in young Korean males reported inversely associated cola intake and whole body, whole femur, and femoral neck bone mineral density. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calvo, M.S.; Dunford, E.K.; Uribarri, J. Industrial Use of Phosphate Food Additives: A Mechanism Linking Ultra-Processed Food Intake to Cardiorenal Disease Risk? Nutrients 2023, 15, 3510. https://doi.org/10.3390/nu15163510
Calvo MS, Dunford EK, Uribarri J. Industrial Use of Phosphate Food Additives: A Mechanism Linking Ultra-Processed Food Intake to Cardiorenal Disease Risk? Nutrients. 2023; 15(16):3510. https://doi.org/10.3390/nu15163510
Chicago/Turabian StyleCalvo, Mona S., Elizabeth K. Dunford, and Jaime Uribarri. 2023. "Industrial Use of Phosphate Food Additives: A Mechanism Linking Ultra-Processed Food Intake to Cardiorenal Disease Risk?" Nutrients 15, no. 16: 3510. https://doi.org/10.3390/nu15163510





