Obesity, Insulin Resistance, Caries, and Periodontitis: Syndemic Framework
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Data Collection
2.3. Latent Variables
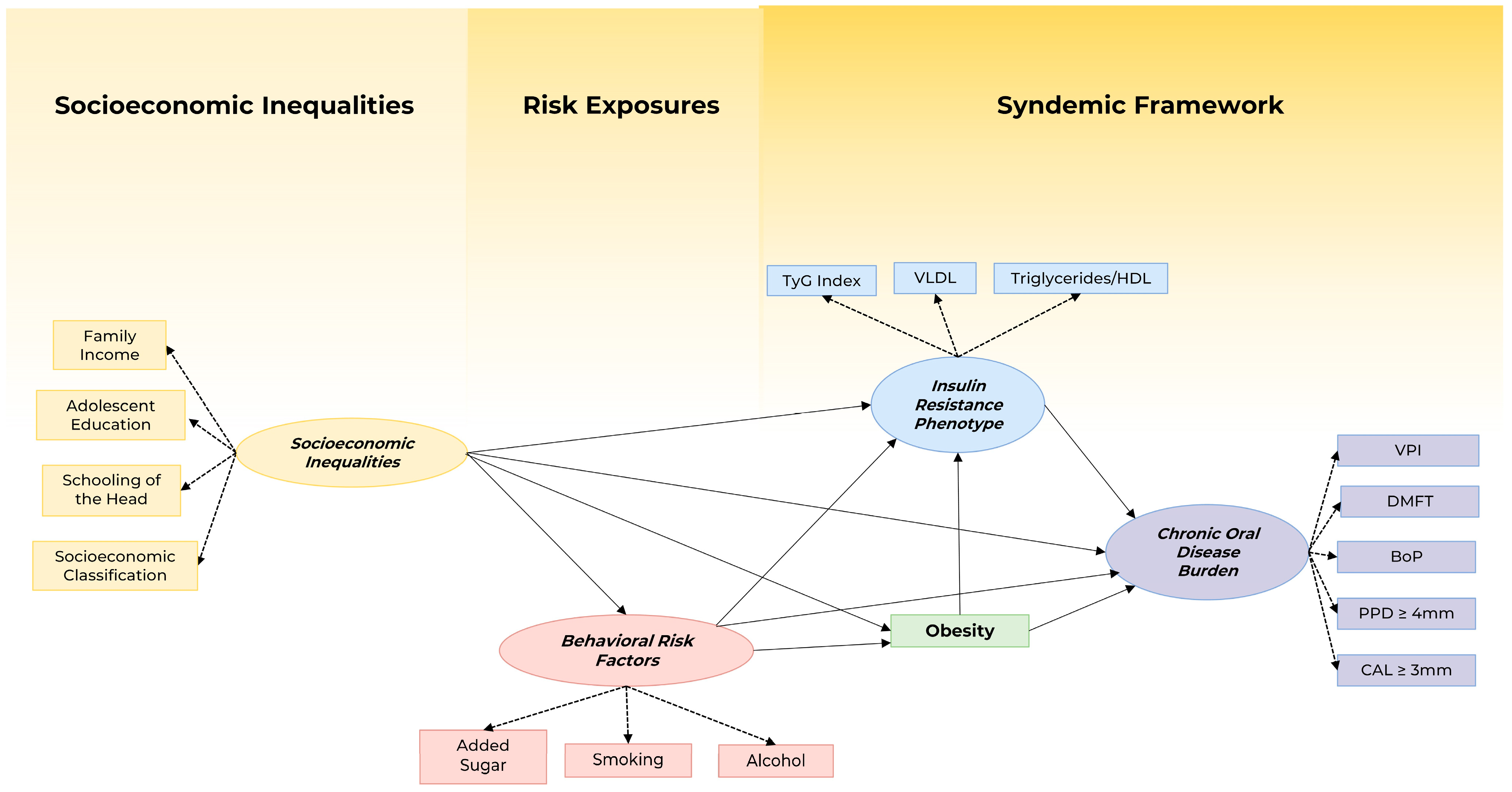
2.4. Theoretical Model
2.5. Statistical Analysis
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singer, M.; Bulled, N.; Ostrach, B.; Mendenhall, E. Syndemics and the Biosocial Conception of Health. Lancet 2017, 389, 941–950. [Google Scholar] [CrossRef]
- Mendenhall, E.; Kohrt, B.A.; Norris, S.A.; Ndetei, D.; Prabhakaran, D. Non-Communicable Disease Syndemics: Poverty, Depression, and Diabetes among Low-Income Populations. Lancet 2017, 389, 951–963. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Network, Global Burden of Disease Collaborative. Global Burden of Disease Study 2019 (GBD 2019); Institute of Health Metrics and Evaluation (IHME). 2020. Available online: http://ghdx.healthdata.org/gbd-results-tool (accessed on 1 June 2023).
- Peres, M.A.; Macpherson, L.M.D.; Weyant, R.J.; Daly, B.; Venturelli, R.; Mathur, M.R.; Listl, S.; Celeste, R.K.; Guarnizo-Herreño, C.C.; Kearns, C.; et al. Oral Diseases: A Global Public Health Challenge. Lancet 2019, 394, 249–260. [Google Scholar] [CrossRef]
- Carmo, C.D.S.S.; Ribeiro, M.R.C.C.; Teixeira, J.X.P.P.; Alves, C.M.C.C.; Franco, M.M.; França, A.K.T.C.T.C.; Benatti, B.B.; Cunha-Cruz, J.; Ribeiro, C.C.C.C.; Cunha-Cruz, J.; et al. Added Sugar Consumption and Chronic Oral Disease Burden among Adolescents in Brazil. J. Dent. Res. 2018, 97, 508–514. [Google Scholar] [CrossRef] [PubMed]
- Nyvad, B.; Takahashi, N. Integrated Hypothesis of Dental Caries and Periodontal Diseases. J. Oral Microbiol. 2020, 12, 1710953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aragno, M.; Mastrocola, R. Dietary Sugars and Endogenous Formation of Advanced Glycation Endproducts: Emerging Mechanisms of Disease. Nutrients 2017, 9, 385. [Google Scholar] [CrossRef] [Green Version]
- Costa, S.A.; Nascimento, G.G.; Colins, P.M.G.; Alves, C.M.C.; Thomaz, E.B.A.F.; Carvalho Souza, S.D.F.; da Silva, A.A.M.; Ribeiro, C.C.C. Investigating Oral and Systemic Pathways between Unhealthy and Healthy Dietary Patterns to Periodontitis in Adolescents: A Population-Based Study. J. Clin. Periodontol. 2022, 49, 580–590. [Google Scholar] [CrossRef]
- Phillips, C.M.; Chen, L.W.; Heude, B.; Bernard, J.Y.; Harvey, N.C.; Duijts, L.; Mensink-Bout, S.M.; Polanska, K.; Mancano, G.; Suderman, M.; et al. Dietary Inflammatory Index and Non-Communicable Disease Risk: A Narrative Review. Nutrients 2019, 11, 1873. [Google Scholar] [CrossRef] [Green Version]
- Lima, G.Q.T.; Brondani, M.A.; da Silva, A.A.M.; do Carmo, C.D.S.; da Silva, R.A.; Ribeiro, C.C.C. Serum Levels of Proinflammatory Cytokines Are High in Early Childhood Caries. Cytokine 2018, 111, 490–495. [Google Scholar] [CrossRef]
- WHO. Guideline: Sugars Intake for Adults and Children; World Health Organ: Geneva, Switzerland, 2015; Volume 26, pp. 34–36. ISBN 978-92-4-154902-8. [Google Scholar]
- Martens, L.; De Smet, S.; Yusof, M.Y.P.M.; Rajasekharan, S.; Martens, L.; De Smet, S.; Yusof, M.Y.P.M.; Rajasekharan, S.; Martens, L.; De Smet, S.; et al. Association between Overweight/Obesity and Periodontal Disease in Children and Adolescents: A Systematic Review and Meta-Analysis. Eur. Arch. Paediatr. Dent. 2017, 18, 69–82. [Google Scholar] [CrossRef]
- Ribeiro, C.C.C.; Silva, M.C.B.D.; Nunes, A.M.M.; Thomaz, E.B.D.A.F.; Carmo, C.D.S.; Ribeiro, M.R.C.; Silva, A.A.M.D. Overweight, Obese, Underweight, and Frequency of Sugar Consumption as Risk Indicators for Early Childhood Caries in Brazilian Preschool Children. Int. J. Paediatr. Dent. 2017, 27, 532–539. [Google Scholar] [CrossRef] [PubMed]
- Araújo, S.M.P.; da Silva, G.Q.T.L.; Costa, E.L.; Nunes, A.M.M.; Ribeiro, C.C.C. Pathways in the Association between Added Sugar Consumption, Obesity in Mother-Child Dyads, and Chronic Oral Disease Burden in Early Childhood. Eur. J. Oral Sci. 2022, 130, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Leite, F.R.M.; Peres, K.G.; Do, L.G.; Demarco, F.F.; Peres, M.A.A. Prediction of Periodontitis Occurrence: Influence of Classification and Sociodemographic and General Health Information. J. Periodontol. 2017, 88, 731–743. [Google Scholar] [CrossRef] [PubMed]
- Demmer, R.T.; Jacobs, D.R.; Desvarieux, M. Periodontal Disease and Incident Type 2 Diabetes: Results from the First National Health and Nutrition Examination Survey and Its Epidemiologic Follow-up Study. Diabetes Care 2008, 31, 1373–1379. [Google Scholar] [CrossRef] [Green Version]
- Ladeira, L.L.C.; Leite, F.R.M.; Nascimento, G.G.; Saraiva, M.D.C.; Brondani, M.A.; Moreira, A.R.O.; Ribeiro, C.C.C. Precursors of Insulin Resistance Underlying Periodontitis in Adolescents Aged 17–18 Years. Oral Dis. 2022. [Google Scholar] [CrossRef]
- Liu, J.; Zong, X.; Vogtmann, E.; Cao, C.; James, A.S.; Chan, A.T.; Rimm, E.B.; Hayes, R.B.; Colditz, G.A.; Michaud, D.S.; et al. Tooth Count, Untreated Caries and Mortality in US Adults: A Population-Based Cohort Study. Int. J. Epidemiol. 2022, 51, 1291–1303. [Google Scholar] [CrossRef]
- Costa, S.A.; Ribeiro, C.C.C.; Leite, F.R.M.; Peres, M.A.; Souza, S.D.F.C.; Nascimento, G.G. Chronic Oral Diseases Burden: The Confluence of Caries and Periodontitis throughout Life. J. Clin. Periodontol. 2023, 50, 452–462. [Google Scholar] [CrossRef]
- Barbosa, J.M.A.; da Silva, A.A.M.; Batista, R.F.L.; Salgado, B.J.L.; Nascimento, J.X.P.T.; Simões, V.M.F.; Ribeiro, M.J.S.; Barbieri, M.A.; Ferraro, A.A.; Ribeiro, C.C.C. Insulin Resistance Phenotype Is Associated with Vascular Risk Phenotype at the End of the Second Decade of Life: A Population-Based Study. Cardiovasc. Diabetol. 2022, 21, 284. [Google Scholar] [CrossRef]
- Confortin, S.C.; Ribeiro, M.R.C.; Barros, A.J.D.; Menezes, A.M.B.; Horta, B.L.; Victora, C.G.; Barros, F.C.; Gonçalves, H.; Bettiol, H.; Dos Santos, I.S.; et al. RPS Brazilian Birth Cohort Consortium (Ribeirão Preto, Pelotas and São Luís): History, Objectives and Methods. Cad. Saude Publica 2021, 37. [Google Scholar] [CrossRef]
- Moretti-Pires, R.O.; Corradi-Webster, C.M. Adaptação e Validação Do Alcohol Use Disorder Identification Test (AUDIT) Para População Ribeirinha Do Interior Da Amazônia, Brasil. Cad. Saude Publica 2011, 27, 497–509. [Google Scholar] [CrossRef]
- Schneider, B.C.; Motta, J.V.D.S.; Muniz, L.C.; Bielemann, R.M.; Madruga, S.W.; Orlandi, S.P.; Gigante, D.P.; Assunção, M.C.F. Desenho de Um Questionário de Frequência Alimentar Digital Autoaplicado Para Avaliar o Consumo Alimentar de Adolescentes e Adultos Jovens: Coortes de Nascimentos de Pelotas, Rio Grande Do Sul. Rev. Bras. Epidemiol. 2016, 19, 419–432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vos, M.B.; Kaar, J.L.; Welsh, J.A.; Van Horn, L.V.; Feig, D.I.; Anderson, C.A.M.; Patel, M.J.; Cruz Munos, J.; Krebs, N.F.; Xanthakos, S.A.; et al. Added Sugars and Cardiovascular Disease Risk in Children: A Scientific Statement From the American Heart Association. Circulation 2017, 135, e1017–e1034. [Google Scholar] [CrossRef] [Green Version]
- WHO. Obesity: Preventing and Managing the Global Epidemic: Report of a WHO Consultation; World Health Organization Technical Report Series 894: i–xii; WHO: Geneva, Switzerland, 2000; pp. 1–253. [Google Scholar]
- Ainamo, J.; Bay, I. Problems and Proposals for Recording Gingivitis and Plaque. Int. Dent. J. 1975, 25, 229–235. [Google Scholar]
- Kline, R.B. Principles and Practice of Structural Equation Modeling, 3rd ed.; The Gilford Press: New York, NY, USA, 2011; Volume 3, ISBN 9781606238776. [Google Scholar]
- Nascimento, G.G.; Leite, F.R.M.M.; Peres, K.G.; Demarco, F.F.; Corrêa, M.B.; Peres, M.A. Metabolic Syndrome and Periodontitis: A Structural Equation Modeling Approach. J. Periodontol. 2019, 90, 655–662. [Google Scholar] [CrossRef] [PubMed]
- Ladeira, L.L.C.; Nascimento, G.G.; Leite, F.R.M.; Alves-Costa, S.; Alves, C.M.C.; Thomaz, E.B.A.F.; Cury, J.A. Sugar Intake above International Recommendations Is Associated with a Heavy Oral Disease Burden. Oral Dis. 2022; ahead of print. [Google Scholar]
- Caprio, S.; Perry, R.; Kursawe, R. Adolescent Obesity and Insulin Resistance: Roles of Ectopic Fat Accumulation and Adipose Inflammation. Gastroenterology 2017, 152, 1638–1646. [Google Scholar] [CrossRef]
- Seneviratne, C.J.; Zhang, C.F.; Samaranayake, L.P. Dental Plaque Biofilm in Oral Health and Disease. Chin. J. Dent. Res. 2011, 14, 87–94. [Google Scholar] [PubMed]
- Nascimento, G.G.; Baelum, V.; Dahlen, G.; Lopez, R. Methodological Issues in Assessing the Association between Periodontitis and Caries among Adolescents. Community Dent. Oral Epidemiol. 2018, 46, 303–309. [Google Scholar] [CrossRef]
- Moreira, A.R.O.O.; Batista, R.F.L.L.; Ladeira, L.L.C.C.; Thomaz, E.B.A.F.A.F.; Alves, C.M.C.C.; Saraiva, M.C.; Silva, A.A.M.M.; Brondani, M.A.; Ribeiro, C.C.C.C. Higher Sugar Intake Is Associated with Periodontal Disease in Adolescents. Clin. Oral Investig. 2021, 25, 983–991. [Google Scholar] [CrossRef]
- Wolf, T.G.; Cagetti, M.G.; Fisher, J.-M.; Seeberger, G.K.; Campus, G. Non-Communicable Diseases and Oral Health: An Overview. Front. Oral Health 2021, 2, 725460. [Google Scholar] [CrossRef]
- Reitsma, M.B.; Kendrick, P.J.; Ababneh, E.; Abbafati, C.; Abbasi-Kangevari, M.; Abdoli, A.; Abedi, A.; Abhilash, E.S.; Abila, D.B.; Aboyans, V.; et al. Spatial, Temporal, and Demographic Patterns in Prevalence of Smoking Tobacco Use and Attributable Disease Burden in 204 Countries and Territories, 1990–2019: A Systematic Analysis from the Global Burden of Disease Study 2019. Lancet 2021, 397, 2337–2360. [Google Scholar] [CrossRef]
- Hach, M.; Christensen, L.B.; Lange, T.; Hvidtfeldt, U.A.; Danielsen, B.; Diderichsen, F.; Osler, M.; Prescott, E.; Andersen, I. Social Inequality in Tooth Loss, the Mediating Role of Smoking and Alcohol Consumption. Community Dent. Oral Epidemiol. 2019, 47, 416–423. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.K.; Appel, L.J.; Brands, M.; Howard, B.V.; Lefevre, M.; Lustig, R.H.; Sacks, F.; Steffen, L.M.; Wylie-Rosett, J. Dietary Sugars Intake and Cardiovascular Health a Scientific Statement from the American Heart Association. Circulation 2009, 120, 1011–1020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knorst, J.K.; Sfreddo, C.S.; de F. Meira, G.; Zanatta, F.B.; Vettore, M.V.; Ardenghi, T.M. Socioeconomic Status and Oral Health-related Quality of Life: A Systematic Review and Meta-analysis. Community Dent. Oral Epidemiol. 2021, 49, 95–102. [Google Scholar] [CrossRef]
- Sfreddo, C.S.; Moreira, C.H.C.; Nicolau, B.; Ortiz, F.R.; Ardenghi, T.M. Socioeconomic Inequalities in Oral Health-Related Quality of Life in Adolescents: A Cohort Study. Qual. Life Res. 2019, 28, 2491–2500. [Google Scholar] [CrossRef] [PubMed]
- Bloch, K.V.; Klein, C.H.; Szklo, M.; Kuschnir, M.C.C.; Abreu, G.D.A.; Barufaldi, L.A.; Veiga, G.V.D.; Schaan, B.; da Silva, T.L.N.; Moraes, A.J.P.; et al. ERICA: Prevalences of Hypertension and Obesity in Brazilian Adolescents. Rev. Saude Publica 2016, 50, 1s–10s. [Google Scholar] [CrossRef]
- Lula, E.C.; Ribeiro, C.C.; Hugo, F.N.; Alves, C.M.; Silva, A.A. Added Sugars and Periodontal Disease in Young Adults: An Analysis of NHANES III Data. Am. J. Clin. Nutr. 2014, 100, 1182–1187. [Google Scholar] [CrossRef] [Green Version]
- Leite, F.R.M.; Nascimento, G.G.; Scheutz, F.; López, R. Effect of Smoking on Periodontitis: A Systematic Review and Meta-Regression. Am. J. Prev. Med. 2018, 54, 831–841. [Google Scholar] [CrossRef]
- Wang, J.; Lv, J.; Wang, W.; Jiang, X. Alcohol Consumption and Risk of Periodontitis: A Meta-Analysis. J. Clin. Periodontol. 2016, 43, 572–583. [Google Scholar] [CrossRef]
- Roesler, A.; Rojas, N.; Falbe, J. Sugar-Sweetened Beverage Consumption, Perceptions, and Disparities in Children and Adolescents. J. Nutr. Educ. Behav. 2021, 53, 553–563. [Google Scholar] [CrossRef]
- Nordestgaard, B.G.; Langsted, A.; Mora, S.; Kolovou, G.; Baum, H.; Bruckert, E.; Watts, G.F.; Sypniewska, G.; Wiklund, O.; Borén, J.; et al. Fasting Is Not Routinely Required for Determination of a Lipid Profile: Clinical and Laboratory Implications Including Flagging at Desirable Concentration Cut-Points-a Joint Consensus Statement from the European Atherosclerosis Society and European Federa. Eur. Heart J. 2016, 37, 1944–1958. [Google Scholar] [CrossRef] [Green Version]
- American Diabetes Association 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2021. Diabetes Care 2021, 44, S15–S33. [Google Scholar] [CrossRef] [PubMed]
- Nordestgaard, B.G. A Test in Context: Lipid Profile, Fasting Versus Nonfasting. J. Am. Coll. Cardiol. 2017, 70, 1637–1646. [Google Scholar] [CrossRef] [PubMed]
- WHO. Adolescents and Mental Health; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
| Variables | N | % | Mean | Standard Deviation |
|---|---|---|---|---|
| Income, Brazilian minimum monthly wage | ||||
| ≥5 | 285 | 11.33 | - | - |
| 3 to <5 | 338 | 13.44 | - | - |
| 1 to <3 | 1079 | 42.90 | - | - |
| <1 | 797 | 31.69 | - | - |
| Missing | 16 | 0.64 | ||
| Educational level of the adolescent | ||||
| College (incomplete) | 672 | 26.72 | - | - |
| High school | 1758 | 69.90 | - | - |
| Middle school | 83 | 3.30 | - | - |
| Missing | 2 | 0.08 | - | - |
| Educational level of the head of the family | ||||
| College | 325 | 12.92 | - | - |
| Incomplete college | 81 | 3.22 | - | - |
| High school | 1260 | 50.10 | - | - |
| Middle school | 563 | 22.39 | - | - |
| Illiterate | 286 | 11.37 | - | - |
| Family economic class (ABEP) | ||||
| A | 94 | 4.22 | - | - |
| B | 565 | 25.37 | - | - |
| C and D | 1118 | 50.20 | - | - |
| E | 450 | 20.21 | ||
| Sex | ||||
| Male | 1197 | 47.59 | - | - |
| Female | 1318 | 52.41 | - | - |
| Adolescent age | ||||
| 18 years | 1742 | 69.26 | - | - |
| 19 years | 773 | 30.74 | - | - |
| Added sugar | ||||
| <25 g | 466 | 18.65 | - | - |
| 25 g a 49.9 g | 705 | 28.21 | - | - |
| 50 g a 74.9 g | 526 | 21.05 | - | - |
| >75 g | 802 | 32.09 | - | - |
| Smoking | ||||
| Yes | 89 | 3.56 | - | - |
| No | 2414 | 96.44 | - | - |
| Risk of alcohol abuse | ||||
| High risk | 488 | 19.43 | - | - |
| Low risk | 2.023 | 80.57 | - | - |
| Height (cm) | 166.81 | 9.11 | - | - |
| Weight (kg) | 61.48 | 13.13 | - | - |
| Body Mass Index (BMI) | ||||
| Non-obese (BMI < 25 kg/m2) | 1905 | 75.75 | - | - |
| Overweight (BMI ≥ 25 to <30 kg/m2) | 459 | 18.25 | - | - |
| Obese (BMI ≥ 30 kg/m2) | 151 | 6.00 | - | - |
| Triglycerides | - | - | 91.01 | 49.28 |
| HDL | - | - | 49.37 | 11.92 |
| Blood glucose level | - | - | 91.98 | 15.78 |
| Triglycerides /HDL | - | - | 2.05 | 1.72 |
| VLDL | - | - | 18.18 | 9.73 |
| TyG | - | - | 8.22 | 0.46 |
| Number of decayed teeth | - | - | 1.58 | 2.14 |
| Visible plaque index (%) | ||||
| <15% | 952 | 39.97 | - | - |
| ≥15% | 1430 | 60.03 | - | - |
| Bleeding on probing | - | - | 11.65 | 6.67 |
| Periodontal probing depth ≥ 4 | - | - | 1.17 | 2.47 |
| Clinical attachment level ≥ 3 mm | - | - | 13.98 | 7.20 |
| Estimators | Expected Indices | Model Indices |
|---|---|---|
| X2 * | 475.064 | |
| Degrees of freedom | 120 | |
| p value X2 | 0.0000 | |
| RMSEA † | <0.05 | 0.067 |
| 90% CI ‡ | <0.08 | 0.64–0.070 |
| p § | >0.05 | 0.890 |
| CFI || | >0.90 | 0.938 |
| TLI # | >0.90 | 0.920 |
| Latent Variable | Standardized Coefficient | Standardized Error | p |
|---|---|---|---|
| Socioeconomic Inequalities | |||
| Household income | 0.621 | 0.026 | <0.001 |
| Educational level of the adolescent | 0.529 | 0.023 | <0.001 |
| Educational level of the head of the family | 0.712 | 0.024 | <0.001 |
| Economic class | 0.854 | 0.027 | <0.001 |
| Behavioral Risk Factors | |||
| Added Sugar | 0.221 | 0.038 | <0.001 |
| Smoking | 0.995 | 0.098 | <0.001 |
| Alcohol abuse | 0.628 | 0.066 | <0.001 |
| Insulin Resistance Phenotype | |||
| Triglycerides/HDL | 0.770 | 0.052 | <0.001 |
| VLDL | 0.902 | 0.061 | <0.001 |
| TyG Index | 0.939 | 0.063 | <0.001 |
| Chronic Oral Disease Burden | |||
| Decayed component (DMFT Index) | 0.335 | 0.018 | <0.001 |
| Visible Plaque Index (VPI Index) | 0.605 | 0.015 | <0.001 |
| Bleeding on probing | 0.516 | 0.018 | <0.001 |
| Periodontal probing depth ≥ 4 mm | 0.674 | 0.013 | <0.001 |
| Clinical attachment level ≥ 3 mm | 0.672 | 0.013 | <0.001 |
| Explanatory Variables | Outcome | Standardized Coefficient | Standardized Error | p |
|---|---|---|---|---|
| Socioeconomic Inequalities | Chronic Oral Diseases Burden | 0.222 | 0.026 | <0.001 |
| Socioeconomic Inequalities | Obesity | −0.099 | 0.032 | 0.010 |
| Obesity | Insulin Resistance Phenotype | 0.098 | 0.027 | <0.001 |
| Obesity | Chronic Oral Diseases Burden | 0.089 | 0.033 | 0.007 |
| Insulin Resistance Phenotype | Chronic Oral Diseases Burden | 0.052 | 0.025 | 0.033 |
| Behavioral Risk Factors | Chronic Oral Diseases Burden | 0.102 | 0.041 | 0.013 |
| Sex | Chronic Oral Diseases Burden | −0.141 | 0.024 | <0.001 |
| Sex | Behavioral Risk Factors | −0.216 | 0.036 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ladeira, L.L.C.; Nascimento, G.G.; Leite, F.R.M.; Alves-Costa, S.; Barbosa, J.M.A.; Alves, C.M.C.; Thomaz, E.B.A.F.; Batista, R.F.L.; Ribeiro, C.C.C. Obesity, Insulin Resistance, Caries, and Periodontitis: Syndemic Framework. Nutrients 2023, 15, 3512. https://doi.org/10.3390/nu15163512
Ladeira LLC, Nascimento GG, Leite FRM, Alves-Costa S, Barbosa JMA, Alves CMC, Thomaz EBAF, Batista RFL, Ribeiro CCC. Obesity, Insulin Resistance, Caries, and Periodontitis: Syndemic Framework. Nutrients. 2023; 15(16):3512. https://doi.org/10.3390/nu15163512
Chicago/Turabian StyleLadeira, Lorena Lúcia Costa, Gustavo Giacomelli Nascimento, Fábio Renato Manzolli Leite, Silas Alves-Costa, Janaína Maiana Abreu Barbosa, Claudia Maria Coelho Alves, Erika Barbara Abreu Fonseca Thomaz, Rosangela Fernandes Lucena Batista, and Cecilia Claudia Costa Ribeiro. 2023. "Obesity, Insulin Resistance, Caries, and Periodontitis: Syndemic Framework" Nutrients 15, no. 16: 3512. https://doi.org/10.3390/nu15163512
APA StyleLadeira, L. L. C., Nascimento, G. G., Leite, F. R. M., Alves-Costa, S., Barbosa, J. M. A., Alves, C. M. C., Thomaz, E. B. A. F., Batista, R. F. L., & Ribeiro, C. C. C. (2023). Obesity, Insulin Resistance, Caries, and Periodontitis: Syndemic Framework. Nutrients, 15(16), 3512. https://doi.org/10.3390/nu15163512









