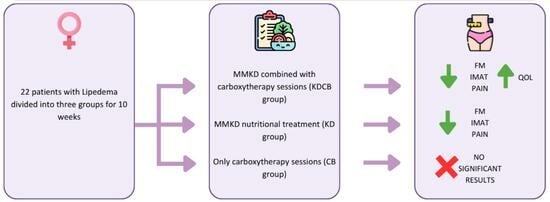
Modified Mediterranean-Ketogenic Diet and Carboxytherapy as Personalized Therapeutic Strategies in Lipedema: A Pilot Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects and Study Design
2.2. Dietary Assessment and Intervention
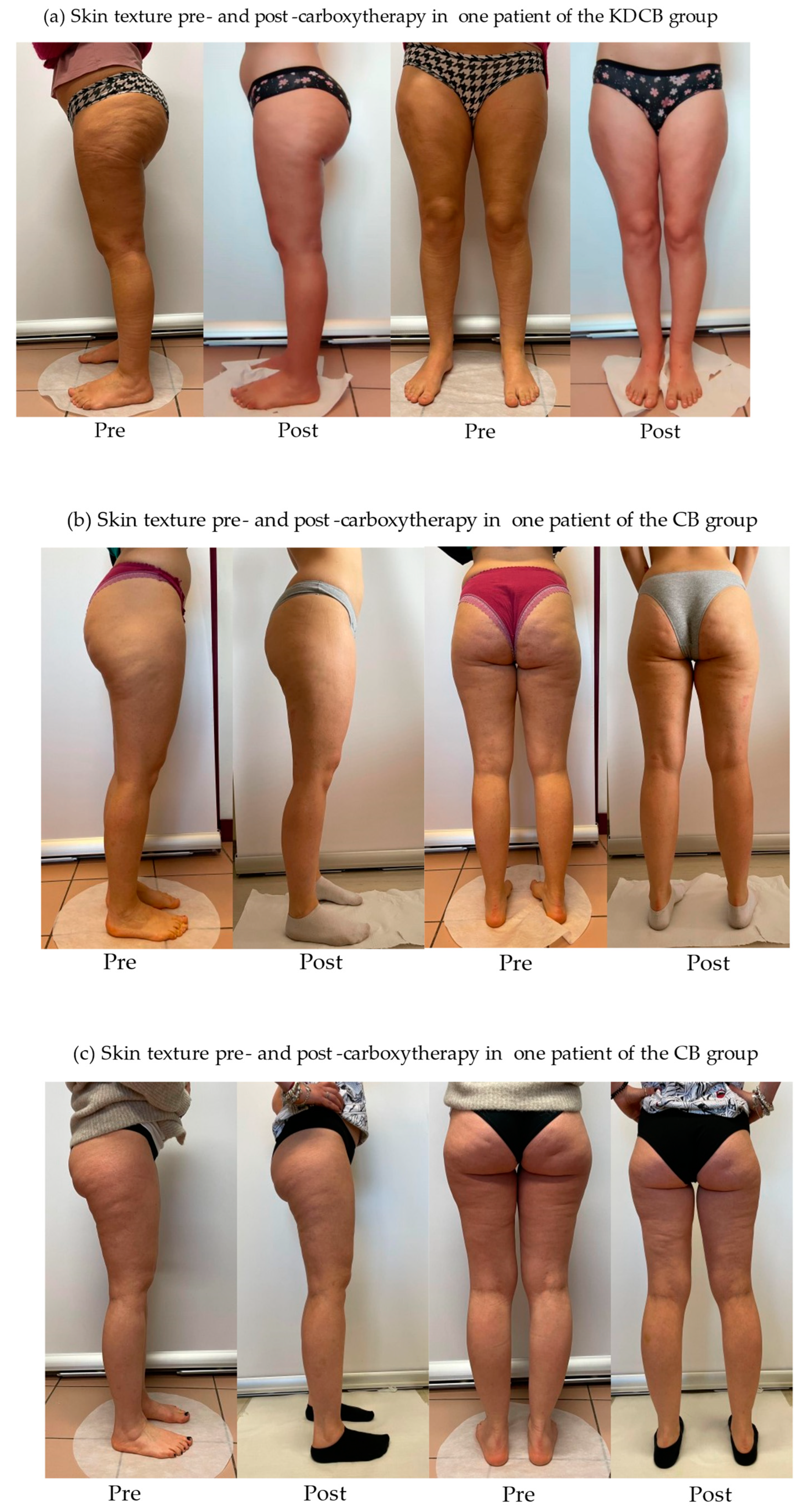
2.3. Carboxytherapy
2.4. Anthropometrics, Body Composition, and Basal Metabolism
2.5. EUROQOL-5 Dimension (EQ-5D)
2.6. Fibromyalgia Tests
2.6.1. Fibromyalgia Assessment Status (FAS)
2.6.2. Fibromyalgia Severity Scale (FSS)
2.6.3. Revised Fibromyalgia Impact Questionnaire (FIQR)
2.7. Difficulties in Emotional Regulation Scale (DERS)
2.8. Yale Food Addiction Scale 2.0 (YFAS 2.0)
2.9. Statistical Analysis
3. Results
3.1. Dietary Components
3.2. Sample Description, Anthropometrics, and Body Composition
3.3. Quality of Life, Fibromyalgia, and Psychometric Tests
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Euro-Qol 5D Indicare quali delle seguenti affermazioni descrive meglio il suo stato di salute oggi, segnando con una crocetta (☒) una sola casella di ciascun gruppo | |||||||||||
| Capacità di movimento Non ho difficoltà nel camminare Ho qualche difficoltà nel camminare Sono costretto/a a letto | ☐ ☐ ☐ | ||||||||||
| Cura della persona Non ho difficoltà nel prendermi cura di me stesso Ho qualche difficoltà nel lavarmi o vestirmi Non sono in grado di lavarmi o vestirmi | ☐ ☐ ☐ | ||||||||||
| Attività abituali (per es. lavoro, studio, lavori domestici, attività familiari o di svago) Non ho difficoltà nello svolgimento delle attività abituali Ho qualche difficoltà nello svolgimento delle attività abituali Non sono in grado di svolgere le mie attività abituali | ☐ ☐ ☐ | ||||||||||
| Dolore, Fastidio o malessere Non provo alcun dolore o fastidio Provo dolore o fastidio moderati Provo estremo dolore o fastidio | ☐ ☐ ☐ | ||||||||||
| Ansia o Depressione Non sono ansioso o depresso Sono moderatamente ansioso o depresso Sono estremamente ansioso o depresso | ☐ ☐ ☐ | ||||||||||
Per aiutarla ad esprimere il suo stato di salute attuale abbiamo disegnato una scala graduate (simile ad un termometro) sulla quale il migliore stato di salute immaginabile è contrassegnato dal numero 100 e il peggiore dallo 0. Vorremmo che indicasse su questa scala quale è, secondo lei, il livello del suo stato di salute oggi, tracciando una linea dal riquadro sottostante fino al punto che corrisponde al suo stato di salute attuale. | Migliore stato di salute immaginabile  Peggiore stato di salute immaginabile | ||||||||||
| Fibromyalgia Assessment Status | |||||||||||
| (1) Quale numero da 0 a 10 descrive nel miglioro modo il livello di fatica che hai provato nella scorsa settimana? | |||||||||||
| Nessuna stanchezza |  1 |  2 |  3 |  4 |  5 |  6 |  7 |  8 |  9 |  10 | La peggior stanchezza |
| (2) Quanti problemi ha avuto per dormire nella scorsa settimana? | |||||||||||
| Nessun problema |  1 |  2 |  3 |  4 |  5 |  6 |  7 |  8 |  9 |  10 | Numerosi problemi |
| (3) Si prega di indicare sotto la sensazione di dolore da o a 4 che hai provato nella settimana passata per ciascuna parte del corpo inserendo una X nelle caselle. Si prega di essere sicuri di contrassegnare entrambi i lati destro e sinistro separatamente. | |||||||||||
 | |||||||||||
| Fibromyalgia Severity Scale |
 |
 |
 |
 |
| DERS Istruzioni Utilizzando la seguente scala di valori, le chiediamo di segnare quanto spesso le seguenti affermazioni possono essere applicate alla sua esperienza, indicando il numero appropiato a fianco di ogni item. 1 Quasi mai (0–10%); 2 A volte (11–35%); 3 Circa la metà delle volte (36–65%); 4 Molte volte (66–90%); 5 Quasi sempre (91–100%). | Quasi sempre | Molte volte | Circa la metà delle volte | A volte | Quasi mai | |
| 1 | Sono sereno riguardo a ciò che provo | 1 | 2 | 3 | 4 | 5 |
| 2 | Presto attenzione a come mi sento | 1 | 2 | 3 | 4 | 5 |
| 3 | Vivo le mie emozioni come travolgenti e fuori dal controllo | 5 | 4 | 3 | 2 | 1 |
| 4 | Non ho idea di come mi sento | 5 | 4 | 3 | 2 | 1 |
| 5 | Ho difficoltà a dare un senso a ciò che provo | 5 | 4 | 3 | 2 | 1 |
| 6 | Presto attenzione alle mie emozioni | 1 | 2 | 3 | 4 | 5 |
| 7 | So esattamente come mi sento | 1 | 2 | 3 | 4 | 5 |
| 8 | Mi interessa come mi sento | 1 | 2 | 3 | 4 | 5 |
| 9 | Sono confuso riguardo a ciò che provo | 5 | 4 | 3 | 2 | 1 |
| 10 | Quando sono turbato, riconosco le mie emozioni | 1 | 2 | 3 | 4 | 5 |
| 11 | Quando sono turbato, mi arrabbio con me stesso perché mi sento in quel modo | 5 | 4 | 3 | 2 | 1 |
| 12 | Quando sono turbato mi imbarazza sentirmi in quel modo | 5 | 4 | 3 | 2 | 1 |
| 13 | Quando sono turbato, ho delle difficoltà a completare il mio lavoro | 5 | 4 | 3 | 2 | 1 |
| 14 | Quando sono turbato, perdo il controllo | 5 | 4 | 3 | 2 | 1 |
| 15 | Quando sono turbato, credo che rimarrò in quello stato per molto tempo | 5 | 4 | 3 | 2 | 1 |
| 16 | Quando sono turbato, credo che finirò per sentirmi depresso | 5 | 4 | 3 | 2 | 1 |
| 17 | Quando sono turbato, credo che i miei sentimenti siano validi e importanti | 1 | 2 | 3 | 4 | 5 |
| 18 | Quando sono turbato, faccio fatica a focalizzarmi su altre cose | 5 | 4 | 3 | 2 | 1 |
| 19 | Quando sono turbato, mi sento senza controllo | 5 | 4 | 3 | 2 | 1 |
| 20 | Quando sono turbato, posso comunque finire le cose che devo fare | 1 | 2 | 3 | 4 | 5 |
| 21 | Quando sono turbato, mi vergogno con me stesso perché mi sento in quel modo | 5 | 4 | 3 | 2 | 1 |
| 22 | Quando sono turbato, so che alla fine posso trovare un modo per sentirmi meglio | 1 | 2 | 3 | 4 | 5 |
| 23 | Quando sono turbato, mi sento debole | 5 | 4 | 3 | 2 | 1 |
| 24 | Quando sono turbato, sento di poter avere ancora il controllo dei miei comportamenti | 1 | 2 | 3 | 4 | 5 |
| 25 | Quando sono turbato, mi sento in colpa perchè mi sento in quel modo | 5 | 4 | 3 | 2 | 1 |
| 26 | Quando sono turbato, ho delle difficoltà a concentrarmi | 5 | 4 | 3 | 2 | 1 |
| 27 | Quando sono turbato, ho delle difficoltà nel controllare i miei comportamenti | 5 | 4 | 3 | 2 | 1 |
| 28 | Quando sono turbato, credo che non ci sia niente che io possa fare per sentirmi meglio | 5 | 4 | 3 | 2 | 1 |
| 29 | Quando sono turbato, mi irrito con me stesso perché mi sento in quel modo | 5 | 4 | 3 | 2 | 1 |
| 30 | Quando sono turbato, inizio a sentirmi molto male con me stesso | 5 | 4 | 3 | 2 | 1 |
| 31 | Quando sono turbato, credo che crogiolarmi in questa emozione sia l’unica cosa che io possa fare | 5 | 4 | 3 | 2 | 1 |
| 32 | Quando sono turbato, perdo il controllo sui miei comportamenti | 5 | 4 | 3 | 2 | 1 |
| 33 | Quando sono turbato, faccio fatica a pensare a qualcosa di diverso | 5 | 4 | 3 | 2 | 1 |
| 34 | Quando sono turbato, mi prendo del tempo per riflettere su quello che sto provando veramente | 1 | 2 | 3 | 4 | 5 |
| 35 | Quando sono turbato, mi ci vuole molto tempo per sentirmi meglio | 5 | 4 | 3 | 2 | 1 |
| 36 | Quando sono turbato, le mie emozioni sono travolgenti | 5 | 4 | 3 | 2 | 1 |
| I-YFAS 2.0 | |||||||||
| ISTRUZIONI | |||||||||
| Il questionario vuole indagare sulle sue abitudini alimentari nell’ultimo anno. Alcune persone hanno difficoltà a controllare l’assunzione di determinati cibi come: -Dolci quali gelati, cioccolato, ciambelle, biscotti, torte, caramelle -Amidi quali pane bianco, pasta e riso -Snack salati quali patatine, crackers ecc -Cibi grassi quali hamburger, pancetta, pizza, patatine fritte ecc -Bevande gassate quali coca cola, fanta ecc Nelle domande la dizione “certi cibi” si riferisce a qualsiasi tipo di cibo menzionato sopra o comunque a qualsiasi tipo di cibo che ritenga possa essere coinvolto. | Ogni Giorno | 4–6 Volte a settimana | 2–3 Volte a Settimana | 1 Volta a settimana | 2–3 Volte al Mese | 1 Volta al Mese | Non Mensilemtente | Mai | |
| 1 | Quando inizio a mangiare “certi cibi” finisco per mangiare molto più di quanto pianificato. | 7 | 6 | 5 | 4 | 3 | 2 | 1 | 0 |
| 2 | Continuo a mangiare “certi cibi” nonostante non sia più affamato. | 7 | 6 | 5 | 4 | 3 | 2 | 1 | 0 |
| 3 | Mangio fino a sentirmi male fisicamente. | 7 | 6 | 5 | 4 | 3 | 2 | 1 | 0 |
| 4 | Non mangiare “certi cibi” o ridurne certi altri è qualcosa che mi preoccupa. | 7 | 6 | 5 | 4 | 3 | 2 | 1 | 0 |
| 5 | Trascorro tanto tempo sentendomi pieno o affaticato per aver mangiato troppo. | 7 | 6 | 5 | 4 | 3 | 2 | 1 | 0 |
| 6 | Mi trovo costantemente a mangiare “certi cibi” durante tutto il giorno. | 7 | 6 | 5 | 4 | 3 | 2 | 1 | 0 |
| 7 | Trovo il modo di procurarmi “certi cibi” quando non sono disponibili. Per esempio, sarei disposto ad allungare il mio percorso di guida verso un negozio per comprarli, anche quando ho altre opzioni disponibili a casa. | 7 | 6 | 5 | 4 | 3 | 2 | 1 | 0 |
| 8 | Ho mangiato “certi cibi” così spesso o in quantità tale che ho smesso di fare altre cose importanti, come lavorare o passare il tempo con la mia famiglia o con gli amici. | 7 | 6 | 5 | 4 | 3 | 2 | 1 | 0 |
| 9 | Ho avuto problemi con la mia famiglia o con i miei amici per aver mangiato troppo. | 7 | 6 | 5 | 4 | 3 | 2 | 1 | 0 |
| 10 | Ho evitato la scuola, il lavoro o attività sociali per paura di mangiare in eccesso lì | 7 | 6 | 5 | 4 | 3 | 2 | 1 | 0 |
| 11 | Quando ho ridotto o smesso di mangiare “certi cibi” mi sono sentito irritabile, nervoso/a o triste. | 7 | 6 | 5 | 4 | 3 | 2 | 1 | 0 |
| 12 | Quando ho avuto sintomi fisici, per non aver mangiato “certi cibi”, li ho mangiati per sentirmi meglio. | 7 | 6 | 5 | 4 | 3 | 2 | 1 | 0 |
| 13 | Quando ho avuto disturbi emotivi, per non aver mangiato “certi cibi”, li ho mangiati per sentirmi meglio. | 7 | 6 | 5 | 4 | 3 | 2 | 1 | 0 |
| 14 | Quando ho ridotto o smesso di mangiare “certi cibi” ho avuto sintomi fisici. Per esempio ho avuto mal di testa o stanchezza. | 7 | 6 | 5 | 4 | 3 | 2 | 1 | 0 |
| 15 | Quando ho ridotto o smesso di mangiare “certi cibi” ho avuto gran voglia di mangiarli. | 7 | 6 | 5 | 4 | 3 | 2 | 1 | 0 |
| 16 | La mia condotta alimentare mi ha causato molto malessere. | 7 | 6 | 5 | 4 | 3 | 2 | 1 | 0 |
| 17 | Ho avuto problemi significativi nella mia vita a causa del cibo e della alimentazione. Questi problemi possono essere relazionati con la mia routine, il lavoro, la scuola, gli amici, la famiglia o la salute. | 7 | 6 | 5 | 4 | 3 | 2 | 1 | 0 |
| 18 | Mi sono sentito male per aver mangiato in eccesso, tanto da non fare altre cose importanti. Tra cui lavorare o passare il tempo con la famiglia e gli amici. | 7 | 6 | 5 | 4 | 3 | 2 | 1 | 0 |
| 19 | Mangiare in eccesso ha interferito nel adempimento dei miei doveri familiari o nel fare i servizi domestici. | 7 | 6 | 5 | 4 | 3 | 2 | 1 | 0 |
| 20 | Ho evitato il lavoro, la scuola o le attività perché in queste situazioni non potevo mangiare “certi cibi”. | 7 | 6 | 5 | 4 | 3 | 2 | 1 | 0 |
| 21 | Ho evitato occasioni/incontri sociali perché la gente non avrebbe approvato quanto mangio. | 7 | 6 | 5 | 4 | 3 | 2 | 1 | 0 |
| 22 | Nonostante sapessi che la mia alimentazione avrebbe causato disturbi emotivi, ho continuato a mangiare “certi cibi”. | 7 | 6 | 5 | 4 | 3 | 2 | 1 | 0 |
| 23 | Nonostante sapessi che la mia alimentazione mi avrebbe causato problemi fisici, ho continuato a mangiare “certi cibi” | 7 | 6 | 5 | 4 | 3 | 2 | 1 | 0 |
| 24 | Mangiare la stessa quantità di cibo non mi da più tanta soddisfazione come prima. | 7 | 6 | 5 | 4 | 3 | 2 | 1 | 0 |
| 25 | Realmente ho voluto ridurre o smettere di mangiare “certi cibi”, però semplicemente non ci sono riuscito. | 7 | 6 | 5 | 4 | 3 | 2 | 1 | 0 |
| 26 | Avevo bisogno di mangiare ogni volta di più per raggiungere la sensazione che volevo raggiungere mangiando. Ciò includeva, ridurre le emozioni negative (come la tristezza) o aumentare le emozioni positive (come il piacere). | 7 | 6 | 5 | 4 | 3 | 2 | 1 | 0 |
| 27 | Non ho reso molto a lavoro o a scuola perché stavo mangiando troppo. | 7 | 6 | 5 | 4 | 3 | 2 | 1 | 0 |
| 28 | Ho continuato a mangiare “certi cibi”, nonostante sapessi che era dannoso per la mia salute. Per esempio, ho continuato a mangiare dolci pur avendo il diabete, o cibi grassi, pur avendo malattie cardiovascolari. | 7 | 6 | 5 | 4 | 3 | 2 | 1 | 0 |
| 29 | Ho avuto impulsi tanto forti per mangiare “certi cibi” che non potevo pensare ad altro. | 7 | 6 | 5 | 4 | 3 | 2 | 1 | 0 |
| 30 | Ho avuto desideri tanto intensi per “certi cibi” che sentivo che avrei dovuto mangiarli nell’immediato. | 7 | 6 | 5 | 4 | 3 | 2 | 1 | 0 |
| 31 | Ho provato a ridurre o smettere di mangiare “certi cibi”, però non ho avuto successo. | 7 | 6 | 5 | 4 | 3 | 2 | 1 | 0 |
| 32 | Ho provato a ridurre o smettere di mangiare “certi cibi” ed ho fallito. | 7 | 6 | 5 | 4 | 3 | 2 | 1 | 0 |
| 33 | Sono stato tanto distratto/a mangiando che ho rischiato di ferirmi (ad esempio guidando l’auto, attraversando la strada, maneggiando macchinari). | 7 | 6 | 5 | 4 | 3 | 2 | 1 | 0 |
| 34 | Sono stato tanto distratto/a pensando al cibo che ho rischiato di ferirmi (ad esempio guidando l’auto, attraversando la strada, maneggiando macchinari). | 7 | 6 | 5 | 4 | 3 | 2 | 1 | 0 |
| 35 | I miei amici o familiari sono stati molto preoccupati perché ho esagerato con il cibo. | 7 | 6 | 5 | 4 | 3 | 2 | 1 | 0 |
References
- Buck, D.W.; Herbst, K.L. Lipedema: A relatively common disease with extremely common misconceptions. Plast. Reconstr. Surg-Glob. Open 2016, 4, e1043. [Google Scholar] [CrossRef]
- Di Renzo, L.; Cinelli, G.; Romano, L.; Zomparelli, S.; De Santis, G.L.; Nocerino, P.; Bigioni, G.; Arsini, L.; Cenname, G.; Pujia, A.; et al. Potential effects of a modified mediterranean diet on body composition in lipoedema. Nutrients 2021, 13, 358. [Google Scholar] [CrossRef] [PubMed]
- Susan, P.; Masino, A.; Ruskin, D.N. Ketogenic Diets and Pain. Bone 2008, 23, 993–1001. [Google Scholar] [CrossRef]
- Athinarayanan, S.J.; Adams, R.N.; Hallberg, S.J.; McKenzie, A.L.; Bhanpuri, N.H.; Campbell, W.W.; Volek, J.S.; Phinney, S.D.; McCarter, J.P. Long-term effects of a novel continuous remote care intervention including nutritional ketosis for the management of type 2 diabetes: A 2-year nonrandomized clinical trial. Front. Endocrinol. 2019, 10, 348. [Google Scholar] [CrossRef] [PubMed]
- McKenzie, A.L.; Hallberg, S.J.; Creighton, B.C.; Volk, B.M.; Link, T.M.; Abner, M.K.; Glon, R.M.; McCarter, J.P.; Volek, J.S.; Phinney, S.D. A novel intervention including individualized nutritional recommendations reduces hemoglobin A1c level, medication use, and weight in type 2 diabetes. JMIR Diabetes 2017, 2, e5. [Google Scholar] [CrossRef]
- Volek, J.S.; Fernandez, M.L.; Feinman, R.D.; Phinney, S.D. Dietary carbohydrate restriction induces a unique metabolic state positively affecting atherogenic dyslipidemia, fatty acid partitioning, and metabolic syndrome. Prog. Lipid Res. 2008, 47, 307–318. [Google Scholar] [CrossRef] [PubMed]
- Keith, L.; Seo, C.; Rowsemitt, C.; Pfeffer, M.; Wahi, M.; Staggs, M.; Dudek, J.; Gower, B.; Carmody, M. Ketogenic diet as a potential intervention for lipedema. Med. Hypotheses 2021, 146, 110435. [Google Scholar] [CrossRef]
- Puchalska, P.; Crawford, P.A. Multi-dimensional roles of ketone bodies. Physiol. Behav. 2019, 176, 139–148. [Google Scholar] [CrossRef]
- Cooper, M.A.; Menta, B.W.; Perez-Sanchez, C.; Jack, M.M.; Khan, Z.W.; Ryals, J.M.; Winter, M.; Wright, D.E. A ketogenic diet reduces metabolic syndrome-induced allodynia and promotes peripheral nerve growth in mice. Exp. Neurol. 2018, 306, 149–157. [Google Scholar] [CrossRef]
- Dudek, J.E.; Białaszek, W.; Ostaszewski, P.; Smidt, T. Depression and appearance-related distress in functioning with lipedema. Psychol. Health Med. 2018, 23, 846–853. [Google Scholar] [CrossRef]
- Murphy, P.; Likhodii, S.; Nylen, K.; Burnham, W.M. The antidepressant properties of the ketogenic diet. Biol. Psychiatry 2004, 56, 981–983. [Google Scholar] [CrossRef]
- Sadala, A.Y.; Machado, A.F.P.; Liebano, R.E. Effects of transcutaneous electrical nerve stimulation on pain intensity during application of carboxytherapy in patients with cellulite: A randomized placebo-controlled trial. J. Cosmet. Dermatol. 2018, 17, 1175–1181. [Google Scholar] [CrossRef]
- Alam, M.; Sadhwani, D.; Geisler, A.; Aslam, I.; Makin, I.R.S.; Schlessinger, D.I.; Disphanurat, W.; Pongprutthipan, M.; Voravutinon, N.; Weil, A.; et al. Subcutaneous infiltration of carbon dioxide (carboxytherapy) for abdominal fat reduction: A randomized clinical trial. J. Am. Acad. Dermatol. 2018, 79, 320–326. [Google Scholar] [CrossRef] [PubMed]
- Brandi, C.; D’Aniello, C.; Grimaldi, L.; Caiazzo, E.; Stanghellini, E. Carbon dioxide therapy: Effects on skin irregularity and its use as a complement to liposuction. Aesthetic Plast. Surg. 2004, 28, 222–225. [Google Scholar] [CrossRef] [PubMed]
- Buscemi, S.; Rosafio, G.; Vasto, S.; Massenti, F.M.; Grosso, G.; Galvano, F.; Rini, N.; Barile, A.M.; Maniaci, V.; Cosentino, L.; et al. Validation of a food frequency questionnaire for use in Italian adults living in Sicily. Int. J. Food Sci. Nutr. 2015, 66, 426–438. [Google Scholar] [CrossRef]
- Colica, C.; Merra, G.; Gasbarrini, A.; De Lorenzo, A.; Cioccoloni, G.; Gualtieri, P.; Perrone, M.A.; Bernardini, S.; Bernardo, V.; Di Renzo, L.; et al. Efficacy and safety of very-low-calorie ketogenic diet: A double blind randomized crossover study. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 2274–2289. [Google Scholar] [PubMed]
- Alberti-Fidanza, A.; Fidanza, F. Mediterranean Adequacy Index of Italian diets. Public Health Nutr. 2004, 7, 937–941. [Google Scholar] [CrossRef]
- Varlaro, F.P.; Manzo, V.; Mugnaini, G.; Bisacci, C. Carboxytherapy: Effects on microcirculation and its use in the treatment of severe lymphedema. Acta Phlebol. 2007, 8, 79–91. [Google Scholar]
- Lee, G.S.K. Quality survey on efficacy of carboxytherapy for localized lipolysis. J. Cosmet. Dermatol. 2016, 15, 484–492. [Google Scholar] [CrossRef]
- Hartmann, B.R.; Bassenge, E.; Pittler, M. Effect of carbon dioxide-enriched water and fresh water on the cutaneous microcirculation and oxygen tension in the skin of the foot. Angiology 1997, 48, 337–343. [Google Scholar] [CrossRef]
- World Health Organization. Obesity. Available online: https://www.who.int/health-topics/obesity (accessed on 3 July 2023).
- Di Renzo, L.; Bertoli, A.; Bigioni, M.; Gobbo, V.; Premrov, M.G.; Calabrese, V.; Di Daniele, N.; De Lorenzo, A. Body composition and -174G/C interleukin-6 promoter gene polymorphism: Association with progression of insulin resistance in normal weight obese syndrome. Curr. Pharm. Des. 2008, 14, 2699–2706. [Google Scholar] [CrossRef]
- De Lorenzo, A.; Romano, L.; Di Renzo, L.; Di Lorenzo, N.; Cenname, G.; Gualtieri, P. Obesity: A preventable, treatable, but relapsing disease. Nutrition 2020, 71, 110615. [Google Scholar] [CrossRef]
- Di Renzo, L.; Bigioni, M.; Bottini, F.G.; Del Gobbo, V.; Premrov, M.G.; Cianci, R.; De Lorenzo, A. Normal Weight Obese syndrome: Role of single nucleotide polymorphism of IL-1 5Ralpha and MTHFR 677C-->T genes in the relationship between body composition and resting metabolic rate. Eur. Rev. Med. Pharmacol. Sci. 2006, 10, 235–245. [Google Scholar]
- De Lorenzo, A.; Di Renzo, L.; Morini, P.; de Miranda, R.C.; Romano, L.; Colica, C. New equations to estimate resting energy expenditure in obese adults from body composition. Acta Diabetol. 2018, 55, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, J.S.; Hayes, A. Clinimetrics: The EuroQol-5 Dimension (EQ-5D). J. Physiother. 2020, 66, 133. [Google Scholar] [CrossRef] [PubMed]
- Salaffi, F.; Di Carlo, M.; Bazzichi, L.; Atzeni, F.; Govoni, M.; Biasi, G.; Di Franco, M.; Mozzani, F.; Gremese, E.; Dagna, L.; et al. Definition of fibromyalgia severity: Findings from a cross-sectional survey of 2339 Italian patients. Rheumatol 2021, 60, 728–736. [Google Scholar] [CrossRef] [PubMed]
- Lee, M. Clinimetrics: The Revised Fibromyalgia Impact Questionnaire. J. Physiother. 2021, 67, 220–221. [Google Scholar] [CrossRef]
- Hallion, L.S.; Steinman, S.A.; Tolin, D.F.; Diefenbach, G.J. Psychometric properties of the difficulties in emotion regulation scale (DERS) and its short forms in adults with emotional disorders. Front. Psychol. 2018, 9, 539. [Google Scholar] [CrossRef]
- Semborski, S.; Henwood, B.; Rhoades, H.; Mason, T.; Wenzel, S.; Rice, E. Construct, concurrent, and real-world predictive validity of the Difficulties in Emotion Regulation (DERS-18) among young adults with history of homelessness. Psychol. Assess. 2021, 33, 385–394. [Google Scholar] [CrossRef]
- Bjureberg, J.; Ljótsson, B.; Tull, M.; Hedman-Lagerlöf, E.; Sahlin, H.; Lundh, L.-G.; Bjärehed, J.; DiLillo, D.; Messmanmoore, T.L.; Gumpert, C.H.; et al. In Emotion Regulation Scale: The DERS-16. J. Psychopathol. Behav. Assess. 2016, 38, 284–296. [Google Scholar] [CrossRef]
- Koehler, A.; Aguirre, T.; Schulte, E.; Bowman, R.; Struwe, L. Secondary analysis of YFAS 2.0 symptom counts, impairment/distress, and food addiction severity in adults with overweight/obesity. Eat. Weight. Disord. 2021, 26, 2393–2399. [Google Scholar] [CrossRef] [PubMed]
- Hauck, C.; Cook, B.; Ellrott, T. Food addiction, eating addiction and eating disorders. Proc. Nutr. Soc. 2020, 79, 103–112. [Google Scholar] [CrossRef]
- Todd, M. Diagnosis and management of lipoedema in the community. Br. J. Community Nurs. 2016, 21, S6–S12. [Google Scholar] [CrossRef] [PubMed]
- Herbst, K.L. Rare adipose disorders (RADs) masquerading as obesity. Acta Pharmacol. Sin. 2012, 33, 155–172. [Google Scholar] [CrossRef] [PubMed]
- Ghods, M.; Georgiou, I.; Schmidt, J.; Kruppa, P. Disease progression and comorbidities in lipedema patients: A 10-year retrospective analysis. Dermatol. Ther. 2020, 33, e14534. [Google Scholar] [CrossRef]
- De Lorenzo, A.; Bernardini, S.; Gualtieri, P.; Cabibbo, A.; Perrone, M.A.; Giambini, I.; Di Renzo, L. Mediterranean meal versus Western meal effects on postprandial ox-LDL, oxidative and inflammatory gene expression in healthy subjects: A randomized controlled trial for nutrigenomic approach in cardiometabolic risk. Acta Diabetol. 2017, 54, 141–149. [Google Scholar] [CrossRef]
- Di Daniele, N.; Di Renzo, L.; Noce, A.; Iacopino, L.; Ferraro, P.M.; Rizzo, M.; Sarlo, F.; Domino, E.; De Lorenzo, A. Effects of Italian Mediterranean organic diet vs. low-protein diet in nephropathic patients according to MTHFR genotypes. J. Nephrol. 2014, 27, 529–536. [Google Scholar] [CrossRef]
- Nagpal, R.; Neth, B.J.; Wang, S.; Craft, S.; Yadav, H. Modified Mediterranean-ketogenic diet modulates gut microbiome and short-chain fatty acids in association with Alzheimer’s disease markers in subjects with mild cognitive impairment. EBioMedicine 2019, 47, 529–542. [Google Scholar] [CrossRef]
- Cannataro, R.; Michelini, S.; Ricolfi, L.; Caroleo, M.C.; Gallelli, L.; De Sarro, G.; Onorato, A.; Cione, E. Management of Lipedema with Ketogenic Diet: 22-Month Follow-Up. Life 2021, 11, 1402. [Google Scholar] [CrossRef]
- Pianez, L.R.; Custódio, F.S.; Guidi, R.M.; de Freitas, J.N.; Sant’Ana, E. Effectiveness of carboxytherapy in the treatment of cellulite in healthy women: A pilot study. Clin. Cosmet. Investig. Dermatol. 2016, 9, 183–190. [Google Scholar] [CrossRef]
- La Torre, Y.S.D.; Wadeea, R.; Rosas, V.; Herbst, K.L. Lipedema: Friend and foe. Horm. Mol. Biol. Clin. Investig. 2018, 33, 20170076. [Google Scholar] [CrossRef] [PubMed]
- Wold, L.E.; Hines, E.A.; Allen, E.V. Lipedema of the legs; a syndrome characterized by fat legs and edema. Ann. Intern. Med. 1951, 34, 1243–1250. [Google Scholar] [CrossRef] [PubMed]
- Brandi, C.; Campana, M.; Russo, F.; Brafa, A.; Nisi, G.; Grimaldi, L.; D’aniello, C. Carbon dioxide: Maybe not the only one but an efficient and secure gas for treating local adiposities. Aesthetic Plast. Surg. 2012, 36, 218–219. [Google Scholar] [CrossRef]
- Koutná, N. Carboxytherapy--a new non-invasive method in aesthetic medicine. Cas. Lek. Cesk 2006, 145, 841–843. [Google Scholar] [PubMed]
- Di Renzo, L.; Di Pierro, D.; Bigioni, M.; Sodi, V.; Galvano, F.; Cianci, R.; La Fauci, L.; De Lorenzo, A. Is antioxidant plasma status in humans a consequence of the antioxidant food content influence? Eur. Rev. Med. Pharmacol. Sci. 2007, 11, 185–192. [Google Scholar]
- Al-Wardat, M.; Alwardat, N.; De Santis, G.L.; Zomparelli, S.; Gualtieri, P.; Bigioni, G.; Romano, L.; Di Renzo, L. The association between serum vitamin D and mood disorders in a cohort of lipedema patients. Horm. Mol. Biol. Clin. Investig. 2021, 42, 351–355. [Google Scholar] [CrossRef]

| Media T0 | Media T1 | p-Value | |
|---|---|---|---|
| Energy (Kcal) | 1551.69 [329.96] | 1512.5 [140.73] | 0.617 |
| Proteins (% Kcal) | 22.88 [7.12] | 22.81 [0.7] | 0.969 |
| Vegetable proteins (% Kcal) | 22.52 [10.17] | 20.08 [2.12] | 0.373 |
| Animal proteins (% Kcal) | 65.96 [9.93] | 77.95 [2.07] | <0.001 * |
| Carbohydrates (% Kcal) | 28.9 [11.85] | 5.95 [0.67] | <0.001 * |
| Sugars (% Kcal) | 22.68 [13.79] | 3.5 [0.26] | <0.001 * |
| Fiber (g) | 18.32 [5.97] | 14.17 [0.9] | 0.011 * |
| Lipids (% Kcal) | 47.84 [10.36] | 71.23 [0.93] | <0.001 * |
| SFA (% Kcal) | 14.92% [9.93%] | 15.70% [0.82%] | 0.759 |
| PUFA (% Kcal) | 5.10% [1.93%] | 8.55% [0.51%] | <0.001 * |
| MUFA (% Kcal) | 22.80% [4.96%] | 40.60% [0.70%] | <0.001 * |
| Vit C (mg) | 154.41 [72.32] | 101.34 [10.21] | 0.007 * |
| Vit D (μcg) | 2.42 [1.89] | 4.91 [0.68] | <0.001 * |
| Vit E (mg) | 14.94 [5.75] | 22.87 [1.81] | <0.001 * |
| MAI | 0.87 [0.65] | 2.63 [0.75] | <0.001 * |
| Sample T0 | |
|---|---|
| Total (n) | 30 |
| Sex (M/F) | 30 (F) |
| Age (years) | 46 [7.4] |
| Height (cm) | 160.65 [6.2] |
| Weight (kg) | 73.8 [17] |
| BMI (kg/m2) | 28.6 [6.5] |
| KDCB T0 | KDCB T1 | p-Value | KD T0 | KD T1 | p-Value | CB T0 | CB T1 | p-Value | |
|---|---|---|---|---|---|---|---|---|---|
| Weight (Kg) | 72.64 [21.17] | 69.29 [21.5] | 0.009 * | 74.26 [11.53] | 70.73 [11.55] | 0.003 * | 76.78 [24.88] | 76.07 [24.78] | 0.168 |
| BMI (Kg/m2) | 28.33 [7.3] | 27.02 [7.51] | 0.008 * | 27.18 [4.66] | 25.88 [4.39] | 0.006 * | 30.68 [10.93] | 30.4 [10.92] | 0.211 |
| Waist Circumference (cm) | 80.5 [16.06] | 77.38 [15.26] | 0.069 | 82.25 [10.94] | 79.19 [10.52] | 0.03 * | 83.37 [17.03] | 83.02 [16.57] | 0.527 |
| Hip Circumference (cm) | 107.41 [13.4] | 104.94 [13.04] | 0.02 * | 109.66 [7.87] | 105.19 [8.54] | 0.0001 * | 112.32 [20.8] | 113.55 [19.93] | 0.239 |
| DXA Parameters | KDCB T0 | KDCB T1 | p-Value | KD T0 | KD T1 | p-Value | CB T0 | CB T1 | p-Value |
|---|---|---|---|---|---|---|---|---|---|
| Total fat tissue (%) | 36.39 [9.21] | 34.38 [9.6] | 0.012 * | 38.03 [7.69] | 36.35 [9.83] | 0.044 * | 38.78 [9.52] | 37.42 [10.43] | 0.153 |
| Total fat tissue (g) | 26,825.88 [15,049.11] | 24,619.63 [15,214.81] | 0.005 * | 29,135.88 [9149.12] | 26,489.17 [11,013.63] | 0.019 * | 32,070.5 [18,361.24] | 29,948.5 [17,215.04] | 0.064 |
| Total leg fat tissue (%) | 42.44 [8.45] | 40.49 [8.93] | 0.020 * | 46.08 [6.02] | 44.97 [8.03] | 0.075 | 45.63 [6.37] | 45.27 [5.98] | 0.598 |
| Total leg fat tissue (g) | 13,008.63 [5692.33] | 11,702.88 [5722.94] | 0.001 * | 14,616.63 [4120.1] | 13,264.5 [4021.63] | 0.015 * | 14,243.5 [6219.5] | 14,108.33 [6473.1] | 0.673 |
| Total arm fat tissue (g) | 2177.25 [682.81] | 2266 [955.18] | 0.676 | 2336.38 [884.28] | 2167.5 [959.96] | 0.664 | 2557.67 [1551.81] | 2674.67 [1820.62] | 0.564 |
| Android total fat tissue (%) | 37.63 [12.71] | 33.75 [13.56] | 0.0011 * | 41.44 [10.09] | 35.47 [17.34] | 0.029 * | 41.67 [11.91] | 40.97 [12.45] | 0.666 |
| Gynoid total fat tissue (%) | 43.95 [7.54] | 40.91 [7.91] | 0.003 * | 46.66 [5.92] | 43.27 [8.63] | 0.030 * | 47.55 [6.4] | 46.48 [6.55] | 0.314 |
| Total lean mass (g) | 41,295.75 [10,954.4] | 40,645.25 [10,826.55] | 0.243 | 43,512.38 [4267.88] | 41,546 [5058.08] | 0.139 | 43,379 [7137.34] | 42,949.67 [6532.6] | 0.530 |
| Total leg lean mass (g) | 15,772.88 [3054.57] | 15,197.63 [3291.54] | 0.114 | 15,725 [2284.37] | 14,888.33 [1940.51] | 0.397 | 15,160.55 [3177.59] | 15,301.67 [3907.32] | 0.815 |
| Total arm lean mass (g) | 3488.5 [596.14] | 3833.5 [398.06] | 0.121 | 3509 [723.16] | 3634.17 [333.77] | 0.426 | 3580 [700.9] | 3921.17 [831.92] | 0.346 |
| IMAT | 0.94 [0.45] | 0.83 [0.45] | 0.026 * | 1.17 [0.43] | 1.05 [0.48] | 0.007 * | 1.16 [0.61] | 1.1 [0.62] | 0.111 |
| ASMMI | 7.54 [0.87] | 7.45 [1.08] | 0.585 | 7.03 [0.8] | 6.63 [0.41] | 0.384 | 7.42 [1.5] | 7.62 [1.7] | 0.538 |
| KDCB T0 | KDCB T1 | p-Value | KD T0 | KD T1 | p-Value | CB T0 | CB T1 | p-Value | |
|---|---|---|---|---|---|---|---|---|---|
| EuroQ_tot | 45.14 [11.98] | 35 [16.41] | 0.141 | 63.63 [38.65] | 75.71 [31.42] | 0.729 | 46.83 [33.2] | 56.67 [27.51] | 0.067 |
| FAS | 12.51 [5.88] | 8.63 [6.06] | 0.049 * | 16.88 [6.71] | 16.44 [6.32] | 0.860 | 10.9 [7.25] | 15.2 [2.11] | 0.155 |
| FIQR_tot | 41.43 [24.52] | 31.33 [21.42] | 0.032 * | 66.5 [42.14] | 64.52 [40.32] | 0.730 | 51.06 [36.26] | 66.39 [23.59] | 0.138 |
| FSS | 12.29 [8.42] | 9.63 [7.82] | 0.119 | 16.38 [8.6] | 16 [5.86] | 0.643 | 12.17 [9.41] | 15.83 [8.13] | 0.175 |
| DERS tot | 81.14 [22.45] | 76.63 [24.77] | 0.408 | 84.38 [29.13] | 79.71 [25.34] | 0.250 | 70.67 [16.27] | 67.83 [13.36] | 0.586 |
| YFAS level of FA | 0.86 [1.46] | 0.38 [1.06] | 0.356 | 0.5 [1.07] | 1.14 [1.35] | 0.280 | 0.17 [0.41] | 0.67 [1.21] | 0.363 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Renzo, L.; Gualtieri, P.; Zomparelli, S.; De Santis, G.L.; Seraceno, S.; Zuena, C.; Frank, G.; Cianci, R.; Centofanti, D.; De Lorenzo, A. Modified Mediterranean-Ketogenic Diet and Carboxytherapy as Personalized Therapeutic Strategies in Lipedema: A Pilot Study. Nutrients 2023, 15, 3654. https://doi.org/10.3390/nu15163654
Di Renzo L, Gualtieri P, Zomparelli S, De Santis GL, Seraceno S, Zuena C, Frank G, Cianci R, Centofanti D, De Lorenzo A. Modified Mediterranean-Ketogenic Diet and Carboxytherapy as Personalized Therapeutic Strategies in Lipedema: A Pilot Study. Nutrients. 2023; 15(16):3654. https://doi.org/10.3390/nu15163654
Chicago/Turabian StyleDi Renzo, Laura, Paola Gualtieri, Samanta Zomparelli, Gemma Lou De Santis, Silvia Seraceno, Claudia Zuena, Giulia Frank, Rossella Cianci, Domenico Centofanti, and Antonino De Lorenzo. 2023. "Modified Mediterranean-Ketogenic Diet and Carboxytherapy as Personalized Therapeutic Strategies in Lipedema: A Pilot Study" Nutrients 15, no. 16: 3654. https://doi.org/10.3390/nu15163654
APA StyleDi Renzo, L., Gualtieri, P., Zomparelli, S., De Santis, G. L., Seraceno, S., Zuena, C., Frank, G., Cianci, R., Centofanti, D., & De Lorenzo, A. (2023). Modified Mediterranean-Ketogenic Diet and Carboxytherapy as Personalized Therapeutic Strategies in Lipedema: A Pilot Study. Nutrients, 15(16), 3654. https://doi.org/10.3390/nu15163654