Associations of Adherence to the 2018 World Cancer Research Fund and the American Institute for Cancer Research Dietary Recommendations with Gut Microbiota and Inflammation Levels
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. World Cancer Research Fund/American Institute for Cancer Research Diet Adherence Score
2.3. Collection of Fecal and Blood Samples
2.4. DNA Extraction
2.5. 16S rDNA Amplicon Pyrosequencing
2.6. Sequence Analysis
2.7. Bioinformatics Analysis
2.8. Assessment of Inflammatory Biomarkers
3. Statistical Analysis
4. Results
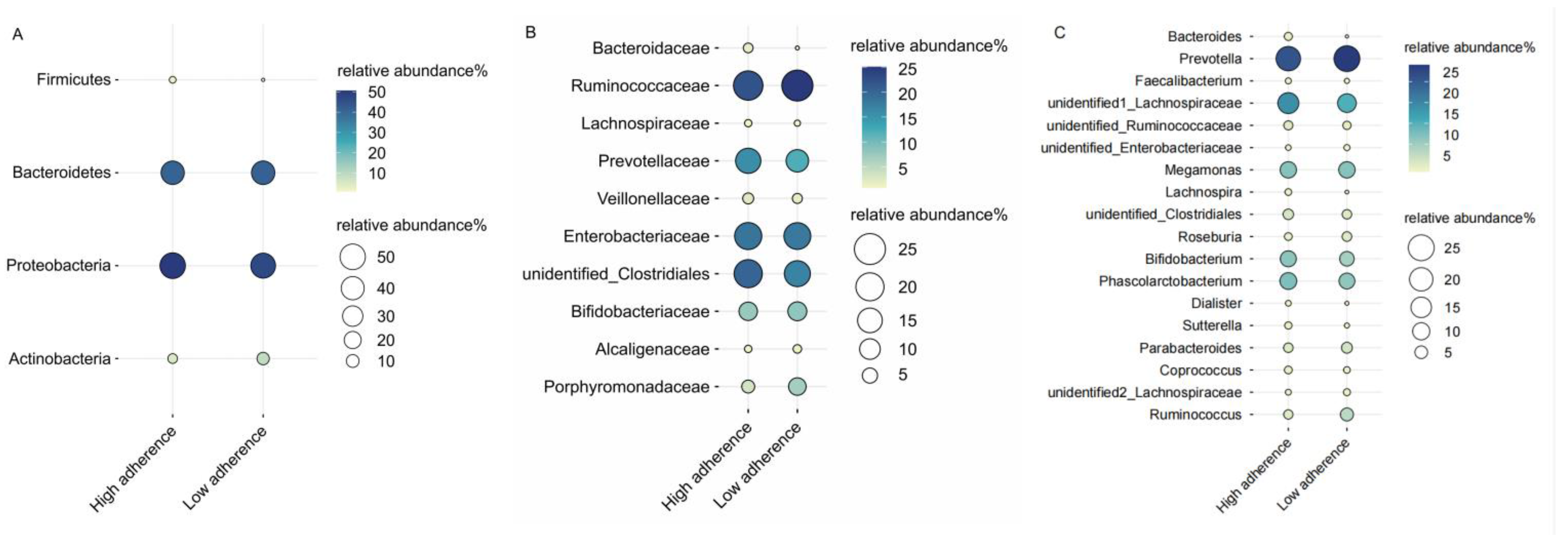
4.1. WCRF/AICR Dietary Adherence and Gut Microbiota
4.2. WCRF/AICR Dietary Adherence and Inflammation
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Conteduca, V.; Sansonno, D.; Russi, S.; Dammacco, F. Precancerous colorectal lesions (Review). Int. J. Oncol. 2013, 43, 973–984. [Google Scholar] [CrossRef] [PubMed]
- Keum, N.; Giovannucci, E. Global burden of colorectal cancer: Emerging trends, risk factors and prevention strategies. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 713–732. [Google Scholar] [CrossRef] [PubMed]
- De Almeida, C.V.; de Camargo, M.R.; Russo, E.; Amedei, A. Role of diet and gut microbiota on colorectal cancer immunomodulation. World J. Gastroenterol. 2019, 25, 151–162. [Google Scholar] [CrossRef] [PubMed]
- García-Montero, C.; Fraile-Martínez, O.; Gómez-Lahoz, A.M.; Pekarek, L.; Castellanos, A.J.; Noguerales-Fraguas, F.; Coca, S.; Guijarro, L.G.; García-Honduvilla, N.; Asúnsolo, A.; et al. Nutritional Components in Western Diet Versus Mediterranean Diet at the Gut Microbiota–Immune System Interplay. Implications for Health and Disease. Nutrients 2021, 13, 699. [Google Scholar] [CrossRef]
- Schröder, L.; Kaiser, S.; Flemer, B.; Hamm, J.; Hinrichsen, F.; Bordoni, D.; Rosenstiel, P.; Sommer, F. Nutritional Targeting of the Microbiome as Potential Therapy for Malnutrition and Chronic Inflammation. Nutrients 2020, 12, 3032. [Google Scholar] [CrossRef]
- World Cancer Research Fund/American Institute for Cancer Research. Diet, Nutrition, Physical Activity and Cancer: A Global Perspective. Available online: http://dietandcancerreport.org (accessed on 11 April 2023).
- Kenkhuis, M.F.; Mols, F.; van Roekel, E.H.; Breedveld-Peters, J.J.L.; Breukink, S.O.; Janssen-Heijnen, M.L.G.; Keulen, E.T.P.; van Duijnhoven, F.J.B.; Weijenberg, M.P.; Bours, M.J.L. Longitudinal Associations of Adherence to the World Cancer Research Fund/American Institute for Cancer Research (WCRF/AICR) Lifestyle Recommendations with Quality of Life and Symptoms in Colorectal Cancer Survivors up to 24 Months Post-Treatment. Cancers 2022, 14, 417. [Google Scholar] [CrossRef]
- Kenkhuis, M.-F.; van der Linden, B.W.A.; Breedveld-Peters, J.J.L.; Koole, J.L.; van Roekel, E.H.; Breukink, S.O.; Mols, F.; Weijenberg, M.P.; Bours, M.J.L. Associations of the dietary World Cancer Research Fund/American Institute for Cancer Research (WCRF/AICR) recommendations with patient-reported outcomes in colorectal cancer survivors 2–10 years post-diagnosis: A cross-sectional analysis. Br. J. Nutr. 2021, 125, 1188–1200. [Google Scholar] [CrossRef]
- Schroeder, J.; Reitz, L.K.; Vieira, F.G.K.; da Silva, E.L.; Di Pietro, P.F. Low to moderate adherence to 2018 diet and physical exercise recommendations of the World Cancer Research Fund/American Institute for Cancer Research is associated with prooxidant biochemical profile in women undergoing adjuvant breast cancer treatment. Nutr. Res. 2023, 109, 1–11. [Google Scholar] [CrossRef]
- Song, R.; Petimar, J.; Wang, M.; Tabung, F.K.; Song, M.; Liu, L.; Lee, D.H.; Giovannucci, E.L.; Zhang, X.; Smith-Warner, S.A. Adherence to the World Cancer Research Fund/American Institute for Cancer Research Cancer Prevention Recommendations and Colorectal Cancer Survival. Cancer Epidemiol. Biomark. Prev. 2021, 30, 1816–1825. [Google Scholar] [CrossRef]
- Holthuijsen, D.D.B.; Bours, M.J.L.; Roekel, E.H.V.; Breukink, S.O.; Janssen-Heijnen, M.L.G.; Keulen, E.T.P.; Ueland, P.M.; Midttun, Ø.; Brezina, S.; Gigic, B.; et al. Longitudinal Associations of Adherence to the Dietary World Cancer Research Fund/American Institute for Cancer Research (WCRF/AICR) and Dutch Healthy Diet (DHD) Recommendations with Plasma Kynurenines in Colorectal Cancer Survivors after Treatment. Nutrients 2022, 14, 5151. [Google Scholar] [CrossRef]
- Quagliariello, V.; D’Aiuto, G.; Iaffaioli, R.V.; Berretta, M.; Buccolo, S.; Iovine, M.; Paccone, A.; Cerrone, F.; Bonanno, S.; Nunnari, G.; et al. Reasons why COVID-19 survivors should follow dietary World Cancer Research Fund/American Institute for Cancer Research (WCRF/AICR) recommendations: From hyper-inflammation to cardiac dysfunctions. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 3898–3907. [Google Scholar] [CrossRef]
- Morimoto, Y.; Beckford, F.; Cooney, R.V.; Franke, A.A.; Maskarinec, G. Adherence to cancer prevention recommendations and antioxidant and inflammatory status in premenopausal women. Br. J. Nutr. 2015, 114, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Tabung, F.K.; Fung, T.T.; Chavarro, J.E.; Smith-Warner, S.A.; Willett, W.C.; Giovannucci, E.L. Associations between adherence to the World Cancer Research Fund/American Institute for Cancer Research cancer prevention recommendations and biomarkers of inflammation, hormonal, and insulin response. Int. J. Cancer 2017, 140, 764–776. [Google Scholar] [CrossRef] [PubMed]
- Laura, A.B.; Vich, V.A.; Floris, I.; Valerie, C.; Ranko, G.; Vera, P.; Cisca, W.; Alexander, K.; Marjo, J.E.C.-K.; Jingyuan, F.; et al. Long-term dietary patterns are associated with pro-inflammatory and anti-inflammatory features of the gut microbiome. Gut 2021, 70, 1287. [Google Scholar] [CrossRef]
- Magklis, E.; Howe, L.D.; Johnson, L. Eating Style and the Frequency, Size and Timing of Eating Occasions: A cross-sectional analysis using 7-day weighed dietary records. Sci. Rep. 2019, 9, 15133. [Google Scholar] [CrossRef]
- Ostan, R.; Guidarelli, G.; Giampieri, E.; Lanzarini, C.; Berendsen, A.A.M.; Januszko, O.; Jennings, A.; Lyon, N.; Caumon, E.; Gillings, R.; et al. Cross-Sectional Analysis of the Correlation between Daily Nutrient Intake Assessed by 7-Day Food Records and Biomarkers of Dietary Intake among Participants of the NU-AGE Study. Front. Physiol. 2018, 9, 1359. [Google Scholar] [CrossRef]
- Vitale, M.; Bruno, V.; D’Abbronzo, G.; Rivellese, A.A.; Bozzetto, L.; Scidà, G.; Annuzzi, G. Evaluation of eating habits by 7-day food record: Web-PC vs. traditional paper format. Int. J. Food Sci. Nutr. 2023, 74, 580–587. [Google Scholar] [CrossRef]
- Esposito, G.; Turati, F.; Serraino, D.; Crispo, A.; Negri, E.; Parazzini, F.; La Vecchia, C. Adherence to the World Cancer Research Fund/American Institute for Cancer Research recommendations and endometrial cancer risk: A multicentric case-control study. Br. J. Nutr. 2023, 129, 2133–2141. [Google Scholar] [CrossRef]
- Shams-White, M.M.; Brockton, N.T.; Mitrou, P.; Romaguera, D.; Brown, S.; Bender, A.; Kahle, L.L.; Reedy, J. Operationalizing the 2018 World Cancer Research Fund/American Institute for Cancer Research (WCRF/AICR) Cancer Prevention Recommendations: A Standardized Scoring System. Nutrients 2019, 11, 1572. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Pena, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Jiang, W. Application of high-throughput sequencing in understanding human oral microbiome related with health and disease. Front. Microbiol. 2014, 5, 508. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.R.; Pop, M.; DeBoy, R.T.; Eckburg, P.B.; Turnbaugh, P.J.; Samuel, B.S.; Gordon, J.I.; Relman, D.A.; Fraser-Liggett, C.M.; Nelson, K.E. Metagenomic analysis of the human distal gut microbiome. Science 2006, 312, 1355–1359. [Google Scholar] [CrossRef] [PubMed]
- Rognes, T.; Flouri, T.; Nichols, B.; Quince, C.; Mahe, F. VSEARCH: A versatile open source tool for metagenomics. PeerJ 2016, 4, e2584. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Cheng, Y.; Shao, L.; Ling, Z. Alterations of the Predominant Fecal Microbiota and Disruption of the Gut Mucosal Barrier in Patients with Early-Stage Colorectal Cancer. BioMed Res. Int. 2020, 2020, 2948282. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhao, H.; Wu, D.; Cao, D.; Ma, W. A comprehensive analysis of the microbiota composition and gene expression in colorectal cancer. BMC Microbiol. 2020, 20, 308. [Google Scholar] [CrossRef]
- Baldelli, V.; Scaldaferri, F.; Putignani, L.; Del Chierico, F. The Role of Enterobacteriaceae in Gut Microbiota Dysbiosis in Inflammatory Bowel Diseases. Microorganisms 2021, 9, 697. [Google Scholar] [CrossRef]
- Lupp, C.; Robertson, M.L.; Wickham, M.E.; Sekirov, I.; Champion, O.L.; Gaynor, E.C.; Finlay, B.B. Host-Mediated Inflammation Disrupts the Intestinal Microbiota and Promotes the Overgrowth of Enterobacteriaceae. Cell Host Microbe 2007, 2, 204. [Google Scholar] [CrossRef]
- Martinez-Medina, M.; Aldeguer, X.; Gonzalez-Huix, F.; Acero, D.; Garcia-Gil, L.J. Abnormal microbiota composition in the ileocolonic mucosa of Crohn’s disease patients as revealed by polymerase chain reaction-denaturing gradient gel electrophoresis. Inflamm. Bowel Dis. 2006, 12, 1136–1145. [Google Scholar] [CrossRef]
- Ridlon, J.M.; Harris, S.C.; Bhowmik, S.; Kang, D.-J.; Hylemon, P.B. Consequences of bile salt biotransformations by intestinal bacteria. Gut Microbes 2016, 7, 22–39. [Google Scholar] [CrossRef]
- Ridlon, J.M.; Alves, J.M.; Hylemon, P.B.; Bajaj, J.S. Cirrhosis, bile acids and gut microbiota. Gut Microbes 2013, 4, 382–387. [Google Scholar] [CrossRef] [PubMed]
- Vega, L.; Bohórquez, L.; Ramírez, J.D.; Muñoz, M. Do we need to change our perspective about gut biomarkers? A public data mining approach to identify differentially abundant bacteria in intestinal inflammatory diseases. Front. Cell. Infect. Microbiol. 2022, 12, 918237. [Google Scholar] [CrossRef] [PubMed]
- Hooda, S.; Boler, B.M.V.; Serao, M.C.R.; Brulc, J.M.; Staeger, M.A.; Boileau, T.W.; Dowd, S.E.; Fahey, G.C.; Swanson, K.S. 454 Pyrosequencing Reveals a Shift in Fecal Microbiota of Healthy Adult Men Consuming Polydextrose or Soluble Corn Fiber. J. Nutr. 2012, 142, 1259–1265. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.T.T.; Cousin, F.J.; Lynch, D.B.; Menon, R.; Brulc, J.; Brown, J.R.M.; O’Herlihy, E.; Butto, L.F.; Power, K.; Jeffery, I.B.; et al. Prebiotic supplementation in frail older people affects specific gut microbiota taxa but not global diversity. Microbiome 2019, 7, 39. [Google Scholar] [CrossRef]
- Gan, L.; Zhao, Y.; Mahmood, T.; Guo, Y. Effects of dietary vitamins supplementation level on the production performance and intestinal microbiota of aged laying hens. Poult. Sci. 2020, 99, 3594–3605. [Google Scholar] [CrossRef]
- Amabebe, E.; Robert, F.O.; Agbalalah, T.; Orubu, E.S.F. Microbial dysbiosis-induced obesity: Role of gut microbiota in homoeostasis of energy metabolism. Br. J. Nutr. 2020, 123, 1127–1137. [Google Scholar] [CrossRef]
- Lane, M.; Howland, G.; West, M.; Hockey, M.; Marx, W.; Loughman, A.; O’Hely, M.; Jacka, F.; Rocks, T. The effect of ultra-processed very low-energy diets on gut microbiota and metabolic outcomes in individuals with obesity: A systematic literature review. Obes. Res. Clin. Pract. 2020, 14, 197–204. [Google Scholar] [CrossRef]
- Cuevas-Sierra, A.; Milagro, F.I.; Aranaz, P.; Martinez, J.A.; Riezu-Boj, J.I. Gut Microbiota Differences According to Ultra-Processed Food Consumption in a Spanish Population. Nutrients 2021, 13, 2710. [Google Scholar] [CrossRef]
- Beilharz, J.E.; Kaakoush, N.O.; Maniam, J.; Morris, M.J. The effect of short-term exposure to energy-matched diets enriched in fat or sugar on memory, gut microbiota and markers of brain inflammation and plasticity. Brain Behav. Immun. 2016, 57, 304–313. [Google Scholar] [CrossRef]
- Hexun, Z.; Miyake, T.; Maekawa, T.; Mori, H.; Yasukawa, D.; Ohno, M.; Nishida, A.; Andoh, A.; Tani, M. High abundance of Lachnospiraceae in the human gut microbiome is related to high immunoscores in advanced colorectal cancer. Cancer Immunol. Immunother. 2023, 72, 315–326. [Google Scholar] [CrossRef]
- Abdugheni, R.; Wang, W.-Z.; Wang, Y.-J.; Du, M.-X.; Liu, F.-L.; Zhou, N.; Jiang, C.-Y.; Wang, C.-Y.; Wu, L.; Ma, J.; et al. Metabolite profiling of human-originated Lachnospiraceae at the strain level. iMeta 2022, 1, e58. [Google Scholar] [CrossRef]
- Niemela, O.; Aalto, M.; Bloigu, A.; Bloigu, R.; Halkola, A.S.; Laatikainen, T. Alcohol Drinking Patterns and Laboratory Indices of Health: Does Type of Alcohol Preferred Make a Difference? Nutrients 2022, 14, 4529. [Google Scholar] [CrossRef]
- Orio, L.A.-O.; Antón, M.; Rodríguez-Rojo, I.C.; Correas, Á.; García-Bueno, B.; Corral, M.; de Fonseca, F.R.; García-Moreno, L.M.; Maestú, F.; Cadaveira, F. Young alcohol binge drinkers have elevated blood endotoxin, peripheral inflammation and low cortisol levels: Neuropsychological correlations in women. Addict. Biol. 2018, 23, 1130–1144. [Google Scholar] [CrossRef] [PubMed]
- Pascual, M.; Montesinos, J.; Marcos, M.; Torres, J.-L.; Costa-Alba, P.; García-García, F.; Laso, F.-J.; Guerri, C. Gender differences in the inflammatory cytokine and chemokine profiles induced by binge ethanol drinking in adolescence. Addict. Biol. 2017, 22, 1829–1841. [Google Scholar] [CrossRef] [PubMed]
- Boughanem, H.; Ruiz-Limon, P.; Crujeiras, A.B.; de Luque, V.; Tinahones, F.J.; Macias-Gonzalez, M. 25-Hydroxyvitamin D status is associated with interleukin-6 methylation in adipose tissue from patients with colorectal cancer. Food Funct. 2021, 12, 9620–9631. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, L.; Nielsen, H.J.; Christensen, I.J. Evaluation of a 92 multiplex protein panel in detection of colorectal cancer and high-risk adenoma in 784 symptomatic individuals. Cancer Biomark. 2021, 32, 73–84. [Google Scholar] [CrossRef]
- Hillmer, A.T.; Nadim, H.; Devine, L.; Jatlow, P.; O’Malley, S.S. Acute alcohol consumption alters the peripheral cytokines IL-8 and TNF-α. Alcohol 2020, 85, 95–99. [Google Scholar] [CrossRef]
- Tan, R.; Dong, H.; Chen, Z.; Jin, M.; Yin, J.; Li, H.; Shi, D.; Shao, Y.; Wang, H.; Chen, T.; et al. Intestinal Microbiota Mediates High-Fructose and High-Fat Diets to Induce Chronic Intestinal Inflammation. Front. Cell. Infect. Microbiol. 2021, 11, 654074. [Google Scholar] [CrossRef]
- Rahman, I.; Biswas, S.K.; Kirkham, P.A. Regulation of inflammation and redox signaling by dietary polyphenols. Biochem. Pharmacol. 2006, 72, 1439–1452. [Google Scholar] [CrossRef]
- Yahfoufi, N.; Alsadi, N.; Jambi, M.; Matar, C. The Immunomodulatory and Anti-Inflammatory Role of Polyphenols. Nutrients 2018, 10, 1618. [Google Scholar] [CrossRef]
- Nestares, T.; Martin-Masot, R.; Flor-Alemany, M.; Bonavita, A.; Maldonado, J.; Aparicio, V.A. Influence of Ultra-Processed Foods Consumption on Redox Status and Inflammatory Signaling in Young Celiac Patients. Nutrients 2021, 13, 156. [Google Scholar] [CrossRef] [PubMed]
- Valentina, L.G.; Ana, C.; Tamara, Š.; Niall, J.D.; Domagoj, K.; Helena, D.; Ana, C.; Frano, V.; Mario, F.; Richard, S.G.; et al. Extensive weight loss can reduce immune age by altering IgG N-glycosylation. medRxiv 2020. [Google Scholar] [CrossRef]
- Ortega, R.M.; Perez-Rodrigo, C.; Lopez-Sobaler, A.M. Dietary assessment methods: Dietary records. Nutr. Hosp. 2015, 31, 38–45. [Google Scholar] [CrossRef] [PubMed]
| WCRF/AICR Diet Score in Categories | p | ||
|---|---|---|---|
| Low Adherence (0 to <3 Points) | High Adherence (3 to <5 Points) | ||
| N | 41 | 110 | |
| Age (years) | 58.49 ± 7.53 | 62.39 ± 6.73 | 0.003 |
| Sex (%) | 0.001 | ||
| Men | 31(75.6) | 50(45.4) | |
| Women | 10(24.4) | 60(54.6) | |
| BMI (kg/m2) | 24.34 ± 3.06 | 24.00 ± 3.02 | 0.537 |
| BMI (%) | 0.858 | ||
| Underweight (<18.5 kg/m2) | 1(2.4) | 5(4.6) | |
| Normal weight (18.5–24.9 kg/m2) | 24(58.5) | 63(57.3) | |
| Overweight (25–29.9 kg/m2) | 14(34.2) | 39(35.5) | |
| Obese (≥30 kg/m2) | 2(4.9) | 3(2.7) | |
| Smoking status (%) | 0.002 | ||
| Never | 23(56.1) | 85(77.3) | |
| Current | 14(34.2) | 11(10.0) | |
| Former | 4(9.8) | 14(12.7) | |
| Drinking status (%) | <0.0001 | ||
| Never | 13(31.7) | 79(71.8) | |
| Current | 20(48.8) | 20(18.2) | |
| Former | 8(19.5) | 11(10.0) | |
| Moderate-to-vigorous physical activity (min/week) (%) | 0.113 | ||
| <150 | 32(78.0) | 71(64.6) | |
| 1150 | 9(22.0) | 39(35.4) | |
| Comorbidities (%) * | 0.002 | ||
| 0 | 15(36.6) | 19(17.3) | |
| 1 | 4(9.7) | 39(35.4) | |
| 92 | 22(53.7) | 52(47.3) | |
| Yogurt consumption (%) | 0.948 | ||
| Yes | 21(51.2) | 57(51.8) | |
| No | 20(48.8) | 53(48.2) | |
| Long-term use of anti-inflammatory drugs (%) | 0.267 | ||
| Yes | 24(58.5) | 75(68.2) | |
| No | 17(41.5) | 35(31.8) | |
| Adenoma (%) | 0.372 | ||
| Yes | 24(58.5) | 73(66.4) | |
| No | 17(41.5) | 37(33.6) | |
| 2018 WCRF/AICR Recommendations a | Operationalization/Comments | Score | Adherence | |
|---|---|---|---|---|
| n | % | |||
| 1. Eat a diet rich in whole grains, vegetables, and fruit | Fruits and vegetables (g/day) | |||
| ≥400 | 0.5 | 75 | 49.7 | |
| 200 to <400 | 0.25 | 42 | 27.8 | |
| <200 | 0 | 34 | 22.5 | |
| Total fiber (g/day) | ||||
| ≥30 | 0.5 | 4 | 2.6 | |
| 15 to <30 | 0.25 | 28 | 18.5 | |
| <15 | 0 | 119 | 78.8 | |
| 2. Limit consumption of ‘fast foods’ and other processed foods high in fat, starches, or sugars | Percentage of total energy from adapted ultra-processed foods | |||
| Tertile 1 | 1 | 114 | 75.5 | |
| Tertile 2 | 0.5 | 30 | 19.9 | |
| Tertile 3 | 0 | 7 | 4.6 | |
| 3. Limit consumption of red and processed meat | Total red meat (g/week) and processed meat (g/week) | |||
| Red meat <500 and processed meat <21 | 1 | 68 | 45.0 | |
| Red meat <500 and processed meat 21 to <100 | 0.5 | 30 | 19.9 | |
| Red meat ≥500 or processed meat ≥100 | 0 | 53 | 35.1 | |
| 4. Limit consumption of sugar-sweetened drinks | Total sugar-sweetened drinks (g/day) | |||
| 0 | 1 | 142 | 94.0 | |
| 0 to ≤250 | 0.5 | 9 | 6.0 | |
| >250 | 0 | 0 | 0.0 | |
| 5. Limit alcohol consumption | Alcoholic drinks (n/week) | |||
| 0 | 1 | 92 | 60.9 | |
| 0 to ≤7 | 0.5 | 57 | 37.8 | |
| >7 | 0 | 2 | 1.3 | |
| Levels | Taxa | WCRF/AICR Diet Score | Continuous WCRF/AICR Diet Score | |
|---|---|---|---|---|
| Low Adherence (0 to <3 Points, n = 41) | High Adherence (3 to <5 Points, n = 110) | |||
| Phylum | Firmicutes | reference | 0.012(−0.054, 0.078) | 0.011(−0.034, 0.055) |
| Bacteroidetes | reference | 0.005(−0.064, 0.073) | −0.005(−0.051, 0.041) | |
| Proteobacteria | reference | −0.041(−0.073, −0.009) | −0.009(−0.031, 0.013) | |
| Actinobacteria | reference | 0.019(−0.007, 0.045) | 0.001(−0.017, 0.019) | |
| Family | Bacteroidaceae | reference | −0.013(−0.086, 0.061) | 0.004(−0.046, 0.053) |
| Ruminococcaceae | reference | 0.007(−0.041, 0.054) | 0.009(−0.023, 0.041) | |
| Lachnospiraceae | reference | 0.005(−0.036, 0.046) | 0.006(−0.022, 0.033) | |
| Prevotellaceae | reference | 0.031(−0.066, 0.129) | 0.006(−0.060, 0.071) | |
| Veillonellaceae | reference | 0.007(−0.024, 0.038) | −0.001(−0.021, 0.020) | |
| Enterobacteriaceae | reference | −0.035(−0.067, −0.003) | −0.006(−0.028, 0.016) | |
| unidentified_Clostridiales | reference | −0.001(−0.011, 0.009) | −0.001(−0.007, 0.006) | |
| Bifidobacteriaceae | reference | 0.016(−0.007, 0.039) | −0.001(−0.016, 0.015) | |
| Alcaligenaceae | reference | −0.002(−0.008, 0.004) | −0.001(−0.004, 0.004) | |
| Porphyromonadaceae | reference | 0.006(−0.002, 0.015) | 0.001(−0.005, 0.006) | |
| Genus | Bacteroides | reference | −0.013(−0.086, 0.061) | 0.004(−0.046, 0.053) |
| Prevotella | reference | 0.031(−0.066, 0.129) | 0.006(−0.060, 0.071) | |
| Faecalibacterium | reference | 0.003(−0.026, 0.031) | 0.009(−0.010, 0.029) | |
| unidentified_Lachnospiraceae | reference | −0.002(−0.026, 0.022) | 0.005(−0.011, 0.021) | |
| unidentified_Ruminococcaceae | reference | 0.004(−0.020, 0.029) | 0.003(−0.013, 0.020) | |
| unidentified_Enterobacteriaceae | reference | −0.029(−0.055, −0.003) | −0.006(−0.024, 0.012) | |
| Megamonas | reference | −0.012(−0.040, 0.015) | −0.010(−0.028, 0.009) | |
| Lachnospira | reference | 0.010(−0.004, 0.023) | 0.003(−0.007, 0.012) | |
| unidentified_Clostridiales | reference | −0.001(−0.011, 0.009) | −0.001(−0.007, 0.006) | |
| Roseburia | reference | −0.009(−0.020, 0.002) | −0.005(−0.012, 0.003) | |
| Bifidobacterium | reference | 0.016(−0.007, 0.039) | −0.001(−0.016, 0.015) | |
| Phascolarctobacterium | reference | 0.003(−0.026, 0.031) | 0.009(−0.010, 0.029) | |
| Dialister | reference | 0.001(−0.006, 0.007) | −0.001(−0.006, 0.003) | |
| Sutterella | reference | 0.009(−0.003, 0.020) | 0.002(−0.006, 0.010) | |
| Parabacteroides | reference | 0.008(−0.001, 0.017) | 0.004(−0.002, 0.010) | |
| Coprococcus | reference | 0.005(−0.002, 0.013) | 0.001(−0.004, 0.007) | |
| unidentified_Lachnospiraceae | reference | −0.002(−0.007, 0.002) | −0.001(−0.003, 0.002) | |
| Ruminococcus | reference | 0.001(−0.006, 0.007) | −0.001(−0.006, 0.003) | |
| Levels | Taxa | WCRF/AICR Diet Score | ||||
|---|---|---|---|---|---|---|
| R1-Vegetables, Fruits, and Whole Grains Intake | R2-Limit Fast Foods | R3-Limit Red and Processed Meat | R4-Limit Sugary Drinks | R5-Limit Alcohol | ||
| Phylum | Firmicutes | 0.012(−0.084, 0.109) | 0.033(−0.066, 0.133) | 0.002(−0.059, 0.062) | −0.168(−0.391, 0.055) | 0.053(−0.068, 0.173) |
| Bacteroidetes | 0.019(−0.081, 0.119) | −0.003(−0.107, 0.100) | 0.002(−0.061, 0.065) | 0.133(−0.099, 0.365) | −0.108(−0.232, 0.015) | |
| Proteobacteria | −0.042(−0.089, 0.006) | −0.031(−0.080, 0.019) | 0.009(−0.021, 0.039) | 0.011(−0.101, 0.122) | 0.009(−0.051, 0.069) | |
| Actinobacteria | 0.005(−0.034, 0.043) | −0.006(−0.045, 0.034) | −0.010(−0.034, 0.014) | 0.004(−0.086, 0.094) | 0.046(−0.002, 0.093) | |
| Family | Bacteroidaceae | −0.070(−0.176, 0.037) | 0.149(0.040, 0.257) | −0.027(−0.095, 0.040) | 0.019(−0.232, 0.269) | 0.021(−0.114, 0.155) |
| Ruminococcaceae | 0.033(−0.036, 0.103) | 0.008(−0.064, 0.080) | 0.000(−0.044, 0.044) | −0.057(−0.219, 0.105) | 0.022(−0.065, 0.109) | |
| Lachnospiraceae | −0.016(−0.076, 0.044) | 0.021(−0.041, 0.083) | 0.005(−0.033, 0.042) | −0.155(−0.292, −0.018) | 0.065(−0.010, 0.139) | |
| Prevotellaceae | 0.088(−0.054, 0.229) | −0.123(−0.269, 0.023) | 0.043(−0.047, 0.132) | 0.086(−0.246, 0.419) | −0.110(−0.288, 0.068) | |
| Veillonellaceae | −0.005(−0.050, 0.040) | 0.015(−0.032, 0.062) | −0.001(−0.030, 0.027) | 0.052(−0.053, 0.157) | −0.026(−0.083, 0.030) | |
| Enterobacteriaceae | −0.037(−0.085, 0.010) | −0.032(−0.081, 0.017) | 0.011(−0.019, 0.041) | 0.028(−0.083, 0.140) | 0.011(−0.049, 0.071) | |
| unidentified_Clostridiales | 0.007(−0.007, 0.022) | −0.006(−0.021, 0.009) | −0.001(−0.010, 0.008) | −0.011(−0.045, 0.023) | 0.004(−0.015, 0.022) | |
| Bifidobacteriaceae | 0.005(−0.029, 0.038) | −0.008(−0.042, 0.027) | −0.009(−0.030, 0.012) | 0.003(−0.075, 0.081) | 0.034(−0.007, 0.076) | |
| Alcaligenaceae | −0.004(−0.012, 0.005) | 0.004(−0.005, 0.013) | 0.000(−0.005, 0.006) | −0.007(−0.027, 0.013) | 0.000(−0.011, 0.011) | |
| Porphyromonadaceae | −0.001(−0.013, 0.012) | −0.002(−0.016, 0.011) | −0.002(−0.010, 0.006) | 0.010(−0.020, 0.039) | 0.010(−0.006, 0.026) | |
| Genus | Bacteroides | −0.070(−0.176, 0.037) | 0.149(0.040, 0.257) | −0.027(−0.095, 0.040) | 0.019(−0.232, 0.269) | 0.021(−0.114, 0.155) |
| Prevotella | 0.088(−0.054, 0.229) | −0.123(−0.269, 0.023) | 0.043(−0.047, 0.132) | 0.086(−0.246, 0.419) | −0.110(−0.288, 0.068) | |
| Faecalibacterium | 0.031(−0.010, 0.073) | 0.019(−0.024, 0.062) | 0.003(−0.023, 0.029) | −0.052(−0.149, 0.045) | −0.004(−0.056, 0.048) | |
| unidentified_Lachnospiraceae | −0.014(−0.048, 0.021) | 0.021(−0.015, 0.056) | 0.006(−0.015, 0.028) | −0.079(−0.159, 0.001) | 0.025(−0.018, 0.068) | |
| unidentified_Ruminococcaceae | −0.002(−0.037, 0.034) | −0.009(−0.046, 0.028) | 0.005(−0.017, 0.028) | −0.011(−0.094, 0.073) | 0.023(−0.022, 0.067) | |
| unidentified_Enterobacteriaceae | −0.036(−0.074, 0.003) | −0.022(−0.062, 0.018) | 0.008(−0.016, 0.032) | 0.017(−0.074, 0.108) | 0.009(−0.040, 0.058) | |
| Megamonas | −0.002(−0.042, 0.038) | −0.015(−0.057, 0.026) | −0.003(−0.029, 0.022) | 0.033(−0.060, 0.127) | −0.044(−0.094, 0.005) | |
| Lachnospira | 0.004(−0.017, 0.024) | 0.004(−0.017, 0.025) | 0.002(−0.011, 0.015) | −0.024(−0.071, 0.023) | 0.008(−0.017, 0.033) | |
| unidentified_Clostridiales | 0.007(−0.007, 0.022) | −0.006(−0.021, 0.009) | −0.001(−0.010, 0.008) | −0.011(−0.045, 0.023) | 0.004(−0.015, 0.022) | |
| Roseburia | −0.015(−0.031, 0.001) | −0.005(−0.022, 0.011) | −0.004(−0.014, 0.007) | −0.029(−0.066, 0.009) | 0.019(−0.001, 0.039) | |
| Bifidobacterium | 0.005(−0.029, 0.038) | −0.008(−0.042, 0.027) | −0.009(−0.030, 0.012) | 0.003(−0.075, 0.081) | 0.034(−0.007, 0.076) | |
| Phascolarctobacterium | 0.013(0.001, 0.026) | 0.012(−0.001, 0.026) | 0.001(−0.007, 0.009) | −0.001(−0.031, 0.030) | −0.011(−0.027, 0.005) | |
| Dialister | 0.002(−0.015, 0.019) | 0.004(−0.014, 0.021) | 0.000(−0.011, 0.011) | 0.010(−0.029, 0.050) | 0.004(−0.017, 0.025) | |
| Sutterella | −0.004(−0.012, 0.005) | 0.005(−0.004, 0.014) | 0.000(−0.005, 0.006) | −0.008(−0.029, 0.012) | 0.000(−0.011, 0.011) | |
| Parabacteroides | −0.001(−0.013, 0.012) | −0.002(−0.016, 0.011) | −0.002(−0.010, 0.006) | 0.010(−0.020, 0.039) | 0.010(−0.006, 0.026) | |
| Coprococcus | 0.009(−0.002, 0.020) | 0.003(−0.009, 0.014) | −0.003(−0.010, 0.004) | −0.010(−0.036, 0.015) | 0.008(−0.006, 0.021) | |
| unidentified_Lachnospiraceae | −0.002(−0.008, 0.004) | 0.001(−0.005, 0.006) | 0.001(−0.003, 0.004) | −0.009(−0.023, 0.005) | 0.003(−0.005, 0.010) | |
| Ruminococcus | 0.003(−0.006, 0.013) | 0.001(−0.008, 0.011) | −0.006(−0.012, 0.000) | 0.004(−0.018, 0.026) | 0.004(−0.008, 0.016) | |
| WCRF/AICR Diet Score | Continuous WCRF/AICR Diet Score | |||
|---|---|---|---|---|
| Biomarkers | Low Adherence (0 to <3 Points, n = 24) | High Adherence (3 to <5 Points, n = 73) | ||
| IL-6 (pg/mL) | Total (n = 97) | reference | 0.143(−0.333, 0.619) | 0.140(−0.174, 0.454) |
| Men (n = 58) | reference | 0.167(−0.270, 0.603) | 0.071(−0.254, 0.396) | |
| Women (n = 39) | reference | 0.259(−1.137, 1.654) | 0.193(−0.564, 0.950) | |
| IL-8 (pg/mL) | Total (n = 97) | reference | −0.062(−0.867, 0.743) | −0.097(−0.629, 0.435) |
| Men (n = 58) | reference | −0.326(−1.220, 0.568) | −0.162(−0.827, 0.503) | |
| Women (n = 39) | reference | 0.458(−1.602, 2.518) | −0.110(−1.234, 1.013) | |
| IgA (g/L) | Total (n = 97) | reference | −0.111(−0.299, 0.078) | −0.024(−0.150, 0.102) |
| Men (n = 58) | reference | −0.102(−0.344, 0.139) | −0.022(−0.202, 0.159) | |
| Women (n = 39) | reference | −0.188(−0.597, 0.220) | −0.035(−0.261, 0.190) | |
| IgG (g/L) | Total (n = 97) | reference | −0.188(−0.597, 0.220) | −0.021(−0.081, 0.040) |
| Men (n = 58) | reference | −0.052(−0.159, 0.054) | −0.047(−0.126, 0.032) | |
| Women (n = 39) | reference | −0.052(−0.223, 0.120) | −0.010(−0.104, 0.084) | |
| IgM (g/L) | Total (n = 97) | reference | −0.039(−0.288, 0.211) | −0.038(−0.203, 0.127) |
| Men (n = 58) | reference | −0.090(−0.384, 0.204) | −0.124(−0.340, 0.091) | |
| Women (n = 39) | reference | 0.271(−0.318, 0.861) | 0.069(−0.255, 0.393) | |
| FCP (ug/g) | Total (n = 97) | reference | −0.519(−1.288, 0.251) | −0.239(−0.751, 0.273) |
| Men (n = 58) | reference | −0.796(−1.735, 0.144) | −0.638(−1.331, 0.055) | |
| Women (n = 39) | reference | 0.408(−1.219, 2.034) | 0.526(−0.339, 1.390) | |
| WCRF/AICR Diet Score | ||||||
|---|---|---|---|---|---|---|
| Biomarkers | R1-Vegetables, Fruits, and Whole Grains Intake | R2-Limit Fast Foods | R3-Limit Red and Processed Meat | R4-Limit Sugary Drinks | R5-Limit Alcohol | |
| IL-6 (pg/mL) | Total (n = 97) | −0.204(−0.895, 0.486) | −0.188(−0.949, 0.574) | 0.318(−0.101, 0.738) | −0.007(−1.744, 1.731) | 0.437(−0.491, 1.364) |
| Men (n = 58) | −0.358(−1.165, 0.449) | −0.371(−1.235, 0.493) | 0.080(−0.344, 0.505) | −0.400(−2.254, 1.455) | 0.918(0.161, 1.675) | |
| Women (n = 39) | −0.883(−2.024, 0.258) | −0.239(−1.511, 1.032) | 0.427(−0.388, 1.242) | −0.816(−3.951, 2.319) | −7.095(−11.286, −2.903) | |
| IL-8 (pg/mL) | Total (n = 97) | 0.086(−1.101, 1.273) | −0.695(−1.995, 0.605) | −0.092(−0.822, 0.637) | 0.464(−2.515, 3.444) | 0.278(−1.311, 1.867) |
| Men (n = 58) | 0.125(−1.652, 1.902) | −0.588(−2.481, 1.306) | −0.433(−1.353, 0.487) | 0.603(−3.449, 4.655) | 0.659(−0.976, 2.294) | |
| Women (n = 39) | −0.283(−2.197, 1.630) | −0.977(−2.990, 1.035) | −0.092(−1.428, 1.245) | −1.059(−6.106, 3.988) | −7.965(−14.700, −1.230) | |
| IgA (g/L) | Total (n = 97) | −0.079(−0.354, 0.195) | −0.090(−0.392, 0.213) | −0.001(−0.170, 0.168) | 0.049(−0.641, 0.739) | 0.034(−0.338, 0.406) |
| Men (n = 58) | −0.128(−0.602, 0.347) | −0.191(−0.697, 0.315) | −0.013(−0.261, 0.236) | 0.356(−0.725, 1.437) | 0.078(−0.366, 0.523) | |
| Women (n = 39) | −0.081(−0.503, 0.341) | 0.045(−0.407, 0.497) | −0.017(−0.312, 0.278) | −0.314(−1.425, 0.797) | −0.019(−1.506, 1.467) | |
| IgG (g/L) | Total (n = 97) | −0.094(−0.224, 0.036) | −0.068(−0.212, 0.076) | 0.005(−0.076, 0.086) | −0.072(−0.402, 0.257) | 0.070(−0.106, 0.246) |
| Men (n = 58) | −0.251(−0.450, −0.052) | −0.226(−0.443, −0.008) | 0.017(−0.095, 0.128) | −0.111(−0.597, 0.374) | 0.078(−0.118, 0.274) | |
| Women (n = 39) | −0.067(−0.241, 0.107) | 0.061(−0.126, 0.247) | −0.003(−0.126, 0.120) | −0.135(−0.597, 0.328) | −0.047(−0.666, 0.572) | |
| IgM (g/L) | Total (n = 97) | −0.064(−0.428, 0.301) | 0.013(−0.389, 0.415) | −0.054(−0.278, 0.170) | 0.051(−0.865, 0.967) | −0.130(−0.619, 0.359) |
| Men (n = 58) | −0.177(−0.759, 0.404) | −0.063(−0.687, 0.562) | −0.075(−0.380, 0.229) | −0.409(−1.736, 0.917) | −0.275(−0.809, 0.259) | |
| Women (n = 39) | 0.080(−0.524, 0.684) | 0.025(−0.621, 0.672) | 0.059(−0.362, 0.480) | 0.801(−0.765, 2.367) | 0.754(−1.370, 2.879) | |
| FCP (ug/g) | Total (n = 97) | −0.752(−1.874, 0.369) | 0.140(−1.109, 1.390) | −0.237(−0.932, 0.458) | 0.851(−1.989, 3.691) | 0.083(−1.436, 1.602) |
| Men (n = 58) | −1.255(−3.148, 0.639) | −0.545(−2.606, 1.516) | −0.703(−1.690, 0.284) | 0.560(−3.846, 4.966) | −0.174(−1.948, 1.599) | |
| Women (n = 39) | −0.218(−1.880, 1.445) | 0.933(−0.808, 2.674) | 0.518(−0.625, 1.661) | 1.363(−3.002, 5.727) | 0.585(−5.264, 6.434) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, D.; Meng, S.; Li, J.; Zhao, J.; Wang, Y.; Du, M.; Wang, Y.; Lu, W.; Zhu, Y. Associations of Adherence to the 2018 World Cancer Research Fund and the American Institute for Cancer Research Dietary Recommendations with Gut Microbiota and Inflammation Levels. Nutrients 2023, 15, 3705. https://doi.org/10.3390/nu15173705
Wang D, Meng S, Li J, Zhao J, Wang Y, Du M, Wang Y, Lu W, Zhu Y. Associations of Adherence to the 2018 World Cancer Research Fund and the American Institute for Cancer Research Dietary Recommendations with Gut Microbiota and Inflammation Levels. Nutrients. 2023; 15(17):3705. https://doi.org/10.3390/nu15173705
Chicago/Turabian StyleWang, Dan, Sijia Meng, Jiqiu Li, Jing Zhao, Yu Wang, Meizhi Du, Yuan Wang, Wenli Lu, and Yun Zhu. 2023. "Associations of Adherence to the 2018 World Cancer Research Fund and the American Institute for Cancer Research Dietary Recommendations with Gut Microbiota and Inflammation Levels" Nutrients 15, no. 17: 3705. https://doi.org/10.3390/nu15173705
APA StyleWang, D., Meng, S., Li, J., Zhao, J., Wang, Y., Du, M., Wang, Y., Lu, W., & Zhu, Y. (2023). Associations of Adherence to the 2018 World Cancer Research Fund and the American Institute for Cancer Research Dietary Recommendations with Gut Microbiota and Inflammation Levels. Nutrients, 15(17), 3705. https://doi.org/10.3390/nu15173705





