MCT-Induced Ketosis and Fiber in Rheumatoid Arthritis (MIKARA)—Study Protocol and Primary Endpoint Results of the Double-Blind Randomized Controlled Intervention Study Indicating Effects on Disease Activity in RA Patients
Abstract
:1. Introduction
2. Materials and Methods
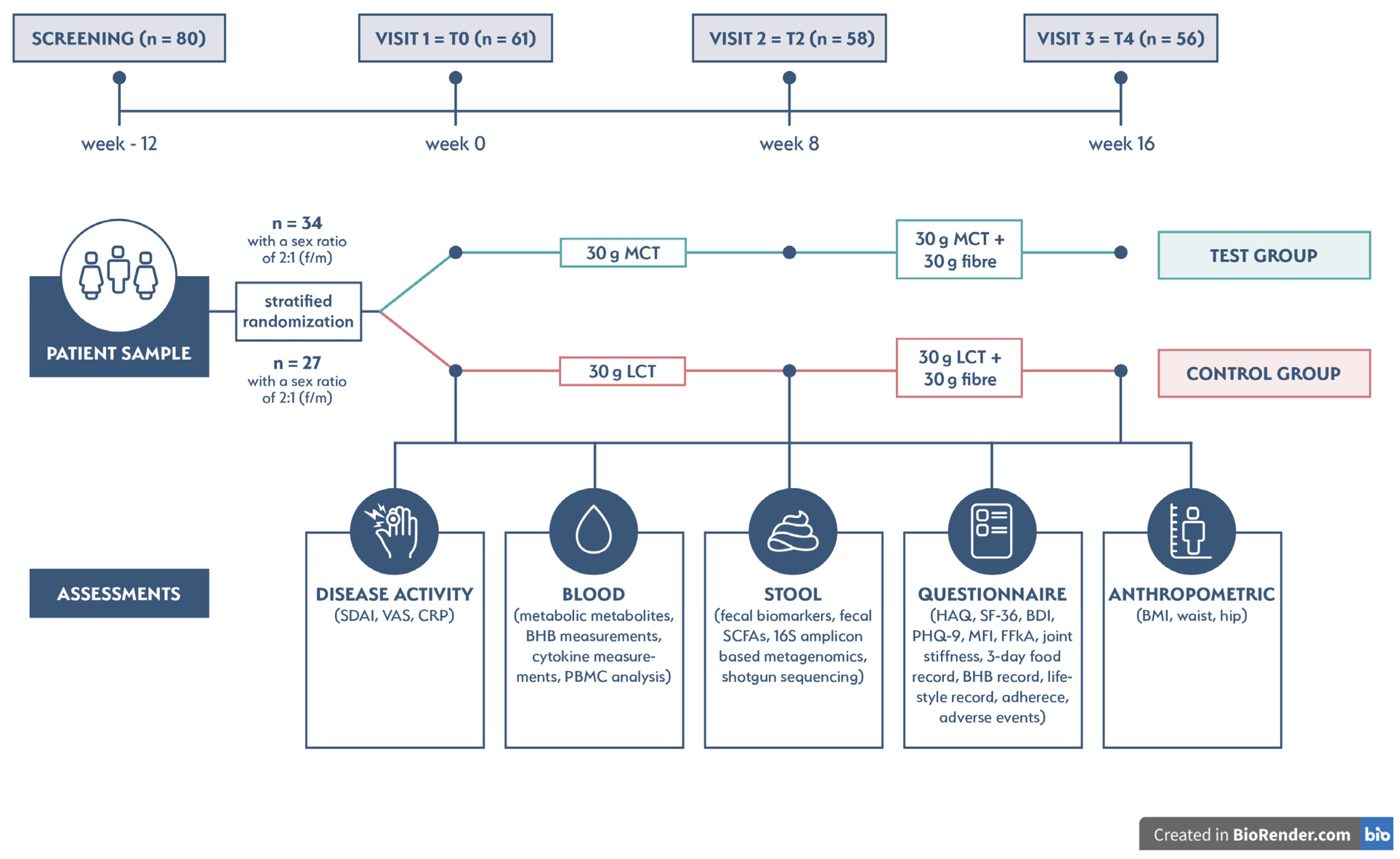
2.1. Clinical Trial Design and Eligibility Criteria
2.2. Randomization, Sample Size, and Intervention
2.3. Study Outcomes
2.4. Measurements
2.4.1. Disease Activity
2.4.2. BHB Levels
2.4.3. Adherence
2.5. Ethical Approval and Trial Registration
2.6. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Primary Outcome
3.3. Secondary Outcomes
3.3.1. SDAI
3.3.2. BHB, CRP, and VAS
3.3.3. Correlations between SDAI, BHB, CRP, and VAS
3.3.4. Linear Mixed Models
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Smolen, J.S.; Feist, E.; Fatenejad, S.; Grishin, S.A.; Korneva, E.V.; Nasonov, E.L.; Samsonov, M.Y.; Fleischmann, R.M. Olokizumab versus Placebo or Adalimumab in Rheumatoid Arthritis. N. Engl. J. Med. 2022, 387, 715–726. [Google Scholar] [CrossRef] [PubMed]
- Aletaha, D.; Smolen, J.S. Diagnosis and Management of Rheumatoid Arthritis: A Review. JAMA 2018, 320, 1360–1372. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Yu, J.; Jiao, W.; Chen, G.; Liu, L.; Zhang, M.; Wu, D. Scientific Knowledge of Rheumatoid Arthritis: A Bibliometric Analysis from 2011 to 2020. J. Pain Res. 2022, 15, 2761–2772. [Google Scholar] [CrossRef]
- Romão, V.C.; Fonseca, J.E. Etiology and Risk Factors for Rheumatoid Arthritis: A State-of-the-Art Review. Front. Med. 2021, 8, 689698. [Google Scholar] [CrossRef] [PubMed]
- Sparks, J.A. Rheumatoid Arthritis. Ann. Intern. Med. 2019, 170, Itc1–Itc16. [Google Scholar] [CrossRef]
- Smolen, J.S.; Landewé, R.B.M.; Bergstra, S.A.; Kerschbaumer, A.; Sepriano, A.; Aletaha, D.; Caporali, R.; Edwards, C.J.; Hyrich, K.L.; Pope, J.E.; et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2022 update. Ann. Rheum. Dis. 2023, 82, 3–18. [Google Scholar] [CrossRef]
- Gioia, C.; Lucchino, B.; Tarsitano, M.G.; Iannuccelli, C.; Di Franco, M. Dietary Habits and Nutrition in Rheumatoid Arthritis: Can Diet Influence Disease Development and Clinical Manifestations? Nutrients 2020, 12, 1456. [Google Scholar] [CrossRef]
- Guan, C.M.; Beg, S. Diet as a Risk Factor for Rheumatoid Arthritis. Cureus 2023, 15, e39273. [Google Scholar] [CrossRef] [PubMed]
- Papandreou, P.; Gioxari, A.; Daskalou, E.; Grammatikopoulou, M.G.; Skouroliakou, M.; Bogdanos, D.P. Mediterranean Diet and Physical Activity Nudges versus Usual Care in Women with Rheumatoid Arthritis: Results from the MADEIRA Randomized Controlled Trial. Nutrients 2023, 15, 676. [Google Scholar] [CrossRef]
- Walrabenstein, W.; Wagenaar, C.A.; van de Put, M.; van der Leeden, M.; Gerritsen, M.; Twisk, J.W.R.; van der Esch, M.; van Middendorp, H.; Weijs, P.J.M.; Roorda, L.D.; et al. A multidisciplinary lifestyle program for metabolic syndrome-associated osteoarthritis: The “Plants for Joints” randomized controlled trial. Osteoarthr. Cartil. 2023; online ahead of print. [Google Scholar] [CrossRef]
- Schönenberger, K.A.; Schüpfer, A.C.; Gloy, V.L.; Hasler, P.; Stanga, Z.; Kaegi-Braun, N.; Reber, E. Effect of Anti-Inflammatory Diets on Pain in Rheumatoid Arthritis: A Systematic Review and Meta-Analysis. Nutrients 2021, 13, 4221. [Google Scholar] [CrossRef]
- Ciaffi, J.; Mitselman, D.; Mancarella, L.; Brusi, V.; Lisi, L.; Ruscitti, P.; Cipriani, P.; Meliconi, R.; Giacomelli, R.; Borghi, C.; et al. The Effect of Ketogenic Diet on Inflammatory Arthritis and Cardiovascular Health in Rheumatic Conditions: A Mini Review. Front. Med. 2021, 8, 792846. [Google Scholar] [CrossRef]
- Youm, Y.H.; Nguyen, K.Y.; Grant, R.W.; Goldberg, E.L.; Bodogai, M.; Kim, D.; D’Agostino, D.; Planavsky, N.; Lupfer, C.; Kanneganti, T.D.; et al. The ketone metabolite β-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nat. Med. 2015, 21, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Mao, L.; Kitani, A.; Strober, W.; Fuss, I.J. The Role of NLRP3 and IL-1β in the Pathogenesis of Inflammatory Bowel Disease. Front. Immunol. 2018, 9, 2566. [Google Scholar] [CrossRef] [PubMed]
- Yurista, S.R.; Chong, C.R.; Badimon, J.J.; Kelly, D.P.; de Boer, R.A.; Westenbrink, B.D. Therapeutic Potential of Ketone Bodies for Patients with Cardiovascular Disease: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2021, 77, 1660–1669. [Google Scholar] [CrossRef] [PubMed]
- Operto, F.F.; Labate, A.; Aiello, S.; Perillo, C.; de Simone, V.; Rinaldi, R.; Coppola, G.; Pastorino, G.M.G. The Ketogenic Diet in Children with Epilepsy: A Focus on Parental Stress and Family Compliance. Nutrients 2023, 15, 1058. [Google Scholar] [CrossRef]
- Liao, T.H.; Hamosh, P.; Hamosh, M. Fat digestion by lingual lipase: Mechanism of lipolysis in the stomach and upper small intestine. Pediatr. Res. 1984, 18, 402–409. [Google Scholar] [CrossRef]
- Pereira, E.; Fernandes, J.M.; Gonçalves, R.; Pinheiro, A.C.; Salomé Duarte, M.; Madalena Alves, M.; Meirelles, A.J.A.; Maximo, G.J.; Vicente, A.A. Evaluating the in vitro digestion of lipids rich in medium-chain fatty acids (MCFAs) using dynamic and static protocols. Food Chem. 2023, 406, 135080. [Google Scholar] [CrossRef]
- Augustin, K.; Khabbush, A.; Williams, S.; Eaton, S.; Orford, M.; Cross, J.H.; Heales, S.J.R.; Walker, M.C.; Williams, R.S.B. Mechanisms of action for the medium-chain triglyceride ketogenic diet in neurological and metabolic disorders. Lancet Neurol. 2018, 17, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Marten, B.; Pfeuffer, M.; Schrezenmeir, J. Medium-chain triglycerides. Int. Dairy J. 2006, 16, 1374–1382. [Google Scholar] [CrossRef]
- Juby, A.G.; Cunnane, S.C.; Mager, D.R. Refueling the post COVID-19 brain: Potential role of ketogenic medium chain triglyceride supplementation: An hypothesis. Front. Nutr. 2023, 10, 1126534. [Google Scholar] [CrossRef]
- Machate, D.J.; Figueiredo, P.S.; Marcelino, G.; Guimarães, R.C.A.; Hiane, P.A.; Bogo, D.; Pinheiro, V.A.Z.; Oliveira, L.C.S.; Pott, A. Fatty Acid Diets: Regulation of Gut Microbiota Composition and Obesity and Its Related Metabolic Dysbiosis. Int. J. Mol. Sci. 2020, 21, 4093. [Google Scholar] [CrossRef] [PubMed]
- Rial, S.A.; Karelis, A.D.; Bergeron, K.F.; Mounier, C. Gut Microbiota and Metabolic Health: The Potential Beneficial Effects of a Medium Chain Triglyceride Diet in Obese Individuals. Nutrients 2016, 8, 281. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, R.K.; Sapra, L.; Mishra, P.K. Osteometabolism: Metabolic Alterations in Bone Pathologies. Cells 2022, 11, 3943. [Google Scholar] [CrossRef] [PubMed]
- Lucas, S.; Omata, Y.; Hofmann, J.; Böttcher, M.; Iljazovic, A.; Sarter, K.; Albrecht, O.; Schulz, O.; Krishnacoumar, B.; Krönke, G.; et al. Short-chain fatty acids regulate systemic bone mass and protect from pathological bone loss. Nat. Commun. 2018, 9, 55. [Google Scholar] [CrossRef]
- Wallimann, A.; Magrath, W.; Pugliese, B.; Stocker, N.; Westermann, P.; Heider, A.; Gehweiler, D.; Zeiter, S.; Claesson, M.J.; Richards, R.G.; et al. Butyrate Inhibits Osteoclast Activity In Vitro and Regulates Systemic Inflammation and Bone Healing in a Murine Osteotomy Model Compared to Antibiotic-Treated Mice. Mediat. Inflamm. 2021, 2021, 8817421. [Google Scholar] [CrossRef] [PubMed]
- Montalvany-Antonucci, C.C.; Duffles, L.F.; de Arruda, J.A.A.; Zicker, M.C.; de Oliveira, S.; Macari, S.; Garlet, G.P.; Madeira, M.F.M.; Fukada, S.Y.; Andrade, I., Jr.; et al. Short-chain fatty acids and FFAR2 as suppressors of bone resorption. Bone 2019, 125, 112–121. [Google Scholar] [CrossRef]
- Tajik, N.; Frech, M.; Schulz, O.; Schälter, F.; Lucas, S.; Azizov, V.; Dürholz, K.; Steffen, F.; Omata, Y.; Rings, A.; et al. Targeting zonulin and intestinal epithelial barrier function to prevent onset of arthritis. Nat. Commun. 2020, 11, 1995. [Google Scholar] [CrossRef]
- Friščić, J.; Dürholz, K.; Chen, X.; Engdahl, C.; Möller, L.; Schett, G.; Zaiss, M.M.; Hoffmann, M.H. Dietary Derived Propionate Regulates Pathogenic Fibroblast Function and Ameliorates Experimental Arthritis and Inflammatory Tissue Priming. Nutrients 2021, 13, 1643. [Google Scholar] [CrossRef]
- Zaiss, M.M.; Jones, R.M.; Schett, G.; Pacifici, R. The gut-bone axis: How bacterial metabolites bridge the distance. J. Clin. Investig. 2019, 129, 3018–3028. [Google Scholar] [CrossRef]
- Lin, T.Y.; Liu, H.W.; Hung, T.M. The Ketogenic Effect of Medium-Chain Triacylglycerides. Front. Nutr. 2021, 8, 747284. [Google Scholar] [CrossRef]
- Traul, K.A.; Driedger, A.; Ingle, D.L.; Nakhasi, D. Review of the toxicologic properties of medium-chain triglycerides. Food Chem. Toxicol. 2000, 38, 79–98. [Google Scholar] [CrossRef]
- Shcherbakova, K.; Schwarz, A.; Apryatin, S.; Karpenko, M.; Trofimov, A. Supplementation of Regular Diet with Medium-Chain Triglycerides for Procognitive Effects: A Narrative Review. Front. Nutr. 2022, 9, 934497. [Google Scholar] [CrossRef]
- Vandenberghe, C.; St-Pierre, V.; Fortier, M.; Castellano, C.A.; Cuenoud, B.; Cunnane, S.C. Medium Chain Triglycerides Modulate the Ketogenic Effect of a Metabolic Switch. Front. Nutr. 2020, 7, 3. [Google Scholar] [CrossRef] [PubMed]
- Cummings, J.H.; Macfarlane, G.T.; Englyst, H.N. Prebiotic digestion and fermentation. Am. J. Clin. Nutr. 2001, 73, 415s–420s. [Google Scholar] [CrossRef]
- Häger, J.; Bang, H.; Hagen, M.; Frech, M.; Träger, P.; Sokolova, M.V.; Steffen, U.; Tascilar, K.; Sarter, K.; Schett, G.; et al. The Role of Dietary Fiber in Rheumatoid Arthritis Patients: A Feasibility Study. Nutrients 2019, 11, 2392. [Google Scholar] [CrossRef] [PubMed]
- Aletaha, D.; Martinez-Avila, J.; Kvien, T.K.; Smolen, J.S. Definition of treatment response in rheumatoid arthritis based on the simplified and the clinical disease activity index. Ann. Rheum. Dis. 2012, 71, 1190–1196. [Google Scholar] [CrossRef]
- Heidt, C.; Fobker, M.; Newport, M.; Feldmann, R.; Fischer, T.; Marquardt, T. Beta-Hydroxybutyrate (BHB), Glucose, Insulin, Octanoate (C8), and Decanoate (C10) Responses to a Medium-Chain Triglyceride (MCT) Oil with and without Glucose: A Single-Center Study in Healthy Adults. Nutrients 2023, 15, 1148. [Google Scholar] [CrossRef] [PubMed]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting linear mixed-effects models using lme4. arXiv 2014, arXiv:1406.5823, preprint. [Google Scholar]
- Heidt, C.; Kämmerer, U.; Marquardt, T.; Reuss-Borst, M. Nutrition Patterns and Their Gender Differences among Rheumatoid Arthritis Patients: A Descriptive Study. Nutrients 2022, 15, 95. [Google Scholar] [CrossRef]
- Heidt, C.; Kämmerer, U.; Fobker, M.; Rüffer, A.; Marquardt, T.; Reuss-Borst, M. Assessment of Intestinal Permeability and Inflammation Bio-Markers in Patients with Rheumatoid Arthritis. Nutrients 2023, 15, 2386. [Google Scholar] [CrossRef]
- Kjeldsen-Kragh, J.; Haugen, M.; Borchgrevink, C.F.; Laerum, E.; Eek, M.; Mowinkel, P.; Hovi, K.; Førre, O. Controlled trial of fasting and one-year vegetarian diet in rheumatoid arthritis. Lancet 1991, 338, 899–902. [Google Scholar] [CrossRef]
- Müller, H.; de Toledo, F.W.; Resch, K.L. Fasting followed by vegetarian diet in patients with rheumatoid arthritis: A systematic review. Scand. J. Rheumatol. 2001, 30, 1–10. [Google Scholar] [CrossRef]
- Hartmann, A.M.; Dell’Oro, M.; Spoo, M.; Fischer, J.M.; Steckhan, N.; Jeitler, M.; Häupl, T.; Kandil, F.I.; Michalsen, A.; Koppold-Liebscher, D.A.; et al. To eat or not to eat-an exploratory randomized controlled trial on fasting and plant-based diet in rheumatoid arthritis (NutriFast-Study). Front. Nutr. 2022, 9, 1030380. [Google Scholar] [CrossRef]
- Kovács, Z.; Brunner, B.; Ari, C. Beneficial Effects of Exogenous Ketogenic Supplements on Aging Processes and Age-Related Neurodegenerative Diseases. Nutrients 2021, 13, 2197. [Google Scholar] [CrossRef]
- Newman, J.C.; Verdin, E. β-Hydroxybutyrate: A Signaling Metabolite. Annu. Rev. Nutr. 2017, 37, 51–76. [Google Scholar] [CrossRef]
- Zhu, H.; Bi, D.; Zhang, Y.; Kong, C.; Du, J.; Wu, X.; Wei, Q.; Qin, H. Ketogenic diet for human diseases: The underlying mechanisms and potential for clinical implementations. Signal Transduct. Target. Ther. 2022, 7, 11. [Google Scholar] [CrossRef]
- Wang, X.; Wu, X.; Liu, Q.; Kong, G.; Zhou, J.; Jiang, J.; Wu, X.; Huang, Z.; Su, W.; Zhu, Q. Ketogenic Metabolism Inhibits Histone Deacetylase (HDAC) and Reduces Oxidative Stress After Spinal Cord Injury in Rats. Neuroscience 2017, 366, 36–43. [Google Scholar] [CrossRef]
- Cavaleri, F.; Bashar, E. Potential Synergies of β-Hydroxybutyrate and Butyrate on the Modulation of Metabolism, Inflammation, Cognition, and General Health. J. Nutr. Metab. 2018, 2018, 7195760. [Google Scholar] [CrossRef]
- Rahman, M.; Muhammad, S.; Khan, M.A.; Chen, H.; Ridder, D.A.; Müller-Fielitz, H.; Pokorná, B.; Vollbrandt, T.; Stölting, I.; Nadrowitz, R.; et al. The β-hydroxybutyrate receptor HCA2 activates a neuroprotective subset of macrophages. Nat. Commun. 2014, 5, 3944. [Google Scholar] [CrossRef]
- Tański, W.; Świątoniowska-Lonc, N.; Tabin, M.; Jankowska-Polańska, B. The Relationship between Fatty Acids and the Development, Course and Treatment of Rheumatoid Arthritis. Nutrients 2022, 14, 1030. [Google Scholar] [CrossRef]
- Huang, L.; Gao, L.; Chen, C. Role of Medium-Chain Fatty Acids in Healthy Metabolism: A Clinical Perspective. Trends Endocrinol. Metab. 2021, 32, 351–366. [Google Scholar] [CrossRef]
- Zhao, J.; Hu, J.; Ma, X. Sodium caprylate improves intestinal mucosal barrier function and antioxidant capacity by altering gut microbial metabolism. Food Funct. 2021, 12, 9750–9762. [Google Scholar] [CrossRef]
- Luscombe, V.B.; Lucy, D.; Bataille, C.J.R.; Russell, A.J.; Greaves, D.R. 20 Years an Orphan: Is GPR84 a Plausible Medium-Chain Fatty Acid-Sensing Receptor? DNA Cell Biol. 2020, 39, 1926–1937. [Google Scholar] [CrossRef]
- Vandenberghe, C.; St-Pierre, V.; Pierotti, T.; Fortier, M.; Castellano, C.A.; Cunnane, S.C. Tricaprylin Alone Increases Plasma Ketone Response More Than Coconut Oil or Other Medium-Chain Triglycerides: An Acute Crossover Study in Healthy Adults. Curr. Dev. Nutr. 2017, 1, e000257. [Google Scholar] [CrossRef]
- Courchesne-Loyer, A.; Lowry, C.M.; St-Pierre, V.; Vandenberghe, C.; Fortier, M.; Castellano, C.A.; Wagner, J.R.; Cunnane, S.C. Emulsification Increases the Acute Ketogenic Effect and Bioavailability of Medium-Chain Triglycerides in Humans: Protein, Carbohydrate, and Fat Metabolism. Curr. Dev. Nutr. 2017, 1, e000851. [Google Scholar] [CrossRef]
- Harvey, C.; Schofield, G.M.; Williden, M. The use of nutritional supplements to induce ketosis and reduce symptoms associated with keto-induction: A narrative review. PeerJ 2018, 6, e4488. [Google Scholar] [CrossRef]
- Cunnane, S.C.; Trushina, E.; Morland, C.; Prigione, A.; Casadesus, G.; Andrews, Z.B.; Beal, M.F.; Bergersen, L.H.; Brinton, R.D.; de la Monte, S.; et al. Brain energy rescue: An emerging therapeutic concept for neurodegenerative disorders of ageing. Nat. Rev. Drug Discov. 2020, 19, 609–633. [Google Scholar] [CrossRef]
- Calatayud, M.; Van den Abbeele, P.; Ghyselinck, J.; Marzorati, M.; Rohs, E.; Birkett, A. Comparative Effect of 22 Dietary Sources of Fiber on Gut Microbiota of Healthy Humans in vitro. Front. Nutr. 2021, 8, 700571. [Google Scholar] [CrossRef]
- Ge, Q.; Li, H.Q.; Zheng, Z.Y.; Yang, K.; Li, P.; Xiao, Z.Q.; Xiao, G.M.; Mao, J.W. In vitro fecal fermentation characteristics of bamboo insoluble dietary fiber and its impacts on human gut microbiota. Food Res. Int. 2022, 156, 111173. [Google Scholar] [CrossRef]
- Juby, A.G.; Brocks, D.R.; Jay, D.A.; Davis, C.M.J.; Mager, D.R. Assessing the Impact of Factors that Influence the Ketogenic Response to Varying Doses of Medium Chain Triglyceride (MCT) Oil. J. Prev. Alzheimers Dis. 2021, 8, 19–28. [Google Scholar] [CrossRef]
- Latif, F.A.A.; Ghazali, W.S.W.; Mohamad, S.M.; Lee, L.K. High fiber multigrain supplementation improved disease activity score, circulating inflammatory and oxidative stress biomarkers in rheumatoid arthritis (RA) patients: A randomized human clinical trial. J. Funct. Foods 2023, 100, 105392. [Google Scholar]
- Smolen, J.S.; Breedveld, F.C.; Schiff, M.H.; Kalden, J.R.; Emery, P.; Eberl, G.; van Riel, P.L.; Tugwell, P. A simplified disease activity index for rheumatoid arthritis for use in clinical practice. Rheumatology 2003, 42, 244–257. [Google Scholar] [CrossRef]
- Janke, K.; Kiefer, C.; McGauran, N.; Richter, B.; Krause, D.; Wieseler, B. A systematic comparison of different composite measures (DAS 28, CDAI, SDAI, and Boolean approach) for determining treatment effects on low disease activity and remission in rheumatoid arthritis. BMC Rheumatol. 2022, 6, 82. [Google Scholar] [CrossRef]




| Variable | Control Group | Test Group |
|---|---|---|
| n | 27 | 34 |
| Female, n (%) | 18 (67%) | 23 (68%) |
| Age, years (median, IQR) | 63.5 (56.2–70.8) | 60.5 (56–70) |
| Disease duration, years (median, IQR) | 3.5 (1.5–17) | 1.3 (0.5–5.3) |
| IgM-RF positive, n (%) | 9 (33) | 12 (35) |
| ACPA positive, n (%) | 8 (30) | 9 (27) |
| SDAI, units (median, IQR) | 10.16 (6.89–13.60) | 13.72 (6.83–18.36) |
| CRP, mg/dL (median, IQR) | 0.2 (0.1–0.57) | 0.2 (0.07–0.44) |
| Pain (VAS), score (median, IQR) | 3 (2–4) | 3.5 (2–5) |
| Methotrexate, n (%) | 8 (30) | 11 (32) |
| Other (cs)-DMARDs, n (%) | 3 (11) | 4 (18) |
| (ts)-DMARDs, n (%) | 1 (4) | 2 (6) |
| Biologicals, n (%) | 8 (30) | 5 (15) |
| Glucocorticoids, n (%) | 7 (26) | 9 (27) |
| N | Control Group | Test Group | p-Value |
|---|---|---|---|
| 56 | 1.28 (−1.78–5.76) 1 | 6.51 (2.87–11.35) 1 | <0.05 |
| 1.75 ± 7.11 2 | 7.18 ± 6.66 2 |
| SDAI Units | Control Group | Test Group | p-Value |
|---|---|---|---|
| T0 (n = 61) | 10.16 (6.69–13.04) 1 | 13.72 (6.38–18.82) 1 | 0.24 |
| 11.36 ± 7.80 2 | 13.91 ± 8.91 2 | ||
| T2 (n = 58) | 7.04 (4.21–11.57) 1 | 6.76 (4.06–9.53) 1 | 0.57 |
| 9.48 ± 7.52 2 | 8.14 ± 6.03 2 | ||
| T4 (n = 56) | 7.27 (5.08–14.02) 1 | 5.12 (2.21–7.85) 1 | 0.03 |
| 9.84 ± 7.17 2 | 6.38 ± 5.94 2 |
| BHB (mmol/L) | Control Group | Test Group | p-Value |
|---|---|---|---|
| T0 (n = 61) | 0.15 (0.10–0.20) 1 | 0.10 (0.10–0.10) 1 | 0.04 |
| 0.15 ± 0.06 2 | 0.12 ± 0.05 2 | ||
| T2 (n = 58) | 0.19 (0.15–0.26) 1 | 0.50 (0.39–0.61) 1 | <0.001 |
| 0.20 ± 0.07 2 | 0.51 ± 0.15 2 | ||
| T4 (n = 56) | 0.23 (0.2–0.31) 1 | 0.49 (0.34–0.54) 1 | <0.001 |
| 0.26 ± 0.10 2 | 0.47 ± 0.14 2 | ||
| CRP (mg/dL) | |||
| T0 (n = 61) | 0.16 (0.11–0.57) 1 | 0.19 (0.06–0.40) 1 | 0.47 |
| 0.58 ± 1.0 2 | 0.38 ± 0.59 2 | ||
| T2 (n = 58) | 0.20 (0.11–0.64) 1 | 0.17 (0.09–0.41) 1 | 0.55 |
| 0.38 ± 0.36 2 | 0.58 ± 1.18 2 | ||
| T4 (n = 56) | 0.17 (0.09–0.52) 1 | 0.21 (0.07–0.36) 1 | 0.74 |
| 0.74 ± 1.94 2 | 0.49 ± 0.85 2 | ||
| VASpatient (cm) | |||
| T0 (n = 61) | 3.0 (2.0–4.0) 1 | 3.5 (2.0–5.0) 1 | 0.73 |
| 3.5 ± 2.2 2 | 3.6 ± 1.9 2 | ||
| T2 (n = 58) | 2.5 (1.6–4.9) 1 | 2.8 (1.9–3.3) 1 | 0.80 |
| 3.0 ± 2.2 2 | 2.8 ± 1.8 2 | ||
| T4 (n = 56) | 2.5 (2.0–4.0) 1 | 2.0 (1.0–3.0) 1 | 0.34 |
| 2.9 ± 2.1 2 | 2.4 ± 1.8 2 |
| Predictors | Model 1 | Model 2 | Model 3 | ||||
|---|---|---|---|---|---|---|---|
| Coefficient (β) | 95% CI | Coefficient (β) | 95% CI | Coefficient (β) | 95% CI | ||
| Fixed effect | Intercept | 10.97 *** | 8.02–13.93 | 12.68 *** | 8.52–16.84 | 12.85 *** | 9.63–16.08 |
| Time point (TP) | −0.41 | −1.08–0.26 | −1.50 ** | −2.50–−0.50 | −1.49 ** | −2.53–−0.44 | |
| formulation (test group) | 2.37 | −1.77–6.50 | 1.96 | −2.89–6.81 | 1.78 | −2.22–5.77 | |
| TP and formulation (test group) | −1.42 ** | −2.31–−0.52 | −1.72 ** | −2.86–0.58 | −1.78 ** | −2.81–0.74 | |
| BHB | −9.00 | −26.01–8.01 | −10.2 ** | −17.63–2.78 | |||
| BHB and TP | 5.11 * | −1.17–9.06 | 5.19 ** | −1.53–8.85 | |||
| BHB and formulation (test group) | −1.23 | −17.37–14.91 | |||||
| Random effect | σ2 | 11.38 | 9.92 | 9.86 | |||
| Marginal R2/conditional R2 | R2 | 0.084/0.814 | 0.107/0.820 | 0.107/0.820 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heidt, C.; Pons-Kühnemann, J.; Kämmerer, U.; Marquardt, T.; Reuss-Borst, M. MCT-Induced Ketosis and Fiber in Rheumatoid Arthritis (MIKARA)—Study Protocol and Primary Endpoint Results of the Double-Blind Randomized Controlled Intervention Study Indicating Effects on Disease Activity in RA Patients. Nutrients 2023, 15, 3719. https://doi.org/10.3390/nu15173719
Heidt C, Pons-Kühnemann J, Kämmerer U, Marquardt T, Reuss-Borst M. MCT-Induced Ketosis and Fiber in Rheumatoid Arthritis (MIKARA)—Study Protocol and Primary Endpoint Results of the Double-Blind Randomized Controlled Intervention Study Indicating Effects on Disease Activity in RA Patients. Nutrients. 2023; 15(17):3719. https://doi.org/10.3390/nu15173719
Chicago/Turabian StyleHeidt, Christina, Jörn Pons-Kühnemann, Ulrike Kämmerer, Thorsten Marquardt, and Monika Reuss-Borst. 2023. "MCT-Induced Ketosis and Fiber in Rheumatoid Arthritis (MIKARA)—Study Protocol and Primary Endpoint Results of the Double-Blind Randomized Controlled Intervention Study Indicating Effects on Disease Activity in RA Patients" Nutrients 15, no. 17: 3719. https://doi.org/10.3390/nu15173719
APA StyleHeidt, C., Pons-Kühnemann, J., Kämmerer, U., Marquardt, T., & Reuss-Borst, M. (2023). MCT-Induced Ketosis and Fiber in Rheumatoid Arthritis (MIKARA)—Study Protocol and Primary Endpoint Results of the Double-Blind Randomized Controlled Intervention Study Indicating Effects on Disease Activity in RA Patients. Nutrients, 15(17), 3719. https://doi.org/10.3390/nu15173719






