Hesperetin-7-O-glucoside/β-cyclodextrin Inclusion Complex Induces Acute Vasodilator Effect to Inhibit the Cold Sensation Response during Localized Cold-Stimulate Stress in Healthy Human Subjects: A Randomized, Double-Blind, Crossover, and Placebo-Controlled Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design, Study Participants, and Preliminary Section Criteria
2.2. Study Test Materials, Supplementation, and Dosages
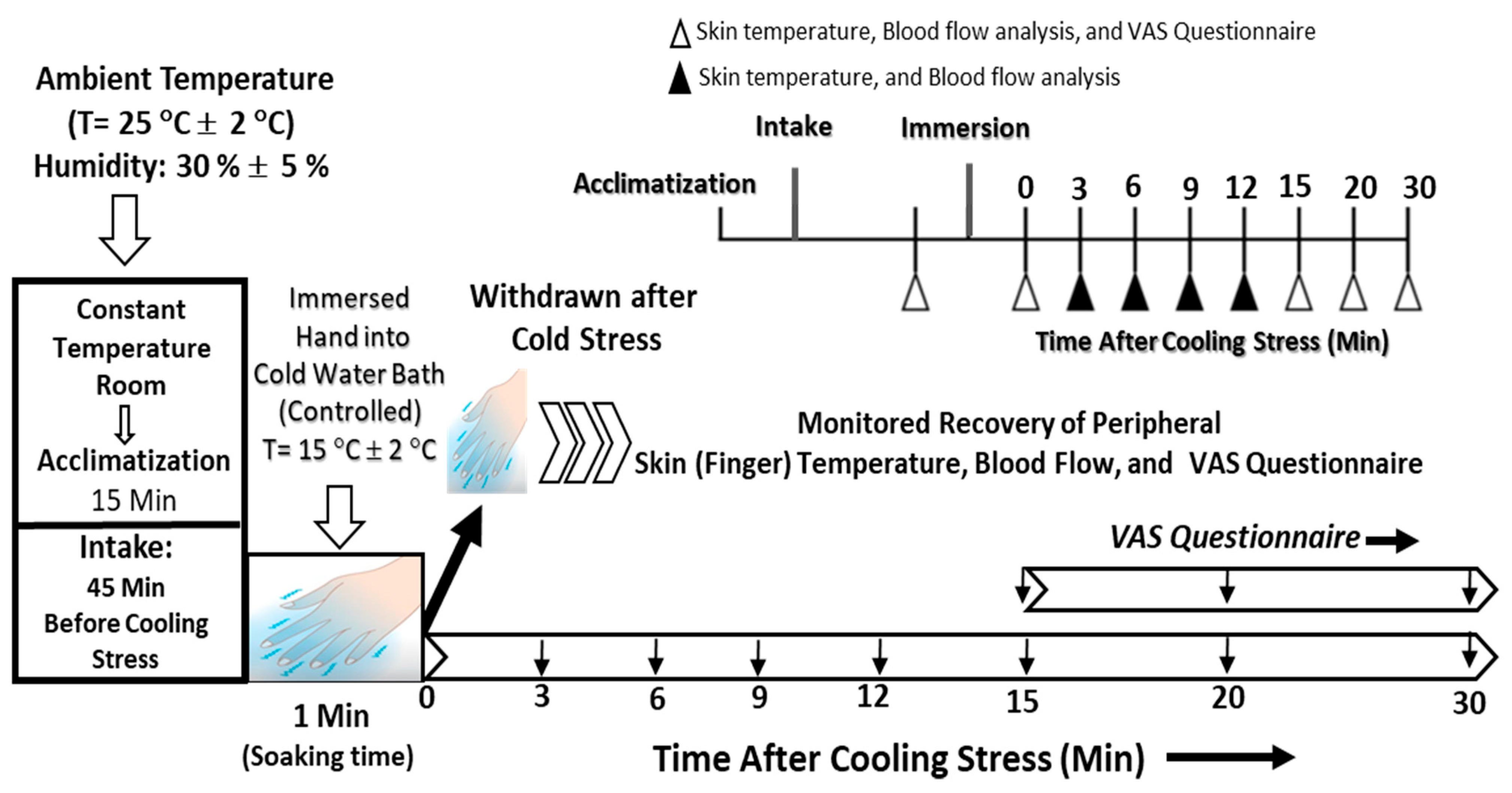
2.3. Study Protocol, Procedure, Instrumentation, and Measurements
2.4. Subjective Symptom Assessments
2.5. Data Processing and Statistical Analyses
3. Results
3.1. Study Characteristics and Procedural Analyses
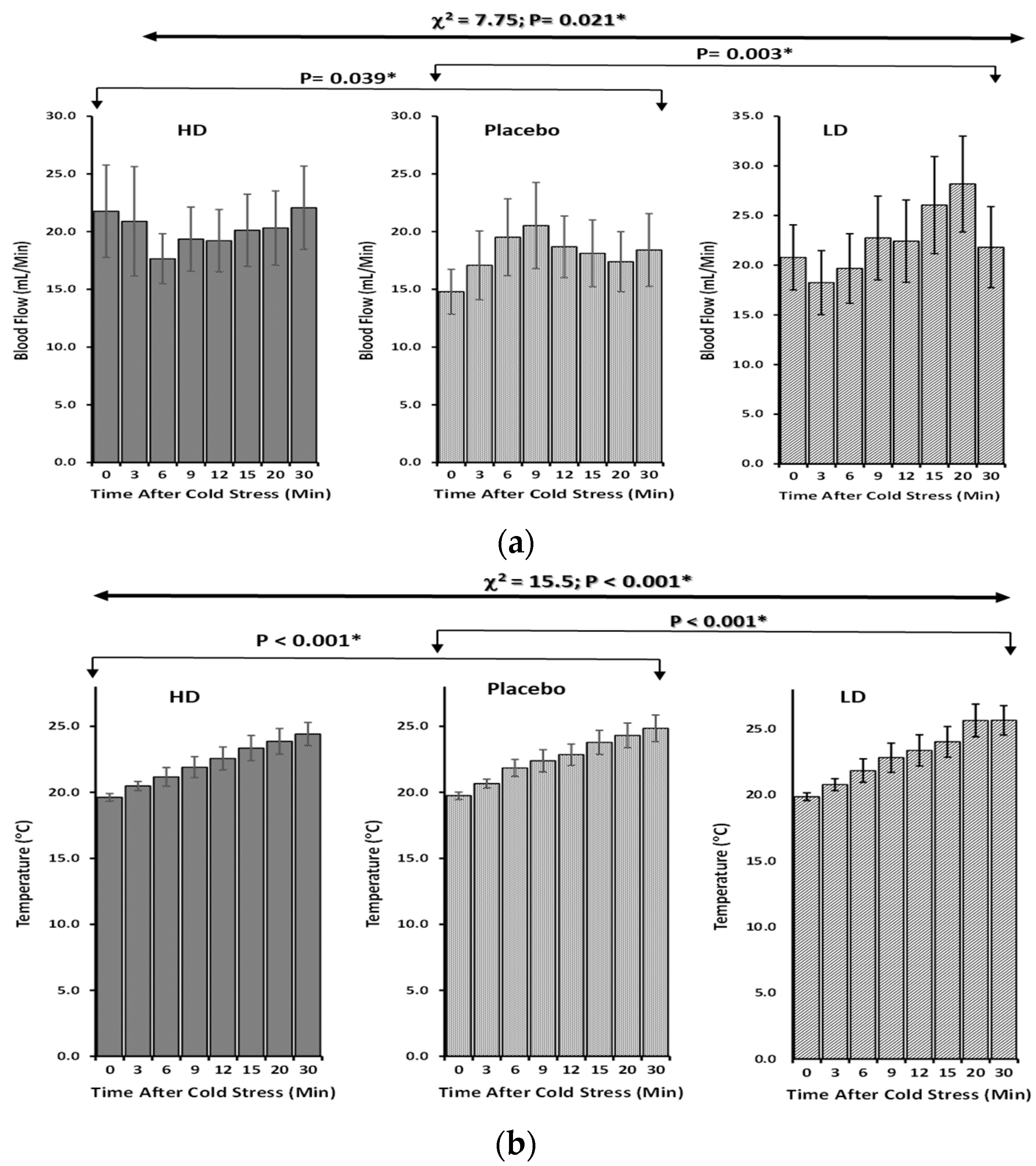
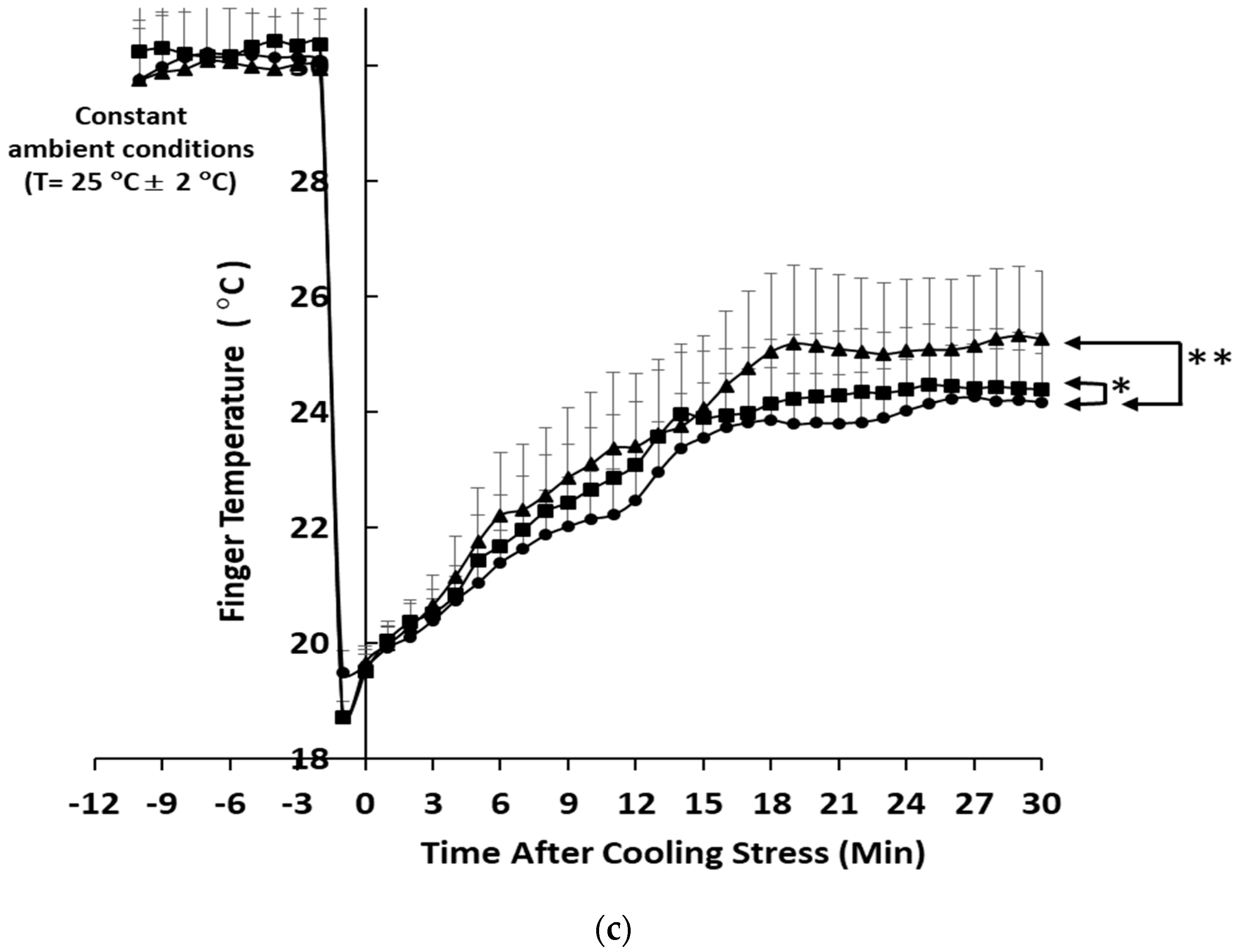
3.2. Skin Peripheral Blood Flow and Temperature Profiles upon Cold-Stimulated Stress
3.3. Cold Sensation Response: A Subjective Questionnaire
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brown, D.J.; Brugger, H.; Boyd, J.; Paal, P. Accidental hypothermia. N. Engl. J. Med. 2012, 367, 1930–1938. [Google Scholar] [CrossRef] [PubMed]
- Cheung, S.S. Responses of the hands and feet to cold exposure. Temperature 2015, 2, 105–120. [Google Scholar] [CrossRef] [PubMed]
- Cheshire, W.P., Jr. Thermoregulatory disorders and illness related to heat and cold stress. Auton. Neurosci. 2016, 196, 91–104. [Google Scholar] [CrossRef] [PubMed]
- DeGroot, D.W.; Gallimore, R.P.; Thompson, S.M.; Kenefick, R.W. Extremity cooling for heat stress mitigation in military and occupational settings. J. Therm. Biol. 2013, 38, 305–310. [Google Scholar] [CrossRef]
- DeGroot, D.W.; Rappole, C.A.; McHenry, P.; Englert, R.M. Seasonal trends for environmental illness incidence in the US Army. Mil. Med. 2022, 187, e672–e677. [Google Scholar] [CrossRef]
- Juopperi, K.; Hassi, J.; Ervasti, O.; Drebs, A.; Näyhä, S. Incidence of frostbite and ambient temperature in Finland, 1986–1995. A national study based on hospital admissions. Int. J. Circumpolar Health 2002, 61, 352–362. [Google Scholar] [CrossRef]
- Norrbrand, L.; Kölegård, R.; Keramidas, M.E.; Mekjavic, I.B.; Eiken, O. No association between hand and foot temperature responses during local cold stress and rewarming. Eur. J. Appl. Physiol. 2017, 117, 1141–1153. [Google Scholar] [CrossRef]
- Danzl, D. Hypothermia. In Seminars in Respiratory and Critical Care Medicine; Thieme Medical Publishers, Inc.: New York, NY, USA, 2002; Volume 23, pp. 57–68. [Google Scholar]
- Davey, M.; Eglin, C.; House, J.; Tipton, M. The contribution of blood flow to the skin temperature responses during a cold sensitivity test. Eur. J. Appl. Physiol. 2013, 113, 2411–2417. [Google Scholar] [CrossRef]
- Eglin, C.M.; Golden, F.S.; Tipton, M.J. Cold sensitivity test for individuals with non-freezing cold injury: The effect of prior exercise. Extrem. Physiol. Med. 2013, 2, 16. [Google Scholar] [CrossRef]
- Ruijs, A.C.; Jaquet, J.B.; Brandsma, M.; Daanen, H.A.; Hovius, S.E. Application of infrared thermography for the analysis of rewarming in patients with cold intolerance. Scand. J. Plast. Reconstr. Surg. Hand Surg. 2008, 42, 206–210. [Google Scholar] [CrossRef]
- Brändström, H.; Grip, H.; Hallberg, P.; Grönlund, C.; Ängquist, K.A.; Giesbrecht, G.G. Hand cold recovery responses before and after 15 months of military training in a cold climate. Aviat. Space Environ. Med. 2008, 79, 904–908. [Google Scholar] [CrossRef] [PubMed]
- Tokunaga, H.; Munakata, K.; Katayama, K.; Yamaguchi, R.; Imoto, S.; Miyano, S.; Watanabe, K. Clinical data mining related to the Japanese kampo concept “hie”(oversensitivity to coldness) in men and pre-and postmenopausal women. Evid.-Based Complement. Altern. Med. 2014, 2014, 832824. [Google Scholar] [CrossRef] [PubMed]
- Yoshino, T.; Katayama, K.; Munakata, K.; Horiba, Y.; Yamaguchi, R.; Imoto, S.; Miyano, S.; Watanabe, K. Statistical analysis of hie (cold sensation) and hiesho (cold disorder) in kampo clinic. Evid.-Based Complement. Altern. Med. 2013, 2013, 398458. [Google Scholar] [CrossRef] [PubMed]
- Joris, P.J.; Mensink, R.P.; Adam, T.C.; Liu, T.T. Cerebral blood flow measurements in adults: A review on the effects of dietary factors and exercise. Nutrients 2018, 10, 530. [Google Scholar] [CrossRef] [PubMed]
- Iwatani, S.; Yamamoto, N. Functional food products in Japan: A review. Food Sci. Hum. Wellness 2019, 8, 96–101. [Google Scholar] [CrossRef]
- Ullah, A.; Munir, S.; Badshah, S.L.; Khan, N.; Ghani, L.; Poulson, B.G.; Emwas, A.H.; Jaremko, M. Important flavonoids and their role as a therapeutic agent. Molecules 2020, 25, 5243. [Google Scholar] [CrossRef]
- Gonzales, G.B.; Smagghe, G.; Grootaert, C.; Zotti, M.; Raes, K.; Camp, J.V. Flavonoid interactions during digestion, absorption, distribution and metabolism: A sequential structure–activity/property relationship-based approach in the study of bioavailability and bioactivity. Drug Metab. Rev. 2015, 47, 175–190. [Google Scholar] [CrossRef]
- Kay, C.D.; Pereira-Caro, G.; Ludwig, I.A.; Clifford, M.N.; Crozier, A. Anthocyanins and flavanones are more bioavailable than previously perceived: A review of recent evidence. Annu. Rev. Food Sci. Technol. 2017, 8, 155–180. [Google Scholar] [CrossRef]
- Gonzales, G.B. In vitro bioavailability and cellular bioactivity studies of flavonoids and flavonoid-rich plant extracts: Questions, considerations and future perspectives. Proc. Nutr. Soc. 2017, 76, 175–181. [Google Scholar] [CrossRef]
- Rodriguez-Mateos, A.; Vauzour, D.; Krueger, C.G.; Shanmuganayagam, D.; Reed, J.; Calani, L.; Mena, P.; Del Rio, D.; Crozier, A. Bioavailability, bioactivity and impact on health of dietary flavonoids and related compounds: An update. Arch. Toxicol. 2014, 88, 1803–1853. [Google Scholar] [CrossRef]
- Rees, A.; Dodd, G.F.; Spencer, J.P. The effects of flavonoids on cardiovascular health: A review of human intervention trials and implications for cerebrovascular function. Nutrients 2018, 10, 1852. [Google Scholar] [CrossRef] [PubMed]
- Perez, A.; Gonzalez-Manzano, S.; Jimenez, R.; Perez-Abud, R.; Haro, J.M.; Osuna, A.; Santos-Buelga, C.; Duarte, J.; Perez-Vizcaino, F. The flavonoid quercetin induces acute vasodilator effects in healthy volunteers: Correlation with beta-glucuronidase activity. Pharmacol. Res. 2014, 89, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Markos, F.; Ruane O’Hora, T.; Noble, M.I. What is the mechanism of flow-mediated arterial dilatation? Clin. Exp. Pharmacol. Physiol. 2013, 40, 489–494. [Google Scholar] [CrossRef] [PubMed]
- Dobiaš, L.; Petrová, M.; Vojtko, R.; Kristová, V. Long-term treatment with hesperidin improves endothelium-dependent vasodilation in femoral artery of spontaneously hypertensive rats: The involvement of NO-synthase and Kv channels. Phytother. Res. 2016, 30, 1665–1671. [Google Scholar] [CrossRef]
- Shastry, S.; Dietz, N.M.; Halliwill, J.R.; Reed, A.S.; Joyner, M.J. Effects of nitric oxide synthase inhibition on cutaneous vasodilation during body heating in humans. J. Appl. Physiol. 1998, 85, 830–834. [Google Scholar] [CrossRef]
- Chiou, C.S.; Lin, J.W.; Kao, P.F.; Liu, J.C.; Cheng, T.H.; Chan, P. Effects of hesperidin on cyclic strain-induced endothelin-1 release in human umbilical vein endothelial cells. Clin. Exp. Pharmacol. Physiol. 2008, 35, 938–943. [Google Scholar] [CrossRef]
- Rizza, S.; Muniyappa, R.; Iantorno, M.; Kim, J.A.; Chen, H.; Pullikotil, P.; Senese, N.; Tesauro, M.; Lauro, D.; Cardillo, C.; et al. Citrus polyphenol hesperidin stimulates production of nitric oxide in endothelial cells while improving endothelial function and reducing inflammatory markers in patients with metabolic syndrome. J. Clin. Endocrinol. Metab. 2011, 96, E782–E792. [Google Scholar] [CrossRef]
- Liu, L.; Xu, D.M.; Cheng, Y.Y. Distinct effects of naringenin and hesperetin on nitric oxide production from endothelial cells. J. Agric. Food Chem. 2008, 56, 824–829. [Google Scholar] [CrossRef]
- Li, C.; Schluesener, H. Health-promoting effects of the citrus flavanone hesperidin. Crit. Rev. Food Sci. Nutr. 2017, 57, 613–631. [Google Scholar] [CrossRef]
- Takumi, H.; Fujishima, N.; Shiraishi, K.; Mori, Y.; Ariyama, A.; Kometani, T.; Hashimoto, S.; Nadamoto, T. Effects of α-glucosylhesperidin on the peripheral body temperature and autonomic nervous system. Biosci. Biotechnol. Biochem. 2010, 74, 707–715. [Google Scholar] [CrossRef]
- Morishita, N.; Ogihara, S.; Endo, S.; Mitsuzumi, H.; Ushio, S. Effects of glucosyl hesperidin on skin blood flow and temperature: A randomized, double-blind, placebo-controlled, crossover study. Shinryo Shinyaku 2020, 57, 129–134. [Google Scholar]
- Takumi, H.; Nakamura, H.; Shimizu, T.; Harada, R.; Kometani, T.; Nadamoto, T.; Mukai, R.; Murota, K.; Kawai, Y.; Terao, J. Bioavailability of orally administered water-dispersible hesperetin and its effect on peripheral vasodilatation in human subjects: Implication of endothelial functions of plasma conjugated metabolites. Food Funct. 2012, 3, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, M.P.; Moriwaki, M.; Minoura, K.; Timm, D.; Abe, A.; Kito, K. Structural Investigation of Hesperetin-7-O-Glucoside Inclusion Complex with β-Cyclodextrin: A Spectroscopic Assessment. Molecules 2022, 27, 5395. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, M.P.; Moriwaki, M.; Uguri, K.; Kito, K.; Timm, D.; Abe, A. Improved bioavailability of hesperetin 7-O-glucoside inclusion complex with β-cyclodextrin in Sprague-Dawley rats and healthy humans. J. Funct. Foods 2023, 107, 105708. [Google Scholar] [CrossRef]
- Goodyear, M.D.; Krleza-Jeric, K.; Lemmens, T. The declaration of Helsinki. BMJ 2007, 335, 624–625. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, K.; Kojima, T.; Matsumoto, C.; Kamegai, S.; Oyama, T.; Shibagaki, Y.; Muramoto, H.; Kawasaki, T.; Fujinaga, H.; Takahashi, K.; et al. Identification of a predictive biomarker for the beneficial effect of a Kampo (Japanese traditional) medicine keishibukuryogan in rheumatoid arthritis patients. Clin. Biochem. 2007, 40, 1113–1121. [Google Scholar] [CrossRef] [PubMed]
- Moriwaki, M.; Kito, K.; Nakagawa, R.; Tominaga, E.; Kapoor, M.P.; Matsumiya, Y.; Fukuhara, T.; Yamagata, H.; Katsumata, T.; Minegawa, K. Mutagenic, Acute, and Subchronic Toxicity Studies of the Hesperetin-7-Glucoside–β-Cyclodextrin Inclusion Complex. Int. J. Toxicol. 2023, 42, 50–62. [Google Scholar] [CrossRef]
- O’Leary, K.A.; Day, A.J.; Needs, P.W.; Sly, W.S.; O’Brien, N.M.; Williamson, G. Flavonoid glucuronides are substrates for human liver β-glucuronidase. FEBS Lett. 2001, 503, 103–106. [Google Scholar] [CrossRef]
- Guo, X.; Li, K.; Guo, A.; Li, E. Intestinal absorption and distribution of naringin, hesperidin, and their metabolites in mice. J. Funct. Foods 2020, 74, 104158. [Google Scholar] [CrossRef]
- Alba, B.K.; Castellani, J.W.; Charkoudian, N. Cold-induced cutaneous vasoconstriction in humans: Function, dysfunction and the distinctly counterproductive. Exp. Physiol. 2019, 104, 1202–1214. [Google Scholar] [CrossRef]
- Stephens, D.P.; Aoki, K.; Kosiba, W.A.; Johnson, J.M. Nonnoradrenergic mechanism of reflex cutaneous vasoconstriction in men. Am. J. Physiol.-Heart Circ. Physiol. 2001, 280, H1496–H1504. [Google Scholar] [CrossRef] [PubMed]
- Kellogg, D.L., Jr. In vivo mechanisms of cutaneous vasodilation and vasoconstriction in humans during thermoregulatory challenges. J. Appl. Physiol. 2006, 100, 1709–1718. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, F. Oral vitamin C enhances the adrenergic vasoconstrictor response to local cooling in human skin. J. Appl. Physiol. 2012, 112, 1689–1697. [Google Scholar] [CrossRef]
- Yamazaki, F.; Sone, R.; Zhao, K.; Alvarez, G.E.; Kosiba, W.A.; Johnson, J.M. Rate dependency and role of nitric oxide in the vascular response to direct cooling in human skin. J. Appl. Physiol. 2006, 100, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, F. Local ascorbate administration inhibits the adrenergic vasoconstrictor response to local cooling in the human skin. J. Appl. Physiol. 2010, 108, 328–333. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Kang, D.; Xu, J.; Lake, M.; Hogan, J.O.; Sun, C.; Walter, K.; Yao, B.; Kim, D. Species differences and molecular determinant of TRPA1 cold sensitivity. Nat. Commun. 2013, 4, 2501. [Google Scholar] [CrossRef]
- Hodges, G.J.; Zhao, K.; Kosiba, W.A.; Johnson, J.M. The involvement of nitric oxide in the cutaneous vasoconstrictor response to local cooling in humans. J. Physiol. 2006, 574, 849–857. [Google Scholar] [CrossRef]
- Johnson, J.M.; Kellogg, D.L., Jr. Thermoregulatory and thermal control in the human cutaneous circulation. Front. Biosci. 2010, 2, 825–853. [Google Scholar]
- Johnson, J.M.; Yen, T.C.; Zhao, K.; Kosiba, W.A. Sympathetic, sensory, and nonneuronal contributions to the cutaneous vasoconstrictor response to local cooling. Am. J. Physiol.-Heart Circ. Physiol. 2005, 288, H1573–H1579. [Google Scholar] [CrossRef]
- Kodji, X.; Aubdool, A.A.; Brain, S.D. Evidence for physiological and pathological roles for sensory nerves in the microvasculature and skin. Curr. Res. Transl. Med. 2016, 64, 195–201. [Google Scholar] [CrossRef]
- Ahmad, T.; Testani, J.M. Haemoconcentration as a treatment goal in heart failure: Ready for prime time? Eur. J. Heart Fail. 2017, 19, 237–240. [Google Scholar] [CrossRef]
- Kim, D.S.; Lim, S.B. Semi-continuous subcritical water extraction of flavonoids from Citrus unshiu peel: Their antioxidant and enzyme inhibitory activities. Antioxidants 2020, 9, 360. [Google Scholar] [CrossRef]
- Khan, A.; Ikram, M.; Hahm, J.R.; Kim, M.O. Antioxidant and anti-inflammatory effects of citrus flavonoid hesperetin: Special focus on neurological disorders. Antioxidants 2020, 9, 609. [Google Scholar] [CrossRef] [PubMed]
- Jayaraman, R.; Subramani, S.; Abdullah, S.H.S.; Udaiyar, M. Antihyperglycemic effect of hesperetin, a citrus flavonoid, extenuates hyperglycemia and exploring the potential role in antioxidant and antihyperlipidemic in streptozotocin-induced diabetic rats. Biomed. Pharmacother. 2018, 97, 98–106. [Google Scholar] [CrossRef]
- Parhiz, H.; Roohbakhsh, A.; Soltani, F.; Rezaee, R.; Iranshahi, M. Antioxidant and anti-inflammatory properties of the citrus flavonoids hesperidin and hesperetin: An updated review of their molecular mechanisms and experimental models. Phytother. Res. 2015, 29, 323–331. [Google Scholar] [CrossRef]
- Nishino, S.; Fujiki, Y.; Sato, T.; Kato, Y.; Shirai, R.; Oizumi, H.; Yamamoto, M.; Ohbuchi, K.; Miyamoto, Y.; Mizoguchi, K.; et al. Hesperetin, a citrus flavonoid, ameliorates inflammatory cytokine-mediated inhibition of oligodendroglial cell morphological differentiation. Neurol. Int. 2022, 14, 471–487. [Google Scholar] [CrossRef]
- Roohbakhsh, A.; Parhiz, H.; Soltani, F.; Rezaee, R.; Iranshahi, M. Neuropharmacological properties and pharmacokinetics of the citrus flavonoids hesperidin and hesperetin—A mini-review. Life Sci. 2014, 113, 1–6. [Google Scholar] [CrossRef]
- Wu, J.; Qian, Y.; Chen, C.; Feng, F.; Pan, L.; Yang, L.; Wang, C. Hesperetin exhibits anti-inflammatory effects on chondrocytes via the AMPK pathway to attenuate anterior cruciate ligament transection-induced osteoarthritis. Front. Pharmacol. 2021, 12, 735087. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, F.; Takahara, K.; Sone, R.; Johnson, J.M. Influence of hyperoxia on skin vasomotor control in normothermic and heat-stressed humans. J. Appl. Physiol. 2007, 103, 2026–2033. [Google Scholar] [CrossRef] [PubMed]
- Drouin, A.; Thorin, E. Flow-induced dilation is mediated by Akt-dependent activation of endothelial nitric oxide synthase-derived hydrogen peroxide in mouse cerebral arteries. Stroke 2009, 40, 1827–1833. [Google Scholar] [CrossRef]
- Green, D.J.; Dawson, E.A.; Groenewoud, H.M.; Jones, H.; Thijssen, D.H. Is flow-mediated dilation nitric oxide mediated? A meta-analysis. Hypertension 2014, 63, 376–382. [Google Scholar] [CrossRef]
- Friebe, A.; Koesling, D. Regulation of nitric oxide-sensitive guanylyl cyclase. Circ. Res. 2003, 93, 96–105. [Google Scholar] [CrossRef]
- Yamamoto, M.; Jokura, H.; Hashizume, K.; Ominami, H.; Shibuya, Y.; Suzuki, A.; Hase, T.; Shimotoyodome, A. Hesperidin metabolite hesperetin-7-O-glucuronide, but not hesperetin-3′-O-glucuronide, exerts hypotensive, vasodilatory, and anti-inflammatory activities. Food Funct. 2013, 4, 1346–1351. [Google Scholar] [CrossRef]
- Imperatrice, M.; Cuijpers, I.; Troost, F.J.; Sthijns, M.M. Hesperidin Functions as an Ergogenic Aid by Increasing Endothelial Function and Decreasing Exercise-Induced Oxidative Stress and Inflammation, Thereby Contributing to Improved Exercise Performance. Nutrients 2022, 14, 2955. [Google Scholar] [CrossRef]
- Yamamoto, M.; Suzuki, A.; Jokura, H.; Yamamoto, N.; Hase, T. Glucosyl hesperidin prevents endothelial dysfunction and oxidative stress in spontaneously hypertensive rats. Nutrition 2008, 24, 470–476. [Google Scholar] [CrossRef]
- Guerrero, L.; Castillo, J.; Quinones, M.; Garcia-Vallve, S.; Arola, L.; Pujadas, G.; Muguerza, B. Inhibition of angiotensin-converting enzyme activity by flavonoids: Structure-activity relationship studies. PLoS ONE 2012, 7, e49493. [Google Scholar] [CrossRef]
- Pan, Y.; Thapa, D.; Baldissera, L.; Argunhan, F.; Aubdool, A.A.; Brain, S.D. Relevance of TRPA1 and TRPM8 channels as vascular sensors of cold in the cutaneous microvasculature. Pflügers Arch.-Eur. J. Physiol. 2018, 470, 779–786. [Google Scholar] [CrossRef]
- Julius, D. TRP channels and pain. Annu. Rev. Cell Dev. Biol. 2013, 29, 355–384. [Google Scholar] [CrossRef]
- Laing, R.J.; Dhaka, A. ThermoTRPs and pain. Neurosci. 2016, 22, 171–187. [Google Scholar] [CrossRef]
- Palkar, R.; Lippoldt, E.K.; McKemy, D.D. The molecular and cellular basis of thermosensation in mammals. Curr. Opin. Neurobiol. 2015, 34, 14–19. [Google Scholar] [CrossRef]
- Kandel, E.R.; Schwartz, J.H.; Jessell, T.; Siegelbaum, S.A.; Hudspeth, A.J. Principles of Neural Science; McGraw Hill Professional: New York, NY, USA, 2013. [Google Scholar]
- Wang, H.; Siemens, J. TRP ion channels in thermosensation, thermoregulation and metabolism. Temperature 2015, 2, 178–187. [Google Scholar] [CrossRef]
- Buijs, T.J.; McNaughton, P.A. The role of cold-sensitive ion channels in peripheral thermosensation. Front. Cell. Neurosci. 2020, 14, 262. [Google Scholar] [CrossRef]
- Zakharian, E.; Cao, C.; Rohacs, T. Gating of transient receptor potential melastatin 8 (TRPM8) channels activated by cold and chemical agonists in planar lipid bilayers. J. Neurosci. 2010, 30, 12526–12534. [Google Scholar] [CrossRef]
- McKemy, D.D.; Neuhausser, W.M.; Julius, D. Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature 2002, 416, 52–58. [Google Scholar] [CrossRef]
- De La Peña, E.; Mälkiä, A.; Cabedo, H.; Belmonte, C.; Viana, F. The contribution of TRPM8 channels to cold sensing in mammalian neurones. J. Physiol. 2005, 567, 415–426. [Google Scholar] [CrossRef]
- Karashima, Y.; Talavera, K.; Everaerts, W.; Janssens, A.; Kwan, K.Y.; Vennekens, R.; Nilius, B.; Voets, T. TRPA1 acts as a cold sensor in vitro and in vivo. Proc. Natl. Acad. Sci. USA 2009, 106, 1273–1278. [Google Scholar] [CrossRef]
- Skagen, C.; Løvsletten, N.G.; Asoawe, L.; Al-Karbawi, Z.; Rustan, A.C.; Thoresen, G.H.; Haugen, F. Functional expression of the thermally activated transient receptor potential channels TRPA1 and TRPM8 in human myotubes. J. Therm. Biol. 2023, 116, 103623. [Google Scholar] [CrossRef]
- Caspani, O.; Heppenstall, P.A. TRPA1 and cold transduction: An unresolved issue? J. Gen. Physiol. 2009, 133, 245–249. [Google Scholar] [CrossRef]
- Knowlton, W.M.; Bifolck-Fisher, A.; Bautista, D.M.; McKemy, D.D. TRPM8, but not TRPA1, is required for neural and behavioral responses to acute noxious cold temperatures and cold-mimetics in vivo. Pain 2010, 150, 340–350. [Google Scholar] [CrossRef]
- Kaneko, Y.; Szallasi, A. Transient receptor potential (TRP) channels: A clinical perspective. Br. J. Pharmacol. 2014, 171, 2474–2507. [Google Scholar]
- Zhang, Z.M.; Wu, X.L.; Zhang, G.Y.; Ma, X.; He, D.X. Functional food development: Insights from TRP channels. J. Funct. Foods 2019, 56, 384–394. [Google Scholar] [CrossRef]




| Characteristics Parameters | Group A | Group B | Group C | All Subjects |
|---|---|---|---|---|
| (n = 7) | (n = 7) | (n = 6) | (n = 20) | |
| Gender (M/F) | 2/5 | 1/6 | 1/5 | 4/16 |
| Age (Years) | 49.00 ± 7.14 | 46.00 ± 10.75 | 47.83 ± 8.13 | 47.60 ± 8.47 |
| Height (Cm) | 163.43 ± 6.83 | 160.29 ± 5.57 | 157.95 ± 5.23 | 160.77 ± 6.08 |
| Weight (Kg) | 57.39 ± 7.81 | 58.17 ± 6.13 | 55.02 ± 6.04 | 56.95 ± 6.52 |
| BMI (Kg/m2) | 21.43 ± 1.79 | 22.70 ± 2.79 | 22.10 ± 2.72 | 22.07 ± 2.39 |
| Systolic blood pressure (mmHg) | 119.86 ± 6.07 | 118.14 ± 6.79 | 116.67 ± 9.18 | 118.30 ± 7.08 |
| Diastolic blood pressure (mmHg) | 78.71 ± 6.02 | 74.29 ± 4.39 | 77.50 ± 6.28 | 76.80 ± 5.63 |
| Pulse rate (bpm) | 63.00 ± 6.56 | 63.86 ± 12.35 | 75.83 ± 7.03 | 67.15 ± 10.43 |
| Blood flow recovery ratio | 0.30 ± 0.69 | 0.40 ± 1.34 | 0.28 ± 0.64 | 0.33 ± 0.91 |
| Skin temperature recovery ratio | 0.29 ± 0.16 | 0.26 ± 0.10 | 0.24 ± 0.06 | 0.26 ± 0.11 |
| Total protein (g/dL) | 7.17 ± 0.39 | 7.06 ± 0.42 | 7.22 ± 0.33 | 7.15 ± 0.37 |
| Total bilirubin (mg/dL) | 0.81 ± 0.30 | 0.76 ± 0.14 | 0.82 ± 0.30 | 0.80 ± 0.24 |
| ALP (U/L) | 67.71 ± 23.65 | 56.00 ± 12.45 | 82.50 ± 15.22 | 68.05 ± 20.15 |
| LD (U/L) | 151.8 ± 18.78 | 156.4 ± 26.15 | 164.7 ± 13.59 | 157.3 ± 20.11 |
| AST (GOT) (U/L) | 20.14 ± 3.34 | 18.29 ± 3.82 | 19.50 ± 4.23 | 19.30 ± 3.67 |
| ALT (GPT) (U/L) | 13.71 ± 5.09 | 16.14 ± 5.87 | 17.67 ± 6.65 | 15.75 ± 5.78 |
| γ-GT (γ-GTP) (U/L) | 18.29 ± 8.86 | 20.29 ± 7.80 | 41.00 ± 24.92 | 25.80 ± 17.68 |
| Creatine kinase (U/L) | 84.71 ± 25.71 | 86.29 ± 41.95 | 73.00 ± 29.82 | 81.75 ± 32.15 |
| Total cholesterol (mg/dL) | 210.1± 19.18 | 189.7 ± 42.59 | 208.5 ± 26.49 | 202.5 ± 31.09 |
| Triglycerides (mg/dL) | 68.14 ± 17.39 | 83.29 ± 86.70 | 56.50 ± 31.80 | 69.95 ± 53.47 |
| HDL cholesterol (mg/dL) | 70.43 ± 12.49 | 70.43 ± 19.49 | 73.67 ± 15.13 | 71.40 ± 15.23 |
| LDL cholesterol (mg/dL) | 128.7 ± 18.63 | 101.1 ± 36.70 | 123.2 ± 17.05 | 117.4 ± 27.69 |
| Urea nitrogen (mg/dL) | 11.57 ± 2.32 | 14.01 ± 5.34 | 12.93 ± 3.38 | 12.84 ± 3.85 |
| Creatinine (mg/dL) | 0.67 ± 0.13 | 0.61 ± 0.15 | 0.59 ± 0.12 | 0.63 ± 0.13 |
| Uric acid (mg/dL) | 4.73 ± 1.55 | 4.24 ± 1.05 | 4.37 ± 0.72 | 4.45 ± 1.14 |
| Glucose (g/dL) | 81.71 ± 5.74 | 79.57 ± 5.62 | 81.50 ± 7.40 | 80.9 ± 5.98 |
| HbA1C (%) | 5.30 ± 0.26 | 5.39 ± 0.21 | 5.28 ± 0.38 | 5.33 ± 0.28 |
| Leukocytes (WBC) (µL−1) | 5666 ± 1312 | 5710 ± 1466 | 6038 ± 1663 | 5793 ± 1406 |
| Erythrocytes (RBC) × 104 (µL−1) | 465.0 ± 32.11 | 446.6 ± 27.20 | 464.7 ± 24.39 | 458.5 ± 28.21 |
| Hemoglobin (dL) | 14.10 ± 1.26 | 13.21 ± 0.38 | 13.95 ± 0.56 | 13.75 ± 0.89 |
| Hct (%) | 43.66 ± 3.60 | 40.04 ± 1.87 | 42.53 ± 1.81 | 42.06 ± 2.92 |
| MCV (fL) | 94.0 ± 5.48 | 90.00 ± 6.66 | 91.83 ± 5.15 | 91.95 ± 5.78 |
| MCH (pg) | 30.3 ± 0.1.26 | 29.70 ± 1.94 | 30.07 ± 1.87 | 30.02 ± 1.64 |
| MCHC (%) | 32.31 ± 1.10 | 33.03 ± 0.62 | 32.83 ± 0.81 | 32.72 ± 0.88 |
| Platelet 104 (µL−1) | 28.67 ± 11.09 | 24.69 ± 2.61 | 28.92 ± 10.28 | 27.35 ± 8.53 |
| Time after Cooling Stress | HEPT7G/βCD Inclusion Complex | Placebo (β-CD) | |
|---|---|---|---|
| High Dose (n = 20) | Low Dose (n = 20) | (n = 20) | |
| Min | Mean ± SD | Mean ± SD | Mean ± SD |
| 0 | 21.77 ± 17.89 | 20.79 ± 14.66 | 14.80 ± 8.67 |
| 3 | 20.90 ± 21.21 | 18.25 ± 14.45 | 17.08 ± 13.31 |
| 6 | 17.66 ± 9.71 | 19.67 ± 15.60 | 19.52 ± 14.89 |
| 9 | 19.35 ± 12.45 | 22.75 ± 18.87 | 20.53 ± 16.72 |
| 12 | 19.21 ± 12.08 | 22.43 ± 18.54 | 18.69 ± 11.96 |
| 15 | 20.12 ± 13.99 | 26.06 ± 21.90 | 18.13 ± 12.95 |
| 20 | 20.32 ± 14.33 | 28.17 ± 21.61 | 17.39 ± 11.61 |
| 30 | 22.07 ± 16.11 | 21.81 ± 18.27 | 18.41 ± 14.10 |
| Friedman Rank Test Repeated Measure (Non-Parametric) | χ2-Value: 7.75 p-Value: 0.021 * | ||
| Durbin–Conover (Post-hoc Test) † | HD vs. P | LD vs. P | HD vs. LD |
| Z-Score | 2.28 | 3.58 | 1.3 |
| p-Value | 0.039 * | 0.003 * | 0.214 |
| Time after Cooling Stress | HEPT7G/βCD Inclusion Complex | Placebo (β-CD) | |
|---|---|---|---|
| High Dose (n = 20) | Low Dose (n = 20) | (n = 20) | |
| Min | Mean ± SD | Mean ± SD | Mean ± SD |
| 0 | 19.62 ± 1.30 | 19.87 ± 1.43 | 19.80 ± 1.25 |
| 3 | 20.48 ± 1.53 | 20.79 ± 2.03 | 20.73 ± 1.53 |
| 6 | 21.18 ± 3.15 | 21.85 ± 3.99 | 21.96 ± 2.91 |
| 9 | 21.91 ± 3.56 | 22.83 ±4.94 | 22.53 ± 3.75 |
| 12 | 22.57 ± 3.92 | 23.38 ± 5.30 | 22.99 ± 3.62 |
| 15 | 23.35 ± 4.29 | 24.03 ± 5.19 | 23.96 ± 4.07 |
| 20 | 23.86 ± 4.34 | 25.65 ± 5.53 | 24.52 ± 4.17 |
| 30 | 24.42 ± 3.92 | 25.67 ± 4.99 | 25.09 ± 4.50 |
| Friedman Rank Test Repeated Measure (Non-Parametric) | χ2-Value: 15.5 p-Value: <0.001 ** | ||
| Durbin-Conover (Post-hoc Test) † | HD vs. P | LD vs. P | HD vs. LD |
| Z-Score | 12.02 | 9.9 | 21.92 |
| p-Value | <0.001 ** | <0.001 ** | <0.001 ** |
| Questionnaire Description (Degree of Coldness) | Time after Cooling Stress | HEPT7G/βCD Inclusion Complex | Placebo (β−CD) | Friedman Rank Test Repeated Measure (Non−Parametric) | † Durbin–Conover (Post−Hoc Test) | |||
|---|---|---|---|---|---|---|---|---|
| High Dose (n = 20) | Low Dose (n = 20) | (n = 20) | Z-Score; p-Value | |||||
| Min | Geo−Mean ± SD | Geo−Mean ± SD | Geo−Mean ± SD | HD vs. P | LD vs. P | HD vs. LD | ||
| 0 | 61.69 ± 28.43 | 62.83 ± 30.24 | 64.38 ± 26.86 | |||||
| Fingertip | 15 | 62.17 ± 24.81 | 56.62 ± 28.20 | 54.92 ± 27.56 | χ2-Value = 5.73 | 1.49 | 2.38 | 3.86 |
| 20 | 54.31 ± 26.15 | 51.58 ± 27.15 | 51.67 ± 32.28 | p-Value = 0.057 # | 0.088 # | 0.055 # | 0.008 * | |
| 30 | 52.33 ± 29.85 | 48.38 ± 30.34 | 47.70 ± 33.78 | |||||
| 0 | 63.85 ± 26.21 | 61.52 ± 31.31 | 60.99 ± 28.21 | |||||
| Hand | 15 | 54.95 ± 29.28 | 53.99 ± 28.47 | 41.01 ± 32.37 | χ2-Value = 0.429 | 0.00 | 0.535 | 0.505 |
| 20 | 40.23 ± 33.34 | 43.90 ± 29.68 | 44.97 ± 33.47 | p-Value = 0.807 | 1.00 | 0.505 | 0.632 | |
| 30 | 42.52 ± 31.99 | 38.18 ± 32.03 | 39.54 ± 32.63 | |||||
| 0 | 36.39 ± 25.50 | 26.77 ± 34.68 | 44.57 ± 28.93 | |||||
| Body | 15 | 36.88 ± 31.02 | 29.31 ± 32.03 | 40.07 ± 31.01 | χ2-Value = 6.50 | 3.54 | 4.95 | 1.41 |
| 20 | 33.20 ± 32.19 | 27.30 ± 31.78 | 36.26 ± 29.69 | p-Value = 0.039 * | 0.012 * | 0.003 * | 0.207 | |
| 30 | 35.70 ± 32.02 | 25.00 ± 31.74 | 33.43 ± 31.07 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kapoor, M.P.; Moriwaki, M.; Abe, A.; Morishima, S.; Ozeki, M.; Sato, N. Hesperetin-7-O-glucoside/β-cyclodextrin Inclusion Complex Induces Acute Vasodilator Effect to Inhibit the Cold Sensation Response during Localized Cold-Stimulate Stress in Healthy Human Subjects: A Randomized, Double-Blind, Crossover, and Placebo-Controlled Study. Nutrients 2023, 15, 3702. https://doi.org/10.3390/nu15173702
Kapoor MP, Moriwaki M, Abe A, Morishima S, Ozeki M, Sato N. Hesperetin-7-O-glucoside/β-cyclodextrin Inclusion Complex Induces Acute Vasodilator Effect to Inhibit the Cold Sensation Response during Localized Cold-Stimulate Stress in Healthy Human Subjects: A Randomized, Double-Blind, Crossover, and Placebo-Controlled Study. Nutrients. 2023; 15(17):3702. https://doi.org/10.3390/nu15173702
Chicago/Turabian StyleKapoor, Mahendra P., Masamitsu Moriwaki, Aya Abe, So Morishima, Makoto Ozeki, and Norio Sato. 2023. "Hesperetin-7-O-glucoside/β-cyclodextrin Inclusion Complex Induces Acute Vasodilator Effect to Inhibit the Cold Sensation Response during Localized Cold-Stimulate Stress in Healthy Human Subjects: A Randomized, Double-Blind, Crossover, and Placebo-Controlled Study" Nutrients 15, no. 17: 3702. https://doi.org/10.3390/nu15173702





