The Critical Exploration into Current Evidence behind the Role of the Nutritional Support in Adult Patients Who Undergo Haematogenic Stem Cell Transplantation
Abstract
:1. Introduction
2. Material and Methods
3. Nutritional Assessment and Screening
4. Enteral versus Parenteral Nutrition
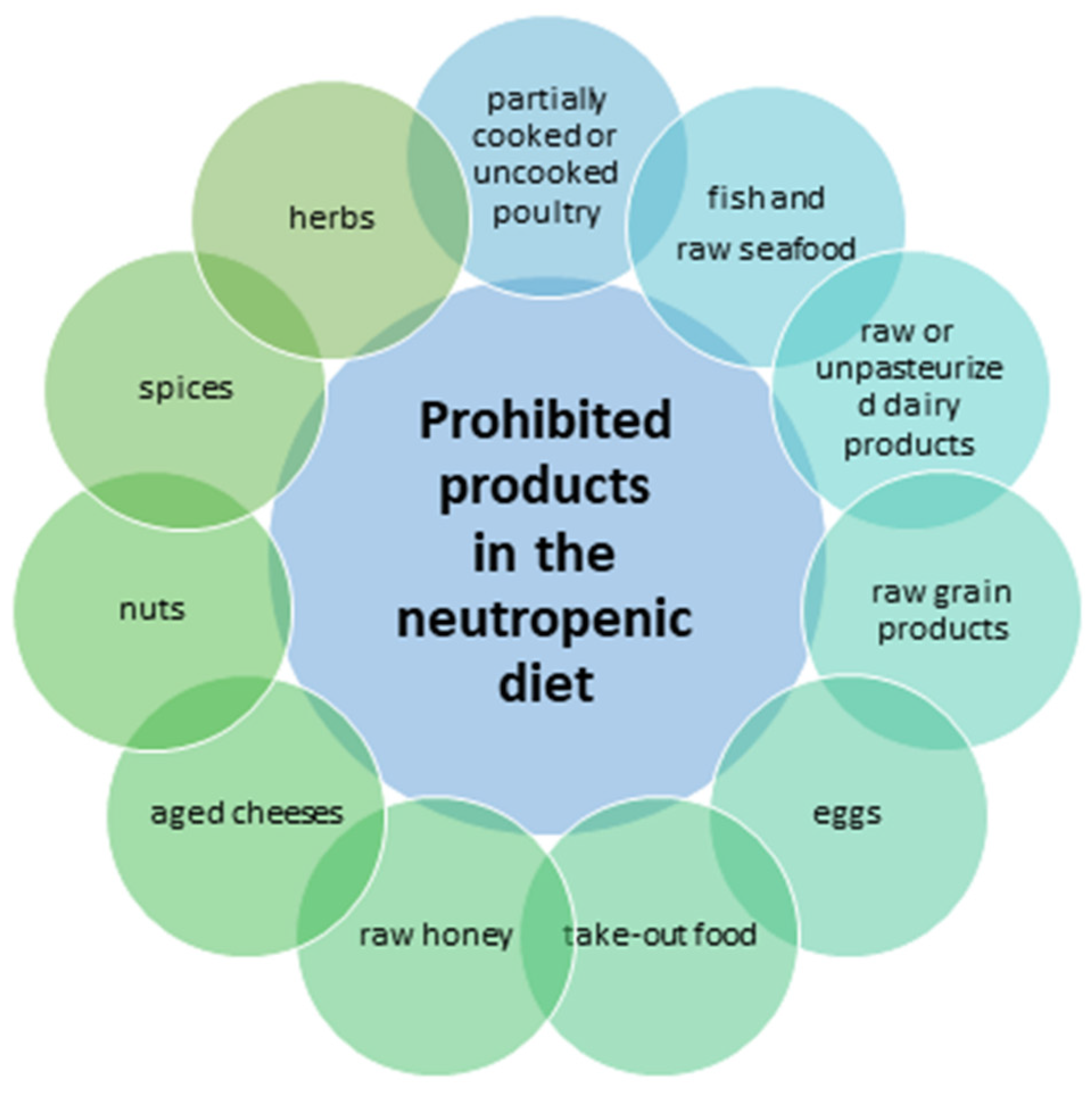
5. Neutropenic Diet
6. Glutamine
7. Polyunsaturated Fatty Acids
8. Vitamin D
9. Vitamin C
10. Gut Microbiome—Probiotics, Prebiotics, Faecal Microbiota Transplantation
11. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Amonoo, H.L.; Harnedy, L.E.; Deary, E.C.; Traeger, L.; Brown, L.A.; Daskalakis, E.P.; Cutler, C.; Kelkar, A.H.; Rosales, R.; Goldschen, L.; et al. Peer Support in Patients with Hematologic Malignancies Undergoing Hematopoietic Stem Cell Transplantation (HSCT): A Qualitative Study. Bone Marrow Transplant. 2022, 57, 1277–1286. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.; Braun, T.M.; Chowdhury, M.; Tewari, M.; Choi, S.W. A Systematic Review of Machine Learning Techniques in Hematopoietic Stem Cell Transplantation (HSCT). Sensors 2020, 20, 6100. [Google Scholar] [CrossRef] [PubMed]
- Aljurf, M.; Rizzo, J.D.; Mohty, M.; Hussain, F.; Madrigal, A.; Pasquini, M.C.; Passweg, J.; Chaudhri, N.; Ghavamzadeh, A.; Solh, H.E.; et al. Challenges and Opportunities for HSCT Outcome Registries: Perspective from International HSCT Registries Experts. Bone Marrow Transplant. 2014, 49, 1016–1021. [Google Scholar] [CrossRef]
- Pagliuca, S.; Michonneau, D.; De Fontbrune, F.S.; Del Galy, A.S.; Xhaard, A.; Robin, M.; De Latour, R.P.; Socie, G. Allogeneic Reactivity–Mediated Endothelial Cell Complications after HSCT: A Plea for Consensual Definitions. Blood Adv. 2019, 3, 2424–2435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cioce, M.; Botti, S.; Lohmeyer, F.M.; Galli, E.; Magini, M.; Giraldi, A.; Garau, P.; Celli, D.; Zega, M.; Sica, S.; et al. Nutritional Status and Quality of Life in Adults Undergoing Allogeneic Hematopoietic Stem Cell Transplantation. Int. J. Hematol. 2022, 116, 266–275. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, C.R.; Hall, A.; Woo, K.M.; Kim, K.M.; Longo, W.; Hematti, P.; Callander, N.; Kenkre, V.P.; Mattison, R.; Juckett, M. Decitabine Induction with Myeloablative Conditioning and Allogeneic Hematopoietic Stem Cell Transplantation in High-Risk Patients with Myeloid Malignancies Is Associated with a High Rate of Infectious Complications. Leuk. Res. 2020, 96, 106419. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, X.; Yang, D.; Pang, A.; Zhang, R.; Ma, Q.; Zhai, W.; He, Y.; Wei, J.; Jiang, E.; et al. The Prognostic Impact of Previously Infectious Complications on Allogeneic Hematopoietic Stem Cell Transplantation for Patients with Severe Aplastic Anemia: A Single-Center, Retrospective Study. Front. Immunol. 2022, 13, 1004787. [Google Scholar] [CrossRef] [PubMed]
- Almakadi, M.; Alahmari, A.; Albeihany, A. Gastrointestinal and Hepatic Considerations in Critically Ill Hematopoietic Stem Cell Transplantation Patient. In Pulmonary and Critical Care Considerations of Hematopoietic Stem Cell Transplantation; Springer: Cham, Switzerland, 2023; pp. 365–371. [Google Scholar] [CrossRef]
- Tarantino, G.; Saraceni, F.; Mancini, G.; Poiani, M.; Maroni, L.; Goteri, G.; Scortechini, I.; Fiorentini, A.; Dubbini, M.V.; Marini, F.; et al. Gastrointestinal Complications after Allogeneic Hematopoietic Stem Cell Transplant: A Multidisciplinary Approach with Early Endoscopic Evaluation. Clin. Hematol. Int. 2021, 3, 161. [Google Scholar] [CrossRef]
- Pasyar, N.; Rambod, M.; Zahedi, F.; Ramzi, M. Pain, Fatigue, Nausea, and Vomiting as the Predictors of Anxiety in Patients Undergoing Hematopoietic Stem Cell Transplantation: A Prospective Cohort Study. Support. Care Cancer 2022, 30, 5871–5879. [Google Scholar] [CrossRef]
- Shouval, R.; Eshel, A.; Dubovski, B.; Kuperman, A.A.; Danylesko, I.; Fein, J.A.; Fried, S.; Geva, M.; Kouniavski, E.; Neuman, H.; et al. Patterns of Salivary Microbiota Injury and Oral Mucositis in Recipients of Allogeneic Hematopoietic Stem Cell Transplantation. Blood Adv. 2020, 4, 2912–2917. [Google Scholar] [CrossRef]
- Patel, P.; Robinson, P.D.; Baggott, C.; Gibson, P.; Ljungman, G.; Massey, N.; Ottaviani, G.; Phillips, R.; Revon-Rivière, G.; Treister, N.; et al. Clinical Practice Guideline for the Prevention of Oral and Oropharyngeal Mucositis in Pediatric Cancer and Hematopoietic Stem Cell Transplant Patients: 2021 Update. Eur. J. Cancer 2021, 154, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Fisher, S.A.; Cutler, A.; Doree, C.; Brunskill, S.J.; Stanworth, S.J.; Navarrete, C.; Girdlestone, J. Mesenchymal Stromal Cells as Treatment or Prophylaxis for Acute or Chronic Graft-versus-Host Disease in Haematopoietic Stem Cell Transplant (HSCT) Recipients with a Haematological Condition. Cochrane Database Syst. Rev. 2019, 2019, 1. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Chen, S.; Yang, P.; Cao, H.; Li, L. The Role of Mesenchymal Stem Cells in Hematopoietic Stem Cell Transplantation: Prevention and Treatment of Graft-versus-Host Disease. Stem Cell. Res. Ther. 2019, 10, 182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eglseer, D.; Seymann, C.; Lohrmann, C.; Hoedl, M. Nutritional Problems and Their Non-pharmacological Treatment in Adults Undergoing Haematopoietic Stem Cell Transplantation—A Systematic Review. Eur. J. Cancer Care 2020, 29, e13298. [Google Scholar] [CrossRef]
- Caccialanza, R.; Lobascio, F.; Cereda, E.; Aprile, G.; Farina, G.; Traclò, F.; Borioli, V.; Caraccia, M.; Turri, A.; De Lorenzo, F.; et al. Cancer-Related Malnutrition Management: A Survey among Italian Oncology Units and Patients’ Associations. Curr. Probl. Cancer 2020, 44, 100554. [Google Scholar] [CrossRef]
- Casirati, A.; Salcedo, I.; Cereda, E.; Chabannon, C.; Ruggeri, A.; Kuball, J.; Clout, R.; Mooyaart, J.E.; Kenyon, M.; Caccialanza, R.; et al. The European Society for Blood and Marrow Transplantation (EBMT) Roadmap and Perspectives to Improve Nutritional Care in Patients Undergoing Hematopoietic Stem Cell Transplantation on Behalf of the Cellular Therapy and Immunobiology Working Party (CTIWP) and the Nurses Group (NG) of the EBMT. Bone Marrow Transplant. 2023, 2023, 1–8. [Google Scholar] [CrossRef]
- Espinoza, M.; Perelli, J.; Olmos, R.; Bertin, P.; Jara, V.; Ramírez, P. Nutritional Assessment as Predictor of Complications after Hematopoietic Stem Cell Transplantation. Rev. Bras. Hematol. Hemoter. 2016, 38, 7–14. [Google Scholar] [CrossRef] [Green Version]
- Fuji, S.; Mori, T.; Khattry, N.; Cheng, J.; Do, Y.R.; Yakushijin, K.; Kohashi, S.; Fukuda, T.; Kim, S.W. Severe Weight Loss in 3 Months after Allogeneic Hematopoietic SCT Was Associated with an Increased Risk of Subsequent Non-Relapse Mortality. Bone Marrow Transpl. 2015, 50, 100–105. [Google Scholar] [CrossRef] [Green Version]
- Yang, P.; Song, Y.; Jing, X.; Ge, Y.; Liu, M.; Tang, F.; Chen, Y.; Li, Q.; Wei, F.; Mao, Y.; et al. Nutritional Assessment in Early Allogenic Hematopoietic Stem Cell Transplant Patients, a Cross-Sectional Study. Nutrition 2023, 75, 1511–1519. [Google Scholar] [CrossRef]
- Limpert, R.; Pan, P.; Wang, L.S.; Chen, X. From Support to Therapy: Rethinking the Role of Nutrition in Acute Graft-versus-Host Disease. Front. Immunol. 2023, 14, 1192084. [Google Scholar] [CrossRef]
- Hemstetter, S.; Fornwalt, R.A.; Stephens, R.S. Multidisciplinary Care and ICU Organization for Hematopoietic Stem Cell Transplantation Patients. In Pulmonary and Critical Care Considerations of Hematopoietic Stem Cell Transplantation; Springer: Cham, Switzerland, 2023; pp. 463–472. [Google Scholar] [CrossRef]
- August, D.A.; Huhmann, M.B.; American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.) Board of Directors. Clinical Guidelines: Nutrition Support Therapy during Adult Anticancer Treatment and in Hematopoietic Cell Transplantation. JPEN J. Parenter. Enter. Nutr. 2009, 33, 472–500. [Google Scholar] [CrossRef]
- Alderwick, H.; Dixon, J. The NHS Long Term Plan. BMJ 2019, 364, 184. [Google Scholar] [CrossRef] [Green Version]
- Sagou, K.; Ozeki, K.; Ukai, S.; Adachi, Y.; Fukushima, N.; Kohno, A. Impact of a Nutritional Risk Index on Clinical Outcomes after Allogeneic Hematopoietic Cell Transplantation. Biol. Blood Marrow Transplant. 2019, 25, 2287–2296. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, A.; Bargetzi, A.; Zueger, N.; Bargetzi, M.; Medinger, M.; Bounoure, L.; Gomes, F.; Stanga, Z.; Mueller, B.; Schuetz, P. Revisiting Nutritional Support for Allogeneic Hematologic Stem Cell Transplantation-a Systematic Review. Bone Marrow Transpl. 2017, 52, 506–513. [Google Scholar] [CrossRef]
- Bozzetti, F.; Arends, J.; Lundholm, K.; Micklewright, A.; Zurcher, G.; Muscaritoli, M. ESPEN Guidelines on Parenteral Nutrition: Non-Surgical Oncology. Clin. Nutr. 2009, 28, 445–454. [Google Scholar] [CrossRef] [PubMed]
- Brotelle, T.; Lemal, R.; Cabrespine, A.; Combal, C.; Hermet, E.; Ravinet, A.; Bay, J.O.; Bouteloup, C. Prevalence of Malnutrition in Adult Patients Previously Treated with Allogeneic Hematopoietic Stem-Cell Transplantation. Clin. Nutr. 2018, 37, 739–745. [Google Scholar] [CrossRef]
- Volchenkov, S.A.; Filatova, L.V.; Lyubimov, S.V.; Zyuzgin, I.S.; Elkhova, S.S.; Zverkova, A.; Ishmatova, I.; Semiglazova, T.Y. Modern Aspects of Nutritional Support during Hematopoietic Stem Cell Transplantation: Review, Experience of the N.N. Petrov National Medical Research Center of Oncology. Pharmateca 2022, 29, 14–22. [Google Scholar] [CrossRef]
- Botti, S.; Liptrott, S.J.; Gargiulo, G.; Orlando, L. Nutritional Support in Patients Undergoing Haematopoietic Stem Cell Transplantation: A Multicentre Survey of the Gruppo Italiano Trapianto Midollo Osseo (GITMO) Transplant Programmes. Ecancermedicalscience 2015, 9, 545. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.Z.; Vigorito, A.C.; de Almeida, A.M.; de Candolo, A.A.; Silva, A.C.L.; de Brandão-Anjos, A.E.P.; de Sá, B.L.; de Souza, C.L.S.; de Castro Junior, C.G.; de Oliveira, J.S.R.; et al. Brazilian Nutritional Consensus in Hematopoietic Stem Cell Transplantation: Graft- versus -Host Disease. Einstein 2020, 18, eAE4799. [Google Scholar] [CrossRef] [Green Version]
- Liu, P.; Wang, B.; Yan, X.; Cai, J.J.; Wang, Y. Comprehensive Evaluation of Nutritional Status before and after Hematopoietic Stem Cell Transplantation in 170 Patients with Hematological Diseases. Chin. J. Cancer Res. 2016, 28, 626–633. [Google Scholar] [CrossRef] [Green Version]
- Akbulut, G.; Yesildemir, O. Overview of Nutritional Approach in Hematopoietic Stem Cell Transplantation: COVID-19 Update. World J. Stem Cells 2021, 13, 1530–1548. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; Min, L.; Chengyuan, L.; Hong, L.; Meng, W.; Chenyi, T.; Jinru, W.; Wei, W.; Hua, L. The Influence of the China GLIM Standards on the Diagnosis of Malnutrition in Patients with Hematopoietic Stem Cell Transplant. Front. Nutr. 2023, 9, 1077442. [Google Scholar] [CrossRef]
- Park, M.Y.; Park, J.Y. Pre- and Post-Transplant Nutritional Assessment in Patients Undergoing Allogeneic Hematopoietic Stem Cell Transplantation. Asian Oncol. Nurs. 2012, 12, 110–116. [Google Scholar] [CrossRef] [Green Version]
- Wang, B.; Yan, X.; Cai, J.; Wang, Y.; Liu, P. Nutritional Assessment with Different Tools in Leukemia Patients after Hematopoietic Stem Cell Transplantation. Chin. J. Cancer Res. 2013, 25, 762. [Google Scholar] [CrossRef] [PubMed]
- Kondo, S.; Nakano, J. Physical Function and Nutrition in Patients with Hematological Malignancies. In Physical Therapy and Research in Patients with Cancer; Springer: Singapore, 2022; pp. 441–460. [Google Scholar] [CrossRef]
- Muscaritoli, M.; Arends, J.; Bachmann, P.; Baracos, V.; Barthelemy, N.; Bertz, H.; Bozzetti, F.; Hütterer, E.; Isenring, E.; Kaasa, S.; et al. ESPEN Practical Guideline: Clinical Nutrition in Cancer. Clin. Nutr. 2021, 40, 2898–2913. [Google Scholar] [CrossRef] [PubMed]
- Barban, J.B.; Simões, B.P.; de Moraes, B.D.G.C.; da Anunciação, C.R.; da Rocha, C.S.; Pintor, D.C.Q.; Guerra, D.C.; Silva, D.A.; Brandão, E.d.C.M.; Kerbauy, F.; et al. Brazilian Nutritional Consensus in Hematopoietic Stem Cell Transplantation: Adults. Einstein 2020, 18, AE4530. [Google Scholar] [CrossRef] [PubMed]
- Ok, Y.; Vural, E.; Alhan, N.; Vurgun, S.; Atas, U.; Yapar, D.; Salim, O.; Undar, L. Lower Body Mass Index and Prognostic Nutritional Index Are Associated with Poor Post-Transplant Outcomes in Lymphoma Patients Undergoing Autologous Stem Cell Transplantation. Oncology 2023. [Google Scholar] [CrossRef]
- Hemrajani, A.; Ismail, Z.; Elms, D.J.; Moore, M.; Smith, S.E.; Hagen, P.; Stiff, P.J.; Tsai, S. Impact of Body Mass Index (BMI) and Weight Changes on Hematopoietic Stem Cell Transplant (HSCT) Outcomes in the Elderly Population. J. Clin. Oncol. 2023, 41, e19058. [Google Scholar] [CrossRef]
- Akbulut, G. Medical Nutritional Therapy in Hematopoietic Stem Cell Transplantation (HSCT). Int. J. Hematol. Oncol. 2013, 23, 65. [Google Scholar] [CrossRef]
- Aoyama, T.; Imataki, O.; Notsu, A.; Yurikusa, T.; Ichimaru, K.; Tsuji, M.; Yoshitsugu, K.; Fukaya, M.; Enami, T.; Ikeda, T. Examination of a Nutritional Treatment Pathway According to Pretreatment Health Status and Stress Levels of Patients Undergoing Hematopoietic Stem Cell Transplantation. PLoS ONE 2022, 17, e0271728. [Google Scholar] [CrossRef]
- Terao, Y.; Nakayama, Y.; Abo, M.; Otobe, Y.; Suzuki, M.; Koyama, S.; Tanaka, S.; Kojima, I.; Haga, N.; Yamada, M. Impact of the Quantity and Quality of the Skeletal Muscle on Survival among Patients Undergoing Allogeneic Hematopoietic Stem Cell Transplantation. Leuk. Res. 2023, 128, 107057. [Google Scholar] [CrossRef]
- Tanaka, S.; Imataki, O.; Kitaoka, A.; Fujioka, S.; Hanabusa, E.; Ohbayashi, Y.; Uemura, M.; Arima, N.; Yamamoto, T. Clinical Impact of Sarcopenia and Relevance of Nutritional Intake in Patients before and after Allogeneic Hematopoietic Stem Cell Transplantation. J. Cancer Res. Clin. Oncol. 2017, 143, 1083–1092. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Zhang, Z.F.; Cai, J.J.; Wang, B.S.; Yan, X. NRS2002 Assesses Nutritional Status of Leukemia Patients Undergoing Hematopoietic Stem Cell Transplantation. Chin. J. Cancer Res. 2012, 24, 299–303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, P.; Yan, X.; Wang, B.S.; Xu, X.D. Three Methods Assess Nutritional Status of Leukemia Patients before Hematopoietic Stem Cell Transplantation. Chin. Med. J. 2012, 125, 440–443. [Google Scholar] [CrossRef]
- Zama, D.; Gori, D.; Muratore, E.; Leardini, D.; Rallo, F.; Turroni, S.; Prete, A.; Brigidi, P.; Pession, A.; Masetti, R. Enteral versus Parenteral Nutrition as Nutritional Support after Allogeneic Hematopoietic Stem Cell Transplantation: A Systematic Review and Meta-Analysis. Transpl. Cell Ther. 2021, 27, 180.e1–180.e8. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, A.; Hoskin, K.; Schuetz, P. Optimization of Nutrition during Allogeneic Hematologic Stem Cell Transplantation. Curr. Opin. Clin. Nutr. Metab. Care 2018, 21, 152–158. [Google Scholar] [CrossRef]
- Gudmundstuen, A.M.; Efficace, F.; Tjønnfjord, G.E.; Skaarud, K.J.; Cottone, F.; Hjermstad, M.J.; Iversen, P.O. The Prognostic Value of Patient-Reported Outcomes in Allogeneic Hematopoietic Stem Cell Transplantation: Exploratory Analysis of a Randomized Nutrition Intervention Trial. Ann. Hematol. 2023, 102, 927–935. [Google Scholar] [CrossRef]
- Kondrup, J.; Ramussen, H.H.; Hamberg, O.; Stanga, Z.; Camilo, M.; Richardson, R.; Elia, M.; Allison, S.; Meier, R.; Plauth, M. Nutritional Risk Screening (NRS 2002): A New Method Based on an Analysis of Controlled Clinical Trials. Clin. Nutr. 2003, 22, 321–336. [Google Scholar] [CrossRef]
- Development, Validation and Implementation of a Program to Detect Malnutrition with NRS-2002 Screening Tool in Patients from the Oncology and Hematology Service|Nutrición Clínica y Dietética Hospitalaria. Available online: https://revista.nutricion.org/index.php/ncdh/article/view/82 (accessed on 1 August 2023).
- Isenring, E.; Elia, M. Which Screening Method Is Appropriate for Older Cancer Patients at Risk for Malnutrition? Nutrition 2015, 31, 594–597. [Google Scholar] [CrossRef]
- Stratton, R.J.; Hackston, A.; Longmore, D.; Dixon, R.; Price, S.; Stroud, M.; King, C.; Elia, M. Malnutrition in Hospital Outpatients and Inpatients: Prevalence, Concurrent Validity and Ease of Use of the “Malnutrition Universal Screening Tool” (‘MUST’) for Adults. Br. J. Nutr. 2004, 92, 799–808. [Google Scholar] [CrossRef] [Green Version]
- Chao, P.C.; Chuang, H.J.; Tsao, L.Y.; Chen, P.Y.; Hsu, C.F.; Lin, H.C.; Chang, C.Y.; Linc, C.F. The Malnutrition Universal Screening Tool (MUST) and a Nutrition Education Program for High Risk Cancer Patients: Strategies to Improve Dietary Intake in Cancer Patients. Biomedicine 2015, 5, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Iyama, S.; Tatsumi, H.; Shiraishi, T.; Yoshida, M.; Tatekoshi, A.; Endo, A.; Ishige, T.; Shiwa, Y.; Ibata, S.; Goto, A.; et al. Possible Clinical Outcomes Using Early Enteral Nutrition in Individuals with Allogeneic Hematopoietic Stem Cell Transplantation: A Single-Center Retrospective Study. Nutrition 2021, 83, 111093. [Google Scholar] [CrossRef] [PubMed]
- Bechtold, M.L.; Brown, P.M.; Escuro, A.; Grenda, B.; Johnston, T.; Kozeniecki, M.; Limketkai, B.N.; Nelson, K.K.; Powers, J.; Ronan, A.; et al. When Is Enteral Nutrition Indicated? J. Parenter. Enter. Nutr. 2022, 46, 1470–1496. [Google Scholar] [CrossRef]
- Fuji, S.; Cheng, J. Nutritional Considerations of Critically Ill Hematopoietic Cell Transplantation Patients. In Pulmonary and Critical Care Considerations of Hematopoietic Stem Cell Transplantation; Springer: Cham, Switzerland, 2023; pp. 449–453. [Google Scholar] [CrossRef]
- Rubin, H.; Mehta, J.; Fong, J.L.; Greenberg, D.; Gruschak, S.; Trifilio, S. Revisiting Infectious Complications Following Total Parenteral Nutrition Use During Hematopoietic Stem Cell Transplantation. J. Adv. Pract. Oncol. 2020, 11, 675. [Google Scholar] [CrossRef] [PubMed]
- Andersen, S.; Staudacher, H.; Weber, N.; Kennedy, G.; Varelias, A.; Banks, M.; Bauer, J. Pilot Study Investigating the Effect of Enteral and Parenteral Nutrition on the Gastrointestinal Microbiome Post-Allogeneic Transplantation. Br. J. Haematol. 2020, 188, 570–581. [Google Scholar] [CrossRef] [PubMed]
- Lemal, R.; Cabrespine, A.; Pereira, B.; Combal, C.; Ravinet, A.; Hermet, E.; Bay, J.O.; Bouteloup, C. Could Enteral Nutrition Improve the Outcome of Patients with Haematological Malignancies Undergoing Allogeneic Haematopoietic Stem Cell Transplantation? A Study Protocol for a Randomized Controlled Trial (the NEPHA Study). Trials 2015, 16, 136. [Google Scholar] [CrossRef]
- Guièze, R.; Lemal, R.; Cabrespine, A.; Hermet, E.; Tournilhac, O.; Combal, C.; Bay, J.O.; Bouteloup, C. Enteral versus Parenteral Nutritional Support in Allogeneic Haematopoietic Stem-Cell Transplantation. Clin. Nutr. 2014, 33, 533–538. [Google Scholar] [CrossRef]
- Atkins, L.; Steer, B.; Ray, H.; Kiss, N. Implementing and Sustaining an Evidence-Based Nutrition Service in a Haematology Unit for Autologous Stem Cell Transplant Patients. Support Care Cancer 2019, 27, 951–958. [Google Scholar] [CrossRef]
- Kiss, N.; Seymour, J.F.; Prince, H.M.; Dutu, G. Challenges and Outcomes of a Randomized Study of Early Nutrition Support during Autologous Stem-Cell Transplantation. Curr. Oncol. 2014, 21, 334–339. [Google Scholar] [CrossRef] [Green Version]
- Farhadfar, N.; Kelly, D.L.; Mead, L.; Nair, S.; Colee, J.; Irizarry Gatell, V.; Murthy, H.S.; Brown, R.A.; Hiemenz, J.W.; Hsu, J.W.; et al. Dietary Intake and Diet Quality of Hematopoietic Stem Cell Transplantation Survivors. Biol. Blood Marrow Transplant. 2020, 26, 1154–1159. [Google Scholar] [CrossRef]
- Chow, R.; Bruera, E.; Arends, J.; Walsh, D.; Strasser, F.; Isenring, E.; Del Fabbro, E.G.; Molassiotis, A.; Krishnan, M.; Chiu, L.; et al. Enteral and Parenteral Nutrition in Cancer Patients, a Comparison of Complication Rates: An Updated Systematic Review and (Cumulative) Meta-Analysis. Support. Care Cancer 2020, 28, 979–1010. [Google Scholar] [CrossRef]
- D’Amico, F.; Biagi, E.; Rampelli, S.; Fiori, J.; Zama, D.; Soverini, M.; Barone, M.; Leardini, D.; Muratore, E.; Prete, A.; et al. Enteral Nutrition in Pediatric Patients Undergoing Hematopoietic SCT Promotes the Recovery of Gut Microbiome Homeostasis. Nutrients 2019, 11, 2958. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Evans, J.C.; Hirani, S.P.; Needle, J.J. Nutritional and Post-Transplantation Outcomes of Enteral versus Parenteral Nutrition in Pediatric Hematopoietic Stem Cell Transplantation: A Systematic Review of Randomized and Nonrandomized Studies. Biol. Blood Marrow Transplant. 2019, 25, e252–e259. [Google Scholar] [CrossRef] [PubMed]
- Ramamoorthy, V.; Rubens, M.; Appunni, S.; Saxena, A.; McGranaghan, P.; Veledar, E.; Viamonte-Ros, A.; Shehadeh, N.; Kaiser, A.; Kotecha, R. Lack of Efficacy of the Neutropenic Diet in Decreasing Infections among Cancer Patients: A Systematic Review. Nutr. Cancer 2019, 72, 1125–1134. [Google Scholar] [CrossRef]
- Fuerst, M.L. Restrictive Diet Causes Unnecessary Burden After Stem Cell Transplant. Oncol. Times 2023, 45, 11. [Google Scholar] [CrossRef]
- Macris, P.C. The Neutropenic Diet: Fact or Fiction? An Update on Evidence-Based Diet Recommendations for Immunocompromised Oncology Patients; Fred Hutchinson Cancer Center: Seattle, WA, USA, 2023. [Google Scholar]
- De Bock, T.; Jacxsens, L.; Maes, F.; Van Meerhaeghe, S.; Reygaerts, M.; Uyttendaele, M. Microbiological Profiling and Knowledge of Food Preservation Technology to Support Guidance on a Neutropenic Diet for Immunocompromised Patients. Front. Microbiol 2023, 14, 1136887. [Google Scholar] [CrossRef] [PubMed]
- Lassiter, M.; Schneider, S.M. A Pilot Study Comparing the Neutropenic Diet to a Non-Neutropenic Diet in the Allogeneic Hematopoietic Stem Cell Transplantation Population. Clin. J. Oncol. Nurs. 2015, 19, 273–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, Y.; Lu, X.; Liu, H. Neutropenic Diet Cannot Reduce the Risk of Infection and Mortality in Oncology Patients with Neutropenia. Front. Oncol. 2022, 12, 836371. [Google Scholar] [CrossRef]
- Carr, S.E.; Halliday, V. Investigating the Use of the Neutropenic Diet: A Survey of U.K. Dietitians. J. Hum. Nutr. Diet. 2015, 28, 510–515. [Google Scholar] [CrossRef]
- Patois, C.; Chen, Y.; Meiselman, H.L.; Barraco, F.; Giboreau, A. Designing Food and Meals for Bone Marrow Transplant Patients with Compromised Immunity. Int. J. Food Des. 2021, 6, 27–52. [Google Scholar] [CrossRef]
- Gurunathan, A.; Patel, N.S.; Freedman, J.L. Mucositis and Pain in the Peri-HSCT Period. In Hematopoietic Stem Cell Transplantation for the Pediatric Hematologist/Oncologist; Springer: Cham, Switzerland, 2017; pp. 209–214. [Google Scholar]
- Yoo, H.C.; Yu, Y.C.; Sung, Y.; Han, J.M. Glutamine Reliance in Cell Metabolism. Exp. Mol. Med. 2020, 52, 1496–1516. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, T.R.; Young, L.S.; Benfell, K.; Scheltinga, M.; Hortos, K.; Bye, R.; Morrow, F.D.; Jacobs, D.O.; Smith, R.J.; Antin, J.H.; et al. Clinical and Metabolic Efficacy of Glutamine-Supplemented Parenteral Nutrition after Bone Marrow Transplantation. A Randomized, Double-Blind, Controlled Study. Ann. Intern. Med. 1992, 116, 821–828. [Google Scholar] [CrossRef] [PubMed]
- Crowther, M.; Avenell, A.; Culligan, D.J. Systematic Review and Meta-Analyses of Studies of Glutamine Supplementation in Haematopoietic Stem Cell Transplantation. Bone Marrow Transpl. 2009, 44, 413–425. [Google Scholar] [CrossRef]
- Alonso Domínguez, M.T. Efectividad de La Glutamina En Nutrición Parenteral En Pacientes Sometidos a Trasplante de Progenitores Hematopoyéticos. Ph.D. Thesis, Universidad de Murcia, Murcia, Spain, 2023. [Google Scholar]
- Sayles, C.; Hickerson, S.C.; Bhat, R.R.; Hall, J.; Garey, K.W.; Trivedi, M.V. Oral Glutamine in Preventing Treatment-Related Mucositis in Adult Patients with Cancer: A Systematic Review. Nutr. Clin. Pract. 2016, 31, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Pytlík, R.; Beneš, P.; Patorková, M.; Chocenská, E.; Gregora, E.; Procházka, B.; Kozák, T. Standardized Parenteral Alanyl-Glutamine Dipeptide Supplementation Is Not Beneficial in Autologous Transplant Patients: A Randomized, Double-Blind, Placebo Controlled Study. Bone Marrow Transpl. 2002, 30, 953–961. [Google Scholar] [CrossRef] [Green Version]
- Mallath, M.K. Nutritional Support and Issues Related to Hematopoietic Stem-Cell Transplantation. In Contemporary Bone Marrow Transplantation; Springer: Cham, Switzerland, 2021; pp. 1–11. [Google Scholar] [CrossRef]
- Yurko-Mauro, K.; Van Elswyk, M.; Teo, L. A Scoping Review of Interactions between Omega-3 Long-Chain Polyunsaturated Fatty Acids and Genetic Variation in Relation to Cancer Risk. Nutrients 2020, 12, 1647. [Google Scholar] [CrossRef] [PubMed]
- Giannattasio, S.; Dri, M.; Merra, G.; Caparello, G.; Rampello, T.; Di Renzo, L. Effects of Fatty Acids on Hematological Neoplasms: A Mini Review. Nutr. Cancer 2021, 74, 1538–1548. [Google Scholar] [CrossRef]
- Moloudizargari, M.; Mortaz, E.; Asghari, M.H.; Adcock, I.M.; Redegeld, F.A.; Garssen, J. Effects of the Polyunsaturated Fatty Acids, EPA and DHA, on Hematological Malignancies: A Systematic Review. Oncotarget 2018, 9, 11858. [Google Scholar] [CrossRef] [Green Version]
- Takatsuka, H.; Takemoto, Y.; Iwata, N.; Suehiro, A.; Hamano, T.; Okamoto, T.; Kanamaru, A.; Kakishita, E. Oral Eicosapentaenoic Acid for Complications of Bone Marrow Transplantation. Bone Marrow Transpl. 2001, 28, 769–774. [Google Scholar] [CrossRef] [Green Version]
- Baena-Gómez, M.A.; de la Torre-Aguilar, M.J.; Aguilera-García, C.M.; Olza, J.; Pérez-Navero, J.L.; Gil-Campos, M. Inflammatory Response Using Different Lipid Parenteral Nutrition Formulas in Children After Hematopoietic Stem Cell Transplantation. Nutr. Cancer 2016, 68, 804–810. [Google Scholar] [CrossRef]
- Lessa, R.C.; de Alves, F.A.; Fortunati, E.; Lu, J. Oral Mucositis in Cancer and Potential Use of Omega-3 Free Fatty Acids in Its Management: A Review. Biomedicines 2021, 9, 1531. [Google Scholar] [CrossRef]
- Sánchez-Lara, K.; Turcott, J.G.; Juárez-Hernández, E.; Nuñez-Valencia, C.; Villanueva, G.; Guevara, P.; De la Torre-Vallejo, M.; Mohar, A.; Arrieta, O. Effects of an Oral Nutritional Supplement Containing Eicosapentaenoic Acid on Nutritional and Clinical Outcomes in Patients with Advanced Non-Small Cell Lung Cancer: Randomised Trial. Clin. Nutr. 2014, 33, 1017–1023. [Google Scholar] [CrossRef] [PubMed]
- Dewey, A.; Baughan, C.; Dean, T.; Higgins, B.; Johnson, I. Eicosapentaenoic Acid (EPA, an Omega-3 Fatty Acid from Fish Oils) for the Treatment of Cancer Cachexia. Cochrane Database Syst. Rev. 2007, 2007, 1. [Google Scholar] [CrossRef] [PubMed]
- Colomer, R.; Moreno-Nogueira, J.M.; García-Luna, P.P.; García-Peris, P.; García-de-Lorenzo, A.; Zarazaga, A.; Quecedo, L.; del Llano, J.; Usán, L.; Casimiro, C. N-3 Fatty Acids, Cancer and Cachexia: A Systematic Review of the Literature. Br. J. Nutr. 2007, 97, 823–831. [Google Scholar] [CrossRef] [Green Version]
- Jabbour, J.; Manana, B.; Zahreddine, A.; Al-Shaar, L.; Bazarbachi, A.; Blaise, D.; El-Cheikh, J. Vitamins and Minerals Intake Adequacy in Hematopoietic Stem Cell Transplant: Results of a Randomized Controlled Trial. Bone Marrow Transpl. 2021, 56, 1106–1115. [Google Scholar] [CrossRef] [PubMed]
- Segon, B.; Lam, L.; Chan, H.Y.; Andersen, S.; Brown, T.; Kenway, D.A.; Bauer, J. Vitamin Requirements during Stem Cell Transplantation: A Systematic Review. Support. Care Cancer 2022, 30, 10391–10405. [Google Scholar] [CrossRef]
- Raoufinejad, K.; Shamshiri, A.R.; Pezeshki, S.; Chahardouli, B.; Hadjibabaie, M.; Jahangard-Rafsanjani, Z.; Gholami, K.; Rajabi, M.; Vaezi, M. Oral Calcitriol in Hematopoietic Recovery and Survival after Autologous Stem Cell Transplantation: A Randomized Clinical Trial. DARU J. Pharm. Sci. 2019, 27, 709. [Google Scholar] [CrossRef]
- Caballero-Velazquez, T.; Montero, I.; Sanchez-Guijo, F.; Parody, R.; Saldana, R.; Valcarcel, D.; Lopez-Godino, O.; Coll, C.F.I.; Cuesta, M.; Carrillo-Vico, A.; et al. Immunomodulatory Effect of Vitamin D after Allogeneic Stem Cell Transplantation: Results of a Prospective Multicenter Clinical Trial. Clin. Cancer Res. 2016, 22, 5673–5681. [Google Scholar] [CrossRef] [Green Version]
- Daloğlu, H.; Uygun, V.; Öztürkmen, S.; Yalçın, K.; Karasu, G.; Yeşilipek, A. Pre-Transplantation Vitamin D Deficiency Increases Acute Graft-versus-Host Disease after Hematopoietic Stem Cell Transplantation in Thalassemia Major Patients. Clin. Transpl. 2023, 37, e14874. [Google Scholar] [CrossRef]
- Hong, S.; Ferraro, C.S.; Hamilton, B.K.; Majhail, N.S. To D or Not to D: Vitamin D in Hematopoietic Cell Transplantation. Bone Marrow Transpl. 2020, 55, 2060–2070. [Google Scholar] [CrossRef]
- Sun, S.; Han, Y.; Lei, Y.; Yu, Y.; Dong, Y.; Chen, J. Hematopoietic Stem Cell: Regulation and Nutritional Intervention. Nutrients 2023, 15, 2605. [Google Scholar] [CrossRef] [PubMed]
- Doseděl, M.; Jirkovský, E.; Macáková, K.; Krčmová, L.K.; Javorská, L.; Pourová, J.; Mercolini, L.; Remião, F.; Nováková, L.; Mladěnka, P. Vitamin C—Sources, Physiological Role, Kinetics, Deficiency, Use, Toxicity, and Determination. Nutrients 2021, 13, 615. [Google Scholar] [CrossRef] [PubMed]
- Bae, M.; Kim, H. The Role of Vitamin C, Vitamin D, and Selenium in Immune System against COVID-19. Molecules 2020, 25, 5346. [Google Scholar] [CrossRef]
- Kashiouris, M.G.; L’heureux, M.; Cable, C.A.; Fisher, B.J.; Leichtle, S.W.; Fowler, A.A. The Emerging Role of Vitamin C as a Treatment for Sepsis. Nutrients 2020, 12, 292. [Google Scholar] [CrossRef] [Green Version]
- van Gorkom, G.N.Y.; Boerenkamp, L.S.; Gijsbers, B.L.M.G.; van Ojik, H.H.; Wodzig, W.K.W.H.; Wieten, L.; Van Elssen, C.H.M.J.; Bos, G.M.J. No Effect of Vitamin C Administration on Neutrophil Recovery in Autologous Stem Cell Transplantation for Myeloma or Lymphoma: A Blinded, Randomized Placebo-Controlled Trial. Nutrients 2022, 14, 4784. [Google Scholar] [CrossRef]
- Carr, A.C.; Spencer, E.; Das, A.; Meijer, N.; Lauren, C.; Macpherson, S.; Chambers, S.T. Patients Undergoing Myeloablative Chemotherapy and Hematopoietic Stem Cell Transplantation Exhibit Depleted Vitamin C Status in Association with Febrile Neutropenia. Nutrients 2020, 12, 1879. [Google Scholar] [CrossRef] [PubMed]
- Urbalejo-Ceniceros, V.I.; Rocha-González, H.I.; Acosta-Maldonado, B.L.; Valero-Saldaña, L.M.; Hernández-Alcantara, A.E.; Pérez-Camargo, D.A. Effect of Vitamin C on Immune Reconstitution after Bone Marrow Transplantation. Int. J. Clin. Pharmacol. Ther. 2022, 60, 384–391. [Google Scholar] [CrossRef] [PubMed]
- Chi, M.; Jiang, T.; He, X.; Peng, H.; Li, Y.; Zhang, J.; Wang, L.; Nian, Q.; Ma, K.; Liu, C. Role of Gut Microbiota and Oxidative Stress in the Progression of Transplant-Related Complications Following Hematopoietic Stem Cell Transplantation. Oxid. Med. Cell. Longev. 2023, 2023, 3532756. [Google Scholar] [CrossRef]
- Raghunathan, V.M.; Sheng, I.; Lim, S.H. Intestinal Dysbiosis and Allogeneic Hematopoietic Progenitor Cell Transplantation. J. Transl. Med. 2016, 14, 335. [Google Scholar] [CrossRef] [Green Version]
- Taur, Y.; Jenq, R.R.; Ubeda, C.; Van Den Brink, M.; Pamer, E.G. Role of Intestinal Microbiota in Transplantation Outcomes. Best Pr. Res. Clin. Haematol. 2015, 28, 155–161. [Google Scholar] [CrossRef] [Green Version]
- Ciernikova, S.; Kasperova, B.; Drgona, L.; Smolkova, B.; Stevurkova, V.; Mego, M. Targeting the Gut Microbiome: An Emerging Trend in Hematopoietic Stem Cell Transplantation. Blood Rev. 2021, 48, 100790. [Google Scholar] [CrossRef]
- Gerbitz, A.; Schultz, M.; Wilke, A.; Linde, H.J.; Schölmerich, J.; Andreesen, R.; Holler, E. Probiotic Effects on Experimental Graft-versus-Host Disease: Let Them Eat Yogurt. Blood 2004, 103, 4365–4367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gorshein, E.; Wei, C.; Ambrosy, S.; Budney, S.; Vivas, J.; Shenkerman, A.; Manago, J.; McGrath, M.K.; Tyno, A.; Lin, Y.; et al. Lactobacillus Rhamnosus GG Probiotic Enteric Regimen Does Not Appreciably Alter the Gut Microbiome or Provide Protection against GVHD after Allogeneic Hematopoietic Stem Cell Transplantation. Clin. Transpl. 2017, 31, e12947. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.A.; Woodfield, M.C.; Boyle, N.; Stednick, Z.; Boeckh, M.; Pergam, S.A. Incidence and Outcomes of Bloodstream Infections among Hematopoietic Cell Transplant Recipients from Species Commonly Reported to Be in Over-the-Counter Probiotic Formulations. Transpl. Infect. Dis. 2016, 18, 699–705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iyama, S.; Sato, T.; Tatsumi, H.; Hashimoto, A.; Tatekoshi, A.; Kamihara, Y.; Horiguchi, H.; Ibata, S.; Ono, K.; Murase, K.; et al. Efficacy of Enteral Supplementation Enriched with Glutamine, Fiber, and Oligosaccharide on Mucosal Injury Following Hematopoietic Stem Cell Transplantation. Case Rep. Oncol. 2014, 7, 692. [Google Scholar] [CrossRef]
- Yazdandoust, E.; Hajifathali, A.; Roshandel, E.; Zarif, M.N.; Pourfathollah, A.A.; Parkhideh, S.; Mehdizadeh, M.; Amini-Kafiabad, S. Gut Microbiota Intervention by Pre and Probiotics Can Induce Regulatory T Cells and Reduce the Risk of Severe Acute GVHD Following Allogeneic Hematopoietic Stem Cell Transplantation. Transpl. Immunol. 2023, 78, 101836. [Google Scholar] [CrossRef]
- Bilinski, J.; Grzesiowski, P.; Sorensen, N.; Madry, K.; Muszynski, J.; Robak, K.; Wroblewska, M.; Dzieciatkowski, T.; Dulny, G.; Dwilewicz-Trojaczek, J.; et al. Fecal Microbiota Transplantation in Patients with Blood Disorders Inhibits Gut Colonization with Antibiotic-Resistant Bacteria: Results of a Prospective, Single-Center Study. Clin. Infect. Dis. 2017, 65, 364–370. [Google Scholar] [CrossRef]
- Webb, B.J.; Brunner, A.; Ford, C.D.; Gazdik, M.A.; Petersen, F.B.; Hoda, D. Fecal Microbiota Transplantation for Recurrent Clostridium Difficile Infection in Hematopoietic Stem Cell Transplant Recipients. Transpl. Infect. Dis. 2016, 18, 628–633. [Google Scholar] [CrossRef]
- Kaźmierczak-Siedlecka, K.; Skonieczna-żydecka, K.; Biliński, J.; Roviello, G.; Iannone, L.F.; Atzeni, A.; Sobocki, B.K.; Połom, K. Gut Microbiome Modulation and Faecal Microbiota Transplantation Following Allogenic Hematopoietic Stem Cell Transplantation. Cancers 2021, 13, 4665. [Google Scholar] [CrossRef]
- Kakihana, K.; Fujioka, Y.; Suda, W.; Najima, Y.; Kuwata, G.; Sasajima, S.; Mimura, I.; Morita, H.; Sugiyama, D.; Nishikawa, H.; et al. Fecal Microbiota Transplantation for Patients with Steroid-Resistant Acute Graft-versus-Host Disease of the Gut. Blood 2016, 128, 2083–2088. [Google Scholar] [CrossRef]
- Spindelboeck, W.; Schulz, E.; Uhl, B.; Kashofer, K.; Aigelsreiter, A.; Zinke-Cerwenka, W.; Mulabecirovic, A.; Kump, P.K.; Halwachs, B.; Gorkiewicz, G.; et al. Repeated Fecal Microbiota Transplantations Attenuate Diarrhea and Lead to Sustained Changes in the Fecal Microbiota in Acute, Refractory Gastrointestinal Graft- versus-Host-Disease. Haematologica 2017, 102, e210–e213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dougé, A.; Ravinet, A.; Corriger, A.; Cabrespine, A.; Wasiak, M.; Pereira, B.; Sokol, H.; Nguyen, S.; Bay, J.O. Protocol: Faecal Microbiota Transplantation to Prevent Complications after Allogeneic Stem Cell Transplantation for Haematological Malignancies: A Study Protocol for a Randomised Controlled Phase-II Trial (the FMT-Allo Study). BMJ Open 2023, 13, e068480. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.A.; Luong, M.K.; Shaw, H.; Nathan, P.; Bataille, V.; Spector, T.D. The Gut Microbiome: What the Oncologist Ought to Know. Br. J. Cancer 2021, 125, 1197–1209. [Google Scholar] [CrossRef]
- Kusakabe, S.; Fukushima, K.; Maeda, T.; Motooka, D.; Nakamura, S.; Fujita, J.; Yokota, T.; Shibayama, H.; Oritani, K.; Kanakura, Y. Pre- and Post-Serial Metagenomic Analysis of Gut Microbiota as a Prognostic Factor in Patients Undergoing Haematopoietic Stem Cell Transplantation. Br. J. Haematol. 2020, 188, 438–449. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Zhang, H.; Lu, X.; Xia, L. Viable Bifidobacterium Tablets for the Prevention of Chemotherapy-/Radiation-Induced Mucositis in Patients Undergoing Haematopoietic Stem Cell Transplantation. Support. Care Cancer 2023, 31, 282. [Google Scholar] [CrossRef]
- Frey-Furtado, L.; Magalhães, I.; Azevedo, M.J.; Sampaio-Maia, B. The Role of Biotics as a Therapeutic Strategy for Oral Mucositis—A Systematic Review. In Probiotics Antimicrob Proteins; Springer: Cham, Switzerland, 2023; pp. 1–14. [Google Scholar]
- Sharma, A.; Tilak, T.; Bakhshi, S.; Raina, V.; Kumar, L.; Chaudhary, S.P.; Sahoo, R.K.; Gupta, R.; Thulkar, S. Lactobacillus Brevis CD2 Lozenges Prevent Oral Mucositis in Patients Undergoing High Dose Chemotherapy Followed by Haematopoietic Stem Cell Transplantation. ESMO Open 2017, 1, e000138. [Google Scholar] [CrossRef] [Green Version]
- Ladas, E.J.; Bhatia, M.; Chen, L.; Sandler, E.; Petrovic, A.; Berman, D.M.; Hamblin, F.; Gates, M.; Hawks, R.; Sung, L.; et al. The Safety and Feasibility of Probiotics in Children and Adolescents Undergoing Hematopoietic Cell Transplantation. Bone Marrow Transpl. 2016, 51, 262–266. [Google Scholar] [CrossRef] [Green Version]
- Nieder, M.L.; Ladas, E.J.; Bhatia, M.; Gates, M.; Hamblin, F.; Petrovic, A.; Chen, L.; Sandler, E. Safety and Feasibility of Administering Lactobacillus Plantarum to Children Undergoing Myeloablative Hematopoietic Cell Transplantation (HCT). Biol. Blood Marrow Transplant. 2015, 21, S256. [Google Scholar] [CrossRef] [Green Version]
- Moraes, B.G.C.; Martins, R.C.R.; Franco, L.A.M.; Lima, V.A.C.C.; Pereira, G.C.O.; Bsc; Santos, J.T.; Fernandes, T.H.; Guimaraes, T.; Rocha, V.G.; et al. 2665. Intestinal Microbiome of Patients Submitted to Hematopoietic Stem Cell Transplantation Using Lactobacillus Plantarum to Decolonized Multidrug-Resistant Bacteria. Open Forum Infect. Dis. 2019, 6, S933–S934. [Google Scholar] [CrossRef]
- Jing, G.; Yun, W.; Huilan, L.; Xia, L. Supplement Yogurt for Patients during Early Phase of Unrelated Cord Blood Transplantation: A Safety and Feasibility Pilot Study. Blood 2017, 130, 5468. [Google Scholar]
- Ritze, Y.; Bárdos, G.; Claus, A.; Ehrmann, V.; Bergheim, I.; Schwiertz, A.; Bischoff, S.C. Lactobacillus Rhamnosus GG Protects against Non-Alcoholic Fatty Liver Disease in Mice. PLoS ONE 2014, 9, e80169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.C.; Wu, C.R.; Huang, T.W. Preventive Effect of Probiotics on Oral Mucositis Induced by Cancer Treatment: A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2022, 23, 13268. [Google Scholar] [CrossRef] [PubMed]
- Ingham, A.C.; Pamp, S.J. Mucosal Microbiotas and Their Role in Stem Cell Transplantation. Apmis 2022, 130, 741–750. [Google Scholar] [CrossRef] [PubMed]
- Taggart, C.; Neumann, N.; Alonso, P.B.; Lane, A.; Pate, A.; Stegman, A.; Stendahl, A.; Davies, S.M.; Dandoy, C.E.; Grimley, M. Comparing a Neutropenic Diet to a Food Safety-Based Diet in Pediatric Patients Undergoing Hematopoietic Stem Cell Transplantation. Biol. Blood Marrow Transpl. 2019, 25, 1382–1386. [Google Scholar] [CrossRef]
| No. | Name of the Screening Tool | Description of the Screening Tool |
|---|---|---|
| 1. | Nutritional Risk Screening 2002 (NRS-2002) | Patients are scored on each of two comorbidities (1) nutritional deterioration and (2) disease severity, according to whether they are absent, mild, moderate, or severe, resulting in a total score of 0–6. Patients with a total score ≥ 3 are classified as being at nutritional risk. Malnutrition was estimated using three variables used in most screening tools: BMI, percentage of recent weight loss, and change in food intake. The variable of age was also considered; when the assessed exceeds 70 years of age, they are assigned 1 additional point. |
| 2. | Malnutrition Universal Screening Tool (MUST) | It is a five-step tool using three independent criteria to determine overall malnutrition risk: current weight status using BMI, unintentional weight loss, and acute disease effect that has induced a phase of nil per os for >5 days. Each variable can be scored on a three-point scale as 0, 1, or 2. The overall risk of malnutrition is set as low (score = 0), medium (score = 1), or high (score > 2). Together, these three criteria are better predictors than each individually. |
| 3. | Malnutrition Screening Tool (MST) | This tool consists of two questions about recent unintentional weight loss and reduced appetite resulting in a reduction in the volume of meals consumed. The first is scored on a 4-point scale according to the number of pounds lost; a positive answer to the second results in a score of 1. The total score ranks from 0 to 5, with patients considered at risk of malnutrition if they score ≥ 2. |
| 4. | Subjective Global Assessment (SGA) | It consists of the patient’s medical history (weight loss, food intake, gastrointestinal symptoms, and functional capacity) and physical examination (subcutaneous fat, muscle atrophy, and oedema). Patients are assessed as well-nourished (A), moderately malnourished (B), or severely malnourished (C). Typically used by clinicians as part of a comprehensive nutrition assessment. |
| Enteral Nutrition | Parental Nutrition | ||
|---|---|---|---|
| Advantages | Flaws | Advantages | Flaws |
|
|
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pawłowski, P.; Pawłowska, P.; Ziętara, K.J.; Samardakiewicz, M. The Critical Exploration into Current Evidence behind the Role of the Nutritional Support in Adult Patients Who Undergo Haematogenic Stem Cell Transplantation. Nutrients 2023, 15, 3558. https://doi.org/10.3390/nu15163558
Pawłowski P, Pawłowska P, Ziętara KJ, Samardakiewicz M. The Critical Exploration into Current Evidence behind the Role of the Nutritional Support in Adult Patients Who Undergo Haematogenic Stem Cell Transplantation. Nutrients. 2023; 15(16):3558. https://doi.org/10.3390/nu15163558
Chicago/Turabian StylePawłowski, Piotr, Paulina Pawłowska, Karolina Joanna Ziętara, and Marzena Samardakiewicz. 2023. "The Critical Exploration into Current Evidence behind the Role of the Nutritional Support in Adult Patients Who Undergo Haematogenic Stem Cell Transplantation" Nutrients 15, no. 16: 3558. https://doi.org/10.3390/nu15163558






