Food Allergens: When Friends Become Foes—Caveats and Opportunities for Oral Immunotherapy Based on Deactivation Methods
Abstract
:1. Introduction
2. Physical Deactivation
2.1. Thermal Treatment
2.2. High-Pressure Treatment
2.3. Pulsed Electric Fields
2.4. Ultrasound and Microwave Irradiations
3. Chemical/Biochemical Deactivation
3.1. Chemo-Enzymatic Treatments
3.2. Use of Phytochemicals
3.3. Allergoids
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cianferoni, A.; Spergel, J.M. Food allergy: Review, classification and diagnosis. Allergol. Int. 2009, 58, 457–466. [Google Scholar] [CrossRef]
- Yu, W.; Freeland, D.M.H.; Nadeau, K.C. Food allergy: Immune mechanisms, diagnosis and immunotherapy. Nat. Rev. Immunol. 2016, 16, 751–765. [Google Scholar] [CrossRef]
- Renz, H.; Allen, K.J.; Sicherer, S.H.; Sampson, H.A.; Lack, G.; Beyer, K.; Oettgen, H.C. Food allergy. Nat. Rev. Dis. Primers 2018, 4, 17098. [Google Scholar] [CrossRef]
- Sicherer, S.H.; Sampson, H.A. Food allergy: A review and update on epidemiology, pathogenesis, diagnosis, prevention, and management. J. Allergy Clin. Immunol. 2018, 141, 41–58. [Google Scholar] [CrossRef]
- Seth, D.; Poowutikul, P.; Pansare, M.; Kamat, D. Food allergy: A review. Pediatr. Ann. 2020, 49, e50–e58. [Google Scholar] [CrossRef]
- Akdis, C.A. The epithelial barrier hypothesis proposes a comprehensive understanding of the origins of allergic and other chronic noncommunicable diseases. J. Allergy Clin. Immunol. 2022, 149, 41–44. [Google Scholar] [CrossRef]
- Pastor-Vargas, C.; Maroto, A.S.; Díaz-Perales, A.; Villalba, M.; Esteban, V.; Ruiz-Ramos, M.; de Alba, M.R.; Vivanco, F.; Cuesta-Herranz, J. Detection of major food allergens in amniotic fluid: Initial allergenic encounter during pregnancy. Pediatr. Allergy Immunol. 2016, 27, 716–720. [Google Scholar] [CrossRef] [PubMed]
- Msallam, R.; Balla, J.; Rathore, A.P.S.; Kared, H.; Malleret, B.; Saron, W.A.A.; Liu, Z.; Hang, J.W.; Dutertre, C.A.; Larbi, A.; et al. Fetal mast cells mediate postnatal allergic responses dependent on maternal IgE. Science 2020, 370, 941–950. [Google Scholar] [CrossRef] [PubMed]
- Rothenberg, M.E. Transferring allergies in the womb. Science 2020, 370, 907–908. [Google Scholar] [CrossRef] [PubMed]
- Ling, Z.; Li, Z.; Liu, X.; Cheng, Y.; Luo, Y.; Tong, X.; Yuan, L.; Wang, Y.; Sun, J.; Li, L.; et al. Altered fecal microbiota composition associated with food allergy in infants. Appl. Environ. Microbiol. 2014, 80, 2546–2554. [Google Scholar] [CrossRef] [PubMed]
- Azad, M.B.; Konya, T.; Guttman, D.S.; Field, C.J.; Sears, M.R.; HayGlass, K.T.; Mandhane, P.J.; Turvey, S.E.; Subbarao, P.; Becker, A.B.; et al. Infant gut microbiota and food sensitization: Associations in the first year of life. Clin. Exp. Allergy 2015, 45, 632–643. [Google Scholar] [CrossRef]
- Bunyavanich, S.; Shen, N.; Grishin, A.; Wood, R.; Burks, W.; Dawson, P.; Jones, S.M.; Leung, D.Y.; Sampson, H.; Sicherer, S.; et al. Early-life gut microbiome composition and milk allergy resolution. Allergy Clin. Immunol. 2016, 138, 1122–1130. [Google Scholar] [CrossRef]
- Eisenstein, M. The microbial ambassadors of the immune system. Nature 2020, 588, S11–S13. [Google Scholar] [CrossRef]
- Couzin-Frankel, J. Toxin or treatment? Science 2018, 362, 278–282. [Google Scholar] [CrossRef] [PubMed]
- Khamsi, R. Food allergies: The psychological toll. Nature 2020, 588, S4–S6. [Google Scholar] [CrossRef] [PubMed]
- Herbert, L.; Marchisotto, M.J.; Vickery, B. Patient’s perspective and needs on novel food allergy tratments in the United States. Curr. Treat. Options Allergy 2021, 8, 1–12. [Google Scholar] [CrossRef]
- Greenhawt, M.; Shaker, M.; Stukus, D.R.; Fleischer, D.M.; Hourihane, J.; Tang, M.L.; Abrams, E.M.; Wang, J.; Bingemann, T.A.; Chan, E.S.; et al. Managing food allergy in schools during the COVID-19 pandemic. J. Allergy Clin. Immunol. Pract. 2020, 8, 2845–2850. [Google Scholar] [CrossRef] [PubMed]
- Food allergy is everyone’s business—Editorial. Nat. Food 2021, 2, 301–302. [CrossRef]
- FASTER Act H. R. 2021, p. 1202. Available online: https://www.congress.gov/bill/117th-congress/house-bill/1202/text (accessed on 24 July 2023).
- Vázquez-Cortés, S.; Jaqueti, P.; Arasi, S.; Machinena, A.; Alvaro-Lozano, M.; Fernández-Rivas, M. Safety of Food Oral Immunotherapy: What we know, and what we need to learn. Immunol. Allergy Clin. N. Am. 2020, 40, 111–133. [Google Scholar] [CrossRef]
- Sabouraud, M.; Biermé, P.; Andre-Gomez, S.-A.; Villard-Truc, F.; Corréard, A.-K.; Garnier, L.; Payot, F.; Braun, C. Oral immunotherapy in food allergies: A practical update for pediatricians. Arch. Pédiatrie 2021, 28, 319–324. [Google Scholar] [CrossRef]
- Flom, J.D.; Sicherer, S.H. Epidemiology of cow’s milk allergy. Nutrients 2019, 11, 1051. [Google Scholar] [CrossRef] [PubMed]
- Aktas, S.; Ergenekon, E.; Ünal, S.; Türkyılmaz, C.; Hirfanoğlu, İ.M.; Atalay, Y. Different presentations of cow’s milk protein allergy during neonatal period. Turk. J. Pediatr. 2017, 59, 322–328. [Google Scholar] [CrossRef] [PubMed]
- Fiocchi, A.; Brozek, J.; Schünemann, H.; Bahna, S.L.; von Berg, A.; Beyer, K.; Bozzola, M.; Bradsher, J.; Compalati, E.; Ebisawa, M.; et al. World’s Allergy Organization (WAO) Diagnosis and rationale for action against cow’s milk allergy (DRACMA) guidelines. Pediatr. Allergy Immunol. Off. Publ. Eur. Soc. Pediatr. Allergy Immunol. 2010, 3, 57–161. [Google Scholar] [CrossRef]
- Seppo, A.E.; Savilahti, E.M.; Berin, M.C.; Sampson, H.A.; Järvinen, K.M. Breast milk IgA to foods has different epitope specificity than serum IgA-Evidence for entero-mammary link for food-specific IgA? Clin. Exp. Allergy J. Br. Soc. Allergy Clin. Immunol. 2017, 47, 1275–1284. [Google Scholar] [CrossRef] [PubMed]
- Vieths, S.; Reese, G.; Barbara-Weber, B.K.; Beyer, K.; Burney, P.; Fernandez-Rivas, M.; Summers, C.; van Ree, R.; Mills, C. The serum bank of EuroPrevall-The prevalence, cost and basis of food allergy across Europe. Food Chem. Toxicol. 2008, 46, S12–S14. [Google Scholar] [CrossRef] [PubMed]
- Grimshaw, K.E.C.; Roberts, G.; Selby, A.; Reich, A.; Butiene, I.; Clausen, M.; Dubakiene, R.; Fiandor, A.; Fiocchi, A.; Grabenhenrich, L.B.; et al. Risk factors for hen’s egg allergy in Europe: EuroPrevall birth cohort. J. Allergy Clin. Immunol. Pract. 2020, 8, 1341–1348. [Google Scholar] [CrossRef]
- Dorofeeva, Y.; Shilovskiy, I.; Tulaeva, I.; Focke-Tejkl, M.; Flicker, S.; Kudlay, D.; Khaitov, M.; Karsonova, A.; Riabova, K.; Karaulov, A.; et al. Past, present and future of allergen immunotherapy vaccines. Allergy 2021, 76, 131–149. [Google Scholar] [CrossRef]
- Leonard, S.A.; Laubach, S.; Wang, J. Integrating oral immunotherapy into clinical practice. J. Allergy Clin. Immunother. 2021, 147, 1–13. [Google Scholar] [CrossRef]
- Maeda, M.; Imai, T.; Ishikawa, R.; Nakamura, T.; Kamiya, T.; Kimura, A.; Fujita, S.; Akashi, K.; Tada, H.; Morita, H.; et al. Effect of oral immunotherapy in children with milk allergy: The ORIMA study. Allergol. Int. 2021, 70, 223–228. [Google Scholar] [CrossRef]
- Kim, E.H.; Perry, T.T.; Wood, R.A.; Leung, D.Y.M.; Berin, M.C.; Burks, A.W.; Cho, C.B.; Jones, S.M.; Scurlock, A.; Sicherer, S.H.; et al. Induction of sustained unresponsiveness after egg oral immunotherapy compared to baked egg therapy in children with egg allergy. J. Allergy Clin. Immunol. 2020, 146, 851–862.e10. [Google Scholar] [CrossRef]
- Remington, B.C.; Baumert, J.L. Risk reduction in peanut immunotherapy. Immunol. Allergy Clin. N. Am. 2020, 40, 187–200. [Google Scholar] [CrossRef]
- FDA. FDA Approves First Drug for Treatment of Peanut Allergy for Children. 2020. Available online: https://www.fda.gov/news-events/press-announcements/fda-approves-first-drug-treatment-peanut-allergy-children (accessed on 24 July 2023).
- Nowak-Wegrzyn, A.; Sampson, H.A. Approaches to therapy in development. In Food Allergy. Adverse Reaction to Foods and Food Additives, 5th ed.; Metcalfe, D.D., Sampson, H.A., Simon, R.A., Lack, G., Eds.; Wiley-Blackwell: New York, NY, USA, 2014; pp. 581–597. [Google Scholar]
- Vazquez-Ortiz, M.; Turner, P.J. Improving the safety of oral immunotherapy for food allergy. Pediatr. Allergy Immunol. 2016, 27, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Chimomas, D.; Hornberger, K.R.; Crews, C.M. Protein degraders enter the clinic-a new approach to cancer therapy. Nat. Rev. Clin. Oncol. 2023, 20, 265–278. [Google Scholar] [CrossRef]
- Nagakura, K.; Yanagida, N.; Nishino, M.; Takahashi, K.; Sato, S.; Ebisawa, M. Randomized controlled trial of oral immunotherapy for children with severe cow’s milk allergy: Heated milk vs. unheated milk. Pediatr. Allergy Immunol. Off. Publ. Eur. Soc. Pediatr. Allergy Immunol. 2020, 13, 100420. [Google Scholar] [CrossRef]
- Mattar, H.; Padfield, P.; Simpson, A.; Mills, E.N.C. The impact of a baked muffin matrix on the bioaccesibility and IgE reactivity of egg and peanut allergens. Food Chem. 2021, 362, 129879. [Google Scholar] [CrossRef]
- Lund, M.N.; Ray, C.A. Control of Maillard reactions in foods: Strategies and chemical mechanisms. J. Agric. Food Chem. 2017, 65, 4537–4552. [Google Scholar] [CrossRef]
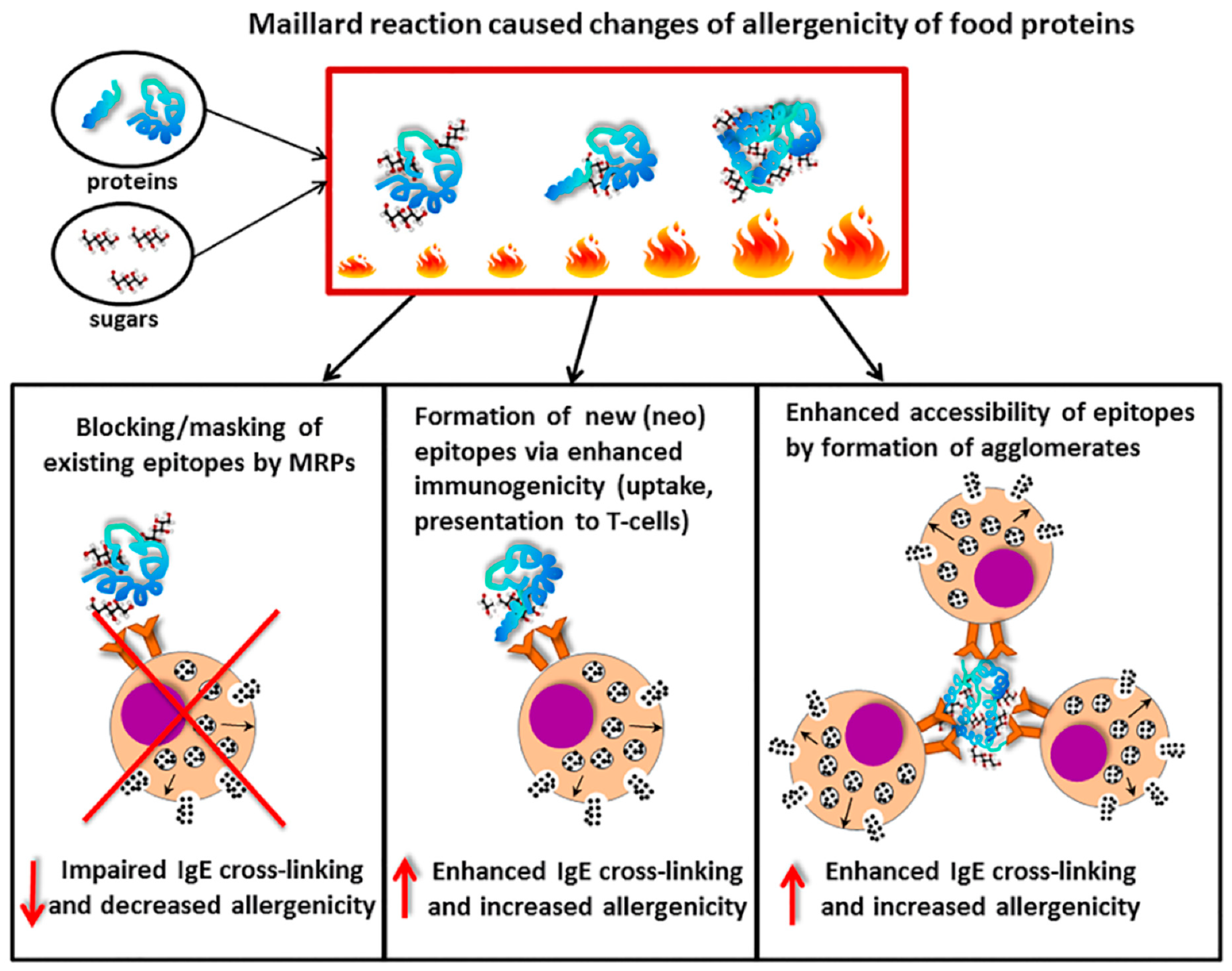
- Teodorowicz, M.; Van Neerven, J.; Savelkoul, H. Food processing: The influence of the Maillard reaction on immunogenicity and allergenicity of food proteins. Nutrients 2017, 9, 835. [Google Scholar] [CrossRef]
- Zenker, H.E.; Raupbach, J.; Boeren, S.; Wichers, H.J.; Hettinga, K.A. The effects of low vs. high temperature dry heating on solubility and digestibility of cow’s milk protein. Food Hydrocoll. 2020, 109, 106098. [Google Scholar] [CrossRef]
- De Jongh, H.H.J.; Taylor, S.L.; Koppelman, S.J. Controlling the aggregation propensity and thereby digestibility of allergens by Maillardation as illustrated for cod fish parvalbumin. J. Biosci. Bioeng. 2011, 111, 204–211. [Google Scholar] [CrossRef]
- Fu, L.; Wang, C.; Wang, J.; Ni, S.; Wang, Y. Maillard reaction with ribose, galacto-oligosaccharide or chitosan-oligosaccharide reduced the allergenicity of shrimp tropomyosin by inducing conformational changes. Food Chem. 2019, 274, 789–795. [Google Scholar] [CrossRef] [PubMed]
- Farjami, T.; Babaei, J.; Nau, F.; Dupont, D.; Madadlou, A. Effects of thermal, non-thermal and emulsification processes on the gastrointestinal digestibility of egg white proteins. Trends Food Sci. Technol. 2020, 107, 45–56. [Google Scholar] [CrossRef]
- Tong, P.; Gao, J.; Chen, H.; Li, X.; Zhang, Y.; Jian, S.; Wichers, H.; Wu, Z.; Yang, A.; Liu, F. Effect of heat treatment on the potential allergenicity and conformational structure of egg allergen ovotransferrin. Food Chem. 2012, 131, 603–610. [Google Scholar] [CrossRef]
- Bloom, K.A.; Huang, F.R.; Bencharitiwong, R.; Bardina, L.; Ross, A.; Sampson, H.A.; Nowak-Węgrzyn, A. Effect of heat treatment on milk and egg proteins allergenicity. Pediatr. Allergy Immunol. 2014, 25, 740–746. [Google Scholar] [CrossRef]
- Vapor, A.; Mendonça, A.; Tomaz, C.T. Processes for reducing egg allergenicity: Advances and different approaches. Food Chem. 2021, 367, 130568. [Google Scholar] [CrossRef]
- Escudero, C.; Sánchez-García, S.; Rodríguez del Río, P.; Pastor-Vargas, C.; García-Fernández, C.; Pérez-Rangel, I. Dehydrated egg white: An allergen source for improving efficacy and safety in the diagnosis and treatment for egg allergy. Pediatr. Allergy Immunol. 2013, 24, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, N.K.; Raghavarao, K.S.M.S.; Balasubramaniam, V.M.; Niranjan, K.; Knorr, D. Opportunities and challenges in high pressure processing of foods. Crit. Rev. Food Sci. Nutr. 2007, 47, 69–112. [Google Scholar] [CrossRef]
- Abera, G. Review on high-pressure processing of foods. Cogent Food Agric. 2019, 5, 1568725. [Google Scholar] [CrossRef]
- Nath, K.G.; Pandiselvam, R.; Sunil, C.K. High-pressure processing: Effect on textural properties of food-A review. J. Food Eng. 2023, 351, 111521. [Google Scholar] [CrossRef]
- Martinez-Monteagudo, S.I.; Saldana, M.D.A. Chemical reactions in food systems at high hydrostatic pressure. Food Eng. Rev. 2014, 6, 105–127. [Google Scholar] [CrossRef]
- Ruiz, G.A.; Xi, B.; Minor, M.; Sala, G.; Van Boekel, M.; Fogliano, V.; Stieger, M. High-pressure-high-temperature processing reduces Maillard reaction and viscosity in whey protein-sugar solutions. J. Agric. Food Chem. 2016, 64, 7208–7215. [Google Scholar] [CrossRef]
- Lee, J.; Choi, E.-J.; Park, S.Y.; Jeon, G.Y.; Jang, J.Y.; Oh, Y.J.; Lim, S.K.; Kwon, M.-S.; Kim, T.-W.; Lee, J.-H.; et al. High-pressure processing of milk alleviates atopic dermatitis in DNCB-induced Balb/c mice. Dairy Sci. Technol. 2016, 96, 67–78. [Google Scholar] [CrossRef]
- Kurpiewska, K.; Biela, A.; Loch, J.I.; Lipowska, J.; Siuda, M.; Lewinski, K. Towards understanding the effect of high pressure on food protein allergenicity: β-lactoglobulin structural studies. Food Chem. 2019, 270, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Bogahawaththa, D.; Buckow, R.; Chandrapala, J.; Vasiljevic, T. Comparison between thermal pasteurization and high pressure processing of bovine skim milk in relation to denaturation and immunogenicity of native milk proteins. Innov. Food Sci. Emerg. Technol. 2018, 47, 301–308. [Google Scholar] [CrossRef]
- Hu, G.; Zheng, Y.; Liu, Z.; Deng, Y.; Zhao, Y. Structure and IgE-binding properties of α-casein treated by high hydrostatic pressure. Food Chem. 2016, 204, 46–55. [Google Scholar] [CrossRef]
- Odani, S.; Kanda, Y.; Hara, T.; Matuno, M.; Suzuki, A. Effects of high hydrostatic pressure treatment on the allergenicity and structure of chicken egg white ovomucoid. High Press. Biosci. Biotechnol. 2007, 1, 252–258. [Google Scholar] [CrossRef]
- Savadkoohi, S.; Bannikova, A.; Mantri, N.; Kasapis, S. Structural properties of condensed ovalbumin systems following application of high pressure. Food Hydrocoll. 2016, 53, 104–114. [Google Scholar] [CrossRef]
- Prando, L.T.; De Souza Melchiors, M.; Torres, T.M.S.; De Oliveira, V.A.; Veneral, J.G.; Castiani, M.A.; de Oliveira, D.; de Oliveira, J.V.; Di Luccio, M. Effect of high pressure and magnetic field treatments on stability of Candida antarctica lipase B (CALB) and lysozyme from chicken egg. Catal. Commun. 2018, 116, 43–47. [Google Scholar] [CrossRef]
- Dhakal, S.; Liu, C.; Zhang, Y.; Roux, K.H.; Sathe, S.K.; Balasubramaniam, V.M. Effect of high pressure processing on the immunoreactivity of almond milk. Food Res. Int. 2014, 62, 215–222. [Google Scholar] [CrossRef]
- Yang, X.; Sun, J.; Tao, J.; Ma, Y.; Wei, J.; Long, F. The allergenic potential of walnuts treated with high pressure and heat in a mouse model of allergy. Innov. Food Sci. Emerg. Technol. 2017, 39, 165–170. [Google Scholar] [CrossRef]
- Liu, K.; Lin, S.; Gao, X.; Wang, S.; Liu, Y.; Liu, Q.; Sun, N. Reduced allergenicity of shrimp (Penaeus vannamei) by altering the protein fold, digestion susceptibility, and allergen epitopes. J. Agric. Food Chem. 2023, 71, 9120–9134. [Google Scholar] [CrossRef]
- Kelso, J.M.; Bardina, L.; Beyer, K. Allergy to canned tuna. J. Allergy Clin. Immunol. 2003, 111, 901. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.-H.; Kung, H.-F.; Lin, C.-S.; Hsieh, C.-Y.; Ou, T.-Y.; Chang, T.-H.; Lee, Y.-C. Impacts of high-pressure processing on quality and shelf-life of yellowfin tuna (Thunnus albacares) stored at 4 °C and 15 °C. Int. J. Food Prop. 2022, 25, 237–251. [Google Scholar] [CrossRef]
- Huang, C.-H.; Hsieh, C.-Y.; Lee, Y.-C.; Ou, T.-Y.; Chang, T.-H.; Lee, S.-H.; Tseng, C.-H.; Tsai, Y.-H. Inhibitory effects of high-hydrostatic pressure processing on growth and histamine formation of histamine-forming bacteria in yellowfin tuna meat during storage. Biology 2022, 11, 702. [Google Scholar] [CrossRef]
- Barba, F.J.; Parniakov, O.; Pereira, S.A.; Wiktor, A.; Grimi, N.; Boussetta, N.; Saraiva, J.A.; Raso, J.; Martin-Belloso, O.; Witrowa-Rajchert, D.; et al. Current applications and new opportunities for the use of pulsed electric fields in food science and industry. Food Res. Int. 2015, 77, 773–798. [Google Scholar] [CrossRef]
- Taha, A.; Casanova, F.; Simonis, P.; Stankevic, V.; Gomaa, M.A.E.; Stirké, A. Pulsed electric field: Fundamentals and effects on the structural and techno-functional properties of dairy and plant proteins. Foods 2022, 11, 1556. [Google Scholar] [CrossRef]
- Johnson, P.E.; Van der Plancken, I.; Balasa, A.; Husband, F.A.; Grauwet, T.; Hendrickx, M.; Knorr, D.; Mills, E.N.C.; Mackie, A.R. High pressure, thermal and pulsed electric-field-induced structural changes in selected food allergens. Mol. Nutr. Food Res. 2010, 54, 1701–1710. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, S.; Ding, J.; Zhong, L.; Sun, N.; Lin, S. Evaluation of the structure-activity relationship between allergenicity and spatial conformation of ovalbumin treated by pulsed electric field. Food Chem. 2022, 388, 133018. [Google Scholar] [CrossRef]
- Aguilo-Aguayo, I.; Soliva-Fortuny, R.; Martin-Belloso, O. Avoiding non-enzymatic browning by high-intensity pulsed electric fields in strawberry, tomato and watermelon juices. J. Food Eng. 2009, 92, 37–43. [Google Scholar] [CrossRef]
- Kentish, S.; Feng, H. Applications of power ultrasound in food processing. Annu. Rev. Food Sci. Technol. 2014, 5, 263–284. [Google Scholar] [CrossRef]
- Bhargava, N.; Mor, R.S.; Kumar, K.; Sharanagat, V.S. Advances in application of ultrasound in food processing: A review. Ultrason. Sonochem. 2021, 70, 105293. [Google Scholar] [CrossRef]
- Ashokkumar, M.; Lee, J.; Zisu, B.; Bhaskarcharya, R.; Palmer, M.; Kentish, S. Hot topic: Sonication increases the heat stability of whey proteins. J. Dairy Sci. 2009, 92, 5353–5356. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Kim, H.; Cadwalladera, K.R.; Feng, H.; Martin, S.E. Sonication in combination with heat and low pressure as an alternative pasteurization treatment–effect on Escherichia coli K12 inactivation and quality of apple cider. Ultrason. Sonochem. 2013, 20, 1131–1138. [Google Scholar] [CrossRef]
- Jiang, L.; Wang, J.; Li, Y.; Wang, Z.; Liang, J.; Wang, R.; Chen, Y.; Ma, W.; Qi, B.; Zhang, M. Effects of ultrasound on the structure and physical properties of black bean protein isolates. Food Res. Int. 2014, 62, 595–601. [Google Scholar] [CrossRef]
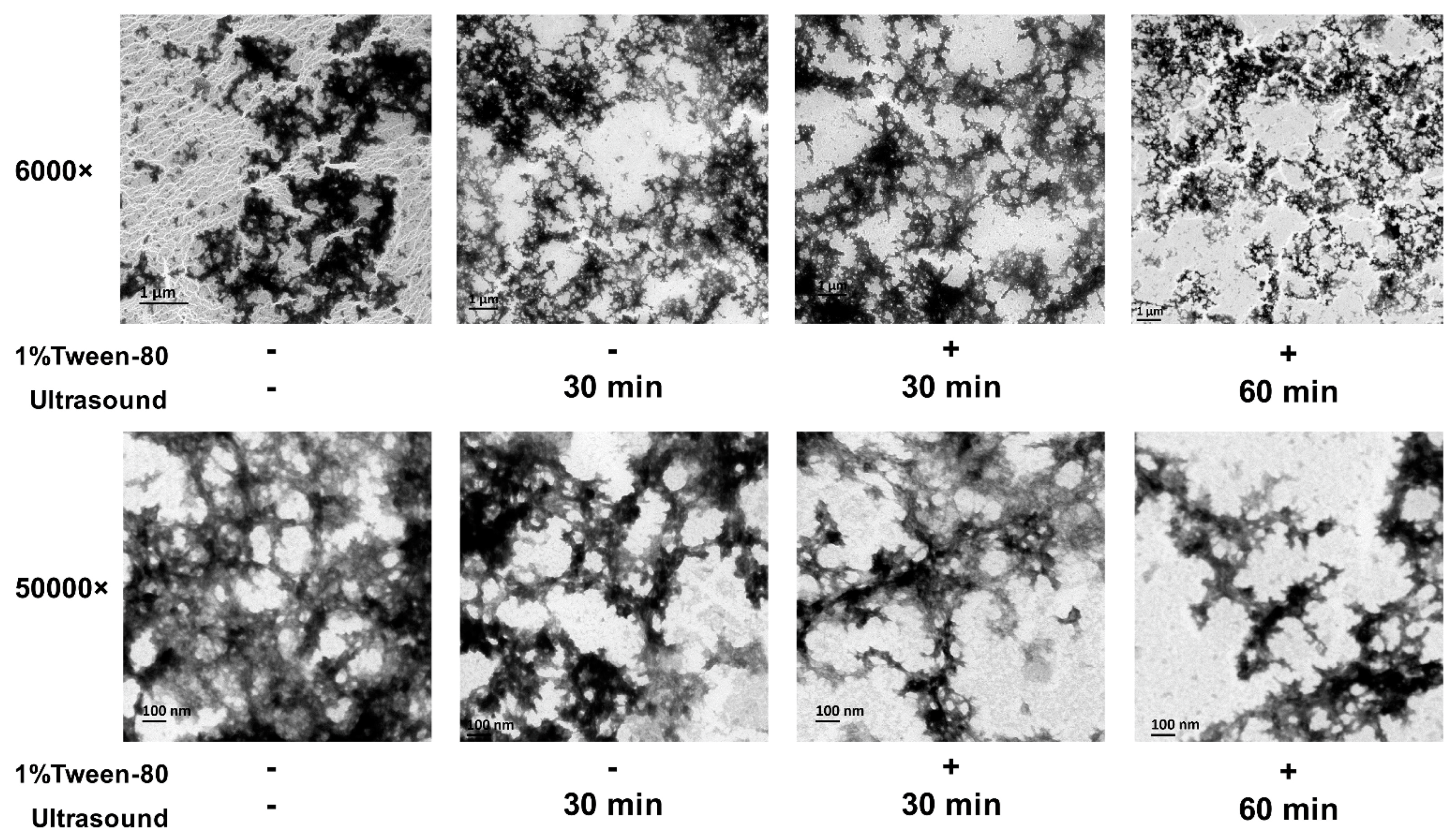
- Wang, C.; Xie, Q.; Wang, Y.; Fu, L. Effect of ultrasound treatment on allergenicity reduction of milk casein via colloid formation. J. Agric. Food Chem. 2020, 68, 4678–4686. [Google Scholar] [CrossRef]
- Wang, J.; Wang, J.; Vanga, S.K.; Raghavan, V. Influence of high-intensity ultrasound on the IgE binding capacity of Act d 2 allergen, secondary structure, and in-vitro digestibility of kiwifruit proteins. Ultrason. Sonochem. 2021, 71, 105409. [Google Scholar] [CrossRef]
- Dong, X.; Wang, J.; Raghavan, V. Impact of microwave processing on the secondary structure, in-vitro protein digestibility and allergenicity of shrimp (Litopenaeus vannamei). Food Chem. 2021, 337, 127811. [Google Scholar] [CrossRef]
- Cravotto, G.; Boffa, L.; Mantegna, S.; Perego, P.; Avogadro, M.; Cintas, P. Improved extraction of vegetable oils under high-intensity ultrasound and/or microwaves. Ultrason. Sonochem. 2008, 15, 898–902. [Google Scholar] [CrossRef] [PubMed]
- Garino, C.; Zitelli, F.; Travaglia, F.; Colsson, J.D.; Cravotto, G.; Arlorio, M. Evaluation of the impact of sequential microwave/ultrasound processing on the IgE binding properties of Pru p 3 in treated peach juice. J. Agric. Food Chem. 2012, 60, 8755–8762. [Google Scholar] [CrossRef]
- Zhu, Y.; Vanga, S.K.; Wang, J.; Raghavan, V. Effects of ultrasonic and microwave processing on avidin assay and secondary structures of egg white protein. Food Bioprocess Technol. 2018, 11, 1974–1984. [Google Scholar] [CrossRef]
- Hacini-Rachinel, F.; Visser, Y.M.; Doucet-Ladevéze, R.; Blanchard, C.; Demont, A.; Perrot, M.; Panchaud, A.; Prioult, G.; Mercenier, A.; Nutten, S. Low-allergenic hydrolyzed egg induces oral tolerance in mice. Int. Arch. Allergy Immunol. 2014, 164, 64–73. [Google Scholar] [CrossRef]
- Lozano-Ojalvo, D.; Molina, E.; López-Fandiño, R. Hypoallergenic hydrolysates of egg white proteins modulate allergen responses induced ex vivo on spleen cells from sensitized mice. Food Res. Int. 2016, 89, 661–669. [Google Scholar] [CrossRef] [PubMed]
- Ueno, H.M.; Kato, T.; Ohnishi, H.; Kawamoto, N.; Kato, Z.; Kaneko, H.; Kondo, N.; Nakano, T. Hypoallergenic casein hydrolysate for peptide-based oral immunotherapy in cow’s milk allergy. J. Allergy Clin. Immunol. 2018, 142, 330–333. [Google Scholar] [CrossRef] [PubMed]
- Porta, R.; Giosafatto, C.V.L.; Di Pierro, P.; Sorrentino, A.; Mariniello, L. Transglutaminase-mediated modification of ovomucoid effects on its trypsin inhibitory activity and antigenic properties. Amino Acids 2013, 44, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Damodaran, S.; Li, Y. A two-step enzymatic modification method to reduce immuno-reactivity of milk proteins. Food Chem. 2017, 237, 724–732. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ni, S.; Wang, C.; Li, X.; Fu, L. Cross-linking of shrimp tropomyosin catalyzed by transglutaminase and tyrosinase produces hypoallergens for potential immunotherapy. Food Funct. 2019, 10, 1609–1618. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Lozano-Ojalvo, D.; Chen, H.; López-Fandiño, R.; Molina, E. Effect of high pressure-assisted crosslinking of ovalbumin and egg white by transglutaminase on their potential allergenicity. Innov. Food Sci. Emerg. Technol. 2015, 29, 143–150. [Google Scholar] [CrossRef]
- Liu, J.; Chen, W.-M.; Shao, Y.-H.; Zhang, J.-L.; Tu, Z.-C. The mechanism of the reduction in allergenic reactivity of bovine α-lactalbumin induced by glycation, phosphorylation and acetylation. Food Chem. 2020, 310, 125853. [Google Scholar] [CrossRef]
- Xu, L.; Gong, Y.; Gern, J.E.; Lucey, J.A. Influence of whey protein hydrolysis in combination with dextran glycation on immunoglobulin E binding capacity with blood sera obtained from patients with a cow milk protein allergy. J. Dairy Sci. 2020, 103, 1141–1150. [Google Scholar] [CrossRef]
- Zhang, Q.; Chen, Q.-H.; He, G.-Q. Effect of ultrasonic-ionic liquid pretreatment on the hydrolysis degree and antigenicity of enzymatic hydrolysates from whey protein. Ultrason. Sonochem. 2020, 63, 104926. [Google Scholar] [CrossRef]
- Shi, J.; Luo, Y.; Xiao, Y.; Li, Z.; Xu, Q.; Yao, M. Effects of fermentation by Lactobacillus casei on the antigenicity and allergenicity of four bovine milk proteins. Int. Dairy J. 2014, 35, 75–80. [Google Scholar] [CrossRef]
- Yao, M.; Xu, Q.; Luo, Y.; Shi, J.; Li, Z. Study on reducing antigenic response and IgE-binding inhibitions of four milk proteins of Lactobacillus casei 1134. J. Sci. Food Agric. 2015, 95, 1303–1312. [Google Scholar] [CrossRef] [PubMed]
- Dhanapala, P.; Withanage-Dona, D.; Tang, M.L.K.; Doran, T.; Suphioglu, C. Hypoallergenic variant of the major egg white allergen Gal d 1 produced by disruption of cysteine bridges. Nutrients 2017, 9, 171. [Google Scholar] [CrossRef]
- Taniguchi, M.; LaRocca, C.A.; Bernat, J.D.; Lindsey, J.S. Digital database of absorption spectra of diverse flavonoids enables structural comparisons and quantitative evaluation. J. Nat. Prod. 2023, 86, 1087–1119. [Google Scholar] [CrossRef] [PubMed]
- Aravind, S.M.; Wichienchot, S.; Tsao, R.; Ramakrishnan, S.; Chakkaravarthi, S. Role of dietary polyphenols on gut microbiota, their metabolites and health benefits. Food Res. Int. 2021, 142, 110189. [Google Scholar] [CrossRef]
- Mukherjee, C.; Chakraborty, S. Study of dietary polyphenols from natural herbal sources for providing protection against human degenerative disorders. Biocatal. Agriultural Biotechnol. 2021, 33, 101956. [Google Scholar] [CrossRef]
- Mihanfar, A.; Mohammad, N.; Roshangar, L.; Khadem-Ansari, M.H. Polyphenols: Natural compounds with promising potential in treating polycystic ovary syndrome. Reprod. Biol. 2021, 21, 100500. [Google Scholar] [CrossRef]
- Labuckas, D.O.; Maestri, D.M.; Perelló, M.; Martínez, M.L.; Lamarque, A.L. Phenolics from walnut (Juglans regia L.) kernels: Antioxidant activity and interactions with proteins. Food Chem. 2008, 107, 607–612. [Google Scholar] [CrossRef]
- Do Valle, I.F.; Roweth, H.G.; Malloy, M.W.; Moco, S.; Barron, D.; Battinelli, E.; Loscalzo, J.; Barabási, A.-L. Network medicine framework shows that proximity of polyphenol targets and disease proteins predicts therapeutic effects of polyphenols. Nat. Food 2021, 2, 143–155. [Google Scholar] [CrossRef]
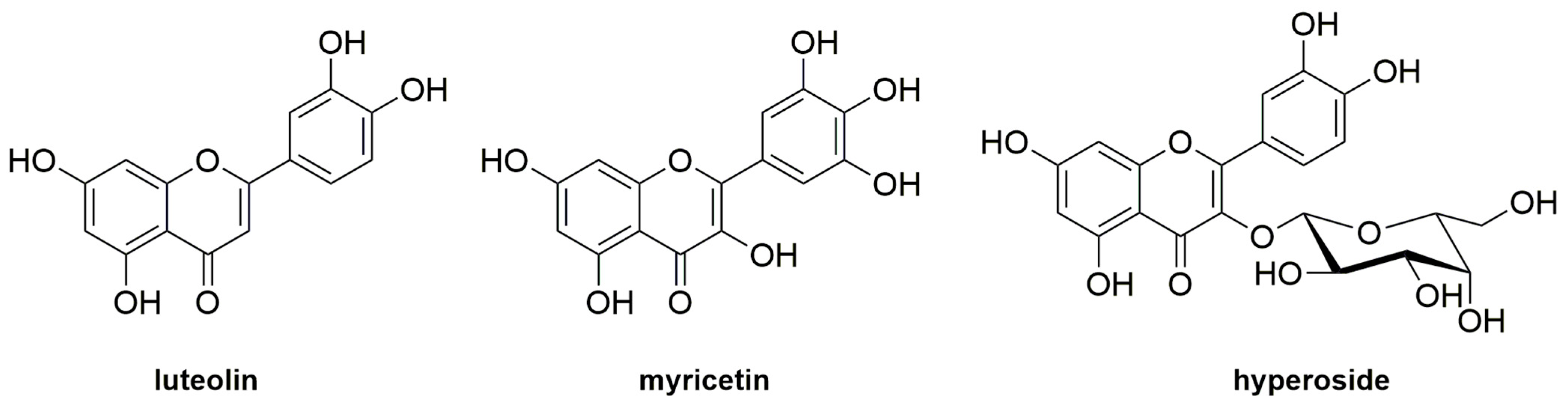
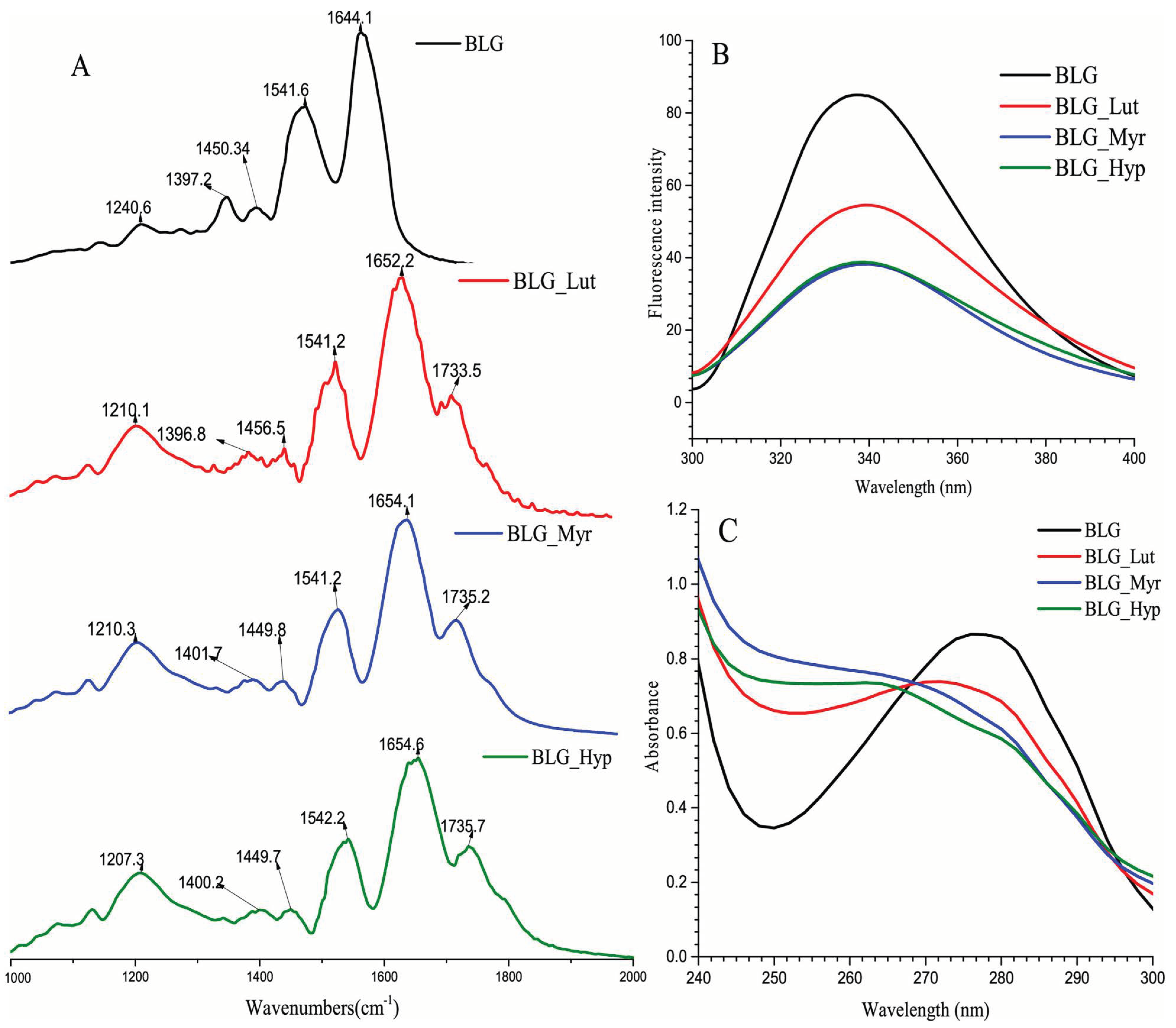
- Liu, J.; Wang, Y.; Tu, Z.-C.; Chen, W.-M.; Yuan, T. Bovine β-lactoglobulin covalent modification by flavonoids: Effect on the allergenicity and human intestinal microbiota. J. Agric. Food Chem. 2021, 69, 6820–6828. [Google Scholar] [CrossRef]
- Lu, Y.; Li, S.; Zhang, T.; Lin, X.; Wu, X. Effect of covalent interaction with chlorogenic acid on the allergenic capacity of ovalbumin. J. Agric. Food Chem. 2018, 66, 9794–9800. [Google Scholar] [CrossRef]
- Xu, H.; Zhang, T.; Lu, Y.; Lin, X.; Hu, X.; Liu, L.; Wu, X. Effect of chlorogenic acid covalent conjugation on the allergenicity, digestability and functional properties of whey protein. Food Chem. 2019, 298, 125024. [Google Scholar] [CrossRef]
- Plundrich, N.J.; Kulis, M.; White, B.L.; Grace, M.H.; Guo, R.; Burks, A.W.; Davis, J.P.; Lila, M.A. Novel strategy to create hypoallergenic peanut protein-polyphenol edible matrices for oral immunotherapy. J. Agric. Food Chem. 2014, 62, 7010–7021. [Google Scholar] [CrossRef] [PubMed]
- Baiano, A. Recovery of biomolecules from food wastes—A review. Molecules 2014, 19, 14821–14842. [Google Scholar] [CrossRef]
- Zema, D.A.; Calabró, P.S.; Folino, A.; Tamburino, V.; Zappia, G.; Zimbone, S.M. Valorisation of citrus processing waste: A review. Waste Manag. 2018, 80, 252–273. [Google Scholar] [CrossRef] [PubMed]
- Ligor, M.; Buszewski, B. Study of VOC distribution in citrus fruits by chromatographic analysis. Anal. Bioanal. Chem. 2003, 376, 668–672. [Google Scholar] [CrossRef]
- Keijer, T.; Bakker, V.; Slootweg, J.C. Circular chemistry to enable a circular economy. Nat. Chem. 2019, 11, 190–195. [Google Scholar] [CrossRef]
- Lila, M.A.; Schneider, M.; Devlin, A.; Plundrich, N.; Laster, S.; Foegeding, E.A. Polyphenol-enriched berry extracts naturally modulate proteins in model foods. Food Funct. 2017, 8, 4760–4767. [Google Scholar] [CrossRef] [PubMed]
- Plundrich, N.J.; Bansode, R.R.; Foegeding, E.A.; Williams, L.L.; Lila, M.A. Protein-bound Vaccinium fruit polyphenols decrease IgE binding to peanut allergens and RBL-2H3 mast cell degranulation in vitro. Food Funct. 2017, 8, 1611–1621. [Google Scholar] [CrossRef]
- Chang, K.; Liu, J.; Jiang, W.; Fan, Y.; Nan, B.; Ma, S.; Zhang, Y.; Liu, B.; Zhang, T. Structural characteristics and foaming properties of ovalbumin-caffeic acid complex. LWT 2021, 146, 111383. [Google Scholar] [CrossRef]
- He, W.; Xu, H.; Lu, Y.; Zhang, T.; Li, S.; Lin, X.; Xu, B.; Wu, X. Function, digestibility and allergenicity assessment of ovalbumin-EGCG conjugates. J. Funct. Foods 2019, 61, 103490. [Google Scholar] [CrossRef]
- Compalati, E.; Incorvaia, C.; Cavaliere, C.; Masieri, S.; Gargiulo, A.; Mistrello, G.; Frati, F. The role of allergoids in allergen immunotherapy: From injective to sublingual route. Eur. Ann. Allergy Clin. Immunol. 2020, 52, 195–204. [Google Scholar] [CrossRef]
- Würtzen, P.A.; Lund, L.; Lund, G.; Holm, J.; Millner, A.; Henmar, H. Chemical modification of birch allergen extract leads to a reduction in allergenicity as well as immunogenicity. Int. Arch. Allergy Immunol. 2007, 144, 287–295. [Google Scholar] [CrossRef]
- Gokmen, N.M.; Ersoy, R.; Gulbahar, O.; Ardeniz, O.; Sin, A.; Unsel, M.; Kokuludag, A. Desensitization effect of preseasonal seven-injection allergoid immunotherapy with olive pollen on basophil activation: The efficacy of olive pollen-specific preseasonal allergoid immunotherapy on basophils. Int. Arch. Allergy Immunol. 2012, 159, 75–82. [Google Scholar] [CrossRef]
- Benito-Villalvilla, C.; Perez-Diego, M.; Subiza, J.L.; Palomares, O. Allergoid-mannan conjugates imprint tolerogenic features in human macrophages. Allergy 2022, 77, 320–323. [Google Scholar] [CrossRef]
- Moya, R.; Odena, M.A.; Gallego, M.; De Oliveira, E.; Carnés, J. Absolute quantification of Bet v 1 in birch polymerized allergenic extracts via mass spectrometry-targeted analysis. Clin. Exp. Allergy 2022, 52, 276–285. [Google Scholar] [CrossRef]
- Carlson, G.; Coop, C. Pollen food allergy syndrome (PFAS): A review of current available literature. Ann. Allergy Asthma Immunol. 2019, 123, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Loo, E.X.L.; Lau, H.X.; Suaini, N.H.A.; Wong, L.S.Y.; Goh, A.E.N.; Teoh, O.H.; Van Bever, H.P.; Shek, L.P.; Lee, B.W.; Tan, K.H.; et al. House dust mite sensitization, eczema, and wheeze increase risk of shellfish sensitization. Pediatr. Allergy Immunol. 2021, 32, 1096–1099. [Google Scholar] [CrossRef] [PubMed]
- Pevec, B.; Pevec, M.R.; Markovic, A.S.; Batista, I. House dust mite subcutaneous immunotherapy does not induce new sensitization to tropomyosin: Does it do the opposite? J. Investig. Allergol. Clin. Immunol. 2014, 24, 29–34. [Google Scholar]
- Furci, F.; Ricciardi, L. Plant food allergy improvement after grass pollen sublingual immunotherapy: A case series. Pathogens 2021, 10, 1412. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gil, M.V.; Fernández-Rivera, N.; Pastor-Vargas, C.; Cintas, P. Food Allergens: When Friends Become Foes—Caveats and Opportunities for Oral Immunotherapy Based on Deactivation Methods. Nutrients 2023, 15, 3650. https://doi.org/10.3390/nu15163650
Gil MV, Fernández-Rivera N, Pastor-Vargas C, Cintas P. Food Allergens: When Friends Become Foes—Caveats and Opportunities for Oral Immunotherapy Based on Deactivation Methods. Nutrients. 2023; 15(16):3650. https://doi.org/10.3390/nu15163650
Chicago/Turabian StyleGil, M. Victoria, Nuria Fernández-Rivera, Carlos Pastor-Vargas, and Pedro Cintas. 2023. "Food Allergens: When Friends Become Foes—Caveats and Opportunities for Oral Immunotherapy Based on Deactivation Methods" Nutrients 15, no. 16: 3650. https://doi.org/10.3390/nu15163650
APA StyleGil, M. V., Fernández-Rivera, N., Pastor-Vargas, C., & Cintas, P. (2023). Food Allergens: When Friends Become Foes—Caveats and Opportunities for Oral Immunotherapy Based on Deactivation Methods. Nutrients, 15(16), 3650. https://doi.org/10.3390/nu15163650





