Protective Effects of Bacteriocin-Producing Lactiplantibacillus plantarum on Intestinal Barrier of Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Strain Cultivation and Bacterial Suspension Preparation
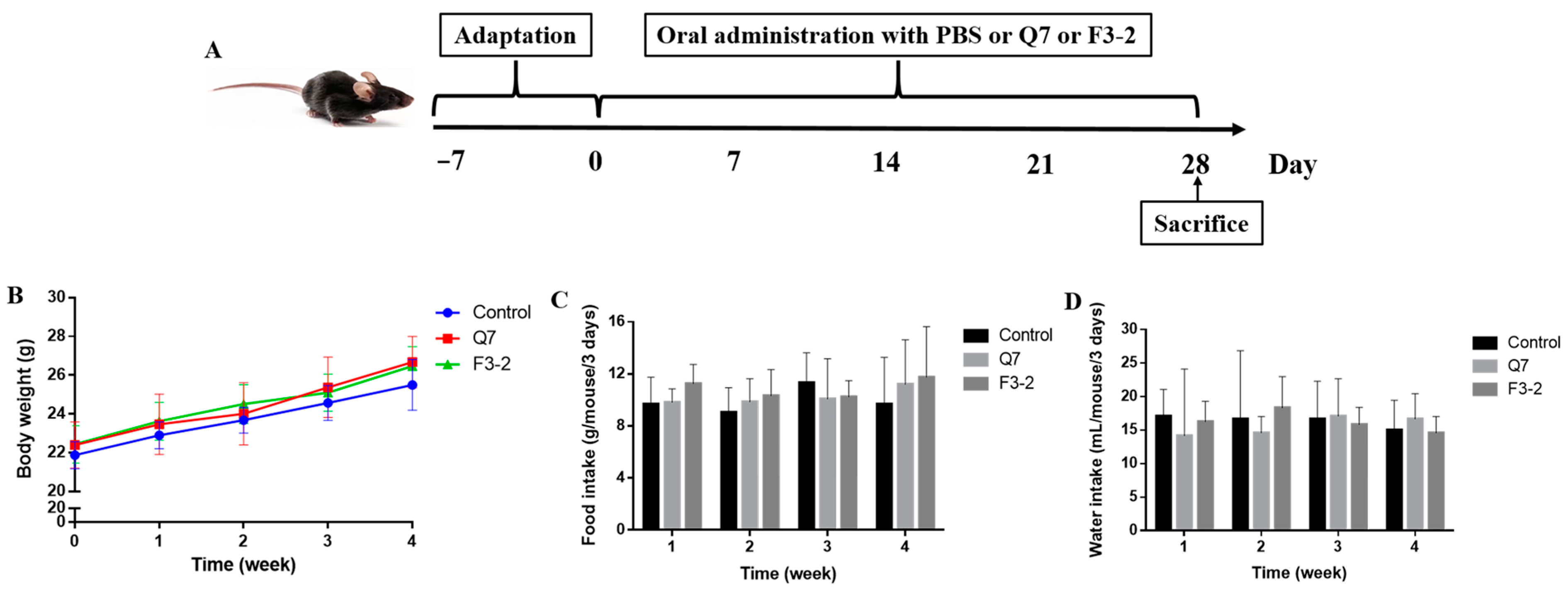
2.2. Animals and Treatments
2.3. Histomorphological and Immunohistochemical Evaluation
2.4. Enzyme-Linked Immunosorbent Assay (ELISA)
2.5. Real-Time Quantitative Polymerase Chain Reaction (RT-qPCR) Analysis
2.6. 16S rRNA Gene Sequencing
2.7. SCFAs Quantification
2.8. Statistical Analysis
3. Results
3.1. Effects of Bacteriocin-Producing L. plantarum on Physiological Indexes of Mice
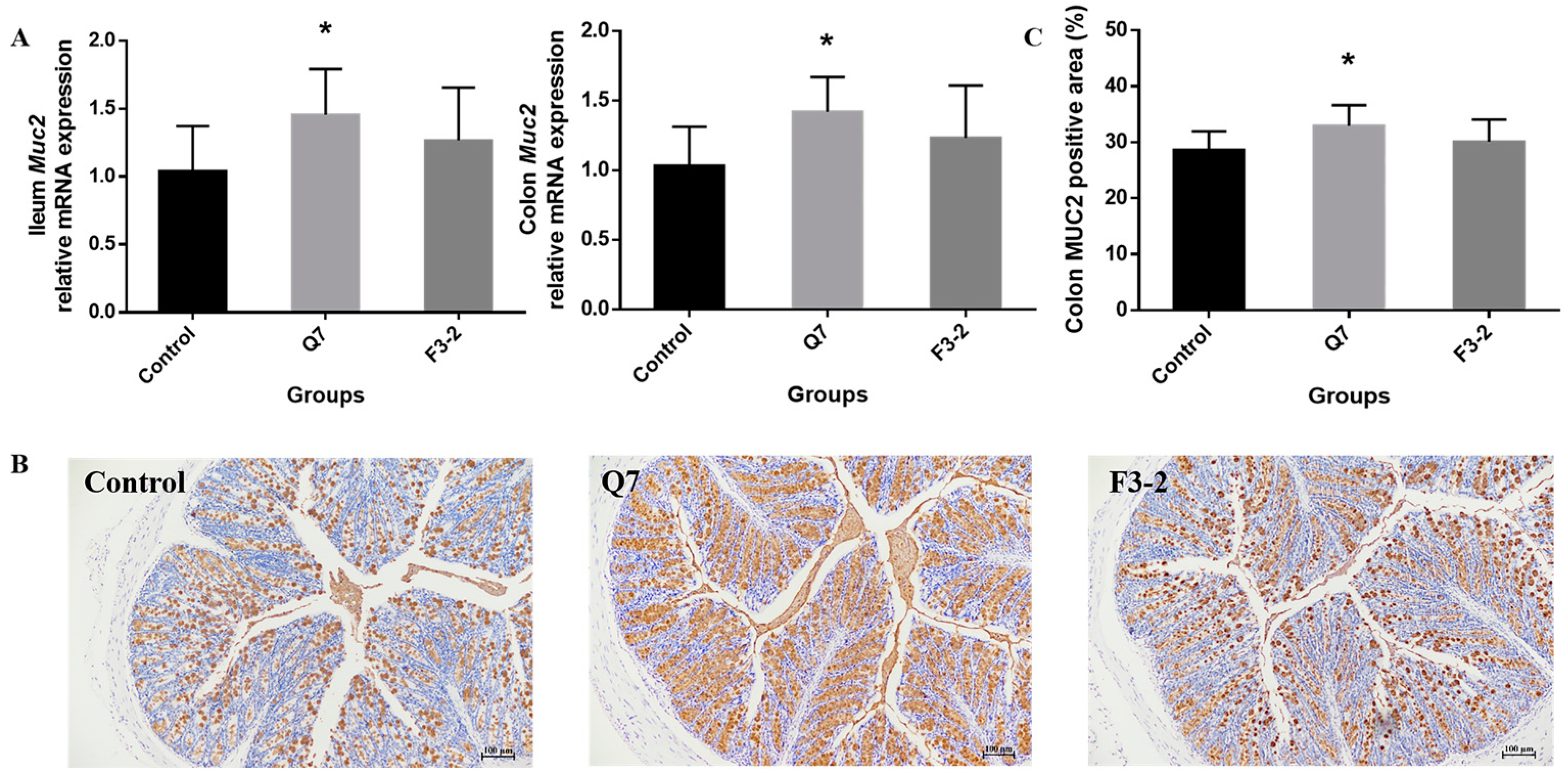
3.2. Effects of Bacteriocin-Producing L. plantarum on Intestinal Chemical Barrier of Mice
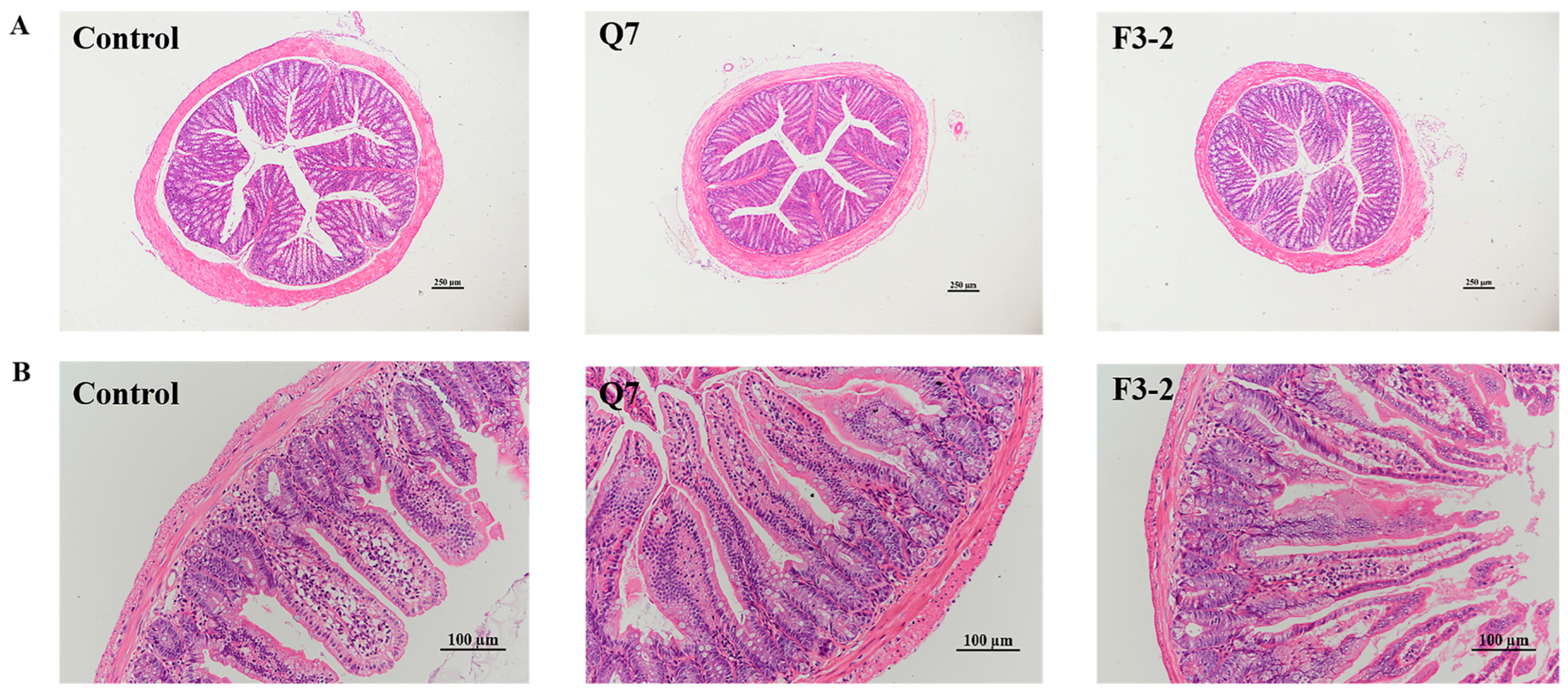
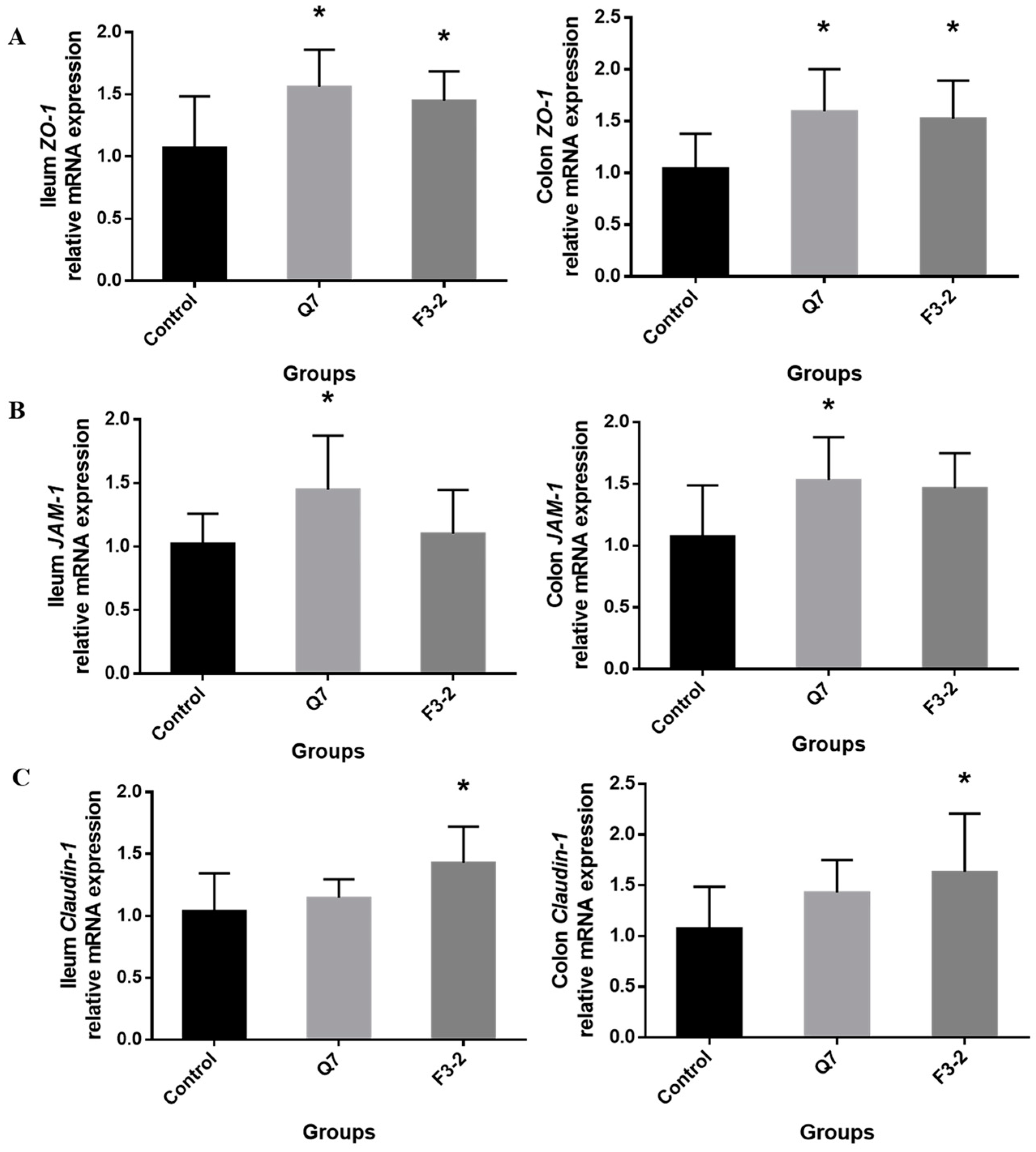
3.3. Effects of Bacteriocin-Producing L. plantarum on Intestinal Physical Barrier of Mice
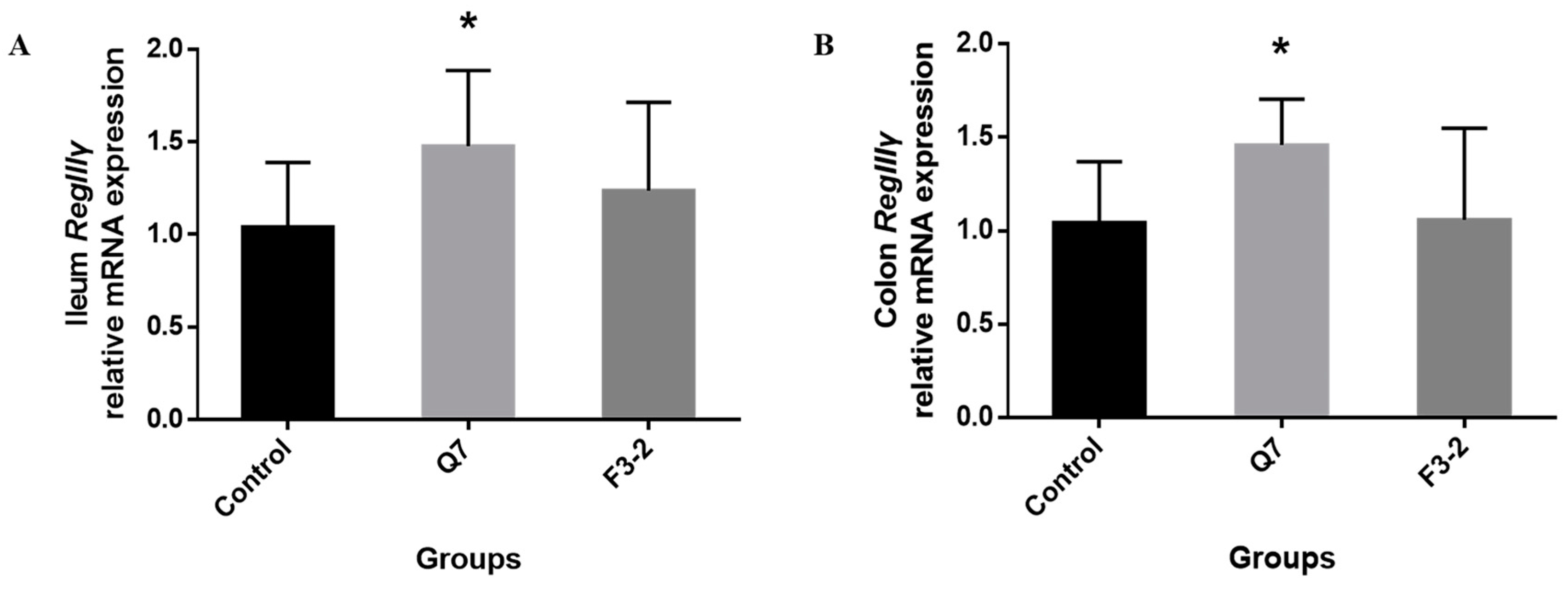
3.4. Effects of Bacteriocin-Producing L. plantarum on Intestinal Immunological Barrier of Mice
3.5. Effects of Bacteriocin-Producing L. plantarum on Intestinal Biological Barrier of Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Singh, T.P.; Natra, B.H. Next-generation probiotics: A promising approach towards designing personalized medicine. Crit. Rev. Microbiol. 2021, 47, 479–498. [Google Scholar] [CrossRef] [PubMed]
- Damián, M.R.; Cortes-Perez, N.G.; Quintana, E.T.; Ortiz-Moreno, A.; Noguez, C.G.; Cruceño-Casarrubias, C.E.; Pardo, M.E.S.; Bermúdez-Humarán, L.G. Functional foods, nutraceuticals and probiotics: A focus on human health. Microorganisms 2022, 10, 1065. [Google Scholar] [CrossRef] [PubMed]
- Vancamelbeke, M.; Vermeire, S. The intestinal barrier: A fundamental role in health and disease. Expert Rev. Gastroent. 2017, 11, 821–834. [Google Scholar] [CrossRef]
- Hao, W.; Hao, C.; Wu, C.; Xu, Y.; Jin, C. Aluminum induced intestinal dysfunction via mechanical, immune, chemical and biological barriers. Chemosphere 2022, 288, 132556. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Wang, B. Role of gut barrier function in the pathogenesis of nonalcoholic fatty liver disease. Gastroenterol. Res. Pract. 2015, 2015, 287348. [Google Scholar] [CrossRef] [Green Version]
- Bron, P.A.; Kleerebezem, M.; Brummer, R.J.; Cani, P.D.; Mercenier, A.; MacDonald, T.T.; Garcia-Ródenas, C.L.; Wells, J.M. Can probiotics modulate human disease by impacting intestinal barrier function? Brit. J. Nutr. 2017, 117, 93–107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fata, G.L.; Weber, P.; Mohajeri, M.H. Probiotics and the gut immune system: Indirect regulation. Probiotics Antimicrob. Proteins 2018, 10, 11–21. [Google Scholar] [CrossRef]
- Chelakkot, C.; Ghim, J.; Ryu, S.H. Mechanisms regulating intestinal barrier integrity and its pathological implications. Exp. Mol. Med. 2018, 50, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Han, X.; Ding, S.; Ma, Y.; Fang, J.; Jiang, H.; Li, Y.; Liu, G. Lactobacillus plantarum and Lactobacillus brevis alleviate intestinal inflammation and microbial disorder induced by ETEC in a murine model. Oxid. Med. Cell. Longev. 2021, 2021, 6867962. [Google Scholar] [CrossRef]
- Van Hemert, S.; Meijerink, M.; Molenaar, D.; Bron, P.A.; de Vos, P.; Kleerebezem, M.; Wells, J.M.; Marco, M.L. Identification of Lactobacillus plantarum genes modulating the cytokine response of human peripheral blood mononuclear cells. BMC Microbiol. 2010, 10, 293. [Google Scholar] [CrossRef] [Green Version]
- Cui, Q.Y.; Tian, X.Y.; Liang, X.; Zhang, Z.; Wang, R.; Zhou, Y.; Yi, H.X.; Gong, P.M.; Lin, K.; Liu, T.J.; et al. Bifidobacterium bifidum relieved DSS-induced colitis in mice potentially by activating the aryl hydrocarbon receptor. Food Funct. 2022, 13, 5115–5123. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, P.; Zhang, X. Probiotics regulate gut microbiota: An effective method to improve immunity. Molecules 2021, 26, 6076. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Ji, Y.; Jung, H.Y.; Park, H.; Kang, J.; Choi, S.H.; Shin, H.; Hyun, C.K.; Kim, K.T.; Holzapfel, W.H. Lactobacillus plantarum HAC01 regulates gut microbiota and adipose tissue accumulation in a diet-induced obesity murine model. Appl. Microbiol. Biotechnol. 2017, 101, 1605–1614. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Teng, K.; Liu, Y.; Cao, Y.; Wang, T.; Ma, C.; Zhang, J.; Zhong, J. Bacteriocins: Potential for human health. Oxid. Med. Cell. Longev. 2021, 2021, 5518825. [Google Scholar] [CrossRef]
- Yoon, J.W.; Kang, S.S. In vitro antibiofilm and anti-inflammatory properties of bacteriocins produced by Pediococcus acidilactici against Enterococcus faecalis. Foodborne Pathog. Dis. 2020, 17, 764–771. [Google Scholar] [CrossRef]
- Kommineni, S.; Bretl, D.J.; Lam, V.; Chakraborty, R.; Hayward, M.; Simpson, P.; Cao, Y.; Bousounis, P.; Kristich, C.J.; Salzman, N.H. Bacteriocin production augments niche competition by enterococci in the mammalian gastrointestinal tract. Nature 2015, 526, 719–722. [Google Scholar] [CrossRef] [Green Version]
- Bai, L.; Kumar, S.; Verma, S.; Seshadri, S. Bacteriocin PJ4 from probiotic lactobacillus reduced adipokine and inflammasome in high fat diet induced obesity. 3 Biotech 2020, 10, 355. [Google Scholar] [CrossRef]
- Bu, Y.; Liu, Y.; Liu, Y.; Wang, S.; Liu, Q.; Hao, H.; Yi, H. Screening and probiotic potential evaluation of bacteriocin-producing Lactiplantibacillus plantarum in vitro. Foods 2022, 11, 1575. [Google Scholar] [CrossRef]
- Han, X.; Guo, J.; You, Y.; Yin, M.; Ren, C.; Zhan, J.; Huang, W. A fast and accurate way to determine short chain fatty acids in mouse feces based on GC-MS. J. Chromatogr. B. 2018, 1099, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Yap, P.G.; Lai, Z.W.; Tan, J.S. Bacteriocins from lactic acid bacteria: Purification strategies and applications in food and medical industries: A review. Beni-Suef Univ. J. Basic Appl. Sci. 2022, 11, 51. [Google Scholar] [CrossRef]
- Dabour, N.; Zihler, A.; Kheadr, E.; Lacroix, C.; Fliss, I. In vivo study on the effectiveness of pediocin PA-1 and Pediococcus acidilactici UL5 at inhibiting Listeria monocytogenes. Int. J. Food Microbiol. 2009, 133, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Heeney, D.; Srisengfa, Y.; Golomb, B.; Griffey, S.; Marco, M. Bacteriocin biosynthesis contributes to the anti-inflammatory capacities of probiotic Lactobacillus plantarum. Benef. Microbes 2018, 9, 333–344. [Google Scholar] [CrossRef]
- Shan, M.; Gentile, M.; Yeiser, J.R.; Walland, A.C.; Bornstein, V.U.; Chen, K.; He, B.; Cassis, L.; Bigas, A.; Cols, M.; et al. Mucus enhances gut homeostasis and oral tolerance by delivering immunoregulatory signals. Science 2013, 342, 447–453. [Google Scholar] [CrossRef] [Green Version]
- Heeney, D.D.; Zhai, Z.; Bendiks, Z.; Barouei, J.; Martinic, A.; Slupsky, C.; Marco, M.L. Lactobacillus plantarum bacteriocin is associated with intestinal and systemic improvements in diet-induced obese mice and maintains epithelial barrier integrity in vitro. Gut Microbes 2019, 10, 382–397. [Google Scholar] [CrossRef] [Green Version]
- Mitra, J.B.; Sharma, V.K.; Kumar, M.; Mukherjee, A. Antimicrobial peptides: Vestiges of past or modern therapeutics? Mini-Rev. Med. Chem. 2020, 20, 183–195. [Google Scholar] [CrossRef] [PubMed]
- Chung, L.K.; Raffatellu, M. GI pros: Antimicrobial defense in the gastrointestinal tract. Semin. Cell Dev. Biol. 2019, 88, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Małaczewska, J.; Kaczorek-Łukowska, E.; Wójcik, R.; Rękawek, W.; Siwicki, A.K. In vitro immunomodulatory effect of nisin on porcine leucocytes. J. Anim. Physiol. Anim. Nutr. 2019, 103, 882–893. [Google Scholar] [CrossRef] [PubMed]
- Cui, S.; Jiang, J.; Li, B.; Ross, R.P.; Stanton, C.; Zhao, J.; Zhang, H.; Yang, B.; Chen, W. Effects of the short-term administration of Pediococcus pentosaceus on physiological characteristics, inflammation, and intestinal microecology in mice. Food Funct. 2021, 12, 1695–1707. [Google Scholar] [CrossRef]
- Qiao, Y.; Qiu, Z.; Tian, F.; Yu, L.; Zhao, J.; Zhang, H.; Zhai, Q.; Chen, W. Effect of bacteriocin-producing Pediococcus acidilactici strains on the immune system and intestinal flora of normal mice. Food Sci. Hum. Well. 2022, 11, 238–246. [Google Scholar] [CrossRef]
- Ahmed, F.; Kerna, N.A.; Tulp, O.L. Managing the F: B ratio in DM; a review of the role of firmicutes and bacteroidetes in diabetes mellitus. Adv. Complement. Altern. Med. 2019, 4, 295–298. [Google Scholar] [CrossRef]
- Shin, N.R.; Whon, T.W.; Bae, J.W. Proteobacteria: Microbial signature of dysbiosis in gut microbiota. Trends Biotechnol. 2015, 33, 496–503. [Google Scholar] [CrossRef]
- Zhou, C.; Zhou, X.; Wen, Z.; Liu, L.; Yang, Z.; Yang, L.; Li, P.; Guo, X.; Mei, X. Compound Fu brick tea modifies the intestinal microbiome composition in high-fat diet-induced obesity mice. Food Sci. Nutr. 2020, 8, 5508–5520. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Yu, Y.; Garcia-Gutierrez, E.; Jin, X.; He, Y.; Wang, L.; Tian, P.; Liu, Z.; Zhao, J.; Zhang, H.; et al. Lactobacillus acidophilus JCM 1132 strain and its mutant with different bacteriocin-producing behaviour have various in situ effects on the gut microbiota of healthy mice. Microorganisms 2019, 8, 49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.; Shin, Y.C.; Kim, T.Y.; Kim, Y.; Lee, Y.S.; Lee, S.H.; Kim, M.N.; O, E.; Kim, K.S.; Kweon, M.N. Mucin degrader Akkermansia muciniphila accelerates intestinal stem cell-mediated epithelial development. Gut Microbes 2021, 13, 1892441. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Huang, M.; Xu, X.; Cao, X.; Chen, H.; Duan, R. Effects of short-term continuous and pulse cadmium exposure on gut histology and microbiota of adult male frogs (Pelophylax nigromaculatus) during pre-hibernation. Environ. Toxicol. Pharmacol. 2022, 94, 103926. [Google Scholar] [CrossRef]
- Henneke, L.; Schlicht, K.; Andreani, N.A.; Hollstein, T.; Demetrowitsch, T.; Knappe, C.; Hartmann, K.; Jensen-Kroll, J.; Rohmann, N.; Pohlschneider, D.; et al. A dietary carbohydrate-gut Parasutterella-human fatty acid biosynthesis metabolic axis in obesity and type 2 diabetes. Gut Microbes 2022, 14, 2057778. [Google Scholar] [CrossRef]
- Riboulet-Bisson, E.; Sturme, M.H.; Jeffery, I.B.; O’Donnell, M.M.; Neville, B.A.; Forde, B.M.; Claesson, M.J.; Harris, H.; Gardiner, G.E.; Casey, P.G.; et al. Effect of Lactobacillus salivarius bacteriocin Abp118 on the mouse and pig intestinal microbiota. PLoS ONE 2012, 7, e31113. [Google Scholar] [CrossRef] [Green Version]
- Gebhart, D.; Lok, S.; Clare, S.; Tomas, M.; Stares, M.; Scholl, D.; Donskey, C.J.; Lawley, T.D.; Govoni, G.R. A modified R-type bacteriocin specifically targeting Clostridium difficile prevents colonization of mice without affecting gut microbiota diversity. mBio 2015, 6, e02368-14. [Google Scholar] [CrossRef] [Green Version]
- Smith, B.J.; Miller, R.A.; Ericsson, A.C.; Harrison, D.C.; Strong, R.; Schmidt, T.M. Changes in the gut microbiome and fermentation products concurrent with enhanced longevity in acarbose-treated mice. BMC Microbiol. 2019, 19, 130. [Google Scholar] [CrossRef] [Green Version]
- Yang, K.; Jian, S.; Guo, D.; Wen, C.; Xin, Z.; Zhang, L.; Kuang, T.; Wen, J.; Yin, Y.; Deng, B. Fecal microbiota and metabolomics revealed the effect of long-term consumption of gallic acid on canine lipid metabolism and gut health. Food Chem. X 2022, 15, 100377. [Google Scholar] [CrossRef]



| Genes | Forward Sequences (5′-3′) | Reverse Sequences (5′-3′) |
|---|---|---|
| β-actin | F: GTGCTATGTTGCTCTAGACTTCG | R: ATGCCACAGGATTCCATACC |
| Muc2 | F: TGCTGACGAGTGGTTGGTGAATG | R: TGATGAGGTGGCAGACAGGAGAC |
| ZO-1 | F: GCTGCCTCGAACCTCTACTC | R: TTGCTCATAACTTCGCGGGT |
| JAM-1 | F: AGTTCGTCCAAGGCAGCACAAC | R: AGAAGGTGACTCGGTCCGCATAG |
| Claudin-1 | F: GCTGGGTTTCATCCTGGCTTCTC | R: CCTGAGCGGTCACGATGTTGTC |
| RegIIIγ | F: GCTTCCTTCCTGTCCTCCATGATC | R: ATCACATCAGCATTGCTCCACTCC |
| TNF-α | F: GCGACGTGGAACTGGCAGAAG | R: GCCACAAGCAGGAATGAGAAGAGG |
| IL-6 | F: ACTTCCATCCAGTTGCCTTCTTGG | R: TTAAGCCTCCGACTTGTGAAGTGG |
| IFN-γ | F: CTGGAGGAACTGGCAAAAGGATGG | R: GACGCTTATGTTGTTGCTGATGGC |
| IL-10 | F: GAGGATCAGCAGGGGCCAGTAC | R: AAGGCAGTCCGCAGCTCTAGG |
| IL-12 | F: TCTTTGATGATGACCCTGTGCCTTG | R: GTGATTCTGAAGTGCTGCGTTGATG |
| IL-1β | F: TCGCAGCAGCACATCAACAAGAG | R: TGCTCATGTCCTCATCCTGGAAGG |
| Groups | Villus Height (μm) | Crypt Depth (μm) | Villus Height/Crypt Depth |
|---|---|---|---|
| Control | 247.57 ± 19.51 b | 87.53 ± 14.44 a | 2.90 ± 0.53 b |
| Q7 | 344.82 ± 20.27 a | 84.41 ± 10.33 a | 4.14 ± 0.58 a |
| F3-2 | 337.36 ± 22.82 a | 85.01 ± 9.94 a | 4.02 ± 0.56 a |
| Groups | Acetic Acid (μg/g) | Propionic Acid (μg/g) | Butyric Acid (μg/g) | Total SCFAs (μg/g) |
|---|---|---|---|---|
| Control | 378.59 ± 83.50 | 242.93 ± 49.57 | 189.53 ± 39.31 | 811.04 ± 48.25 |
| Q7 | 531.15 ± 90.89 * | 320.38 ± 63.08 * | 200.78 ± 49.40 | 1052.31 ± 139.84 ** |
| F3-2 | 480.68 ± 121.61 | 257.81 ± 63.02 | 208.98 ± 35.16 | 947.46 ± 135.75 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bu, Y.; Liu, Y.; Liu, Y.; Cao, J.; Zhang, Z.; Yi, H. Protective Effects of Bacteriocin-Producing Lactiplantibacillus plantarum on Intestinal Barrier of Mice. Nutrients 2023, 15, 3518. https://doi.org/10.3390/nu15163518
Bu Y, Liu Y, Liu Y, Cao J, Zhang Z, Yi H. Protective Effects of Bacteriocin-Producing Lactiplantibacillus plantarum on Intestinal Barrier of Mice. Nutrients. 2023; 15(16):3518. https://doi.org/10.3390/nu15163518
Chicago/Turabian StyleBu, Yushan, Yisuo Liu, Yinxue Liu, Jiayuan Cao, Zhe Zhang, and Huaxi Yi. 2023. "Protective Effects of Bacteriocin-Producing Lactiplantibacillus plantarum on Intestinal Barrier of Mice" Nutrients 15, no. 16: 3518. https://doi.org/10.3390/nu15163518





