Effect of the Nutritional Intervention Program on Body Weight and Selected Cardiometabolic Factors in Children and Adolescents with Excess Body Weight and Dyslipidemia: Study Protocol and Baseline Data
Abstract
1. Introduction
Study Aims and Hypothesis
- (a)
- The nutritional intervention program will induce a reduction in body weight and improve lipid parameters within 8 weeks of the recommended dietotherapy;
- (b)
- The LGI diet may be a more effective form of dietotherapy compared to the ST diet.
2. Methods
2.1. Study Design
2.2. Participants and Sample Estimation
- -
- Size of the general population: The number of children aged 7–18 living in the Mazowieckie Voivodship with overweight/obesity—these were the initial assumptions and criteria for inclusion in the study (n = 140,211).
- -
- Significance level (0.05) and confidence level of 95%, maximum estimation error (5%).
- -
- In the estimates of the sample size, the assumption of repeatability of the examined feature was adopted, i.e., the size of the fraction at the level of 0.039 (3.9%—prevalence of lipid disorders in the population of children in Poland).
2.3. Eligibility Criteria
2.4. Dietary Procedure
2.5. General Procedure
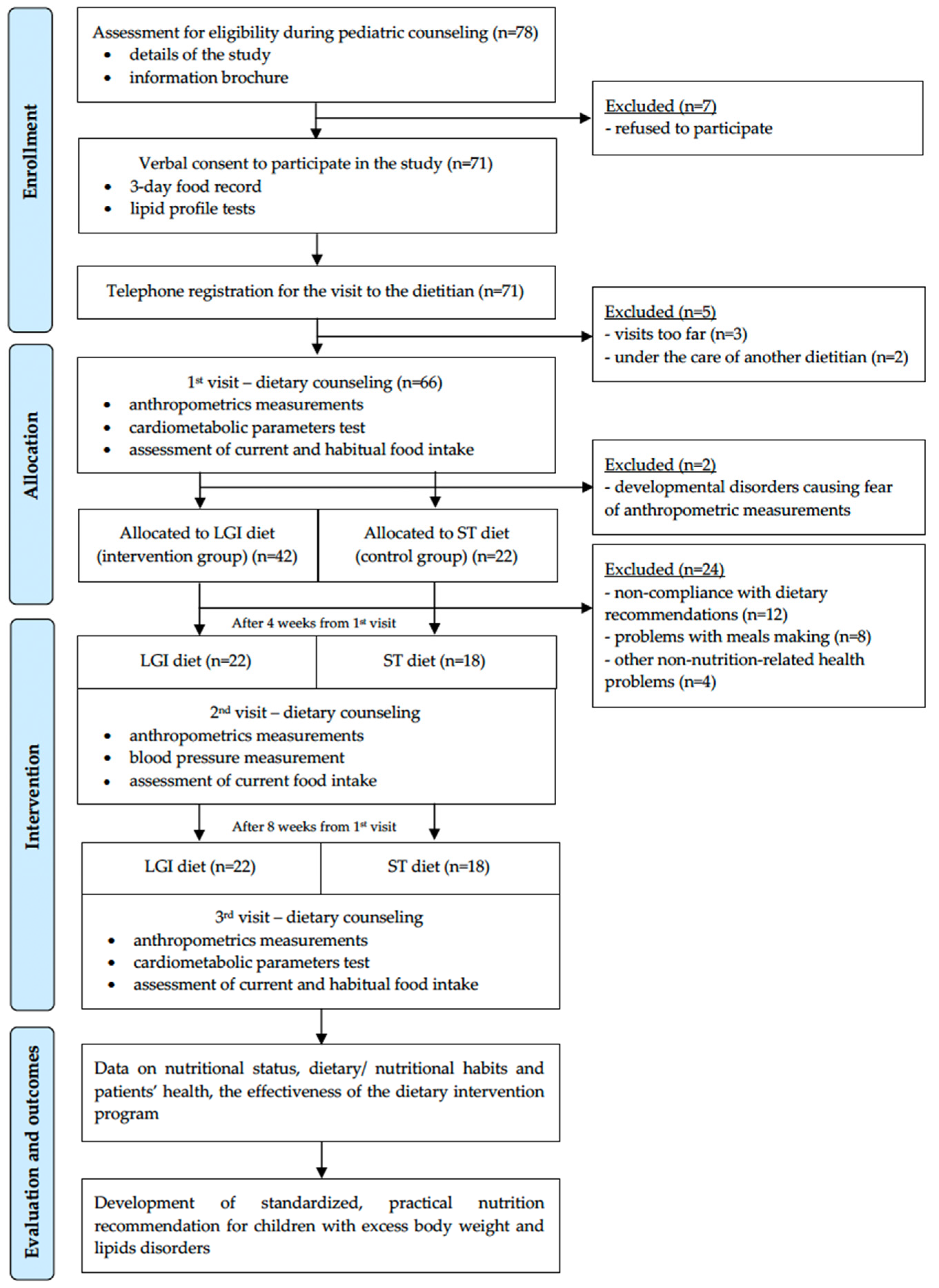
2.5.1. Enrollment
2.5.2. First Visit—Baseline
2.5.3. Second Visit—After 4 Weeks
2.5.4. Third Visit—After 8 Weeks
3. Statistical Analysis
4. Key Findings at the Beginning of the Study
5. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A

References
- World Health Organization (WHO). Obesity and Overweight–Key Facts. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 4 December 2022).
- Gurnani, M.; Birken, C.; Hamilton, J. Childhood obesity. Causes, consequences, and management. Pediatr. Clin. N. Am. 2015, 62, 821–840. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, K.; Sahoo, B.; Choudhury, A.K.; Sofi, N.Y.; Kumar, R.; Bhadoria, A.S. Childhood obesity: Causes and consequences. J Fam. Med. Prim. Care 2015, 4, 187–192. [Google Scholar]
- Lobstein, T.; Jackson-Leach, R. Planning for the worst: Estimates of obesity and comorbidities in school-age children in 2025. Pediatr. Obes. 2016, 11, 321–325. [Google Scholar] [CrossRef] [PubMed]
- Inchley, J.; Currie, D.; Jewell, J.; Breda, J.; Barnekow, V. Adolescent Obesity and Related Behaviours: Trends and Inequalities in the WHO European Region, 2002–2014: Observations from the Health Behaviour in School-Aged Children (HBSC) WHO Collaborative Cross-National Study; World Health Organization Regional Office for Europe: Copenhagen, Denmark, 2017.
- Harton, A.; Myszkowska-Ryciak, J.; Laskowski, W.; Gajewska, D. Prevalence of overweight and obesity among adolescents in Poland. J. Health Inequal. 2019, 5, 180–187. [Google Scholar] [CrossRef]
- Bondyra-Wiśniewska, B.; Myszkowska-Ryciak, J.; Harton, A. Impact of lifestyle intervention programs for children and adolescents with overweight or obesity on body weight and selected cardiometabolic factors–A systematic review. Int. J. Environ. Res. Public Health 2021, 18, 2061. [Google Scholar] [CrossRef]
- Močnik, M.; Marčun Varda, N. Lipid biomarkers and atherosclerosis–Old and new in cardiovascular risk in childhood. Int. J. Mol. Sci. 2023, 24, 2237. [Google Scholar] [CrossRef]
- Karney, A.; Brągoszewska, H.; Soluch, L.; Ołtarzewski, M. Risk factors for atherosclerosis in obese children aged 6–12 years. Dev. Period Med. 2017, 21, 259–265. [Google Scholar]
- Ding, W.; Cheng, H.; Yan, Y.; Zhao, X.; Chen, F.; Huang, G.; Hou, D.; Mi, J. 10-year trends in serum lipid levels and dyslipidemia among children and adolescents from several schools in Beijing, China. J. Epidemiol. 2016, 26, 637–645. [Google Scholar] [CrossRef]
- Koskinen, J.; Juonala, M.; Dwyer, T.; Venn, A.; Thomson, R.; Bazzano, L.; Berenson, G.S.; Sabin, M.A.; Burns, T.L.; Viikari, J.S.A.; et al. Impact of lipid measurements in youth in addition to conventional clinic-based risk factors on predicting preclinical atherosclerosis in adulthood. The International Childhood Cardiovascular Cohort (i3C) Consortium. Circulation 2018, 137, 1246–1255. [Google Scholar] [CrossRef]
- Umer, A.; Kelley, G.A.; Cottrell, L.E.; Giacobbi Jr, P.; Innes, K.E.; Lilly, C.L. Childhood obesity and adult cardiovascular disease risk factors: A systematic review with meta-analysis. BMC Public Health 2017, 17, 683. [Google Scholar] [CrossRef]
- Brzeziński, M.; Metelska, P.; Myśliwiec, M.; Szlagatys-Sidorkiewicz, A. Lipid disorders in children living with overweight and obesity–large cohort study from Poland. Lipids Health Dis. 2020, 19, 47. [Google Scholar] [CrossRef] [PubMed]
- Zafar, M.I.; Mills, K.E.; Zheng, J.; Regmi, A.; Hu, S.Q.; Gou, L.; Chen, L. Low-glycemic index diets as an intervention for diabetes: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2019, 110, 891–902. [Google Scholar] [CrossRef] [PubMed]
- Kulczyński, B.; Gramza-Michałowska, A. Importance of glycemic index and glycemic load in prevention of cardiovascular diseases. Probl. Hig. Epidemiol. 2015, 96, 51–56. [Google Scholar]
- Schwingshackl, L.; Hobl, L.P.; Hoffmann, G. Effects of low glycaemic index/low glycaemic load vs. high glycaemic index/ high glycaemic load diets on overweight/obesity and associated risk factors in children and adolescents: A systematic review and meta-analysis. Nutr. J. 2015, 14, 87. [Google Scholar] [CrossRef]
- Kavey, R.E.; Simons-Morton, D.G.; Jesus, J.M. Expert panel on integrated guidelines for cardiovascular health and risk reduction in children and adolescents: Summary report. Pediatr. Clin. Pract. Guidel. Policies 2016. [Google Scholar] [CrossRef][Green Version]
- Banach, M.; Jankowski, P.; Jóźwiak, J.; Cybulska, B.; Windak, A.; Guzik, T.; Mamcarz, A.; Broncel, M.; Tomasik, T. PoLA/CFPiP/PCS Guidelines for the management of dyslipidaemias for family physicians 2016. Arch. Med. Sci. 2017, 13, 1–45. [Google Scholar] [CrossRef]
- Kułaga, Z.; Różdżyńska-Świątkowska, A.; Grajda, A.; Gurzkowska, B.; Wojtyło, M.; Góźdź, M.; Świąder-Leśniak, A.; Litwin, M. Percentile charts for growth and nutritional status assessment in Polish children and adolescents from birth to 18 year of age. Stand. Med. Pediatr. 2015, 12, 119–135. [Google Scholar]
- Cole, T.J.; Bellizzi, M.C.; Flegal, K.M.; Dietz, W.H. Establishing a standard definition for child overweight and obesity word wide: International survey. BMJ 2000, 320, 1240–1243. [Google Scholar] [CrossRef]
- Wasyluk, W.; Wasyluk, M.; Zwolak, A.; Łuczyk, R.J. Limits of body composition assessment by bioelectrical impedance analysis (BIA). J. Educ. Health Sport 2019, 9, 35–44. [Google Scholar]
- Mialich, M.S.; Faccioli Sicchieri, J.M.; Jordao Junior, A.A. Analysis of body composition: A critical review of the use of bioelectrical impedance analysis. Int. J. Clin. Nutr. 2014, 2, 1–10. [Google Scholar]
- Parillo, M.; Licenziati, M.R.; Vacca, M.; De Marco, D.; Iannuzzi, A. Metabolic changes after a hypocaloric, low-glycemic-index diet in obese children. J. Endocrinol. Investig. 2012, 35, 629–633. [Google Scholar]
- Visuthranukul, C.; Sirimongkol, P.; Prachansuwan, A.; Pruksananonda, C.; Chomtho, S. Low-glycemic index diet may improve insulin sensitivity in obese children. Pediatr. Res. 2015, 78, 567–573. [Google Scholar] [CrossRef] [PubMed]
- Augustin, L.S.A.; Kendall, C.W.C.; Jenkins, D.J.A.; Willett, W.C.; Astrup, A.; Barclay, A.W.; Björck, I.; Brand-Miller, J.C.; Brighenti, F.; Buyken, A.E.; et al. Glycemic index, glycemic load and glycemic response: An International Scientific Consensus Summit from the International Carbohydrate Quality Consortium (ICQC). Nutr. Metab. Cardiovasc. Dis. 2015, 25, 795–815. [Google Scholar] [CrossRef] [PubMed]
- Alman, K.L.; Lister, N.B.; Garnett, S.P.; Gow, M.L.; Aldwell, K.; Jebeile, H. Dietetic management of obesity and severe obesity in children and adolescents: A scoping review of guidelines. Obes. Rev. 2021, 22, e13132. [Google Scholar] [CrossRef] [PubMed]
- Institute of Medicine. Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids; The National Academies Press: Washington, DC, USA, 2005. [Google Scholar]
- Atkinson, F.S.; Foster-Powell, K.; Brand-Miller, J.C. International table of glycemic index and glycemic load values: 2008. Diabetes Care 2008, 31, 2281–2283. [Google Scholar] [CrossRef]
- Jarosz, M. Piramida Zdrowego Żywienia i Stylu Życia Dzieci i Młodzieży. 2009. Available online: https://ncez.pzh.gov.pl/dzieci-i-mlodziez/piramida-zdrowego-zywienia-i-stylu-zycia-dzieci-i-mlodziezy-2/ (accessed on 4 August 2022).
- American College of Cardiology. 2018 Guideline on the management of blood cholesterol. J. Am. Coll. Cardiol. 2018. [Google Scholar] [CrossRef]
- Brantlov, S.; Ward, L.C.; Jødal, L.; Rittig, S.; Lange, A. Critical factors and their impact on bioelectrical impedance analysis in children: A review. J. Med. Eng. Technol. 2017, 41, 22–35. [Google Scholar] [CrossRef]
- World Health Organization (WHO). WHO Guidelines on Physical Activity and Sedentary Behaviour: At a Glance; WHO: Geneva, Switzerland, 2020.
- Centers for Disease Control and Prevention (CDC). National Health and Nutrition Examination Survey (NHANES). Anthropometry Procedures Manual. 2016. Available online: https://wwwn.cdc.gov/nchs/data/nhanes/2015-2016/manuals/2016_Anthropometry_Procedures_Manual.pdf (accessed on 4 August 2022).
- Świąder-Leśniak, A.; Kułaga, Z.; Grajda, A.; Gurzkowska, B.; Góźdź, M.; Wojtyło, M.; Różdżyńska-Świątkowska, A.; Litwin, M. References for waist and hip circumferences in Polish children and adolescents 3–18 year of age. Stand. Med.-Pediatr. 2015, 12, 137–150. [Google Scholar]
- Addo, O.Y.; Himes, J.H.; Zemel, B.S. Reference ranges for midupper arm circumference, upper arm muscle area, and upper arm fat area in US children and adolescents aged 1–20 y. Am. J. Clin. Nutr. 2017, 105, 111–120. [Google Scholar] [CrossRef]
- Kułaga, Z.; Grajda, A.; Gurzkowska, B.; Góźdź, M.; Wojtyło, M.; Świąder, A.; Różdżyńska-Świątkowska, A.; Litwin, M.; OLA Research Group. Centile charts for blood pressure assessment in children and adolescents aged 3–18 years. Stand. Med.-Pediatr. 2013, 1, 22–30. [Google Scholar]
- Kunachowicz, H.; Przygoda, B.; Iwanow, K.; Nadolna, I. Tabele Wartości Odżywczej Produktów Spożywczych i Potraw. Baza Danych–Wersja Pełna; National Institute of Public Health–National Institute of Hygiene: Warsaw, Poland, 2017.
- Wądołowska, L.; Niedźwiedzka, E. Food Frequency Questionnaire with 6 Answers. 2018. Available online: http://www.uwm.edu.pl/edu/lidiawadolowska/ (accessed on 4 August 2022).
- Fang, K.; Mu, M.; Liu, K.; He, Y. Screen time and childhood overweight/obesity: A systematic review and meta-analysis. Child Care Health Dev. 2019, 45, 744–753. [Google Scholar] [CrossRef]
- Pederiva, C.; Capra, M.E.; Viggiano, C.; Rovelli, V.; Banderali, G.; Biasucci, G. Early prevention of atherosclerosis: Detection and management of hypercholesterolaemia in children and adolescents. Life 2021, 11, 345. [Google Scholar] [CrossRef]
- Ho, M.; Garnett, S.P.; Baur, L.A.; Burrows, T.; Stewart, L.; Neve, M.; Collins, C. Impact of dietary and exercise interventions on weight change and metabolic outcomes in obese children and adolescents: A systematic review and meta-analysis of randomized trials. JAMA Pediatr. 2013, 167, 759–768. [Google Scholar] [CrossRef]
- Md. Yusop, N.B.; Mohd Shariff, Z.; Hwu, T.T.; Talib, R.A.; Spurrier, N. The effectiveness of a stage-based lifestyle modification intervention for obese children. BMC Public Health 2018, 18, 299. [Google Scholar] [CrossRef] [PubMed]
- Malik, V.S.; Pan, A.; Willett, W.C.; Hu, F.B. Sugar-sweetened beverages and weight gain in children and adults: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2013, 98, 1084–1102. [Google Scholar] [CrossRef] [PubMed]
- Prada, M.; Saraiva, M.; Garrido, M.V.; Sério, A.; Teixeira, A.; Lopes, D.; Silva, D.A.; Rodrigues, D.L. Perceived associations between excessive sugar intake and health conditions. Nutrients 2022, 14, 640. [Google Scholar] [CrossRef] [PubMed]
- Te Morenga, L.A.; Howatson, A.J.; Jones, R.M.; Mann, J. Dietary sugars and cardiometabolic risk: Systematic review and meta-analyses of randomized controlled trials of the effects on blood pressure and lipids. Am. J. Clin. Nutr. 2014, 100, 65–79. [Google Scholar] [CrossRef]
- Poorolajal, J.; Farbakhsh, F.; Mahjub, H.; Bidarafsh, A.; Babaee, E. How much excess body weight, blood sugar, or age can double the risk of hypertension? Public Health 2016, 133, 14–18. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Guideline: Sugars Intake for Adults and Children; WHO: Geneva, Switzerland, 2015.
- Quadros, T.M.; Gordia, A.P.; Silva, R.C.; Silva, L.R. Predictive capacity of anthropometric indicators for dyslipidemia screening in children and adolescents. J. Pediatr. 2015, 91, 455–463. [Google Scholar] [CrossRef]
| Parameter | Method of Measurement |
|---|---|
| Anthropometrics | |
| Height | No shoes, headgear, or head ornaments. Measured twice with a height meter to the nearest 1 mm. If there were any differences, the results were averaged. Height was compared with the Polish growth reference values [19]. |
| Weight | In light clothes, no shoes, and heavy items in pockets (e.g., phone, wallet). Measurement taken using the professional TANITA MC-780 P MA multi-frequency body composition analyzer with weighing function to the nearest 100 g. Weight was compared with the Polish growth reference values [19]. |
| Waist circumference | Waist circumference was measured in a standing position. Body weight was evenly distributed over both feet. After a few natural breaths, with freely relaxed abdominal muscles, waist circumference was assessed at the midpoint between the lower costal margin and the iliac crest at the end of a normal expiration. Measured twice with an anthropometric tape to the nearest 1 mm. If there were any differences, the results were averaged. Waist circumferences were compared with the Polish reference values [34]. Reference values for healthy children and adolescents: <90th percentile. |
| Hip circumference | Hip circumference was measured in a standing position. Body weight was evenly distributed over both feet. Hip circumference was assessed around the widest part of the buttocks. Measured twice with an anthropometric tape to the nearest 1 mm. If there were any differences, the results were averaged. Hip circumferences were compared with the Polish reference values [34]. Reference values for healthy children and adolescents: <90th percentile. |
| Arm circumference | Arm circumference was measured on a freely lowered non-dominant arm, with relaxed muscles. Arm circumference was assessed at the midpoint between the tips of the shoulder and elbow, in the place where the arm circumference is greatest. Measured twice with an anthropometric tape to the nearest 1 mm. If there were any differences, the results were averaged. Arm circumferences were compared with the percentile reference ranges [35]. Reference values for healthy children and adolescents: <90th percentile. |
| Body composition analysis | Body composition analysis was performed in a standing position. Body weight was evenly distributed over both feet. In light clothes, with no shoes, socks, tights, or heavy items in pockets (e.g., phone, wallet). Measurement taken using the professional TANITA MC-780 P MA multi-frequency body composition analyzer. Before each measurement, the electrodes were thoroughly wiped with an appropriate disinfectant. |
| Cardiometabolics | |
| Lipid profile (including levels of TC, HDL-C, LDL-C, and TG) | Tests performed after referral and under the supervision of the physician by qualified medical personnel in the laboratory. Lipid profile values were compared by pediatrician with the reference ranges according to the American College of Cardiology [30]. Acceptable values for healthy children and adolescents: <170 mg/dL for TC, >45 mg/dL for HDL-C, <110 mg/dL for LDL-C, <75 mg/dL for TG in children aged 0–9 years, and <90 mg/dL for TG in children and adolescents aged 10–19 years. |
| Blood pressure | The measurement was taken using an automatic upper-arm blood pressure monitor intended for children and adolescents. The cuff was placed on the left arm at the level of the heart, with the arm resting on the tabletop, the back resting on the back of the chair, and the feet resting on the floor. The measurement was taken in a sitting position after min, with 10 min of rest, twice at approximately 5 min intervals. Blood pressures were compared with the Polish reference values [36]. Systolic and diastolic blood pressure reference values for healthy children and adolescents: <90th percentile. |
| Food intake | |
| Current food intake | Current food intake was assessed using a 3-day food record before the 1st visit and each day throughout the duration of the diet intervention. Calculations of energy value and all nutrients from the food records were made by the dietitian with the use of a table of nutritional value of food products and dishes [37]. |
| Habitual food intake | Habitual food intake was assessed using the validated Food Frequency Questionnaire (FFQ-6) [38]. FFQ-6 is used to collect information on the frequency of consumption of 62 assortment groups of products, representing 8 main food groups: (1) sweets and snacks, (2) dairy products and eggs, (3) grain products, (4) fats, (5) fruits, (6) vegetables, legumes, and nuts, (7) meat and fish products, and (8) drinks. |
| Variable | Total (n = 64) | LGI Diet (n = 42) | ST Diet (n = 22) | p-Value (Mann–Whitney U Test) |
|---|---|---|---|---|
| Age [years] | 12.78 ± 2.65 | 12.33 ± 2.73 | 13.64 ± 2.32 | ns |
| Birth weight [g] | 3355.94 ± 387.22 | 3398.10 ± 394.91 | 3275.45 ± 367.39 | ns |
| Moderate or high-intensity physical activity [min/day] | 40.12 ± 38.43 | 40.83 ± 36.91 | 38.77 ± 43.04 | ns |
| Screen time [min/day] | 172.77 ± 90.17 | 147.55 ± 82.46 | 220.91 ± 86.13 | 0.004 |
| Anthropometrics | ||||
| Height (cm) | 164.86 ± 16.17 | 164.17 ± 17.09 | 166.19 ± 14.53 | ns |
| Body weight (kg) | 75.66 ± 25.46 | 75.13 ± 28.32 | 76.67 ± 19.39 | ns |
| Body weight-for-age percentile | 94.39 ± 5.87 | 94.45 ± 5.55 | 94.26 ± 6.57 | ns |
| BMI (kg/m2) | 26.94 ± 5.23 | 26.75 ± 5.74 | 27.29 ± 4.16 | ns |
| BMI-for-age percentile | 94.46 ± 4.60 | 94.32 ± 4.70 | 94.72 ± 4.51 | ns |
| Arm circumference (cm) | 29.96 ± 4.38 | 29.83 ± 4.51 | 30.19 ± 4.51 | ns |
| Waist circumference (cm) | 94.00 ± 16.79 | 95.22 ± 17.83 | 91.67 ± 14.72 | ns |
| Hip circumference (cm) | 101.85 ± 13.26 | 99.81 ± 13.57 | 105.75 ± 11.99 | ns |
| WHtR | 0.57 ± 0.06 | 0.58 ± 0.06 | 0.55 ± 0.07 | ns |
| WHR | 0.92 ± 0.08 | 0.95 ± 0.07 | 0.86 ± 0.08 | <0.001 |
| FM (kg) | 25.04 ± 11.56 | 24.55 ± 13.22 | 25.99 ± 7.61 | ns |
| Percent of body fat (%) | 32.11 ± 4.72 | 31.32 ± 5.06 | 33.61 ± 3.67 | 0.004 |
| FFM (kg) | 50.62 ± 15.09 | 50.58 ± 16.42 | 50.69 ± 12.50 | ns |
| TBW (kg) | 37.06 ± 11.05 | 37.03 ± 12.03 | 37.10 ± 9.16 | ns |
| Percent of TBW (%) | 49.68 ± 3.46 | 50.27 ± 3.70 | 48.57 ± 2.69 | 0.004 |
| MM (kg) | 48.04 ± 14.39 | 48.02 ± 15.67 | 48.09 ± 11.92 | ns |
| SMM (kg) | 28.61 ± 8.55 | 28.61 ± 9.31 | 28.61 ± 7.05 | ns |
| Percent of SMM (%) | 38.41 ± 2.62 | 38.81 ± 2.80 | 37.64 ± 2.06 | 0.007 |
| Variable | Total (n = 64) | LGI Diet (n = 42) | ST Diet (n = 22) | p-Value (Chi-Squared Test) | |
|---|---|---|---|---|---|
| Gender | Male | 44/69 | 34 a/81 | 10 b/46 | 0.004 |
| Female | 20/31 | 8/19 | 12/54 | ||
| Birth weight percentile | <90 | 56/88 | 36/86 | 20/91 | ns |
| ≥90 | 8/12 | 6/14 | 2/9 | ||
| BMI interpretation | Overweight | 28/44 | 20/48 | 8/36 | ns |
| Obesity | 36/56 | 22/52 | 14/64 | ||
| WC percentile | <90 | 6/9 | 2/5 | 4/18 | ns |
| ≥90 | 58/91 | 40/95 | 18/82 | ||
| Moderate or high-intensity physical activity—minimum 60 min a day as recommended by WHO [32] | Yes | 18/28 | 14/33 | 4/18 | ns |
| No | 46/72 | 28/67 | 18/82 | ||
| Screen time (hours/day) [39] | <2 | 20/31 | 16/38 | 4/18 | ns |
| ≥2 | 44/69 | 26/62 | 18/82 | ||
| Parent’s level of education | Higher | 34/53 | 24/57 | 10/46 | ns |
| Secondary | 20/31 | 14/33 | 6/27 | ||
| Vocational | 10/16 | 4/10 | 6/27 | ||
| Financial situation | Very good | 6/9 | 2/5 | 4/18 | ns |
| Rather good | 40/63 | 24/57 | 16/73 | ||
| Average | 18/28 | 16/38 | 2/9 | ||
| Place of living | Village | 20/31 | 10/24 | 10/46 | ns |
| Town < 100,000 citizens | 20/31 | 14/33 | 6/27 | ||
| Town ≥ 100,000 citizens | 24/38 | 18/43 | 6/27 | ||
| Variable | Total (n = 64) | LGI Diet (n = 42) | ST Diet (n = 22) | p-Value (Mann–Whitney U Test) |
|---|---|---|---|---|
| SBP (mmHg) | 118.22 ± 8.54 | 117.38 ± 6.99 | 119.82 ± 10.92 | ns |
| SBP-for-age percentile | 70.16 ± 22.44 | 69.52 ± 19.91 | 71.36 ± 27.11 | ns |
| DBP (mmHg) | 71.13 ± 5.38 | 71.90 ± 4.00 | 69.64 ± 7.21 | ns |
| DBP-for-age percentile | 80.94 ± 18.65 | 85.29 ± 9.73 | 72.64 ± 27.36 | ns |
| Heart rate (bpm) | 73.59 ± 5.85 | 73.95 ± 6.37 | 72.91 ± 4.75 | ns |
| TC (mg/dL) | 204.05 ± 41.94 | 203.60 ± 43.41 | 204.90 ± 39.96 | ns |
| HDL-C (mg/dL) | 42.25 ± 12.54 | 43.30 ± 14.67 | 40.24 ± 6.71 | ns |
| LDL-C (mg/dL) | 112.26 ± 19.23 | 113.05 ± 21.33 | 110.74 ± 14.76 | ns |
| TG (mg/dL) | 224.15 ± 112.10 | 208.38 ± 107.90 | 254.25 ± 116.29 | ns |
| Variable | Total (n = 64) | LGI Diet (n = 42) | ST Diet (n = 22) | p-Value (Chi-Squared Test) | |
|---|---|---|---|---|---|
| SBP-for-age percentile | <90 | 48/75 | 32/76 | 16/73 | ns |
| ≥90 | 16/25 | 10/24 | 6/27 | ||
| DBP-for-age percentile | <90 | 42/66 | 24/57 | 18/82 | ns |
| ≥90 | 22/34 | 18/43 | 4/18 | ||
| TC | Acceptable | 14/22 | 10/24 | 4/18 | ns |
| Borderline high * | 24/37 | 14/33 | 10/46 | ||
| High * | 26/41 | 18/43 | 8/36 | ||
| HDL-C | Acceptable | 12/19 | 8/19 | 4/18 | ns |
| Borderline high | 20/31 | 14/33 | 6/27 | ||
| High | 32/50 | 20/48 | 12/55 | ||
| LDL-C | Acceptable | 38/60 | 30 a/71 | 8 a/36 | 0.012 |
| Borderline high | 20/31 | 8 a/19 | 12 b/55 | ||
| High | 6/9 | 4 a/9 | 2 a/9 | ||
| TG | Acceptable | 0/0.0 | 0/0 | 0/0 | ns |
| Borderline high | 8/12 | 8/19 | 0/0 | ||
| High | 56/88 | 34/81 | 22/100 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bondyra-Wiśniewska, B.; Harton, A. Effect of the Nutritional Intervention Program on Body Weight and Selected Cardiometabolic Factors in Children and Adolescents with Excess Body Weight and Dyslipidemia: Study Protocol and Baseline Data. Nutrients 2023, 15, 3646. https://doi.org/10.3390/nu15163646
Bondyra-Wiśniewska B, Harton A. Effect of the Nutritional Intervention Program on Body Weight and Selected Cardiometabolic Factors in Children and Adolescents with Excess Body Weight and Dyslipidemia: Study Protocol and Baseline Data. Nutrients. 2023; 15(16):3646. https://doi.org/10.3390/nu15163646
Chicago/Turabian StyleBondyra-Wiśniewska, Beata, and Anna Harton. 2023. "Effect of the Nutritional Intervention Program on Body Weight and Selected Cardiometabolic Factors in Children and Adolescents with Excess Body Weight and Dyslipidemia: Study Protocol and Baseline Data" Nutrients 15, no. 16: 3646. https://doi.org/10.3390/nu15163646
APA StyleBondyra-Wiśniewska, B., & Harton, A. (2023). Effect of the Nutritional Intervention Program on Body Weight and Selected Cardiometabolic Factors in Children and Adolescents with Excess Body Weight and Dyslipidemia: Study Protocol and Baseline Data. Nutrients, 15(16), 3646. https://doi.org/10.3390/nu15163646






